Meta-analysis of the Cepheid Xpert® CT/NG assay for extragenital detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections
Claire C. Bristow A D , Sheldon R. Morris A , Susan J. Little A , Sanjay R. Mehta A and Jeffrey D. Klausner B CA Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, La Jolla, CA 92093, USA.
B Division of Infectious Diseases, Department of Medicine, University of California Los Angeles, Los Angeles, CA 90095, USA.
C Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, Los Angeles, CA 90095, USA.
D Corresponding author. Email: cbristow@ucsd.edu
Sexual Health 16(4) 314-319 https://doi.org/10.1071/SH18079
Submitted: 12 April 2018 Accepted: 7 March 2019 Published: 12 July 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Background: Most studies evaluating extragenital testing performance for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) detection by the Xpert® CT/NG show high per cent agreement with comparison assays; however, the precision around positive per cent agreement is low and thus the values that have been reported are not highly informative. Therefore, a systematic review was conducted and data from five studies were combined to better assess positive per cent agreement. Methods: The literature indexed on PubMed.gov was searched. Included studies were those that were an evaluation of the Xpert CT/NG assay with rectal and/or pharyngeal specimen types compared with another nucleic acid amplification test (NAAT), the Aptima transcription mediated amplification assay. A full Bayesian method was used for bivariate fixed-effect meta-analysis of positive and negative per cent agreement and pooled estimates (and 95% confidence intervals (CI)) were presented for each. Results: The pooled positive and negative per cent agreement for detection of CT in rectal specimens was 89.72% (95% CI: 84.97%, 93.64%) and 99.23% (95% CI: 98.74%, 99.60%), and in pharyngeal specimens, they were 89.96% (95% CI: 66.38%, 99.72%) and 99.62% (95% CI: 98.95%, 99.95%) respectively. For NG detection in rectal specimens, the pooled positive and negative per cent agreement was 92.75% (95% CI: 87.91%, 96.46%) and 99.75% (95% CI: 99.46%, 99.93%), and in pharyngeal specimens, they were 92.51% (95% CI: 85.84%, 97.18%) and 98.56% (95% CI: 97.69%, 99.23%) respectively. Conclusions: It was found that the Xpert CT/NG assay performed similarly to the Aptima transcription mediated amplification assay for the detection of CT and NG in extragenital specimens. The Xpert assay has the benefit of providing faster results at the point-of-care, thus reducing the turnaround time for results, potentially enabling same-day treatment.
Additional keywords: diagnosis, nucleic acid amplification test, pharyngeal, rectal.
Introduction
Sexually transmissible infections (STIs) of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) continue to place an immense health burden on men and women worldwide. Both CT and NG infections are common STIs accounting for 209 million cases globally each year.1 In the USA alone, over 2 million chlamydial and gonoccocal infections were reported to the USA Centers for Disease Control and Prevention (CDC) in 2016, making those the most common notifiable diseases in the USA.2
Routine screening, timely treatment and partner treatment are mainstays of STI control programs. However, both urogenital and extragenital CT and NG infections are frequently asymptomatic3 and therefore go undetected and untreated if screening tests are not performed. In the absence of appropriate screening, extragenital sites may be important reservoirs for CT and NG in a population, and can serve to perpetuate the spread of these infections. Among men who have sex with men, 65–77% of extragenital NG infections and 75–85% of extragenital CT infections are detected in the absence of urethral infection, warranting routine screening at extragenital sites in addition to urethral screening.4–6 Among women, 14–44% of CT and NG infections may be missed without extragenital screening.7–10
Studies have shown that nucleic acid amplification tests (NAATs) perform better than other tests available (e.g. culture) for CT and NG detection.11–14 The CDC currently recommends NAATs for the detection of CT and NG from all anatomic sites.15 The Xpert® CT/NG assay (Cepheid, Sunnyvale, CA, USA) is a NAAT with sample-to-result instrumentation (on the GeneXpert® system) that can be used in laboratories or at the point-of-care. Similar to other STI NAAT assays, the Xpert CT/NG is Food and Drug Administration (FDA) cleared for use with urogenital specimens,16–18 and has recently been approved for use with extragenital specimens.19
The Xpert CT/NG assay results are obtained in less than 90 min and are displayed on a computer system connected to the test instrument via a data cable. The assay contains internal quality control mechanisms including: a sample processing control; a sample adequacy control; and a probe check control, all of which are included in the Xpert CT/NG assay cartridge. The sample processing control spikes DNA from the non-pathogen, Bacillus globigii, which is then co-extracted and co-amplified with the sample nucleic acid. Detection of this control DNA verifies that binding and elution of target DNA have occurred. The sample adequacy control reagents detect the presence of the single-copy human gene encoding hydroxymethylbilane synthase to monitor whether the sample contains human DNA. Thus, a negative sample adequacy control indicates that inadequate numbers of human cells were present in the sample due to an inadequately collected specimen, sample degradation or insufficient mixing. The probe check control verifies reagent rehydration, polymerase chain reaction tube filling in the cartridge, probe integrity and dye stability.
