Cryoprotective effect of antifreeze protein III on the rabbit ovary
Qin Zeng A # , Kai Wang B # , Li-Bin He A , Ting-Ting Wang A , Xue-Mei Fan C and Wei-Xin Liu A *
A # , Kai Wang B # , Li-Bin He A , Ting-Ting Wang A , Xue-Mei Fan C and Wei-Xin Liu A *
A Key Laboratory of Reproductive Medicine, Sichuan Provincial Maternity and Child Health Care Hospital, The Affiliated Women’s and Children’s Hospital of Chengdu Medical College, Chengdu 610045, China.
B Department of Acute Care Surgery, Sichuan Provincial People’s Hospital, Sichuan Academy of Medical Sciences, Chengdu 610072, China.
C School of Medical and Life Sciences/Reproductive & Women-Children Hospital, Chengdu University of Traditional Chinese Medicine, Chengdu 610041, China.
Handling Editor: Ye Yuan
Reproduction, Fertility and Development 34(9) 645-657 https://doi.org/10.1071/RD21324
Published online: 22 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Context: Ovarian tissue cryopreservation is effective in preserving fertility in cancer patients who have concerns about fertility loss due to cancer treatment. However, ischemia reduces the lifespan of grafts. Microvascular transplantation of cryopreserved whole ovary may allow immediate revascularisation, but the damage incurred during the cryopreservation procedure may cause follicular depletion; hence, preventing chilling injury would help maintain ovarian function.
Aim: This study was designed to investigate the beneficial effects of antifreeze protein III (AFP III) on rabbit ovary cryopreservation.
Methods: Ovaries (n = 25) obtained from 5-month-old female rabbits (n = 13) were frozen by slow freezing and vitrification. Cryoprotectant media were supplemented with and without 1 mg/mL of AFP III. The experiment was divided into five groups: fresh control group (F), slow freezing group (S), slow freezing group with AFP III (AFP III-S), vitrification group (V) and vitrification group with AFP III (AFP III-V). All groups of ovaries were examined by histological characteristics analysis, ultrastructural analysis, apoptosis detection and follicle viability test.
Key results: With slow freezing, the normal rate of change in follicle morphology, density of stromal cells and the survival rate of follicles in the AFP III supplemented group were significantly higher than those in the non-supplemented group, and a lower oocyte apoptotic rate was shown in the AFP III supplemented group. In the vitrification groups, the normal rate of change in follicle morphology and density of stromal cells in the AFP III supplemented group were significantly higher than those in the non-supplemented group, and a lower oocyte apoptotic rate was found in the AFP III supplemented group. But there was no obvious difference in the survival rate of follicles between the two groups. There was also no significant difference in the normal rate of change in follicle morphology, the survival rate of follicles and the apoptotic rate of oocytes between the vitrification and slow freezing groups (P > 0.05), but the density of stromal cells in the vitrification groups was statistically higher than that of the slow freezing group (P < 0.05).
Conclusions: The addition of AFP III in slow freezing and vitrification could improve the cryoprotective effect of ovaries, which was more evident in slow freezing.
Implications: The findings of this study provide a foundation for further research on the effects of AFP III in human ovarian tissue.
Keywords: antifreeze protein III, cancer patients, cryopreservation, infertility, reproduction, slow freezing, vitrification, whole ovaries.
Introduction
With the rapid development of chemotherapy and radiotherapy, the survival rate of cancer patients is greatly improved compared to the past, but unfortunately both chemotherapy and radiotherapy can cause decreased ovarian function or ovarian failure due to depletion. Therefore, fertility preservation of women of reproductive age with cancer is considered as one of the important goals for tumour treatment.
There were some advantages of cryopreservation of ovarian tissue such as preserving reproductive endocrine function of female patients without delaying tumour treatment, and it is the only method of fertility preservation for pre-puberal girls (Petru 2010). In recent years, nonvascular grafting of both slow-frozen (Practice Committee of American Society for Reproductive Medicine 2014) and vitrified (Donnez et al. 2004) ovarian cortex has permitted healthy births. However, a cessation of the function is seen in most cases within 6–9 months after retransplantation (Schmidt et al. 2005). The limited amount of tissue in combination with the extended ischemic period may explain the short lifespan of the graft (Zhang et al. 2011). In a study of sheep ovarian tissue, it was found that 60–70% of follicles were lost after transplantation but only 7% of the loss was dependent on the cryopreservation procedure itself (Baird et al. 1999). Thus, the major loss seems to occur during the warm ischemic period, probably extending over several days until neovascularisation has restored the blood flow to the grafted tissue.
In theory, cryopreservation of intact ovary followed by autologous transplantation using microvascular anastomosis could achieve an immediate blood supply to maximise graft survival. Spontaneous pregnancy after transplantation of an intact frozen–thawed ewe ovary with microvascular anastomosis shows that this technique is promising (Imhof et al. 2006). Unfortunately, follicular loss after vascular transplantation of the whole frozen–thawed ovaries is as severe as after transplantation of frozen–thawed ovarian tissue (Imhof et al. 2006; Courbiere et al. 2009).
Compared with ovarian cortex fragments, cryopreservation of the whole ovary is more difficult because it is larger and the structure is more complicated (Jacobs and Pucci 2013). Imperfect cryopreservation for bulky organs can be a barrier for whole ovary transplantation. The protocol for cryopreserving the whole ovary should be optimised before the procedure can be of clinical significance. In order to alleviate cryoinjury during ovarian cryopreservation and improve survival, we attempted to reduce the cryodamage through lowering the freezing point and preventing ice recrystallisation during the cryopreservation procedure.
Antifreeze proteins (AFP) lower the freezing point of a solution in a non-colligative manner, leading to an increase in the difference between the melting point and the freezing point. This phenomenon is known as thermal hysteresis (Leinala et al. 2002). AFP have been reported to bind to ice crystals and inhibit their growth and recrystallisation to protect cellular membranes in this way (Lee et al. 2012). In 1969, DeVries and colleague isolated the first AFP from Antarctic fish (DeVries and Wohlschlag 1969). Since then, AFP that play a role in survival in below-zero environments have been found in vertebrates, insects, fungi, bacteria and plants (Yeh and Feeney 1996).
