Associations between oral sex practices and frequent mouthwash use in men who have sex with men: implications for gonorrhoea prevention
Tiffany Phillips A C , Christopher K. Fairley A B , Sandra Walker A and Eric P. F. Chow
A C , Christopher K. Fairley A B , Sandra Walker A and Eric P. F. Chow  A B
A B
A Melbourne Sexual Health Centre, Alfred Health, 580 Swanston Street, Melbourne, Vic. 3053, Australia.
B Central Clinical School, Faculty of Medicine, Nursing and Health Sciences, Monash University, 99 Commercial Road, Melbourne, Vic. 3004, Australia.
C Corresponding author. Email: tphillips@mshc.org.au
Sexual Health 16(5) 473-478 https://doi.org/10.1071/SH18131
Submitted: 18 July 2018 Accepted: 30 August 2018 Published: 18 December 2018
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: Rates of gonorrhoea continue to rise among men who have sex with men (MSM) in Australia and worldwide. Recently, it has been proposed that oropharyngeal gonorrhoea may play a role in its onward transmission and that mouthwash use may be an effective intervention for gonorrhoea prevention and control. The objective of this study was to determine the association between specific oral sex practices and frequency of mouthwash use. Methods: A questionnaire-based study was conducted among MSM attending the Melbourne Sexual Health Centre in Australia from March to September 2015. Logistic regression was performed to examine the association between frequent mouthwash use (i.e. daily or weekly mouthwash use) and four oral sex practices (tongue kissing, receptive fellatio with or without ejaculation, and insertive rimming) among MSM. Results: Of the 918 MSM included in the final analysis, 490 men (53.4%) were frequent mouthwash users. Participants aged 24–34 years were 2.13-fold (95% CI 1.52–2.98) and those ≥35 years were 2.64-fold (95% CI 1.83–3.83) more likely to use mouthwash frequently than those aged ≤24 years. The most common oral sex practice was tongue kissing (n = 874; 95.2%), followed by receptive fellatio without ejaculation (n = 839; 91.4%), receptive fellatio with ejaculation (n = 610; 66.5%), then insertive rimming (n = 356; 38.8%). No significant association was found between frequent mouthwash use and tongue kissing, receptive fellatio with or without ejaculation, or insertive rimming with regular or casual male partners in the previous 3 months. Conclusions: Younger MSM are less likely to use mouthwash. There is no association between engaging in oral sex practices and frequent mouthwash use among MSM.
Additional keywords: homosexuality, Neisseria gonorrhoeae, sexual practices, sexually transmissible infection.
Introduction
Rates of gonorrhoea continue to rise among gay, bisexual and other men who have sex with men (MSM) in Australia1 and worldwide.2,3 Apart from the clear morbidity associated with high rates of gonorrhoea, the higher rates of infection increase the risk of resistant strains developing and are the reason that both the World Health Organization and Centers for Disease Control and Prevention have called for collaborative action to reduce the burden of the infection and the use of antibiotics.4