Most studies evaluating extragenital testing performance for CT and NG detection by the Xpert CT/NG show high per cent agreement with comparison assays; however, because of the limited sample sizes of CT-and NG-positive cases, the precision around the positive per cent agreement is low.20–24 Therefore, we aimed to combine data from all published studies of the performance of extragenital testing with Xpert CT/NG to better assess positive per cent agreement.
Methods
We searched the literature indexed on PubMed.gov (https://www.ncbi.nlm.nih.gov/pubmed/) related to Xpert CT/NG extragenital testing evaluations. We used the following search terms: (Xpert OR GeneXpert OR Cepheid) AND (extragenital OR rectal OR pharyngeal OR throat) AND (chlamydia OR gonorrh*) (last search: 1 January 2018). There were no language restrictions; however, only English articles returned in our search. References within articles were reviewed to identify additional relevant studies. Titles, abstracts and full texts were reviewed for all articles, and a study was included if it met the inclusion criteria of being an evaluation of the Xpert CT/NG assay with rectal and/or pharyngeal specimen types compared with another NAAT platform. C. C. Bristow identified and selected articles. A review protocol was not written.
Data were extracted from all included studies, including author, publication year, sample size, sex of participants, specimen types, reference tests and numbers of positive and negative results on each assay into a data extraction spreadsheet. We used SAS v9.4 (Cary, NC, USA) to perform all analyses. We calculated per cent agreement, positive per cent agreement and negative per cent agreement for each study and for combined results. We calculated 95% confidence intervals using the exact binomial method for each study individually. We used a full Bayesian method for bivariate fixed-effect and random-effects meta-analysis of positive per cent agreement and negative per cent agreement with a SAS software procedure (PROC MCMC)25 and present pooled estimates of each. We considered a test of the deviance information criterion (DIC) to assess the best model fit of the fixed-effect and random-effects model. The fixed-effect model was considered to be significantly better (substantial), if its DIC was less than 10 greater than the DIC of the random-effects model; that is, if (DICFIXED − DICRANDOM) <10.26 In addition, we report the between-study heterogeneity of positive per cent agreement and negative per cent agreement by univariate Cochran Q-tests, using the free meta-analytic program, Meta-DiSc, version 1.4.27 We also used the Meta-DiSc program to calculate univariate I2-values for quantifying the between-study heterogeneity. However, the Cochran Q-test and I2-values were not used to determine best model fit.
Results
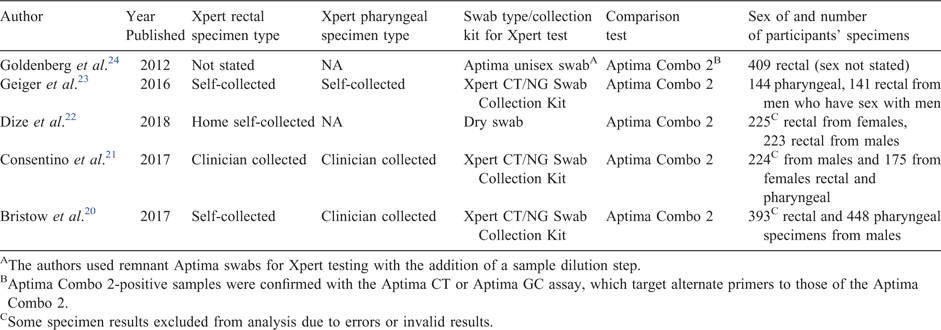
Our search yielded a total of eight publications,4,20–24,28,29 five20–24 of which met our inclusion criteria. We excluded three studies because they were not evaluation studies. Table 1 shows the included studies published between 2012 and 2017, with data from the USA and the UK. In total, the studies included results from 1743 rectal specimens and 986 pharyngeal specimens. All studies used the Aptima transcription mediated amplification assay (Combo 2, Hologic, San Diego, CA, USA) as the comparison test for the Xpert CT/NG.
