Maternal undernutrition during pregnancy and lactation increases transcription factors, ETV5 and GDNF, and alters regulation of apoptosis and heat shock proteins in the testis of adult offspring in the rat
Graciela Pedrana A F , Camila Larrañaga A , Alejandra Diaz A , Helen Viotti A , Paula Lombide A , Daniel Cavestany A , Mark H. Vickers B , Graeme B. Martin C and Deborah M. Sloboda D E
A F , Camila Larrañaga A , Alejandra Diaz A , Helen Viotti A , Paula Lombide A , Daniel Cavestany A , Mark H. Vickers B , Graeme B. Martin C and Deborah M. Sloboda D E
A Facultad de Veterinaria, Universidad de la República, Montevideo, 11600, Uruguay.
B Liggins Institute, University of Auckland, Auckland, 1142, New Zealand.
C UWA School of Agriculture and Environment and UWA Institute of Agriculture, University of Western Australia, Perth, WA 6009, Australia.
D Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, L8S 4L8, Canada.
E Department of Pediatrics, McMaster University, Hamilton, L8S 4L8, Canada, and Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, L8S 4L8, Canada.
F Corresponding author. Email: gpedrana@gmail.com
Reproduction, Fertility and Development 33(7) 484-496 https://doi.org/10.1071/RD20260
Submitted: 30 September 2020 Accepted: 24 March 2021 Published: 22 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
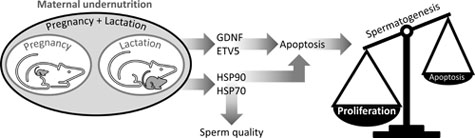
We tested whether changes in Sertoli cell transcription factors and germ cell heat shock proteins (HSPs) are linked to the effects of maternal undernutrition on male offspring fertility. Rats were fed ad libitum with a standard diet (CONTROL) throughout pregnancy and lactation or with 50% of CONTROL intake throughout pregnancy (UNP) or lactation (UNL) or both periods (UNPL). After postnatal Day 21, 10 male pups per group were fed a standard diet ad libitum until postnatal Day 160 when testes were processed for histological, mRNA and immunohistochemical analyses. Compared with CONTROL: caspase-3 was increased in UNP and UNPL (P = 0.001); Bax was increased in UNL (P = 0.002); Bcl-2 (P < 0.0001) was increased in all underfed groups; glial cell line-derived neurotrophic factor (P = 0.002) was increased in UNP and UNL; E twenty-six transformation variant gene 5 and HSP70 were increased, and HSP90 was diminished in all underfed groups (P < 0.0001). It appears that maternal undernutrition during pregnancy and lactation disrupts the balance between proliferation and apoptosis in germ cells, increasing germ cell production and perhaps exceeding the support capacity of the Sertoli cells. Moreover, fertility could be further compromised by changes in meiosis and spermiogenesis mediated by germ cell HSP90 and HSP70.
Keywords: nutrition, fetal programming, transcription factor, testis, Sertoli cell, apoptosis.
References
Au, C. E., Hermo, L., Byrne, E., Smirle, J., Fazel, A., Simon, P. H. G., Kearney, R. E., Cameron, P. H., Smith, C. E., Vali, H., Fernandez-Rodriguez, J., Ma, K., Nilsson, T., and Bergeron, J. J. M. (2015). Expression, sorting, and segregation of Golgi proteins during germ cell differentiation in the testis. Mol. Biol. Cell 26, 4015–4032.| Expression, sorting, and segregation of Golgi proteins during germ cell differentiation in the testis.Crossref | GoogleScholarGoogle Scholar | 25808494PubMed |
Bateson, P., Barker, D., Clutton-Brock, T., Deb, D., D’Udine, B., Foley, R. A., Gluckman, P., Godfrey, K., Kirkwood, T., Lahr, M. M., McNamara, J., Metcalfe, N. B., Monaghan, P., Spencer, H. G., and Sultan, S. E. (2004). Developmental plasticity and human health. Nature 430, 419–421.
| Developmental plasticity and human health.Crossref | GoogleScholarGoogle Scholar | 15269759PubMed |
Beere, H. M. (2004). ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117, 2641–2651.
| ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis.Crossref | GoogleScholarGoogle Scholar | 15169835PubMed |
Bell, A. W., and Greenwood, P. L. (2016). Prenatal origins of postnatal variation in growth, development and productivity of ruminants. Anim. Prod. Sci. 56, 1217–1232.
| Prenatal origins of postnatal variation in growth, development and productivity of ruminants.Crossref | GoogleScholarGoogle Scholar |
Chen, S.-R., and Liu, Y.-X. (2015). Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 149, R159–R167.
| Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling.Crossref | GoogleScholarGoogle Scholar | 25504872PubMed |
Cooke, P. S., Hess, R. A., Simon, L., Schlesser, H. N., Carnes, K., Tyagi, G., Hofmann, M.-C., and Murphy, K. M. (2006). The transcription factor Ets-Related Molecule (ERM) is essential for spermatogonial stem cell maintenance and self-renewal. Anim. Reprod. 3, 98–107.
Dixit, A., and Verkhivker, G. M. (2012). Probing Molecular Mechanisms of the Hsp90 Chaperone: Biophysical Modeling Identifies Key Regulators of Functional Dynamics. PLoS One 7, e37605.
| Probing Molecular Mechanisms of the Hsp90 Chaperone: Biophysical Modeling Identifies Key Regulators of Functional Dynamics.Crossref | GoogleScholarGoogle Scholar | 23284721PubMed |
Domingos, F. F. T., Thomé, R. G., Martinelli, P. M., Sato, Y., Bazzoli, N., and Rizzo, E. (2013). Role of HSP70 in the regulation of the testicular apoptosis in a seasonal breeding teleost Prochilodus argenteus from the São Francisco River, Brazil. Microsc. Res. Tech. 76, 350–356.
| Role of HSP70 in the regulation of the testicular apoptosis in a seasonal breeding teleost Prochilodus argenteus from the São Francisco River, Brazil.Crossref | GoogleScholarGoogle Scholar |
Figueiredo, A. F., França, L. R., Hess, R. A., and Costa, G. (2016). Sertoli cells are capable of proliferation into adulthood in the transition region between the seminiferous tubules and the rete testis in Wistar rats. Cell Cycle 15, 2486–2496.
| Sertoli cells are capable of proliferation into adulthood in the transition region between the seminiferous tubules and the rete testis in Wistar rats.Crossref | GoogleScholarGoogle Scholar | 27420022PubMed |
FitzGerald, U., Gorman, A. M., and Samali, A. (2009). Heat Shock Proteins and the Regulation of Apoptosis. In ‘Heat Shock Proteins in Neural Cells. Neuroscience Intelligence Unit’. (Ed. C. Richter-Landsberg.) pp. 53–66. (Springer New York: New York, NY)
França, L. R., Hess, R. A., Dufour, J. M., Hofmann, M. C., and Griswold, M. D. (2016). The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 4, 189–212.
| The Sertoli cell: One hundred fifty years of beauty and plasticity.Crossref | GoogleScholarGoogle Scholar | 26846984PubMed |
Fukushima, T., Yamamoto, T., Kikkawa, R., Hamada, Y., Komiyama, M., Mori, C., and Horii, I. (2005). Effects of male reproductive toxicants on gene expression in rat testes. J. Toxicol. Sci. 30, 195–206.
| Effects of male reproductive toxicants on gene expression in rat testes.Crossref | GoogleScholarGoogle Scholar | 16141653PubMed |
Furuchi, T., Masuko, K., Nishimune, Y., Obinata, M., and Matsui, Y. (1996). Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development 122, 1703–1709.
| 8674410PubMed |
Gao, Y., Mruk, D. D., and Cheng, C. Y. (2015). Sertoli cells are the target of environmental toxicants in the testis – a mechanistic and therapeutic insight. Expert Opin. Ther. Targets 19, 1073–1090.