It has been proposed that the oropharynx may play a larger role in onward gonorrhoea transmission among MSM.5,6 Studies have shown that gonorrhoea can be cultured in human saliva,7–9 suggesting that saliva is infectious and gonorrhoea could be transmitted via saliva during sex. An Australian study has also demonstrated that use of saliva as a lubricant during anal sex is a risk factor for anorectal gonorrhoea among MSM.10 The risk of gonorrhoea transmission from saliva, coupled with the fact that oropharyngeal gonorrhoea is often asymptomatic, places oropharyngeal gonorrhoea as a potential driver of gonorrhoea prevalence in MSM.5
If oropharyngeal gonorrhoea does play a greater role in transmission than what was previously thought, an intervention aimed at the oropharynx may be beneficial. However, to date, there has been no intervention targeting the oropharynx for gonorrhoea prevention and control. A small Australian randomised controlled trial (RCT) and laboratory study have found that a single dose of antiseptic mouthwash inhibited the growth of Neisseria gonorrhoeae.11 This suggests mouthwash use could potentially be a preventive intervention for gonorrhoea control, and a larger RCT in Australia is underway to confirm this.12
Oral sex practice might be a confounding factor associated with mouthwash use due to personal oral hygiene.13 In order to understand whether frequent mouthwash use is associated with any particular oral sex practice, we undertook an analysis of an earlier study to determine the association between mouthwash use and specific oral sex practices (i.e. tongue kissing, insertive rimming and receptive fellatio).5
Methods
Study population and setting
A case-control study named the ‘GONorrhoea Eradication’ (GONE) study was conducted at the Melbourne Sexual Health Centre (MSHC), Victoria, Australia, between 30 March and 23 September 2015. MSHC is the largest public sexual health service in Victoria, which provided ~39 000 consultations in 2015, of which 38% of the consultations were for MSM.14 The details of the study design and main findings were published elsewhere.15 In brief, the GONE study was a 29-item multiple choice, paper-based questionnaire on mouthwash use and sexual practices with regular and casual partners in the previous 3 months among MSM. The primary aim of the GONE study was to determine the risk factors associated with oropharyngeal gonorrhoea.15 The secondary analysis in the present study examined the association between oral sexual practices and frequent mouthwash use. MSM aged ≥16 years attending the MSHC during the study period who reported having sex with another man in previous 12 months were invited by clinicians to complete the questionnaire. Consent was implied by returning a completed questionnaire. Ethical approval was obtained from the Alfred Hospital Ethics Committee, Melbourne, Victoria, Australia (number 544/14).
Study measures
Eligible men were given a questionnaire that asked about their frequency of mouthwash use, whether they had regular or casual male partners in the previous 3 months, how many regular and casual male partners they had in the previous 3 months, and the oral sex practices they engaged in with these partners. Four sex practices included in the questionnaire were tongue kissing, receptive fellatio (oral-penile sex, partner’s penis in participant’s mouth) with ejaculation, receptive fellatio without ejaculation, and insertive rimming (oral-anal sex, participant’s mouth in or around partner’s anus). For those participants indicating that they had a casual male partner/s in the previous 3 months, they were asked with how many casual partners they performed the previously mentioned sex practices. Demographic characteristics including age, country of birth and HIV status were collected using a computer-assisted self-interview as part of routine care at the MSHC.