|
Figure 1a–d shows the calculated positive per cent agreement and negative per cent agreement for each study along with the pooled positive per cent agreement and the pooled negative per cent agreement. The positive per cent agreement in the studies ranged from 85.7% to 95.5% for the detection of CT in rectal specimens and 88.4% to 100% for the detection of NG in rectal specimens. The negative per cent agreement ranged from 98.3% to 100% for CT in rectal specimens and 99.4% to 100% for NG in rectal specimens.
For the detection of CT in pharyngeal specimens, the positive per cent agreement ranged from 50% to 100% and the negative per cent agreement ranged from 99.5% to 100%. For the detection of NG in pharyngeal specimens, the positive per cent agreement ranged from 77.8% to 97.3% and the negative per cent agreement ranged from 97.5% to 100%.
We found that there was little heterogeneity (determined using DIC of each random-effects and fixed-effect model) between studies and therefore we report pooled estimates of positive and negative per cent agreement from a fixed-effect model. DIC values, Q-test results and I2 values are in the footnotes of Figure 1a–d. The pooled positive per cent agreement and pooled negative per cent agreement for detection of CT in rectal specimens was 89.72% (95% CI: 84.97%, 93.64%) and 99.23% (95% CI: 98.74%, 99.60%) respectively. For NG detection in rectal specimens, the pooled positive per cent agreement and pooled negative per cent agreement was 92.75% (95% CI: 87.91%, 96.46%) and 99.75% (95% CI: 99.46%, 99.93%) respectively.
The pooled positive per cent agreement and the pooled negative per cent agreement for detection of CT in pharyngeal specimens was 89.96% (95% CI: 66.38%, 99.72%) and 99.62% (95% CI: 98.95%, 99.95%) respectively. For the detection of NG in pharyngeal specimens, the pooled positive per cent agreement and pooled negative per cent agreement was 92.51% (95% CI: 85.84%, 97.18%) and 98.56% (95% CI: 97.69%, 99.23%) respectively.
Discussion
CT and NG screening must be conducted using specimens from the anatomic site of exposure if clinicians want to identify those sites as infected. In addition, given most extragenital infections in men and some in women occur in the absence of a urogenital CT or NG infection, there is a need for reliable and accurate screening tests for extragenital specimens. Currently, clinical trials funded by the NIH (clinicaltrials.gov; NCT02870101) have recently been completed to provide data and analyses to support FDA applications for multiple NAAT diagnostic platforms for the detection of pharyngeal and rectal CT and NG.19
In this meta-analysis, we combined evidence from five studies evaluating the Xpert CT/NG with extragenital specimen types using Aptima transcription mediated amplification assay, Combo 2, as the comparator. One study used residual Aptima specimens and required a dilution of the samples to overcome the incompatibility of the Aptima buffer with the Xpert system; however, this study found a good correlation between the two test systems.24 That reference NAAT assay, the Aptima Combo 2, has demonstrated very good sensitivity and specificity ranges for detection of NG and CT in extragenital specimens across several studies of 71–100% and 87.9–100% respectively.30 In all studies we included, there was a lack of precision around estimates of positive per cent agreement because of the small or moderate sample size of positive specimens. Therefore, by combining evidence from all five studies, we were able to achieve narrower confidence intervals around positive per cent agreement. We chose to use a fixed-effect model to generate the pooled estimates of positive per cent agreement after identifying very little heterogeneity between studies. Our findings suggest that the Xpert CT/NG test was similar to another laboratory-based NAAT for the detection of CT and NG in extragenital specimens. We calculated per cent agreement for positive and negative results separately and found that negative per cent agreement was nearly 100% for both organisms at both extragenital sites included in the study. We found that positive per cent agreement was over 89% for both organisms at both extragenital sites. Further studies may be needed to assess what sensitivity value is acceptable in clinical practice in various settings. Under ideal conditions, patient infection status would be determined using an algorithm that includes multiple test assays to ensure that the reference for a test evaluation was as close to the true infection status of each participant as possible. That would control for potential disease status misclassification by the reference test(s). As a limitation, the positive per cent agreement values in this study should be interpreted with caution, as most studies did not include a tiebreaker or confirmatory testing, therefore, the determination of infection status of the anatomic site of each participant does not use an optimal anatomic site infection status determination. However, the clinical trial mentioned above uses multiple assays to determine anatomic site infection status.
