Effects of mobile phone use on semen parameters: a cross-sectional study of 1634 men in China
Shanshan Zhang A B , Fengyi Mo B , Yali Chang B , Shufang Wu B , Qing Ma B , Fan Jin B and Lanfeng Xing B *
B *
A School of Medicine, Zhejiang University, Hangzhou, 310012 Zhejiang Province, People’s Republic of China.
B Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, 310006 Zhejiang Province, People’s Republic of China.
Reproduction, Fertility and Development 34(9) 669-678 https://doi.org/10.1071/RD21234
Published online: 19 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Mobile phones play an irreplaceable role in modern people’s lives. However, the radiofrequency electromagnetic radiation produced by mobile phones has also caused increasing concern. A cross-sectional study was conducted to investigate the effect of radiofrequency electromagnetic radiation produced by mobile phones on semen parameters in 1634 men who underwent semen examination at the Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, China. Analysis of variance and multivariate linear regression were used to explore differences among different groups. A P < 0.05 was considered statistically significant. The results showed significant associations among different groups of daily mobile phone use time and daily duration of phone calls in the percentage of progressively motile spermatozoa (P = 0.004 and P = 0.007), rapid progressively motile spermatozoa (P = 0.012 and P = 0.006) and total motile spermatozoa (P = 0.004 and P = 0.046). After adjustments for the confounding effects of age and body mass index by multiple linear regression, the results showed that the daily duration of mobile phone use had a negative effect on sperm motility. However, there was no statistically significant correlation between daily phone call duration and sperm motility. Therefore, the daily duration of mobile phone use may negatively affect sperm motility and impair male fertility.
Keywords: daily phone call duration, DNA fragmentation, fertility, mobile phone radiation, RF-EMR, sperm concentration, sperm motility, sperm morphology.
Introduction
Globally, infertile couples currently account for 7–15% of married couples of childbearing age (Ying et al. 2017), of which the male factor accounts for approximately 50% (Agarwal et al. 2015). Studies have shown a downward trend in the quality of human sperm, but the reason for the decline is not clear (Levine et al. 2017; Sengupta et al. 2018). A study published in 2017 indicated that the semen quality of young Chinese men has declined significantly over the past 15 years (Huang et al. 2017). The sperm concentration has also declined worldwide by an average of 57% over the past 35 years (Sengupta et al. 2017). Existing studies have confirmed a few factors that can adversely affect semen quality, including infection (Agarwal et al. 2018), dietary factors (Hatch et al. 2018; Falsig et al. 2019), shortened sleep duration (Wise et al. 2018), and antidepressant drug use (Nørr et al. 2016). The effect of radiofrequency electromagnetic radiation (RF-EMR) from mobile phones on semen quality has been the focus of attention in recent years, but the results have been controversial.
There is no denying that mobile phones have become an indispensable part of modern people’s lives because of the great convenience they provide. However, mobile phone use is also one of the main causes of exposure to RF-EMR, and the current public consensus is that mobile phone RF-EMR is a major risk factor for sperm quality decline. The harmful effects of RF-EMR on DNA integrity and various organs, such as the brain and heart, have been previously reported (Agarwal et al. 2008). The World Health Organization (WHO) has officially announced that the use of mobile phones can lead to the occurrence of brain cancer (Baan et al. 2011). La Vigneras et al. (2012) demonstrated the deleterious effects of RF-EMR on testicular stromal cells, seminal tubules, and especially sperm. It was reported that mobile phone radiation could suppress sperm motility and viability (Ghanbari et al. 2013). Oxidative stress is the main cause of sperm dysfunction, leading to male infertility and DNA damage in male germ line (Aitken et al. 2014). This state of oxidative stress occurs in spermatozoa, mainly due to an increase in ROS produced by mitochondria, and Complex III of the mitochondrial electron transport chain (ETC) as the key target of this radiation (Houston et al. 2018). Agarwal et al. (2009) also found that the non-thermal effects of RF-EMR could increase oxidative stress and lead to sperm DNA damage. The latest mate-analysis, which included 39 studies, showed that mobile phone RF-EMR exposure could reduce the motility and viability of mature human sperm in vitro, and the same conclusion was drawn from the pooled results of animal studies (Yu et al. 2021). However, the results of the current research were contradictory. Different studies have suggested that this type of radiation does not affect sperm concentration, motility, or viability in rodents (Trošić et al. 2013). Similarly, Erogul et al. (2006) did not observe any direct correlation between sperm deformation rates in men and increasing mobile phone usage time. However, the effects of RF-EMR generated by mobile phones on semen parameters have mostly been examined in animal experiments and in vitro experiments or observational studies conducted in a small number of people, and confounding factors, such as smoking and drinking, were not excluded in many cross-sectional surveys. Therefore, this study aimed to investigate the influence of mobile phone use on semen parameters based on a relatively large sample size to provide accurate guidance for men of reproductive age to minimise the effect of RF-EMR.
Materials and methods
Study population
In this study, a descriptive cross-sectional design was used to conduct a questionnaire survey among 1634 men who underwent semen examination at the Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, China, from May 30, 2020, to January 13, 2021. All participants were informed of the purpose of the questionnaire and signed an informed consent form. This study was reviewed and approved by the Ethics Committee of Women’s Hospital, School of Medicine, Zhejiang University (No.: IRB-20210111-R).
The inclusion criteria were as follows: (a) men aged between 20 and 40 years; (b) 2–7 days of abstinence; (c) an educational level above primary school (all questionnaires were completed online. To reduce the bias caused by understanding, this criterion was included); and (d) informed and voluntary consent to participate in the research.