Multiple animal studies have reported that supplementation with AFP III increases the survival rate of frozen–thawed spermatozoa [rabbit (Nishijima et al. 2014), chimpanzee (Younis et al. 1998)], embryos [mouse (Jo et al. 2011), rabbit (Nishijima et al. 2014)] and ovarian tissue [mouse (Lee et al. 2015a; Kim et al. 2017)] in some mammalian species, although there is a species-specific optimal AFP dosage. The aims of this study were to investigate the effect of AFP III supplementation during the ovarian slow freezing and vitrification procedures.
Materials and methods
This study was approved by the Ethics Committee in Animal Experimentation of Sichuan Provincial Maternity and Child Health Care Hospital.
Experimental animals and grouping
The experimental design is described in Fig. 1. Thirteen female rabbits aged 4–5 months and weighing 1.8–2.2 kg were used in this study, which were provided by the experimental animal center of Chengdu University of Traditional Chinese Medicine. Five ovaries of three rabbits were selected as the fresh control group (C). The left ovaries of five rabbits were used in the conventional slow freezing group (S). The right ovaries were used in the slow freezing group with the addition of AFP III (AFP III-S). The left ovaries of the other five rabbits were used in the routine vitrification group (V). The right ovaries were used in the vitrification group with the addition of AFP III (AFP III-V).
Whole ovarian acquisition and vascular perfusion
Female rabbits were anesthetised by injection of 1% pentobarbital (3 mL/kg) along the ear vein. After fixation, skin preparation and disinfection, each rabbit underwent a subumbilical midline laparotomy under general anaesthesia. Electrical scalpels were not used. The ovary and ovarian vessels were located (Fig. 2a), then separated from the surrounding tissue. The right ovarian pedicle was dissected by following the lumbo-ovarian vessels up to their junction with the aorta and inferior vena cava. The left ovarian pedicle was dissected by following the lumbo-ovarian vessels up to their junction with the aorta and left renal vein. Each secondary vessel was ligated as near as possible to the vascular pedicle (Fig. 2b), using non-absorbable surgical suture (Jiahe, Taiwan, China), then divided. Care was taken to protect the vascular supply to the tube and ovary. The lumbo-ovarian artery and vein were then ligated and divided as near as possible to the aorta and inferior vena cava or left renal vein, respectively, and the ovary with its pedicle was harvested. They were then placed in a petri dish containing heparinised physiological solution (precooled at 4°C). The ovarian artery was cannulated with the 24 G vein indwelling needle under an anatomical microscope (Olympus CKX41, Tokyo, Japan) as soon as possible and fixed with silk thread (Fig. 3a). The ovary was then perfused with heparinised physiological solution (kept on ice outside the petri dish) at a rate of 1.3 mL/min (Wang 2011) until all the blood cells were removed and the ovaries turned white (Fig. 3b).
|
|
|
|
Slow freezing and thawing of whole ovaries
The ovaries after removing all blood cells by perfusion were immersed in a bath and perfused containing a cryoprotective solution of Leibovitz-15 (L-15; L1518, Sigma, St Louis, USA), 1.5 M dimethyl sulfoxide (DMSO; C6164, Sigma), 20% fetal bovine serum (FBS; 12007c, Sigma) and with/without 1 mg/mL of AFP III (A/F Protein Inc., Waltham, USA) at 4°C. Ovarian perfusion was performed with a pump (Terumo Corporation, Tokyo, Japan) to maintain a flow rate of 1.3 mL/min for 30 min.
The cannula used to perfuse the ovary was then left in place with the aim of facilitating perfusion after thawing, and the ovary was placed in a 1.8 mL cryotube with the slow freezing solution where it was pre-equilibrated at 4°C in a cryoprotective solution bath for 10 min, with gentle shaking. After pre-equilibration, freezing according to the slow freezing method proposed by Martinez-Madrid et al. (2004), the cryotube was placed in a 5100 Cryo 1°C freezing container (Thermo Fisher Scientic, Waltham, USA) precooled at 4°C, then placed in a −80°C freezer. This confers a cooling rate of −1°C/min. After 24 h in the −80°C freezer, the cryotube was transferred to liquid nitrogen.
One week later, the whole ovary was thawed by the rapid thawing method. The cryotube was removed from liquid nitrogen, then melted at room temperature (25°C) for 2 min, immersed in a water bath at 37°C and shaken gently until the ice crystals in the tube melted completely. The ovaries were gently removed from the cryogenic tube and placed in a petri dish containing thawing solution. The cryoprotectant was rinsed out by both immersion and perfusion in three steps (10 min each step) at room temperature, with a reversed sucrose (S1888, Sigma) concentration gradient (0.25 M, 0.1 M and 0 M sucrose) in L-15 medium, to avoid osmotic injuries. It was perfused using the same flow rate described earlier.
Vitrification and warming of whole ovaries
After removing all blood cells by perfusion, the ovaries were perfused via the ovarian artery with the vitrified equilibrium solution that contained L-15 medium, 10% ethylene glycol (293237, Sigma), 10% DMSO and 20% FBS for 15 min, then perfused with the vitrified solution that contained L-15 medium, 20% ethylene glycol, 20% DMSO, 1 M sucrose with and without 1 mg/mL AFP III for another 15 min, and the flow rate was 1.3 mL/min. After perfusion, the whole ovaries were placed in a 1.8 mL cryotube with the vitrified solution, and then directly placed in liquid nitrogen for storage.
For warming, the cryotube was directly transferred from liquid nitrogen, then melted at room temperature for 2 min, immersed in a water bath at 37°C and shaken gently until the ice crystals in the tube melted completely. The ovaries were gently removed from the cryogenic tube and placed in a petri dish containing warming solution. The corresponding warming solution (L-15 + 10% FBS + 0.5 M, 0.25 M or 0 M sucrose) was perfused at the same rate of 1.3 mL/min in the order of decreasing sucrose concentration gradient to activate the cell metabolism of the ovaries (10 min each step).