Our findings demonstrate that Xpert CT/NG assay results were similar to a laboratory-based NAAT assay, Aptima transcription mediated amplification assay, Combo 2, for the detection of CT and NG in extragenital specimens. That Combo 2 NAAT has been laboratory verified for use with extragenital specimens at multiple reference laboratories and thus was used as the reference test in the studies we identified. The Xpert CT/NG assay has the benefit of providing faster results and can be done at the point-of-care,17 thus reducing the turnaround time for results,31–33 potentially enabling same-day treatment.
Conflicts of interest
All authors have received donated Cepheid test kits for research studies in the past 12 months. J. D. Klausner has received donated test kits and grant support for research from Hologic, research grant support and a $2500 speaker’s fee from Cepheid in the past 12 months. S. J. Little has received grants paid to her institution from Gilead Sciences.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers: T32AI007384 (to C. C. Bristow), K01AI136725 (to C. C. Bristow), AI106039 (to S. J. Little), the UCLA Center for AIDS Research (P30AI028697 to J. D. Klausner) and National Institutes of Health UCLA Center for HIV Identification, Prevention and Treatment Services (P30MH058107 to J. D. Klausner). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
[1] World Health Organization. Sexually transmitted infections: fact sheet. Geneva: WHO; 2016. Available online at: http://www.who.int/mediacentre/factsheets/fs110/en/ [verified 18 April 2019].[2] Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2016. Atlanta: Health and Human Services; 2017. Available online at: https://www.cdc.gov/std/stats16/toc.htm [verified 18 April 2019].
[3] Workowski KA, Bolan GA, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64 55–69.
[4] Danby CS, Cosentino LA, Rabe LK, Priest CL, Damare KC, Macio IS, Meyn LA, Wiesenfeld HC, Hillier SL. Patterns of extragenital chlamydia and gonorrhea in women and men who have sex with men reporting a history of receptive anal intercourse. Sex Transm Dis 2016; 43 105–9.
| Patterns of extragenital chlamydia and gonorrhea in women and men who have sex with men reporting a history of receptive anal intercourse.Crossref | GoogleScholarGoogle Scholar | 26766527PubMed |
[5] Patton ME, Kidd S, Llata E, Stenger M, Braxton J, Asbel L, Bernstein K, Gratzer B, Jespersen M, Kerani R, Mettenbrink C, Mohamed M, Pathela P, Schumacher C, Stirland A, Stover J, Tabidze I, Kirkcaldy RD, Weinstock H. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men–STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 2014; 58 1564–70.
| 24647015PubMed |
[6] Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 2014; 41 589–91.
| Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics.Crossref | GoogleScholarGoogle Scholar | 25211252PubMed |
[7] Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. Evidence for increased chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 2015; 60 398–404.
| 25336625PubMed |
[8] Ladd J, Hsieh YH, Barnes M, Quinn N, Jett-Goheen M, Gaydos CA. Female users of internet-based screening for rectal STIs: descriptive statistics and correlates of positivity. Sex Transm Infect 2014; 90 485–90.
| Female users of internet-based screening for rectal STIs: descriptive statistics and correlates of positivity.Crossref | GoogleScholarGoogle Scholar | 24604333PubMed |
[9] Peters RP, Nijsten N, Mutsaers J, Jansen CL, Morre SA, van Leeuwen AP. Screening of oropharynx and anorectum increases prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infection in female STD clinic visitors. Sex Transm Dis 2011; 38 783–7.
| Screening of oropharynx and anorectum increases prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infection in female STD clinic visitors.Crossref | GoogleScholarGoogle Scholar | 21844729PubMed |
[10] Trebach JD, Chaulk CP, Page KR, Tuddenham S, Ghanem KG. Neisseria gonorrhoeae and Chlamydia trachomatis among women reporting extragenital exposures. Sex Transm Dis 2015; 42 233–9.