The exclusion criteria were as follows: (a) a history of genitourinary diseases; (b) liver, kidney or other serious systemic chronic diseases; (c) a history of testicular or epididymal injury; (d) varicocele or other identifiable disease that can affect semen quality; (e) inability to complete sperm extraction by masturbation; (f) smoking; (g) daily drinking; and (h) participation in other clinical studies.
Semen collection and analyses
The participants collected their semen in a special wide-mouth container by masturbating in a private sperm collection room next to the laboratory. After the semen was collected, the sample was placed in an incubator at 37°C and gently mixed or rotated on a two-dimensional shaker. The semen analysis was usually performed 30 min after ejaculation when the semen was liquefied. The liquid status and appearance of the semen were mainly evaluated visually. Semen volume was measured by weighing the semen in a pre-weighed container. Microcell slides were prepared with 10 μL semen samples, and six field or at least 200 spermatozoa were observed. Semen concentration, total sperm count (TSC), motility and morphology were calculated by computer-aided semen analysis (CASA) and validated by technicians. Sperm chromatin structure analysis (SCSA) was used to detect the DNA fragmentation index (DFI). Single-stranded DNA fragments fluoresced red when combined with acridine orange, while intact double-stranded DNA fluoresced green when combined with acridine orange. The proportion of red fluorescent sperm in the total number of sperm was calculated to obtain the sperm DFI. Proprietary software was used to analyse flow cytometer data. Semen analysis was performed according to the WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th Edition (World Health Organization (WHO) 2010). All analyses were performed by experienced technicians at the Andrology Laboratory of the Women’s Hospital, and all technicians were unaware of the research.
The semen parameters observed in this study included the DFI, percentage of normal forms of morphology, volume, sperm concentration, total sperm number, percentage of progressively motile spermatozoa, percentage of rapid progressively motile spermatozoa, percentage of slow progressively motile spermatozoa and percentage of total motile spermatozoa.
Questionnaire
A self-designed questionnaire was used in this study. The questionnaire was formulated by the researchers (SSZ, FYM, FJ and LFX) after consulting relevant studies in the literature and modified after discussion between the research group and five andrology experts. The questionnaire consisted of two parts: basic demographic information and daily habits of mobile phone usage. The subjects were divided into different groups according to the daily habits of mobile phone usage, such as daily duration of mobile phone use (<2 h per day (h/day), 2–4 h/day, 4–6 h/day and >6 h/day), daily phone call duration (<0.5 h/day, 1–2 h/day and >2 h/day), use of earphones while talking on the mobile phone (never, occasionally and always) and the location where the mobile phone was carried (bag, coat pocket, rear trouser pocket and front pants pocket). The questionnaire data were collected online in this study. Before the survey, all researchers were provided with relevant training, including regarding matters needing attention in filling out the questionnaire, providing unified instructions, and questionnaire quality control. The questionnaire was completed online before semen extraction, and the answers were collected and reviewed by the same researchers. To avoid repeat submissions, only one questionnaire could be submitted for each internet protocol address. Upon reviewing the daily questionnaires, incomplete data were eliminated. The semen report was checked by the participant’s medical record number, and the medical history was checked. The results were double-checked and recorded by two researchers.
Statistical analysis
Statistical software (IBM SPSS Statistics, version 25.0; IBM Corp.) was used for statistical analysis of the data. Measurement data are described by the mean plus or minus standard deviation. Due to the large sample size in this study, the dependent variable was a continuous variable, and the independent variable was a combination of classified and ordered variables. Analysis of variance was performed to explore differences among different groups. Multiple linear regression analysis was used to eliminate the influence of potential confounders, including age and body mass index (BMI). P < 0.05 was considered statistically significant.
Results
Basic clinical information of research subjects
In this study, a total of 1712 questionnaires were collected, 78 of which were eliminated because they were invalid, and 1634 participants were included in the final analysis, giving a recovery rate of 95.4%. Table 1 shows the basic clinical information of the participants. The mean age of the subjects was 31.45 ± 3.55 (20–40) years, and the mean BMI was 23.45 ± 3.03 (14.17–35.16) kg/m2. The mean DFI, volume, sperm concentration, total sperm number, percentage of progressively motile spermatozoa and total motile spermatozoa were all within the normal range of WHO guidelines (World Health Organization (WHO) 2010), while the percentage of normal forms of morphology was low (3.20 ± 1.83%).

|
Influence of daily mobile phone use time on semen parameters
According to our results (Table 2), there were significant differences among the different groups of daily mobile phone use time in terms of sperm motility, including the progressively motile spermatozoa rate (P = 0.004), rapid progressively motile spermatozoa rate (P = 0.012) and total motile spermatozoa rate (P = 0.004). The results indicate that sperm motility gradually decreased with increasing mobile phone use duration. However, there were no significant differences in the DFI (P = 0.579), normal forms of morphology (P = 0.170), sperm volume (P = 0.208), concentration (P = 0.689), total sperm number (P = 0.729) or slow progressively motile spermatozoa rate (P = 0.127). Bonferroni pairwise comparison showed significant differences in the percentage of progressively motile spermatozoa between different groups of daily mobile phone use times (P = 0.015 and P = 0.005 for 2–4 h vs 4–6 h and 2–4 h vs >6 h, respectively). Similar results were observed in the percentage of rapid progressive motile spermatozoa (P = 0.048 and P = 0.010 for 2–4 h vs 4–6 h and 2–4 h vs >6 h, respectively) and the percentage of total motile spermatozoa (P = 0.012 and P = 0.006 for 2–4 h vs 4–6 h and 2–4 h vs >6 h, respectively).