Sample preparation
The frozen and thawed ovaries were cut longitudinally in half along the ovarian hilum under an anatomic microscope, and then each half of the ovary was divided evenly in two. The 1/4 ovary was used for ovarian histological examination, 1/4 was used for follicular ultrastructure analysis, 1/4 was used for follicular viability assessment, and 1/4 was used for the follicular apoptosis test.
Histological examination
The ovarian fragments were fixed in 4% paraformaldehyde for 24 h at room temperature. Fixed ovarian fragments were dehydrated, embedded in paraffin wax, and serially sectioned at 4 μm. Every fifth section was mounted and stained with hematoxylin–eosin (H&E). All sections were examined using a light microscope (Olympus CX31, Tokyo, Japan) at a magnification of ×400. The numbers of each type of follicle (due to the small number of antral follicles, they were counted in secondary follicles) were counted in the entire cut ovarian surface. Only follicles with a visible nucleus in the oocyte were counted to avoid double counting. Each type of follicle was categorised according to the following classification (Lundy et al. 1999).
-
Primordial: single layer of attened pre-granulosa cells.
-
Primary: single layer of granulosa cells; one or more being cuboidal cells.
-
Secondary: two or more layers of cuboidal granulosa cells; antrum absent.
-
Antral: multiple layers of cuboidal granulosa cells; antrum present.
The integrity of each follicle was evaluated using the following criteria (Neto et al. 2008).
-
Type I: follicle without any morphological defect. Follicle is regular, with joined follicular cells. Cytoplasm of the oocyte is homogeneous and chromatin is diffused and regular.
-
Type II: follicle with cytoplasmic defect. Cytoplasm of the oocyte is vacuolised or eosinophilic.
-
Type III: follicle with nuclear defect. Nucleus of the oocyte is pycnotic, without apparent nuclear membrane or with an irregular nuclear membrane.
-
Type IV: degenerated follicle. Oocyte with combined cytoplasmic and nuclear defects or follicle with irregular shape or with disjoined follicular cells or with swollen follicular cells.
The proportion of normal follicles was defined as proportions of follicles with good morphology (Type I).
According to the method reported by Faustino et al. (2010), ovarian stroma density was evaluated by calculating the stromal cell per 100 μm2. For each treatment, 10 fields per slide were assessed and the mean number of stromal cells per field was calculated. At least three sections of each ovary were examined.
Follicular ultrastructure
Ovarian samples with a maximum dimension of 1 mm × 1 mm were fixed in 2.5% glutaraldehyde for at least 2 h, then washed in 0.1 mol/L phosphate buffered saline (pH 7.4) three times (10 min each time). After fixing and washing, specimens were post-fixed in 1% osmium tetroxide for 1 h at 37°C, then washed in 0.1 mol/L phosphate buffered saline for 20 min. Subsequently, the samples were dehydrated through a gradient of acetone solutions (70% acetone solution for 15 min, 80% acetone solution for 15 min, 90% acetone solution for 15 min, 100% acetone solution for 10 min) and the tissues were embedded in Spurr (Bosheng Biological Technology Co, Shanghai, China). The samples were cut into approximately 0.5 μm sections, stained with toluidine blue and observed under a light microscope. Then samples with follicles were selected, cut into 50 nm sections and stained with uranyl acetate and lead citrate and examined under a transmission electron microscope (Hitachi H7650, Tokyo, Japan).
We evaluated the integrity of oocytes, granulosa and stromal cells according to previously reported subcellular criteria (Fabbri et al. 2010).
Oocyte apoptosis
Paraffin-embedded ovarian sections were used for terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) analysis using the cell death detection kit conjungated with horseradish peroxidase (Roche, Shanghai, China), according to the manufacturer‘s instructions. Briefly, after deparaffination, rehydratation and washing with phosphate buffered saline, sections were immersed in 10 mM citrate buffer and underwent antigen retrieval in a microwave for 5 min (350 W). Then the slides were incubated with 50 mL of TUNEL reaction mixture and incubated at 37°C for 60 min in the dark inside a humidified chamber.
As a positive control, sections were incubated with DNase I (3000 U/mL in 50 mM Tris–HCl, pH 7.5, 1 mg/mL bovine serum albumin) for 10 min at 15–25°C to induce DNA strand breaks, prior to the labelling procedure. As a negative control, sections were incubated with label solution only (without terminal transferase) instead of the TUNEL reaction mixture.
The apoptotic signal was recorded as positive when dUTP stained the oocyte nucleus brown. The percentage of apoptotic oocytes was calculated as follows: apoptotic oocyte number/total oocyte number × 100%.
Follicular viability assay
Isolation of small follicles
Follicles were isolated according to the method proposed by Neto et al. (2008). Ovarian fragments were finely dissected in TCM 199 at room temperature, incubated with collagenase type I (0.5 mg/mL) at 37°C for 1.5 h and gently pipetted every 30 min. Collagenase action was blocked by the addition of FBS. The suspension was filtered through a 60-mm nylon filter (Biocomma, Shenzhen, China) to recover the small follicles, and centrifuged at 400g for 5 min. The pelleted cells were resuspended with 30 mL Euroflush medium (IMV, L‘Aigle, France).
Viability test using calcein AM and ethidium homodimer I
Follicle viability was evaluated on isolated follicles by the live/dead test using calcein AM and ethidium homodimer I stains [Live/Dead Viability/Cytotoxicity Kit (L-3224, Molecular Probes, Leiden, the Netherlands)]. According to the Live/Dead Viability–Cytotoxicity Kit instructions, live cells are distinguished by the presence of ubiquitous intracellular esterase activity, determined by the enzymatic conversion of the virtually non-fluorescent cell-permeant calcein AM to the intensely fluorescent calcein. The polyanionic dye calcein is well retained within live cells, producing an intense uniform green fluorescence in live cells (ex/em ∼495 nm/∼515 nm). EthD-1 enters cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to nucleic acids, thereby producing a bright red fluorescence in dead cells (ex/em ∼495 nm/∼635 nm). EthD-1 is excluded by the intact plasma membrane of live cells.
The follicles were classified into four categories depending on the percentage of dead granulosa cells (Martinez-Madrid et al. 2004).
-
Live follicles: follicles with the oocyte and all the granulosa cells viable.
-
Minimally damaged follicles: follicles with 10% of dead granulosa cells.