Statistical analysis
Univariable logistic regression was performed to examine the association between frequent mouthwash use and the four oral sexual practices, as well as with demographic characteristics. Age was categorised into three groups (≤24, 25–34, and ≥35 years), as per previous studies,1,16 and a χ2 test for trend was performed to examine the association between the age groups and sexual practices. The total number of male partners (including regular and casual partners in the previous 3 months) was divided by the median; <5 or ≥5. Mouthwash use was categorised into ‘frequent’ and ‘infrequent’, where ‘frequent’ was defined as daily or weekly use and ‘infrequent’ as monthly, less than monthly, less than 6 monthly or never. All statistical analyses were performed using Stata (version 14.2, StataCorp, College Station, TX, USA).
Results
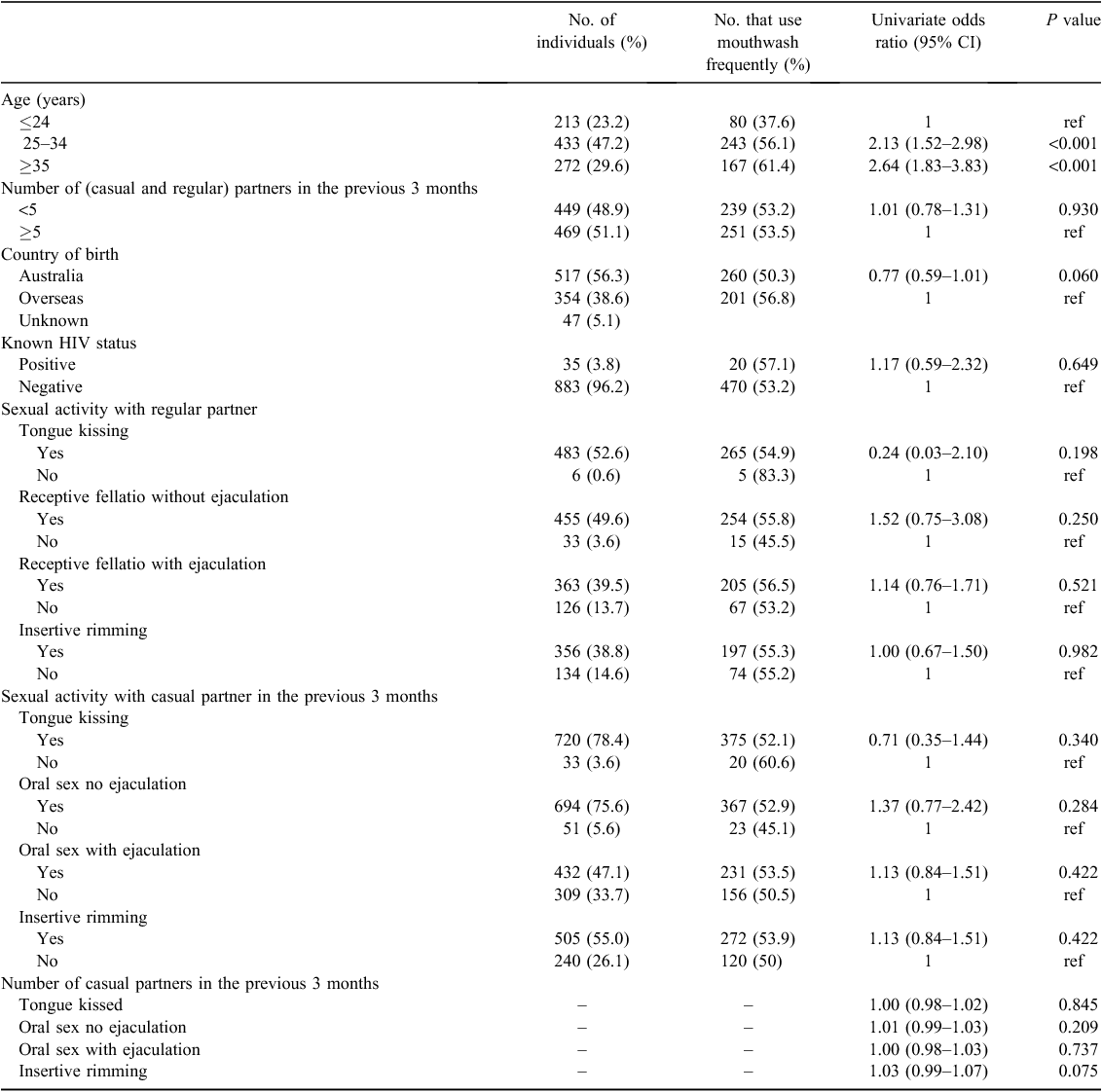
A total of 1150 MSM attending the MSHC were invited to participate in the GONE study, of which 949 returned a questionnaire (82.5%) and 918 (79.8%) were included in the final analysis after excluding those with incomplete questionnaires (Fig. 1). The age of participants ranged from 19 to 77 years, with a median age of 29 (interquartile range (IQR) 25–36) years. The median number of male partners in the previous 3 months was five (IQR 2–9). More than half (n = 517; 56.3%) of the participants were born in Australia, of which 260 men (50.3%) were frequent mouthwash users.

|
Of the 918 MSM, 874 (95.2%) reported tongue kissing with any sexual partner in the previous 3 months, 839 (91.4%) reported receptive fellatio without ejaculation, 610 (66.4%) reported receptive fellatio with ejaculation and 356 (38.8%) performed rimming. All four types of sexual practices did not differ by age group (Fig. 2).

|
There was a significant age pattern for frequent mouthwash use, from 37.6% among men aged ≤24 years to 56.1% among men aged 25–34 years and 61.4% among men aged ≥35 years (Ptrend = <0.001) reporting frequent mouthwash use. However, country of birth and HIV status were not associated with frequent mouthwash use (Table 1). Furthermore, the univariable analyses showed there was no significant association between frequent mouthwash use and tongue kissing, receptive fellatio with or without ejaculation or performing rimming with regular or casual partners.
|
Discussion
This is the first study examining the association between mouthwash use and oral sexual practices, and we found that frequent mouthwash use is not associated with engaging in oral sexual practices (i.e. tongue kissing, receptive fellatio (with or without ejaculation) or performing rimming) with regular or casual sex partners among MSM. Previous studies have shown that younger MSM are at a higher risk of acquiring oropharyngeal gonorrhoea compared with older MSM;1,16 however, the absence of an association between age and oral sexual practices could not explain this association.