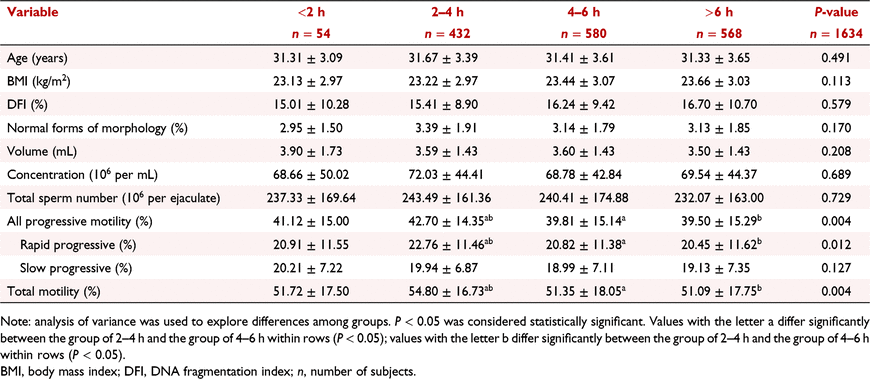
|
Influence of daily duration of phone calls on semen parameters
Table 3 shows that there were significant differences in sperm motility among the three groups of different accumulated daily call times, including the progressively motile spermatozoa rate (P = 0.007), rapid progressively motile spermatozoa rate (P = 0.006) and total motile spermatozoa rate (P = 0.046). Bonferroni pairwise comparison showed significant differences in the percentage of progressively motile spermatozoa (P = 0.005 and P = 0.027 for >2 h vs <0.5 h and >2 h vs 1–2 h) and the percentage of rapid progressive motile spermatozoa (P = 0.004 and P = 0.012 for >2 h vs <0.5 h and >2 h vs 1–2 h) between different groups of daily durations of phone calls. There were also significant differences among the three groups in age (P = 0.003) and BMI (P = 0.001).

|
Influence of earphone use during mobile phone use on semen parameters
As Table 4 shows, regardless of whether earphones were used for mobile phone calls, there were no differences in the DFI, normal forms of morphology, volume, sperm concentration, total sperm number, or sperm motility (P > 0.05).

|
Influence of mobile phone location on semen parameters
As shown in Table 5, there were no differences among the groups in the DFI, normal forms of morphology, volume, sperm concentration, total sperm number or sperm motility (P > 0.05) according to different mobile phone locations.

|
Multiple linear regression results for mobile phone use and semen parameters
As shown in Table 6, factors affecting semen quality from univariate analysis results were included in multiple linear regression analysis. After adjusting for the confounding effects of age and BMI, the results showed that the daily duration of mobile phone use had a statistically significant effect on sperm motility. When the daily duration of mobile phone use increased by 1 unit, the percentage of progressively motile spermatozoa decreased by 1.29% (95% CI: −2.16, −0.43; P = 0.004), the percentage of rapid progressive motile spermatozoa decreased by 0.88% (95% CI: −1.54, −0.21; P = 0.010), and the percentage of total motile spermatozoa decreased by 1.47% (95% CI: −2.49, −0.44; P = 0.005). However, the effect of daily duration of phone calls on semen parameters was no longer significant (P > 0.05).

|
Discussion
There are more than 186 million infertile people in the world, mainly from developing countries (Louis et al. 2013), and male factors account for approximately 50% of these cases of infertility (Agarwal et al. 2015). Sperm concentrations have declined worldwide by an average of 57% in recent years (Sengupta et al. 2017). Huang et al. (2017) analysed sperm samples from 30 636 young Chinese men who donated between 2001 and 2015. In the time period from 2001 and 2005 and from 2011 to 2015, the average sperm concentration dropped from 68 million/mL to 47 million/mL, the progressively motile sperm count dropped from 34 million to 21 million, and the percentage of normal forms of morphology dropped from 31.8% to 10.8%. The average percentage of sperm with normal morphology of the subjects in this study was 3.20% (±1.83), lower than the normal range of WHO guidelines (≥4%), while the average value of other semen parameters was within the normal range, which may be related to the fact that most of the subjects in our study were part of the infertile population.