-
Moderately damaged follicles: follicles with 50% of dead granulosa cells.
-
Dead follicles: follicles with both the oocyte and all the granulosa cells dead.
Live follicles and minimally damaged follicles were considered to survive, the rest were dead follicles, and the follicular survival rate was calculated. Follicular survival rate (%) = the number of surviving follicles from isolated follicles/the number of total follicles isolated × 100%.
Statistical analysis
SPSS (version 20; IBM Corp.) was used for statistical analyses. Statistical analysis was performed using analysis of variance, Student’s t-test or Kruskal–Wallis test for continuous variables, whereas Chi-squared or Fisher’s exact tests were used for categorical variables, and one-way ANOVA was used to judge the difference between the groups, as appropriate. Differences were considered statistically significant for P-values < 0.05.
Results
Proportions of follicle stages and normal morphology
According to the morphological classification and injury criteria, the normal morphology follicle rate of the fresh control group was 88.1% (Fig. 4, Table 1). It was shown that the normal morphology follicle rates in the freeze–thawed groups (S, AFP-S, V and AFP-V) were significantly lower than in the fresh control group. In the slow freezing groups, the normal morphology follicle rate in the AFP-S group was significantly higher than that in the S group (P < 0.05). In the vitrification groups, the normal morphology follicle rate in the AFP-V group was significantly higher than in the V group (P < 0.05). There was no significant difference of normal morphology follicle rate between the S group and the V group. No significant difference was shown between the two freezing methods after adding AFP III (AFP-V group and AFP-S group).

|
Stromal cell density
The density of stromal cells in the fresh control group was 10.9 ± 1.7 cells/100 μm2, which was significantly higher than in the other four groups (P < 0.05). Adding AFP III to the cryoprotectant can significantly increase the density of stromal cells whether in slow freezing or in vitrification (P < 0.05). It was also found that the vitrification group had greater stromal cell density than the slow freezing group (P < 0.05). There was no significant difference between the AFP-S group and the AFP-V group (P > 0.05) (Table 2).

|
Follicular ultrastructural analysis
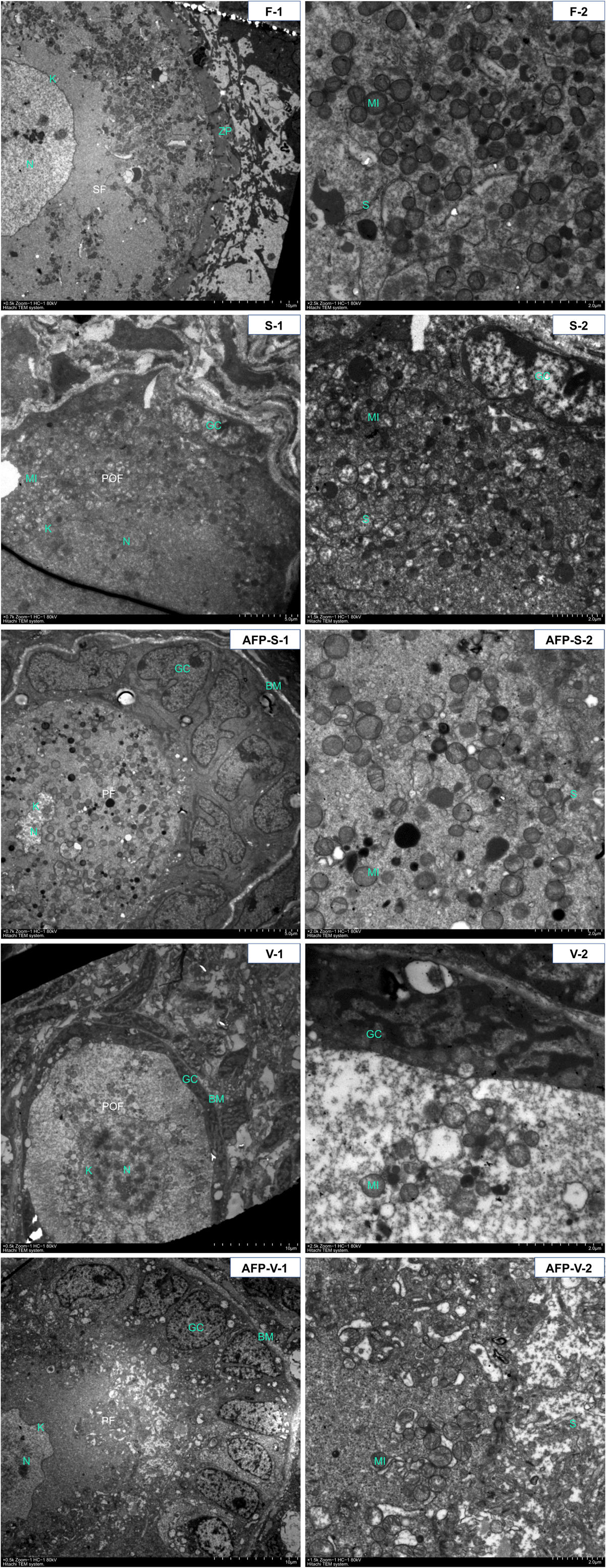
In the fresh control group (Fig. 5F-1, F-2), mitochondria, free ribosomes, rough and smooth endoplasmic reticulum, and Golgi apparatus were observed in the oocyte cytoplasm. In particular, there were a number of mitochondria that appeared typically round, with a low-density matrix, clustered around a dense and amorphous intermitochondrial substance (Fig. 5F-2) in the oocyte cytoplasm. The presence of swollen mitochondrial cristae was rarely found.
The ultrastructure of the oocytes in the AFP-S group (Fig. 5AFP-S-1, AFP-S-2) was similar to those in the fresh control group, the round or ovoid-shaped mitochondria and endoplasmic reticulum were the most abundant organelles in the oocytes. The endoplasmic reticulum was well defined, and the mitochondria exhibited highly organised structures with a condensed matrix and intact cristae. The granulosa cells had a normal morphology and organelle distribution and showed uniform contact with no obvious increase in the level of vacuolisation in the AFP-S group (Fig. 5AFP-S-1). However, great changes were observed in the S group (Fig. 5S-1, S-2), including obvious cell swelling, indistinct intercellular junction of the granulosa cell, irregular oocyte shape, disordered cytoplasmic structure, shrunken nuclear membranes of oocytes, chromatin aggregation and significant reduction of the intracellular mitochondria.