This study had several limitations. First, this study was conducted at one sexual health clinic and may not be generalisable to the entire MSM community. Second, this study asked only about frequency of mouthwash use as part of routine dental care, and did not ask if mouthwash was used before or after specific sex practices. Third, kissing without sex is relative common in MSM, particularly in younger MSM;5 however, we did not collect data on the number of partners that men tongue kissed but did not have sex with. Finally, this study did not ask about condom use during oral sex or the use of dental dams for insertive rimming. It is possible that MSM who use condoms and dental dams are more concerned about personal hygiene and therefore may be more frequent mouthwash users. However, previous studies have shown that using condoms for oral sex13 and dental dams for insertive rimming is rare in the MSM population.17
There is still substantial uncertainty and debate about whether sexual practices involving the mouth, other than penile-oral sex, transmit gonorrhoea, and more research will be required to answer this question.18 Recent studies of MSM couples have shown that of men who were diagnosed with oropharyngeal gonorrhoea, 23–28% of their male regular partners also had oropharyngeal gonorrhoea.19 Furthermore, tongue kissing without sex has been identified as a risk factor for oropharyngeal gonorrhoea in MSM.20
Oropharyngeal gonorrhoea is often asymptomatic21 and has a relatively short duration of infection of ~3–4 months;22 and thus annual screening of oropharyngeal gonorrhoea would not be effective in reducing asymptomatic gonorrhoea cases.23 A novel intervention, such as the use of antiseptic mouthwash, targeting the oropharynx would be warranted for gonorrhoea prevention and control. A previous in vitro study has shown a reduction in the amount of N. gonorrhoeae on the oropharynx after 1 min of exposure to two products of Listerine (Johnson & Johnson, New Brunswick, NJ, USA) mouthwashes,11 and a mathematical model has estimated that mouthwash could potentially reduce oropharyngeal gonorrhoea by 82%, with 50% of mouthwash coverage and an additional 1.5% clearance of infection per use.24 Although the present study estimated half (53.4%) of sexually active MSM use mouthwash daily, further studies are required to identify which brand of mouthwash they use and how they use it (i.e. rinse vs gargle).25 If mouthwash is proven to be effective for gonorrhoea prevention, a public health campaign should be launched to encourage its use. As this study indicates there is no association between performing any specific oral sex practices and mouthwash use, such a campaign should target all MSM regardless of which sex practices they engage in. In addition, it is important to target young MSM specifically because this group has the highest prevalence of oropharyngeal gonorrhoea but with the lowest coverage of frequent mouthwash use.16 However, further studies are required before the implementation of this campaign.26
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors acknowledge Afrizal Afrizal for his assistance with data extraction and Clare Bellhouse for assistance with data collection and entry. This work was supported by the National Health and Medical Research Council (NHMRC) program grant (number 568971). E.P.F. Chow is supported by the NHMRC Early Career Fellowship (number 1091226).
References
[1] Chow EPF, Tomnay J, Fehler G, Whiley D, Read TRH, Denham I, Bradshaw CS, Chen MY, Fairley CK. Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013. Sex Transm Dis 2015; 42 81–7.| Substantial increases in chlamydia and gonorrhea positivity unexplained by changes in individual-level sexual behaviors among men who have sex with men in an Australian sexual health service from 2007 to 2013.Crossref | GoogleScholarGoogle Scholar |
[2] Weston EJ, Kirkcaldy RD, Stenger M, Llata E, Hoots B, Torrone EA. Narrative review: assessment of Neisseria gonorrhoeae infections among men who have sex with men in national and sentinel surveillance systems in the United States. Sex Transm Dis 2018; 45 243–9.
[3] Public Health England. Sexually transmitted infections and screening for chlamydia in England, 2017 – Health Protection Report, Volume 12 Number 20, 8 June 2018. London, UK: Public Health England; 2018. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/713962/hpr2018_AA-STIs_v5.pdf [verified 13 December 2018].