| Neisseria gonorrhoeae and Chlamydia trachomatis among women reporting extragenital exposures.Crossref | GoogleScholarGoogle Scholar | 25868133PubMed |
[11] Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW, Martin DH, Ferrero DV, Schachter J. Performance of the APTIMA Combo 2 assay for the multiplex detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol 2003; 41 304–9.
| Performance of the APTIMA Combo 2 assay for the multiplex detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens.Crossref | GoogleScholarGoogle Scholar | 12517865PubMed |
[12] Gaydos CA, Theodore M, Dalesio N, Wood BJ, Quinn TC. Comparison of three nucleic acid amplification tests for the detection of Chlamydia trachomatis in urine specimens. J Clin Microbiol 2004; 42 3041–5.
| Comparison of three nucleic acid amplification tests for the detection of Chlamydia trachomatis in urine specimens.Crossref | GoogleScholarGoogle Scholar | 15243057PubMed |
[13] Martin DH, Cammarata C, Van Der Pol B, Jones RB, Quinn TC, Gaydos CA, Crotchfelt K, Schachter J, Moncada J, Jungkind D, Turner B, Payton C. Multicenter evaluation of AMPLICOR and automated COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae. J Clin Microbiol 2000; 38 3544–9.
| 11015361PubMed |
[14] Van Der Pol B, Quinn TC, Gaydos CA, Crotchfelt K, Schachter J, Moncada J, Jungkind D, Martin DH, Turner B, Peyton C, Jones RB. Multicenter evaluation of the AMPLICOR and automated COBAS AMPLICOR CT/NG tests for the detection of Chlamydia trachomatis. J Clin Microbiol 2000; 38 1105–12.
| 10699004PubMed |
[15] Association of Public Health Laboratories. APHL/CDC panel summary reports: laboratory diagnostic testing for Chlamydia trachomatis and Neisseria gonorrhoeae, laboratory diagnostic testing for Treponema pallidum. Guidelines for the Laboratory Testing of STDs. Silver Spring: APHL; 2009. Available online at: http://www.aphl.org/aphlprograms/infectious/std/Documents/ID_2009Jan_CTGCLab-Guidelines-Meeting-Report.pdf [verified 18 April 2019].
[16] Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, Gaydos CA. Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol 2013; 51 1611–3.
| Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania.Crossref | GoogleScholarGoogle Scholar | 23486714PubMed |
[17] Gaydos CA. Review of use of a new rapid real-time PCR, the Cepheid GeneXpert(R) (Xpert) CT/NG assay, for Chlamydia trachomatis and Neisseria gonorrhoeae: results for patients while in a clinical setting. Expert Rev Mol Diagn 2014; 14 135–7.
| Review of use of a new rapid real-time PCR, the Cepheid GeneXpert(R) (Xpert) CT/NG assay, for Chlamydia trachomatis and Neisseria gonorrhoeae: results for patients while in a clinical setting.Crossref | GoogleScholarGoogle Scholar | 24450867PubMed |
[18] Gaydos CA, Van Der Pol B, Jett-Goheen M, Barnes M, Quinn N, Clark C, Daniel GE, Dixon PB, Hook EW, CT/NG Study Group. Performance of the Cepheid CT/NG Xpert Rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2013; 51 1666–72.
| Performance of the Cepheid CT/NG Xpert Rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae.Crossref | GoogleScholarGoogle Scholar | 23467600PubMed |
[19] US Food & Drug Administration. FDA news release: FDA Clears first diagnostic tests for extragenital testing for chlamydia and gonorrhea. Silver Spring: FDA; 2019. Available online at: https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea [verified 20 June 2019].