In the vitrification group (Fig. 5V-1, V-2), indistinct intercellular junction and vacuoles were observed in the granulosa cells. Cytoplasmic clearing and focal enlargment of perinuclear cisternae were also shown in oocytes. Numbers of mitochondria were reduced. The endoplasmic reticulum and the Golgi were irregular in shape and swollen. Some minimal damage of the ultrastructure of the oocytes and the granulosa cells, including swollen mitochondrial cristae, vacuolisation and shrunken nuclear membranes, were found in the AFP-V group (Fig. 5AFP-V-1, AFP-V-2).
Oocyte apoptosis
The apoptosis rate of oocytes in the fresh group (13.47%) was significantly lower than that in the other four groups (P < 0.05). Whether in the slow freezing groups or the vitrification group, the apoptotic rate of oocytes was significantly lower in the AFP III added group than in the non-AFP III added group (P < 0.05). There was no obvious difference in the apoptotic rate between the two freezing groups with or without the addition of AFP III (Table 2).
Follicular viability assessment
The survival rate of follicles isolated in the fresh group (79.59%) was significantly higher than that in the other four groups (P < 0.05), according to the follicle survival criterion (Fig. 6) and differential staining (Fig. 6) by follicular viability assessment. In the slow freezing groups, the survival rate of follicles in the AFP-S group was significantly higher than in the S group (61.19% vs 44.23%, P < 0.05). In the vitrification groups, there was no significant difference between the AFP-V group and V group (50.94% vs 41.67%, P > 0.05). No difference was shown between the S group and the V group (P > 0.05).
|
|
Discussion
Our group showed that vitrification of rabbit ovaries was comparable with slow freezing. However, the density of stromal cells in the vitrification group tended to be higher than that of the slow freezing group (there was no significant difference). At present, there are two methods for ovarian cryopreservation: slow freezing and vitrification. In slow freezing, ice crystal formation is minimised by lowering the temperature slowly and using a small dose of cryoprotectant. Vitrification seeks to avoid ice crystal formation by cooling the tissue rapidly and using high doses of cryoprotectants. To date, there is no clear conclusion about which cryopreservation method is more suitable for whole ovarian freezing. Some results have indicated that slow freezing is less efficient than vitrification in whole ovarian freezing studies of rats (Milenkovic et al. 2012), sheep (Imhof et al. 2004), and cows (Courbiere et al. 2006). However, slow freezing may be superior to vitrification in other studies of guinea pig (Xu et al. 2012) and rabbit (Wu 2013). These different results may due to differences in the procedure, for example, cryoprotectant composition and concentrations, exposure times to cryoprotectants and speed of vitrification (Sheikhi et al. 1989).
Ice recrystallisation (IR) is one of the main factors of cell death during cryopreservation (Gage and Baust 1998). IR occurs constantly in nature due to moderate cooling and temperature fluctuations of frozen substances. During cryopreservation, IR causes cell membranes to rupture and causes cell dehydration, resulting in lethal damage to cells and tissues. It is believed that many freeze-tolerant organisms inhabiting cold environments have developed AFP to ensure their survival (Knight et al. 1988). Because AFP are effective at inhibiting IR, they are beneficial for the cryopreservation of cells and tissues (Bagis et al. 2008; Rubinsky et al. 2010; Jo et al. 2012; Kamijima et al. 2013). Because of their complicated structure and large size, IR occurs easily during whole ovarian cryopreservation, leading to damage and death of many follicles (Jacobs and Pucci 2013). The current study evaluated the cryoprotective efficacy of AFP III in the slow freezing and vitrification of whole rabbit ovaries. Addition of AFP III improved the normal follicular morphology, stromal density and follicle viability and decreased the apoptosis of oocytes during the slow freezing and vitrification procedures. Furthermore, the ultrastructure of the follicles was well preserved in the slow freezing group with addition of AFP III.
In terms of follicle preservation, whether ovaries were vitrified or slow-frozen, a higher normal follicular rate was observed in the groups supplemented with 1 mg/mL AFP III compared with the corresponding groups (AFP-S and S; AFP-V and V). It was also shown that in the slow freezing groups the survival rate of follicles was significantly higher in the AFP III group than in the non-addition group. In the vitrification groups, though the surviving follicles in the group with AFP III increased, there was no significant difference in follicular survival rate compared with the vitrification group. It was indicated that AFP III could improve the survival rate of follicles for slow freezing. The mechanism of the cryoprotective effect of AFP III during slow freezing and vitrification in the present study is not clear. However, a protective mechanism suggested by previous studies may be applicable to ovarian tissue cryopreservation (Wang 2000). Jo et al. 2011 suggested that one protective mechanism in mouse oocytes involved preservation of structural and functional integrity associated with the maintenance and recovery of spindle reassembly during vitrification and warming. A potential protective mechanism may involve the interaction of AFP with the function and structure of the cell membrane (Lee et al. 2015b).
Ultrastructural analysis allows assessment of the real integrity of subcellular components and detection of features of cryodamage not observable using light microscopy (Hreinsson et al. 2003; Keros et al. 2009). In fact, transmission electron microscopy evidenced foci of interstitial oedema, likely due to tissue rehydratation occurring during the thawing procedure. This kind of cryodamage is often observed in the cryopreserved ovarian tissue (Hreinsson et al. 2003; Fabbri et al. 2010). In the present study, the ultrastructural analysis of follicles vitrified and slow-frozen without AFP III evidenced irregularly shaped oocyte nuclei with slightly thickened chromatin, broken or swollen mitochondria, vacuoles and clarification of cytoplasm. But we observed that the ultrastructure of follicles in the slow freezing group with AFP III was similar to that in the fresh control group, and some minimal damage of the ultrastructure of the oocytes and the granulosa cells was found in the vitrification group with AFP III. It may be due to the antifreeze effect of AFP III mainly in the inhibition of ice crystal formation (Raymond and DeVries 1977; Yang et al. 1988; Davies and Hew 1990) and recrystallisation in rewarming (Carpenter and Hansen 1992; Chao et al. 1996). The main factor affecting the slow freezing efficiency is the cell damage caused by ice crystal formation.