[4] Wi T, Lahra MM, Ndowa F, Bala M, Dillon J-AE, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017; 14 e1002344
| Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action.Crossref | GoogleScholarGoogle Scholar |
[5] Fairley CK, Hocking JS, Zhang L, Chow EPF. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23 102–4.
| Frequent transmission of gonorrhea in men who have sex with men.Crossref | GoogleScholarGoogle Scholar |
[6] Fairley CK, Zhang L, Chow EPF. New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer? Curr Opin Infect Dis 2018; 31 45–9.
| New thinking on gonorrhoea control in MSM: are antiseptic mouthwashes the answer?Crossref | GoogleScholarGoogle Scholar |
[7] Hutt DM, Judson FN. Epidemiology and treatment of oropharyngeal gonorrhea. Ann Intern Med 1986; 104 655–8.
| Epidemiology and treatment of oropharyngeal gonorrhea.Crossref | GoogleScholarGoogle Scholar |
[8] Chow EPF, Lee D, Tabrizi SN, Phillips S, Snow A, Cook S, Howden BP, Petalotis I, Bradshaw CS, Chen MY, Fairley CK. Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission. Sex Transm Infect 2016; 92 347–9.
| Detection of Neisseria gonorrhoeae in the pharynx and saliva: implications for gonorrhoea transmission.Crossref | GoogleScholarGoogle Scholar |
[9] Hallqvist L, Lindgren S. Gonorrhoea of the throat at a venereological clinic. Incidence and results of treatment. Br J Vener Dis 1975; 51 395–7.
[10] Chow EPF, Cornelisse VJ, Read TRH, Lee D, Walker S, Hocking JS, Chen MY, Bradshaw CS, Fairley CK. Saliva use as a lubricant for anal sex is a risk factor for rectal gonorrhoea among men who have sex with men, a new public health message: a cross-sectional survey. Sex Transm Infect 2016; 92 532–6.
| Saliva use as a lubricant for anal sex is a risk factor for rectal gonorrhoea among men who have sex with men, a new public health message: a cross-sectional survey.Crossref | GoogleScholarGoogle Scholar |
[11] Chow EPF, Howden BP, Walker S, Lee D, Bradshaw CS, Chen MY, Snow A, Cook S, Fehler G, Fairley CK. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study. Sex Transm Infect 2017; 93 88–93.
| Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: a randomised controlled trial and an in vitro study.Crossref | GoogleScholarGoogle Scholar |
[12] Chow EPF, Walker S, Hocking JS, Bradshaw CS, Chen MY, Tabrizi SN, Howden BP, Law MG, Maddaford K, Read TRH, Lewis DA, Whiley DM, Zhang L, Grulich AE, Kaldor JM, Cornelisse VJ, Phillips S, Donovan B, McNulty AM, Templeton DJ, et al A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol. BMC Infect Dis 2017; 17 456
| A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: the OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol.Crossref | GoogleScholarGoogle Scholar |
[13] Walker S, Bellhouse C, Fairley CK, Bilardi JE, Chow EPF. Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission - a qualitative study. PLoS One 2016; 11 e0164033
| Pharyngeal gonorrhoea: the willingness of Australian men who have sex with men to change current sexual practices to reduce their risk of transmission - a qualitative study.Crossref | GoogleScholarGoogle Scholar |
[14] Needleman R, Chow EP, Towns JM, Cornelisse VJ, Yang TZT, Chen MY, Bradshaw CS, Fortune R, Fairley CK. Access to sexual health services after the rapid roll out of the launch of pre-exposure prophylaxis for HIV in Melbourne, Australia: a retrospective cross-sectional analysis. Sex Health 2018; 15 528–32.
| Access to sexual health services after the rapid roll out of the launch of pre-exposure prophylaxis for HIV in Melbourne, Australia: a retrospective cross-sectional analysis.Crossref | GoogleScholarGoogle Scholar |
[15] Cornelisse VJ, Walker S, Phillips T, Hocking JS, Bradshaw CS, Lewis DA, Prestage GP, Grulich AE, Fairley CK, Chow EPF. Risk factors for oropharyngeal gonorrhoea in men who have sex with men: an age-matched case-control study. Sex Transm Infect 2018; 94 359–64.