In this study, we found that the average daily mobile phone use duration was negatively correlated with the progressively motile spermatozoa rate, rapid progressively motile spermatozoa rate, and total motile spermatozoa rate. Sperm motility is crucial to male fertility and has been the focus of recent studies on the effect of RF-EMR from mobile phones on male semen quality. In a study of 371 men who were assessed for infertility, Fejes et al. (2005) found that the time spent on mobile phones was inversely associated with the rapid progressively motile spermatozoa rate and positively associated with the slow progressively motile spermatozoa rate. An in vitro study showed that RF-EMR from mobile phones resulted in a slight decrease in both rapid progressively and slow progressively motile spermatozoa (Erogul et al. 2006). This is similar to our results, except that we did not find an association between mobile phone use duration and slow progressively motile spermatozoa rate. Our results are consistent with the findings of two meta-analyses in 2014 showing that mobile phone use was associated with the total motile spermatozoa rate but not with other semen parameters (Adams et al. 2014; Liu et al. 2014). Clinical data from manual sperm motility assessments and computer-assisted sperm analysis suggest that the distinction between rapidly progressing sperm is biologically and clinically important. The evidence includes in vivo conception (Barratt et al. 1992), artificial insemination (Bollendorf et al. 1996), donor insemination (Irvine and Aitken 1986) and in vitro fertilisation (Sifer et al. 2005). The motility of healthy sperm has forward progressions of at least 25 μm/s, and the percentages of grade A and grade B progressively motile sperm are at least 50%. If these parameters are not met, the sperm may have difficulty passing through cervical mucus, resulting in fertilisation failure (Kumar and Singh 2015). In our study, it was observed that the percentage of all progressive motility and total motility dropped from 42.70 ± 14.35% to 39.50 ± 15.29% and 54.80 ± 16.73% to 51.09 ± 17.75% with a duration of 2–4 h and >6 h of mobile phone use, respectively, and this remained statistically significant after multiple linear regression. The percentages of all progressive motility and total motility decreased (P < 0.05), albeit only slightly. We believe that mobile phone RF-EMR may be the main cause of sperm motility decline. These trends suggest that recent concerns about long-term exposure to RF-EMR from mobile phones should be taken more seriously, given the growing trend of deterioration of the male reproductive system. Thus, the duration of mobile phone use should be reduced in daily life in order to avoid further declines in sperm motility, affecting fertility, especially in men of reproductive age with asthenospermia. The extent of progressive sperm motility is related to pregnancy rates (World Health Organization (WHO) 2010). It was also found that sperm motility was the most significant parameter in predicting the chance of natural conception in a study based on 358 semen samples from a group of men representing the general male population (Larsen et al. 2000). Similarly, the multivariate analysis of an earlier study showed that the best prognostic indicator of fertility was given by the percentage of motile sperm, particularly in patients with primary infertility (Jouannet et al. 1988). In another study, using mobile phones for more than 4 h a day or carrying them in back pant pockets resulted in a slight increase in the DFI (Rago et al. 2013). However, in our study we did not find any difference in the DFI according to mobile phone use duration. In addition, we did not find differences in the percentage of normal forms of morphology, volume, sperm concentration, or total sperm number according to mobile phone use duration, similar to the findings of a US study involving 153 infertile subjects, in which there were no associations between daily mobile phone use duration and semen parameters (Lewis et al. 2017).
The daily phone call duration has been shown to negatively affect the sperm concentration and sperm count in previous studies. In a study assessing infertility in 361 men, the daily phone call duration was found to be associated with a drop in the total sperm number (Agarwal et al. 2008). A study in 2015 also found that a daily talking duration ≥1 h was associated with an abnormal semen concentration (Zilberlicht et al. 2015). A cross-sectional study involving 794 young men in China showed that the mean semen volume, sperm concentration, and total sperm number decreased slightly with increasing daily talking time on a mobile phone (Zhang et al. 2017). Interestingly, in univariate analysis results of this study, the progressively motile spermatozoa rate, rapid progressively motile spermatozoa rate, and total motile spermatozoa rate were significantly different among different groups of daily duration of phone calls, but the differences were no longer significant in the multiple linear regression analysis. Similarly, no differences were found in the DFI, the percentage of normal forms of morphology, volume, sperm concentration, total sperm number, or slow progressively motile spermatozoa rate.
We did not observe any correlation of semen quality with the use of a headset while talking on the phone or the location where the mobile phone was carried. This is consistent with the findings of Lewis et al. (2017), who found no correlation of mobile phone placement or earphone with semen parameters. Studies have shown that the anomalies caused by RF-EMR depend on physical parameters such as exposure time, distance from the source of radiation, power density and penetration depth (Kesari et al. 2018). Front trouser pocket exposure was one of our prior exposures of interest, which was considered to have the greatest biological justification for the potential effects of RF-EMR on testicular function due to proximity, and most men tended to keep their phones in the front pant pockets. In this study, no significant differences were observed among the different groups of mobile phone placement, which was similar to previous studies. A cross-sectional study in China found little correlation between different mobile phone locations (mainly the front pant pocket compared to other locations) and semen parameters (Zhang et al. 2017). Hatch et al. (2021) found little evidence of an overall link between mobile phone use and fertility or semen quality in men. Although mobile phone placement in the front pants pocket was associated with lower fecundability in thinner men with BMI < 25 kg/m2, this association did not increase with prolonged exposure to RF-EMR. There were also some different conclusions. Rago et al. (2013) found that placing the mobile phone in the back pant pocket led to a slight increase in the sperm DNA fragmentation rate. However, we did not obtain a similar result.