Based on previous studies (Lee et al. 2012, 2015a), 1 mg/mL of AFP III was used in this study. Several earlier studies have reported a detrimental effect of AFP, where high AFP concentrations were associated with a destructive effect on cells and tissues. In those studies, AFP at relatively low concentrations enhanced the survival rate of red blood cells, whereas at high concentrations AFP reduced survival rates (Yang et al. 1988; Carpenter and Hansen 1992). A study involving immature mouse oocytes showed that high doses of AFP has a harmful effect on oocyte survival (Jo et al. 2012). In fact, the low concentration of AFP could improve the efficiency of cryopreservation of oocytes [500 ng/mL (Zhang et al. 2009)], embryos [500 ng/mL (Asgari et al. 2015)] and spermatozoa [0.1 ug/mL (Nishijima et al. 2014)]. However, a study found that a high dose of AFP III (20 mg/mL) demonstrated better results than a lower dose (5 mg/mL) for mouse ovarian tissue vitrification (Lee et al. 2015b). Lee et al. (2015a) reported that with three different types of antifreeze proteins (FfIBP, LeIBP, type III) and concentrations (0.1, 1, 10 mg/mL) used in mouse ovarian tissue vitrification and transplantation, no significant differences were observed between the AFP in terms of follicular preservation at each of the developmental stages examined. However, at 10 mg/mL, AFP provided cryoprotective effects in primordial follicle preservation, as compared with that observed in the vitrification control (Lee et al. 2015a). In those ovarian tissue vitrification studies, it seems that high concentration of AFP have advantages over low concentrations. In the present study, the ultrastructure of the follicles and the follicular viability seem to better in the slow freezing group with addition of AFP III than vitrification with AFP III. Appropriate high concentration is important to have a sufficient diffusion of cryoprotectant through the stroma and granulosa cells to oocytes, which may be good for ovarian vitrification because the vitrification technique avoids ice crystal formation by cooling the tissue rapidly and using high doses of cryoprotectants. Therefore, the concentration of AFP III (1 mg/mL) used in the present study may be too low for ovarian vitrification. Further study is necessary to confirm whether AFP at higher doses is harmful, and also to determine a more optimal dose of AFP for whole ovary cryopreservation. Wang (2000) explained that AFP have both protective and destructive actions. It depends on many relevant factors such as composition and concentration of cryoprotectant, type and concentration of AFP, cooling and warming rate, and cell surface features.
In the present study, we showed the cryoprotective effect of AFP III on rabbit ovary cryopreservation. Nevertheless, our study has some limitations. Only the recovery of ovarian function after replanting could give proof of the true capabilities of the cryopreserved and stored ovarian tissue. We did not evaluate the restoration of function after autotransplantation of cryopreserved ovaries. Moreover, we did not investigate the exact mechanism underlying the beneficial effects derived from AFP III during vitrification and slow freezing.
Conclusion
The present study demonstrated that supplementation of AFP III in the cryoprotectant has beneficial effects on the survival of follicle during freezing. AFP III may represent a promising supplementary agent for reducing cryodamage during whole ovary freezing. The findings of this study provide a foundation for further research on the effects of AFP III in human ovarian tissue.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported by the National Natural Science Foundation of China (81072834), Applied Basic Research Project of Sichuan Science and Technology Department (2018JY0050), Key Natural Science Project of Education Department of Sichuan Province (18ZA0155), National Key Research and Development Program (2017YFC0907304), Key Science and Technology Innovation Project in Hospital of Sichuan Provincial Maternity and Child Health Care Hospital (CXZD01-2019) and the Key Medical Science and Technology Research and Development Project of Sichuan Provincial Health Commission (21ZD009).
Acknowledgements
The authors thank Song Lei for excellent technical support and Professor Mengjun Luo for critically reviewing the manuscript.
References
Asgari, F, Valojerdi, MR, Ebrahimi, B, and Fatehi, R (2015). Three dimensional in vitro culture of preantral follicles following slow-freezing and vitrification of mouse ovarian tissue. Cryobiology 71, 529–536.| Three dimensional in vitro culture of preantral follicles following slow-freezing and vitrification of mouse ovarian tissue.Crossref | GoogleScholarGoogle Scholar | 26586099PubMed |
Bagis, H, Tas, A, and Kankavi, O (2008). Determination of the expression of fish antifreeze protein (AFP) in 7th generation transgenic mice tissues and serum. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology 309A, 255–261.
| Determination of the expression of fish antifreeze protein (AFP) in 7th generation transgenic mice tissues and serum.Crossref | GoogleScholarGoogle Scholar |
Baird, DT, Webb, R, Campbell, BK, et al. (1999). Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology 140, 462.
| Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C.Crossref | GoogleScholarGoogle Scholar | 9886858PubMed |
Carpenter, JF, and Hansen, TN (1992). Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proceedings of the National Academy of Sciences of the United States of America 89, 8953–8957.
| Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth.Crossref | GoogleScholarGoogle Scholar | 1409591PubMed |
Chao, H, Davies, PL, and Carpenter, JF (1996). Effects of antifreeze proteins on red blood cell survival during cryopreservation. Journal of Experimental Biology 199, 2071–2076.
| Effects of antifreeze proteins on red blood cell survival during cryopreservation.Crossref | GoogleScholarGoogle Scholar |
Courbiere, B, Odagescu, V, Baudot, A, Massardier, J, Mazoyer, C, Salle, B, and Lornage, J (2006). Cryopreservation of the ovary by vitrification as an alternative to slow-cooling protocols. Fertility and Sterility 86, 1243–1251.
| Cryopreservation of the ovary by vitrification as an alternative to slow-cooling protocols.Crossref | GoogleScholarGoogle Scholar | 16978623PubMed |
Courbiere, B, Caquant, L, Mazoyer, C, Franck, M, Lornage, J, and Salle, B (2009). Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification. Fertility and Sterility 91, 2697–2706.