| Risk factors for oropharyngeal gonorrhoea in men who have sex with men: an age-matched case-control study.Crossref | GoogleScholarGoogle Scholar |
[16] Chow EPF, Walker S, Read TRH, Chen MY, Bradshaw CS, Fairley CK. Self-reported use of mouthwash and pharyngeal gonorrhoea detection by nucleic acid amplification test. Sex Transm Dis 2017; 44 593–5.
| Self-reported use of mouthwash and pharyngeal gonorrhoea detection by nucleic acid amplification test.Crossref | GoogleScholarGoogle Scholar |
[17] Hunt AJ, Weatherburn P, Hickson FCI, Davies PM, McManus TJ, Coxon APM. Changes in condom use by gay men. AIDS Care 1993; 5 439–48.
| Changes in condom use by gay men.Crossref | GoogleScholarGoogle Scholar |
[18] Bernstein KT, Chesson H, Kirkcaldy RD, Marcus JL, Gift TL, Aral SO. Kiss and tell: limited empirical data on oropharyngeal Neisseria gonorrhoeae among men who have sex with men and implications for modeling. Sex Transm Dis 2017; 44 596–8.
| Kiss and tell: limited empirical data on oropharyngeal Neisseria gonorrhoeae among men who have sex with men and implications for modeling.Crossref | GoogleScholarGoogle Scholar |
[19] Cornelisse VJ, Zhang L, Law M, Chen MY, Bradshaw CS, Bellhouse C, Fairley CK, Chow EPF. Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia. BMC Infect Dis 2018; 18 95
| Concordance of gonorrhoea of the rectum, pharynx and urethra in same-sex male partnerships attending a sexual health service in Melbourne, Australia.Crossref | GoogleScholarGoogle Scholar |
[20] Chow EPF, Priest D, Cornelisse V, Read TRH, Bradshaw CS, Chen MY, Fairley CK. Kissing but not sex is the strongest risk factor for oropharyngeal gonorrhoea in men who have sex with men: a cross-sectional survey. [Poster abstract P103] In: Gazzard B, Lundgren J, editors. HIV Medicine, 2018, 19(Suppl 2). Abstracts of the 4th Joint Conference of the British HIV Association (BHIVA) with the British Association for Sexual Health and HIV (BASHH); 17–20 April 2018; Edinburgh, UK. Oxford: John Wiley & Sons; 2018.
[21] Kinghorn G. Pharyngeal gonorrhoea: a silent cause for concern. Sex Transm Infect 2010; 86 413–4.
| Pharyngeal gonorrhoea: a silent cause for concern.Crossref | GoogleScholarGoogle Scholar |
[22] Chow EPF, Camilleri S, Ward C, Huffam S, Chen MY, Bradshaw CS, Fairley CK. Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review. Sex Health 2016; 13 199–204.
[23] Jenness SM, Weiss KM, Goodreau SM, Gift T, Chesson H, Hoover KW, Smith DK, Liu AY, Sullivan PS, Rosenberg ES. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis 2017; 65 712–8.
| Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study.Crossref | GoogleScholarGoogle Scholar |
[24] Zhang L, Regan DG, Chow EPF, Gambhir M, Cornelisse V, Grulich A, Ong J, Lewis DA, Hocking J, Fairley CK. Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash. Sex Transm Dis 2017; 44 586–92.
| Neisseria gonorrhoeae transmission among men who have sex with men: an anatomical site-specific mathematical model evaluating the potential preventive impact of mouthwash.Crossref | GoogleScholarGoogle Scholar |
[25] Lin CTS, Raman R. Comparison of the efficacy between oral rinse, oral gargle, and oral spray. J Prim Care Community Health 2012; 3 80–2.
| Comparison of the efficacy between oral rinse, oral gargle, and oral spray.Crossref | GoogleScholarGoogle Scholar |
[26] Chow EPF, Fairley CK. Could antiseptic mouthwash inhibit pharyngeal Neisseria gonorrhoeae? Further research is required. Sex Transm Infect 2017; 93 403
| Could antiseptic mouthwash inhibit pharyngeal Neisseria gonorrhoeae? Further research is required.Crossref | GoogleScholarGoogle Scholar |