The decrease in sperm quality caused by RF-EMR is likely to be related to oxidative stress due to increased levels of free radicals or superoxide anions, as increases in the superoxide anion concentration can trigger decreases in sperm motility (Agarwal et al. 2009). De Iuliis et al. (2006) found in their study that spontaneous superoxide produced by human spermatozoa originated from nonmitochondrial sources and was shown to result in both severe sperm motility loss and DNA damage. A meta-analysis in 2020 found that the nonthermal effects of RF-EMR were limited to specific rapidly growing and poorly differentiated cells, such as human sperm cells (based on 19 reported experiments, P = 0.002) and human epithelial cells (based on 89 reported experiments, P < 0.0001) (Halgamuge et al. 2020). Previous studies have confirmed that continuous mobile phone use will lead to excessive reactive oxygen species (ROS) production, which will lead to DNA damage in sperm (Kumar et al. 2014). De Iuliis et al. (2009a) found that RF-EMR in both the mobile phone power density and frequency range enhances the production of reactive oxygen species in the mitochondria of human spermatozoa, reducing the motility and vitality of these cells while stimulating the formation of a DNA base adduct and resulting in DNA fragmentation. In the same year, they found in another study that the efficiency of chromatin remodelling and the formation of 8-hydroxy-20-deoxyguanosine (8OHdG) were highly correlated with DNA damage in human spermatozoa (De Iuliis et al. 2009b). Similarly, Kesari et al. (2018) have shown, based on existing research evidence in vitro and in vivo, that RF-EMR may cause oxidative stress and increase the level of ROS, which may lead to infertility. This suggests that exposure to RF-EMR has a negative effect on sperm quality. Several animal and human studies have found that prolonged exposure to RF radiation can lead to a reduced total sperm count and reduced sperm motility (Salama et al. 2010; Adams et al. 2014). However, a few recent studies have found that there seems to be little relationship between the two (Mortazavi et al. 2017). Mobile phones produce low levels of RF radiation in the range of 800–2600 MHz, and human exposure to RF radiation from mobile phones is measured by specific absorption rates (SARs). Some people believe that due to the strict regulatory standards for SAR and the operating power of mobile phones, the increase in heat during mobile phone use has little effect on nearby tissues and is unlikely to affect semen quality. Some in vitro studies have shown that 2.45-GHz RF-EMR has effects on sperm motility and DNA fragmentation in human semen (Avendaño et al. 2012). However, the short-term effects of RF radiation are not sufficient to cause any genomic changes, as such damage may be the result of cumulative effects of repeated exposure (Agarwal et al. 2011). Therefore, there is no final conclusion regarding the effect of RF-EMR from mobile phones on semen quality worldwide, and the effect of short-term direct RF radiation on semen quality in vitro is different from that of long-term RF radiation due to carrying mobile phones.
This study has some limitations. First, since the most common way to collect data on mobile phone use in a cross-sectional study is through patient statements, it is inevitable that there will be some information bias, which may be unavoidable in all observational studies. This study collected information on mobile phone use in the more recent past, and the mobile phone’s built-in software was used to view the relevant information about the users’ mobile phone use situation, likely resulting in less recall bias. In addition, other sources of RF radiation in the environment cannot be explained or excluded (Chiaramello et al. 2019). The subjects of our study were all from the Department of Reproductive Endocrinology, Women’s Hospital, School of Medicine, Zhejiang University, China. Therefore, the subjects of our study were limited, and the influence of regional differences could not be excluded. Sperm vitality is a key standard semen analysis parameter. However, as our study population comprised those who underwent semen examination in the Andrology clinic, most of them were patients who underwent premarital examination or prenatal examination, so the semen indicators of the vast majority of men were normal. Thus, sperm viability is not a routine item in our Andrology clinic. Considering the large differences in sample sizes of different semen indicators, sperm vitality was excluded in this study. However, the results of our study have certain clinical significance, which can provide accurate guidance on mobile phone use for men of reproductive age to minimise the effect of RF-EMR produced by mobile phones on semen quality and provide original data for future related research. This study is a cross-sectional study. The relationship between the study factors and the conclusions is exploratory, and the causal relationship needs to be confirmed by further prospective studies.
Conclusion
Our results suggest that the average daily cell phone use duration may affect sperm motility to some extent, leading to a decrease in sperm motility. Therefore, we recommend that men of reproductive age avoid prolonged durations of using mobile phones. In addition, more well-designed cross-sectional investigations and mechanistic studies are needed in the future to clarify the effects of RF-EMR produced by mobile phones on male semen quality.
Data availability
The data that support this study are available in the article.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was supported by the National Key Research and Development Program of China (grant number 2018YFC1004900).
Author contributions
Conception and design: Lanfeng Xing, Shanshan Zhang, Fengyi Mo. Administrative support: Lanfeng Xing, Fan Jin. Provision of study materials or patients: Shanshan Zhang, Fengyi Mo. Collection and assembly of data: Shanshan Zhang, Yali Chang, Shufang Wu, Qing Ma. Data analysis and interpretation: Shanshan Zhang, Yali Chang. Manuscript writing: all authors. Final approval of manuscript: all authors.
Acknowledgements
We acknowledge Lejun Li, Jingping Li and Zhongyan Liang for their advice.
References
Adams, JA, Galloway, TS, Mondal, D, Esteves, SC, and Mathews, F (2014). Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environment International 70, 106–112.| Effect of mobile telephones on sperm quality: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24927498PubMed |
Agarwal, A, Deepinder, F, Sharma, RK, Ranga, G, and Li, J (2008). Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertility and Sterility 89, 124–128.
| Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study.Crossref | GoogleScholarGoogle Scholar | 17482179PubMed |
Agarwal, A, Desai, NR, Makker, K, Varghese, A, Mouradi, R, Sabanegh, E, and Sharma, R (2009). Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertility and Sterility 92, 1318–1325.
| Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study.Crossref | GoogleScholarGoogle Scholar | 18804757PubMed |
Agarwal, A, Singh, A, Hamada, A, and Kesari, K (2011). Cell phones and male infertility: a review of recent innovations in technology and consequences. International Brazilian Journal of Urology 37, 432–454.
| Cell phones and male infertility: a review of recent innovations in technology and consequences.Crossref | GoogleScholarGoogle Scholar | 21888695PubMed |
Agarwal, A, Mulgund, A, Hamada, A, and Chyatte, MR (2015). A unique view on male infertility around the globe. Reproductive Biology and Endocrinology 13, 37.