| Difficulties improving ovarian functional recovery by microvascular transplantation and whole ovary vitrification.Crossref | GoogleScholarGoogle Scholar | 18440531PubMed |
Davies, PL, and Hew, CL (1990). Biochemistry of fish antifreeze proteins. The FASEB Journal 4, 2460–2468.
| Biochemistry of fish antifreeze proteins.Crossref | GoogleScholarGoogle Scholar | 2185972PubMed |
DeVries, AL, and Wohlschlag, DE (1969). Freezing resistance in some Antarctic fishes. Science 163, 1073–1075.
| Freezing resistance in some Antarctic fishes.Crossref | GoogleScholarGoogle Scholar | 5764871PubMed |
Donnez, J, Dolmans, MM, Demylle, D, et al. (2004). Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. The Lancet 364, 1405–1410.
| Livebirth after orthotopic transplantation of cryopreserved ovarian tissue.Crossref | GoogleScholarGoogle Scholar |
Fabbri, R, Pasquinelli, G, Keane, D, Magnani, V, Paradisi, R, and Venturoli, S (2010). Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reproductive BioMedicine Online 21, 819–828.
| Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum.Crossref | GoogleScholarGoogle Scholar | 21050819PubMed |
Faustino, LR, Santos, RR, Silva, CMG, Pinto, LC, Celestino, JJH, Campello, CC, Figueiredo, JR, and Rodrigues, APR (2010). Goat and sheep ovarian tissue cryopreservation: effects on the morphology and development of primordial follicles and density of stromal cell. Animal Reproduction Science 122, 90–97.
| Goat and sheep ovarian tissue cryopreservation: effects on the morphology and development of primordial follicles and density of stromal cell.Crossref | GoogleScholarGoogle Scholar | 20800393PubMed |
Gage, AA, and Baust, J (1998). Mechanisms of tissue injury in cryosurgery. Cryobiology 37, 171–186.
| Mechanisms of tissue injury in cryosurgery.Crossref | GoogleScholarGoogle Scholar | 9787063PubMed |
Hreinsson, J, Zhang, P, Swahn, ML, Hultenby, K, and Hovatta, O (2003). Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Human Reproduction 18, 2420–2428.
| Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions.Crossref | GoogleScholarGoogle Scholar | 14585896PubMed |
Imhof, M, Hofstetter, G, Bergmeister, H, Rudas, M, Kain, R, Lipovac, M, and Huber, J (2004). Cryopreservation of a whole ovary as a strategy for restoring ovarian function. Journal of Assisted Reproduction and Genetics 21, 459–465.
| Cryopreservation of a whole ovary as a strategy for restoring ovarian function.Crossref | GoogleScholarGoogle Scholar | 15704522PubMed |
Imhof, M, Bergmeiter, H, Lipovac, M, Rudas, M, Hofstetter, G, and Huber, J (2006). Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth. Fertility and Sterility 85, 1208–1215.
| Orthotopic microvascular reanastomosis of whole cryopreserved ovine ovaries resulting in pregnancy and live birth.Crossref | GoogleScholarGoogle Scholar | 16616094PubMed |
Jacobs, LA, and Pucci, DA (2013). Adult survivors of childhood cancer: the medical and psychosocial late effects of cancer treatment and the impact on sexual and reproductive health. The Journal of Sexual Medicine 10, 120–126.
| Adult survivors of childhood cancer: the medical and psychosocial late effects of cancer treatment and the impact on sexual and reproductive health.Crossref | GoogleScholarGoogle Scholar | 23387917PubMed |
Jo, JW, Jee, BC, Lee, JR, et al. (2011). Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertility and Sterility 96, 1239–1245.
| Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 21917250PubMed |
Jo, JW, Jee, BC, Suh, CS, and Kim, SH (2012). The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes. PLoS ONE 7, e37043.
| The beneficial effects of antifreeze proteins in the vitrification of immature mouse oocytes.Crossref | GoogleScholarGoogle Scholar | 22649508PubMed |
Kamijima, T, Sakashita, M, Miura, A, Nishimiya, Y, and Tsuda, S (2013). Antifreeze protein prolongs the life-time of insulinoma cells during hypothermic preservation. PLoS ONE 8, e73643.
| Antifreeze protein prolongs the life-time of insulinoma cells during hypothermic preservation.Crossref | GoogleScholarGoogle Scholar | 24069217PubMed |
Keros, V, Xella, S, Hultenby, K, Pettersson, K, Sheikhi, M, Volpe, A, Hreinsson, J, and Hovatta, O (2009). Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Human Reproduction 24, 1670–1683.
| Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue.Crossref | GoogleScholarGoogle Scholar | 19359339PubMed |
Kim, MK, Kong, HS, Youm, HW, and Jee, BC (2017). Effects of supplementation with antifreeze proteins on the follicular integrity of vitrified-warmed mouse ovaries: comparison of two types of antifreeze proteins alone and in combination. Clinical and Experimental Reproductive Medicine 44, 8–14.
| Effects of supplementation with antifreeze proteins on the follicular integrity of vitrified-warmed mouse ovaries: comparison of two types of antifreeze proteins alone and in combination.Crossref | GoogleScholarGoogle Scholar | 28428938PubMed |
Knight, CA, Hallett, J, and DeVries, AL (1988). Solute effects on ice recrystallization: an assessment technique. Cryobiology 25, 55–60.
| Solute effects on ice recrystallization: an assessment technique.Crossref | GoogleScholarGoogle Scholar | 3349811PubMed |
Lee, SG, Koh, HY, Lee, JH, Kang, S-H, and Kim, HJ (2012). Cryopreservative effects of the recombinant ice-binding protein from the arctic yeast Leucosporidium sp. on red blood cells. Applied Biochemistry and Biotechnology 167, 824–834.
| Cryopreservative effects of the recombinant ice-binding protein from the arctic yeast Leucosporidium sp. on red blood cells.Crossref | GoogleScholarGoogle Scholar | 22622645PubMed |
Lee, J, Kim, SK, Youm, HW, et al. (2015a). Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS ONE 10, e0126252.
| Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation.Crossref | GoogleScholarGoogle Scholar | 25938445PubMed |
Lee, JR, Youm, HW, Lee, HJ, Jee, BC, Suh, CS, and Kim, SH (2015b). Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation. Yonsei Medical Journal 56, 778–784.
| Effect of antifreeze protein on mouse ovarian tissue cryopreservation and transplantation.Crossref | GoogleScholarGoogle Scholar | 25837185PubMed |
Leinala, EK, Davies, PL, Doucet, D, Tyshenko, MG, Walker, VK, and Jia, Z (2002). A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights. Journal of Biological Chemistry 277, 33349–33352.
| A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights.Crossref | GoogleScholarGoogle Scholar |
Lundy, T, Smith, P, O’Connell, A, Hudson, NL, and McNatty, KP (1999). Populations of granulosa cells in small follicles of the sheep ovary. Reproduction 115, 251–262.
| Populations of granulosa cells in small follicles of the sheep ovary.Crossref | GoogleScholarGoogle Scholar |
Martinez-Madrid, B, Dolmans, M-M, Van Langendonckt, A, Defrère, S, and Donnez, J (2004). Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertility and Sterility 82, 1390–1394.
| Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device.Crossref | GoogleScholarGoogle Scholar | 15533365PubMed |
Milenkovic, M, Diaz-Garcia, C, Wallin, A, and Brännström, M (2012). Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertility and Sterility 97, 1176–1182.
| Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification.Crossref | GoogleScholarGoogle Scholar | 22341373PubMed |
Nishijima, K, Tanaka, M, Sakai, Y, et al. (2014). Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology 69, 22–25.
| Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit.Crossref | GoogleScholarGoogle Scholar | 24809634PubMed |
Neto, V, Buff, S, Lornage, J, Bottollier, B, Guérin, P, and Joly, T (2008). Effects of different freezing parameters on the morphology and viability of preantral follicles after cryopreservation of doe rabbit ovarian tissue. Fertility and Sterility 89, 1348–1356.
| Effects of different freezing parameters on the morphology and viability of preantral follicles after cryopreservation of doe rabbit ovarian tissue.Crossref | GoogleScholarGoogle Scholar | 17604027PubMed |
Petru, E (2010). Fertility preservation and infertility treatment in breast cancer patients. Wiener Medizinische Wochenschrift 160, 487–492.
| Fertility preservation and infertility treatment in breast cancer patients.Crossref | GoogleScholarGoogle Scholar | 20972713PubMed |
(2014). Ovarian tissue cryopreservation: a committee opinion. Fertility and Sterility 101, 1237–1243.
| Ovarian tissue cryopreservation: a committee opinion.Crossref | GoogleScholarGoogle Scholar | 24684955PubMed |
Raymond, JA, and DeVries, AL (1977). Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proceedings of the National Academy of Sciences of the United States of America 74, 2589–2593.
| Adsorption inhibition as a mechanism of freezing resistance in polar fishes.Crossref | GoogleScholarGoogle Scholar | 267952PubMed |
Rubinsky, L, Raichman, N, Lavee, J, Frenk, H, Ben-Jacob, E, and Bickler, PE (2010). Antifreeze protein suppresses spontaneous neural activity and protects neurons from hypothermia/re-warming injury. Neuroscience Research 67, 256–259.
| Antifreeze protein suppresses spontaneous neural activity and protects neurons from hypothermia/re-warming injury.Crossref | GoogleScholarGoogle Scholar | 20398707PubMed |
Schmidt, KLT, Andersen, CY, Loft, A, Byskov, AG, Ernst, E, and Andersen, AN (2005). Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Human Reproduction 20, 3539–3546.
| Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation.Crossref | GoogleScholarGoogle Scholar |
Sheikhi, M, Hultenby, K, Niklasson, B, Lundqvist, M, and Hovatta, O (1989). Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Metallurgical Transactions A 20, 1585–1591.
Wang, J-H (2000). A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology 41, 1–9.
| A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation.Crossref | GoogleScholarGoogle Scholar | 11017755PubMed |
Wang XJ (2011) ‘The influence of cryoprotectan perfusion time on the frozen-thawed whole rabbit ovary.’ (Wenzhou Medical College: Zhejiang)
Wu F (2013) ‘A comparative study of the cryopreservation effect for whole rabbit ovary program med freezing and vitrification.’ (Zhejiang: Wenzhou Medical College)
Xu, Z, Wang, X, Wu, Y, Meng, Y, Wu, F, Zhou, N, Chen, W, Ye, B, Liu, J, and Zhou, Y (2012). Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries. Theriogenology 77, 483–491.
| Slow-controlled freezing versus speed-cooling for cryopreservation of whole guinea pig ovaries.Crossref | GoogleScholarGoogle Scholar | 21958638PubMed |
Yang, DSC, Sax, M, Chakrabartty, A, and Hew, CL (1988). Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature 333, 232–237.
| Crystal structure of an antifreeze polypeptide and its mechanistic implications.Crossref | GoogleScholarGoogle Scholar |
Yeh, Y, and Feeney, RE (1996). Antifreeze proteins: structures and mechanisms of function. Chemical Reviews 96, 601–618.
| Antifreeze proteins: structures and mechanisms of function.Crossref | GoogleScholarGoogle Scholar | 11848766PubMed |
Younis, AI, Rooks, B, Khan, S, et al. (1998). The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. Journal of Andrology 19, 207–214.
| 9570745PubMed |
Zhang, J-M, Li, L-X, Yang, Y-X, Liu, X-L, and Wan, X-P (2009). Is caspase inhibition a valid therapeutic strategy in cryopreservation of ovarian tissue? Journal of Assisted Reproduction and Genetics 26, 415–420.
| Is caspase inhibition a valid therapeutic strategy in cryopreservation of ovarian tissue?Crossref | GoogleScholarGoogle Scholar | 19697118PubMed |
Zhang, J-M, Sheng, Y, Cao, YZ, et al. (2011). Cryopreservation of whole ovaries with vascular pedicles: vitrification or conventional freezing? Journal of Assisted Reproduction and Genetics 28, 445–452.
| Cryopreservation of whole ovaries with vascular pedicles: vitrification or conventional freezing?Crossref | GoogleScholarGoogle Scholar | 21287401PubMed |