[20] Bristow CC, McGrath MR, Cohen AC, Anderson LJ, Gordon KK, Klausner JD. Comparative evaluation of 2 nucleic acid amplification tests for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae at extragenital sites. Sex Transm Dis 2017; 44 398–400.
| Comparative evaluation of 2 nucleic acid amplification tests for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae at extragenital sites.Crossref | GoogleScholarGoogle Scholar | 28604481PubMed |
[21] Cosentino LA, Danby CS, Rabe LK, Macio I, Meyn LA, Wiesenfeld HC, Hillier SL. Use of nucleic acid amplification testing for diagnosis of extragenital sexually transmitted infections. J Clin Microbiol 2017; 55 2801–7.
| Use of nucleic acid amplification testing for diagnosis of extragenital sexually transmitted infections.Crossref | GoogleScholarGoogle Scholar | 28679521PubMed |
[22] Dize L, Silver B, Gaydos C. Comparison of the Cepheid GeneXpert CT/NG assay to the Hologic Aptima Combo2 assay for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in self-collected rectal swabs. Diagn Microbiol Infect Dis 2018; 90 83–84.
| 29174733PubMed |
[23] Geiger R, Smith DM, Little SJ, Mehta SR. Validation of the GeneXpert(R) CT/NG assay for use with male pharyngeal and rectal swabs. Austin J HIV AIDS Res 2016; 3 1021
| 27536736PubMed |
[24] Goldenberg SD, Finn J, Sedudzi E, White JA, Tong CY. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol 2012; 50 3867–9.
| Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples.Crossref | GoogleScholarGoogle Scholar | 22993183PubMed |
[25] Menke J. Bivariate random-effects meta-analysis of sensitivity and specificity with the Bayesian SAS PROC MCMC: methodology and empirical evaluation in 50 meta-analyses. Med Decis Making 2013; 33 692–701.
| Bivariate random-effects meta-analysis of sensitivity and specificity with the Bayesian SAS PROC MCMC: methodology and empirical evaluation in 50 meta-analyses.Crossref | GoogleScholarGoogle Scholar | 23475941PubMed |
[26] Menke J. Bayesian bivariate meta-analysis of sensitivity and specificity: summary of quantitative findings in 50 meta-analyses. J Eval Clin Pract 2014; 20 844–52.
| Bayesian bivariate meta-analysis of sensitivity and specificity: summary of quantitative findings in 50 meta-analyses.Crossref | GoogleScholarGoogle Scholar | 24828853PubMed |
[27] Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006; 6 31
| Meta-DiSc: a software for meta-analysis of test accuracy data.Crossref | GoogleScholarGoogle Scholar | 16836745PubMed |
[28] Salow KR, Cohen AC, Bristow CC, McGrath MR, Klausner JD. Comparing mail-in self-collected specimens sent via United States Postal Service versus clinic-collected specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in extra-genital sites. PLoS One 2017; 12 e0189515
| Comparing mail-in self-collected specimens sent via United States Postal Service versus clinic-collected specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in extra-genital sites.Crossref | GoogleScholarGoogle Scholar | 29240781PubMed |
[29] Speers DJ, Chua IJ, Manuel J, Marshall L. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis from pooled rectal, pharyngeal and urine specimens in men who have sex with men. Sex Transm Infect 2018; 94 293–297.
| 29066627PubMed |
[30] Hologic Inc. Aptima Combo 2 Assay Package Insert 502183EN Rev. 004. San Diego, CA, USA: Hologic, Inc.; 2017.
[31] Wingrove I, McOwan A, Nwokolo N, Whitlock G. Diagnostics within the clinic to test for gonorrhoea and chlamydia reduces the time to treatment: a service evaluation. Sex Transm Infect 2014; 90 474
| Diagnostics within the clinic to test for gonorrhoea and chlamydia reduces the time to treatment: a service evaluation.Crossref | GoogleScholarGoogle Scholar | 25118322PubMed |
[32] Bourgeois-Nicolaos N, Jaureguy F, Pozzi-Gaudin S, Masson C, Guillet-Caruba C, Lavisse F, Larmignat P, Benachi A, Picard B, Doucet-Populaire F. Benefits of rapid molecular diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae infections in women attending family planning clinics. Sex Transm Dis 2015; 42 652–3.
| Benefits of rapid molecular diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae infections in women attending family planning clinics.Crossref | GoogleScholarGoogle Scholar | 26462191PubMed |
[33] Whitlock GG, Gibbons DC, Longford N, Harvey MJ, McOwan A, Adams EJ. Rapid testing and treatment for sexually transmitted infections improve patient care and yield public health benefits. Int J STD AIDS 2018; 29 474–482.
| 29059032PubMed |