| A unique view on male infertility around the globe.Crossref | GoogleScholarGoogle Scholar | 25928197PubMed |
Agarwal, A, Rana, M, Qiu, E, AlBunni, H, Bui, AD, and Henkel, R (2018). Role of oxidative stress, infection and inflammation in male infertility. Andrologia 50, e13126.
| Role of oxidative stress, infection and inflammation in male infertility.Crossref | GoogleScholarGoogle Scholar | 30569652PubMed |
Aitken, RJ, Smith, TB, Jobling, MS, Baker, MA, and De Iuliis, GN (2014). Oxidative stress and male reproductive health. Asian Journal of Andrology 16, 31–38.
| Oxidative stress and male reproductive health.Crossref | GoogleScholarGoogle Scholar | 24369131PubMed |
Avendaño, C, Mata, A, Sanchez Sarmiento, CA, and Doncel, GF (2012). Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertility and Sterility 97, 39–45.e2.
| Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation.Crossref | GoogleScholarGoogle Scholar | 22112647PubMed |
Baan, R, Grosse, Y, Lauby-Secretan, B, El Ghissassi, F, Bouvard, V, Benbrahim-Tallaa, L, Guha, N, Islami, F, Galichet, L, and Straif, K (2011). Carcinogenicity of radiofrequency electromagnetic fields. The Lancet Oncology 12, 624–626.
| Carcinogenicity of radiofrequency electromagnetic fields.Crossref | GoogleScholarGoogle Scholar | 21845765PubMed |
Barratt, CLR, McLeod, ID, Dunphy, BC, and Cooke, ID (1992). Prognostic value of two putative sperm function tests: hypo-osmotic swelling and bovine sperm mucus penetration test (Penetrak). Human Reproduction 7, 1240–1244.
| Prognostic value of two putative sperm function tests: hypo-osmotic swelling and bovine sperm mucus penetration test (Penetrak).Crossref | GoogleScholarGoogle Scholar |
Bollendorf, A, Check, JH, and Lurie, D (1996). Evaluation of the effect of the absence of sperm with rapid and linear progressive motility on subsequent pregnancy rates following intrauterine insemination or in vitro fertilization. Journal of Andrology 17, 550–557.
| 8957699PubMed |
Chiaramello, E, Bonato, M, Fiocchi, S, Tognola, G, Parazzini, M, Ravazzani, P, and Wiart, J (2019). Radio frequency electromagnetic fields exposure assessment in indoor environments: a review. International Journal of Environmental Research and Public Health 16, 955.
| Radio frequency electromagnetic fields exposure assessment in indoor environments: a review.Crossref | GoogleScholarGoogle Scholar |
De Iuliis, GN, Wingate, JK, Koppers, AJ, McLaughlin, EA, and Aitken, RJ (2006). Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa. The Journal of Clinical Endocrinology & Metabolism 91, 1968–1975.
| Definitive evidence for the nonmitochondrial production of superoxide anion by human spermatozoa.Crossref | GoogleScholarGoogle Scholar |
De Iuliis, GN, Newey, RJ, King, BV, and Aitken, RJ (2009a). Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 4, e6446.
| Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro.Crossref | GoogleScholarGoogle Scholar | 19649291PubMed |
De Iuliis, GN, Thomson, LK, Mitchell, LA, Finnie, JM, Koppers, AJ, Hedges, A, Nixon, B, and Aitken, RJ (2009b). DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress. Biology of Reproduction 81, 517–524.
| DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2′-deoxyguanosine, a marker of oxidative stress.Crossref | GoogleScholarGoogle Scholar | 19494251PubMed |
Erogul, O, Oztas, E, Yildirim, I, Kir, T, Aydur, E, Komesli, G, Irkilata, HC, Irmak, MK, and Peker, AF (2006). Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Archives of Medical Research 37, 840–843.
| Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study.Crossref | GoogleScholarGoogle Scholar | 16971222PubMed |
Falsig, A-ML, Gleerup, CS, and Knudsen, UB (2019). The influence of omega-3 fatty acids on semen quality markers: a systematic PRISMA review. Andrology 7, 794–803.
| The influence of omega-3 fatty acids on semen quality markers: a systematic PRISMA review.Crossref | GoogleScholarGoogle Scholar |
Fejes, I, Závaczki, Z, Szöllősi, J, Koloszár, S, Daru, J, Kovács, L, and Pál, A (2005). Is there a relationship between cell phone use and semen quality? Archives of Andrology 51, 385–393.
| Is there a relationship between cell phone use and semen quality?Crossref | GoogleScholarGoogle Scholar | 16087567PubMed |
Ghanbari, M, Mortazavi, SB, Khavanin, A, and Khazaei, M (2013). The effects of cell phone waves (900 MHz-GSM band) on sperm parameters and total antioxidant capacity in rats. International Journal of Fertility and Sterility 7, 21–28.
| 24520459PubMed |
Halgamuge, MN, Skafidas, E, and Davis, D (2020). A meta-analysis of in vitro exposures to weak radiofrequency radiation exposure from mobile phones (1990–2015). Environmental Research 184, 109227.
| A meta-analysis of in vitro exposures to weak radiofrequency radiation exposure from mobile phones (1990–2015).Crossref | GoogleScholarGoogle Scholar | 32199316PubMed |
Hatch, EE, Wesselink, AK, Hahn, KA, Michiel, JJ, Mikkelsen, EM, Sorensen, HT, Rothman, KJ, and Wise, LA (2018). Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology 29, 369–378.
| Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort.Crossref | GoogleScholarGoogle Scholar | 29384791PubMed |
Hatch, EE, Willis, SK, Wesselink, AK, Mikkelsen, EM, Eisenberg, ML, Sommer, GJ, Sorensen, HT, Rothman, KJ, and Wise, LA (2021). Male cellular telephone exposure, fecundability, and semen quality: results from two preconception cohort studies. Human Reproduction 36, 1395–1404.
| Male cellular telephone exposure, fecundability, and semen quality: results from two preconception cohort studies.Crossref | GoogleScholarGoogle Scholar | 33564831PubMed |
Houston, BJ, Nixon, B, King, BV, Aitken, RJ, and De Iuliis, GN (2018). Probing the origins of 1,800 MHz radio frequency electromagnetic radiation induced damage in mouse immortalized germ cells and spermatozoa in vitro. Frontiers in Public Health 6, 270.
| Probing the origins of 1,800 MHz radio frequency electromagnetic radiation induced damage in mouse immortalized germ cells and spermatozoa in vitro.Crossref | GoogleScholarGoogle Scholar | 30298125PubMed |
Huang, C, Li, B, Xu, K, Liu, D, Hu, J, Yang, Y, Nie, H, Fan, L, and Zhu, W (2017). Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertility and Sterility 107, 83–88.e2.
| Decline in semen quality among 30,636 young Chinese men from 2001 to 2015.Crossref | GoogleScholarGoogle Scholar | 27793371PubMed |
Irvine, DS, and Aitken, RJ (1986). Predictive value of in-vitro sperm function tests in the context of an AID service. Human Reproduction 1, 539–545.
| Predictive value of in-vitro sperm function tests in the context of an AID service.Crossref | GoogleScholarGoogle Scholar | 3818912PubMed |
Jouannet, P, Ducot, B, Feneux, D, and Spira, A (1988). Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics. International Journal of Andrology 11, 379–394.
| Male factors and the likelihood of pregnancy in infertile couples. I. Study of sperm characteristics.Crossref | GoogleScholarGoogle Scholar | 3235207PubMed |
Kesari, KK, Agarwal, A, and Henkel, R (2018). Radiations and male fertility. Reproductive Biology and Endocrinology 16, 118.
| Radiations and male fertility.Crossref | GoogleScholarGoogle Scholar | 30445985PubMed |
Kumar, N, and Singh, AK (2015). Trends of male factor infertility, an important cause of infertility: a review of literature. Journal of Human Reproductive Sciences 8, 191–196.
| Trends of male factor infertility, an important cause of infertility: a review of literature.Crossref | GoogleScholarGoogle Scholar | 26752853PubMed |
Kumar, S, Nirala, JP, Behari, J, and Paulraj, R (2014). Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian Journal of Experimental Biology 52, 890–897.
| 25241589PubMed |
La Vignera, S, Condorelli, RA, Vicari, E, D’Agata, R, and Calogero, AE (2012). Effects of the exposure to mobile phones on male reproduction: a review of the literature. Journal of Andrology 33, 350–356.
| Effects of the exposure to mobile phones on male reproduction: a review of the literature.Crossref | GoogleScholarGoogle Scholar | 21799142PubMed |
Larsen, L, Scheike, T, Jensen, TK, Bonde, JP, Ernst, E, Hjollund, NH, Zhou, Y, Skakkebaek, NE, and Giwercman, A (2000). Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Human Reproduction 15, 1562–1567.
| Computer-assisted semen analysis parameters as predictors for fertility of men from the general population.Crossref | GoogleScholarGoogle Scholar | 10875866PubMed |
Levine, H, Jørgensen, N, Martino-Andrade, A, Mendiola, J, Weksler-Derri, D, Mindlis, I, Pinotti, R, and Swan, SH (2017). Temporal trends in sperm count: a systematic review and meta-regression analysis. Human Reproduction Update 23, 646–659.
| Temporal trends in sperm count: a systematic review and meta-regression analysis.Crossref | GoogleScholarGoogle Scholar | 28981654PubMed |
Lewis, RC, Minguez-Alarcon, L, Meeker, JD, Williams, PL, Mezei, G, Ford, JB, and Hauser, R (2017). Self-reported mobile phone use and semen parameters among men from a fertility clinic. Reproductive Toxicology 67, 42–47.
| Self-reported mobile phone use and semen parameters among men from a fertility clinic.Crossref | GoogleScholarGoogle Scholar | 27838386PubMed |
Liu, K, Li, Y, Zhang, G, Liu, J, Cao, J, Ao, L, and Zhang, S (2014). Association between mobile phone use and semen quality: a systemic review and meta-analysis. Andrology 2, 491–501.
| Association between mobile phone use and semen quality: a systemic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24700791PubMed |
Louis, JF, Thoma, ME, Sørensen, DN, McLain, AC, King, RB, Sundaram, R, Keiding, N, and Buck Louis, GM (2013). The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 1, 741–748.
| The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample.Crossref | GoogleScholarGoogle Scholar | 23843214PubMed |
Mortazavi, SMJ, Mortazavi, SAR, and Paknahad, M (2017). Self-reported mobile phone use and semen parameters among men from a fertility clinic. Reproductive Toxicology 71, 164.
| Self-reported mobile phone use and semen parameters among men from a fertility clinic.Crossref | GoogleScholarGoogle Scholar | 28188905PubMed |
Nørr, L, Bennedsen, B, Fedder, J, and Larsen, ER (2016). Use of selective serotonin reuptake inhibitors reduces fertility in men. Andrology 4, 389–394.
| Use of selective serotonin reuptake inhibitors reduces fertility in men.Crossref | GoogleScholarGoogle Scholar | 27019308PubMed |
Rago, R, Salacone, P, Caponecchia, L, Sebastianelli, A, Marcucci, I, Calogero, AE, Condorelli, R, Vicari, E, Morgia, G, Favilla, V, Cimino, S, Arcoria, AF, and La Vignera, S (2013). The semen quality of the mobile phone users. Journal of Endocrinological Investigation 36, 970–974.
| The semen quality of the mobile phone users.Crossref | GoogleScholarGoogle Scholar | 23722985PubMed |
Salama, N, Kishimoto, T, and Kanayama, H-O (2010). Effects of exposure to a mobile phone on testicular function and structure in adult rabbit. International Journal of Andrology 33, 88–94.
| Effects of exposure to a mobile phone on testicular function and structure in adult rabbit.Crossref | GoogleScholarGoogle Scholar | 19076254PubMed |
Sengupta, P, Nwagha, U, Dutta, S, Krajewska-Kulak, E, and Izuka, E (2017). Evidence for decreasing sperm count in African population from 1965 to 2015. African Health Sciences 17, 418–427.
| Evidence for decreasing sperm count in African population from 1965 to 2015.Crossref | GoogleScholarGoogle Scholar | 29062337PubMed |
Sengupta, P, Borges, E, Dutta, S, and Krajewska-Kulak, E (2018). Decline in sperm count in European men during the past 50 years. Human & Experimental Toxicology 37, 247–255.
| Decline in sperm count in European men during the past 50 years.Crossref | GoogleScholarGoogle Scholar |
Sifer, C, Sasportes, T, Barraud, V, Poncelet, C, Rudant, J, Porcher, R, Cedrin-Durnerin, I, Martin-Pont, B, Hugues, JN, and Wolf, JP (2005). World Health Organization grade ‘a’ motility and zona-binding test accurately predict IVF outcome for mild male factor and unexplained infertilities. Human Reproduction 20, 2769–2775.
| World Health Organization grade ‘a’ motility and zona-binding test accurately predict IVF outcome for mild male factor and unexplained infertilities.Crossref | GoogleScholarGoogle Scholar | 15958402PubMed |
Trošić, I, Mataušić-Pišl, M, Pavičić, I, and Marjanović, AM (2013). Histological and cytological examination of rat reproductive tissue after short-time intermittent radiofrequency exposure. Archives of Industrial Hygiene and Toxicology 64, 513–519.
| Histological and cytological examination of rat reproductive tissue after short-time intermittent radiofrequency exposure.Crossref | GoogleScholarGoogle Scholar | 24384757PubMed |
Wise, LA, Rothman, KJ, Wesselink, AK, Mikkelsen, EM, Sorensen, HT, McKinnon, CJ, and Hatch, EE (2018). Male sleep duration and fecundability in a North American preconception cohort study. Fertility and Sterility 109, 453–459.
| Male sleep duration and fecundability in a North American preconception cohort study.Crossref | GoogleScholarGoogle Scholar | 29566862PubMed |
World Health Organization (WHO) (2010) ‘WHO Laboratory Manual for the examination and processing of human semen’, 5th edn. (WHO Press: Geneva)
Ying, L, Chen, X, Wu, LH, Shu, J, Wu, X, and Loke, AY (2017). The Partnership And Coping Enhancement Programme for couples undergoing in vitro fertilization treatment: the development of a complex intervention in China. 34, 99–108.
| The Partnership And Coping Enhancement Programme for couples undergoing in vitro fertilization treatment: the development of a complex intervention in China.Crossref | GoogleScholarGoogle Scholar | 27744588PubMed |
Yu, G, Bai, Z, Song, C, Cheng, Q, Wang, G, Tang, Z, and Yang, S (2021). Current progress on the effect of mobile phone radiation on sperm quality: an updated systematic review and meta-analysis of human and animal studies. Environmental Pollution 282, 116952.
| Current progress on the effect of mobile phone radiation on sperm quality: an updated systematic review and meta-analysis of human and animal studies.Crossref | GoogleScholarGoogle Scholar | 33862271PubMed |
Zhang, G, Yan, H, Chen, Q, Liu, K, Ling, X, Sun, L, Zhou, N, Wang, Z, Zou, P, Wang, X, Tan, L, Cui, Z, Zhou, Z, Liu, J, Ao, L, and Cao, J (2017). Reply to comment on “Effects of cell phone use on semen parameters: results from the MARHCS cohort study in Chongqing, China”. Environment International 98, 231–232.
| Reply to comment on “Effects of cell phone use on semen parameters: results from the MARHCS cohort study in Chongqing, China”.Crossref | GoogleScholarGoogle Scholar | 27838118PubMed |
Zilberlicht, A, Wiener-Megnazi, Z, Sheinfeld, Y, Grach, B, Lahav-Baratz, S, and Dirnfeld, M (2015). Habits of cell phone usage and sperm quality – does it warrant attention? Reproductive BioMedicine Online 31, 421–426.
| Habits of cell phone usage and sperm quality – does it warrant attention?Crossref | GoogleScholarGoogle Scholar | 26206279PubMed |