| Sertoli cells are the target of environmental toxicants in the testis – a mechanistic and therapeutic insight.Crossref | GoogleScholarGoogle Scholar | 25913180PubMed |
Govin, J., Caron, C., Escoffier, E., Ferro, M., Kuhn, L., Rousseaux, S., Eddy, E. M., Garin, J., and Khochbin, S. (2006). Post-meiotic shifts in HSPA2/HSP70.2 chaperone activity during mouse spermatogenesis. J. Biol. Chem. 281, 37888–37892.
| Post-meiotic shifts in HSPA2/HSP70.2 chaperone activity during mouse spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 17035236PubMed |
Grad, I., Cederroth, C. R., Walicki, J., Grey, C., Barluenga, S., Winssinger, N., De Massy, B., Nef, S., and Picard, D. (2010). The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 5, e15770.
| The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse.Crossref | GoogleScholarGoogle Scholar | 21209834PubMed |
Hess, R. A., Schaeffer, D. J., Eroschenko, V. P., and Keen, J. E. (1990). Frequency of the stages in the cycle of the seminiferous epithelium in the rat. Biol. Reprod. 43, 517–524.
| Frequency of the stages in the cycle of the seminiferous epithelium in the rat.Crossref | GoogleScholarGoogle Scholar | 2271733PubMed |
Hofmann, M.-C. (2008). Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell. Endocrinol. 288, 95–103.
| Gdnf signaling pathways within the mammalian spermatogonial stem cell niche.Crossref | GoogleScholarGoogle Scholar | 18485583PubMed |
Howie, G. J., Sloboda, D. M., and Vickers, M. H. (2012). Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br. J. Nutr. 108, 298–307.
| Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring.Crossref | GoogleScholarGoogle Scholar | 22018052PubMed |
Ishii, K., Kanatsu-Shinohara, M., Toyokuni, S., and Shinohara, T. (2012). FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development 139, 1734–1743.
| FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation.Crossref | GoogleScholarGoogle Scholar | 22491947PubMed |
Jazwiec, P. A., and Sloboda, D. M. (2019). Nutritional adversity, sex and reproduction: 30 years of DOHaD and what have we learned? J. Endocrinol. 242, T51–T68.
| Nutritional adversity, sex and reproduction: 30 years of DOHaD and what have we learned?Crossref | GoogleScholarGoogle Scholar | 31013473PubMed |
Jha, K. N., Coleman, A. R., Wong, L., Salicioni, A. M., Howcroft, E., and Johnson, G. R. (2013). Heat Shock Protein 90 Functions to Stabilize and Activate the Testis-specific Serine/Threonine Kinases, a Family of Kinases Essential for Male Fertility. J. Biol. Chem. 288, 16308–16320.
| Heat Shock Protein 90 Functions to Stabilize and Activate the Testis-specific Serine/Threonine Kinases, a Family of Kinases Essential for Male Fertility.Crossref | GoogleScholarGoogle Scholar | 23599433PubMed |
Jijiwa, M., Kawai, K., Fukihara, J., Nakamura, A., Hasegawa, M., Suzuki, C., Sato, T., Enomoto, A., Asai, N., Murakumo, Y., and Takahashi, M. (2008). GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells 13, 365–374.
| GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells.Crossref | GoogleScholarGoogle Scholar | 18363967PubMed |
Johnston, D. S., Olivas, E., DiCandeloro, P., and Wright, W. W. (2011). Stage-Specific Changes in GDNF Expression by Rat Sertoli Cells: A Possible Regulator of the Replication and Differentiation of Stem Spermatogonia. Biol. Reprod. 85, 763–769.
| Stage-Specific Changes in GDNF Expression by Rat Sertoli Cells: A Possible Regulator of the Replication and Differentiation of Stem Spermatogonia.Crossref | GoogleScholarGoogle Scholar | 21653894PubMed |
Kamada, S., Kikkawa, U., Tsujimoto, Y., and Hunter, T. (2005). Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s). J. Biol. Chem. 280, 857–860.
| Nuclear translocation of caspase-3 is dependent on its proteolytic activation and recognition of a substrate-like protein(s).Crossref | GoogleScholarGoogle Scholar | 15569692PubMed |
Kennedy, D., Jäger, R., Mosser, D. D., and Samali, A. (2014). Regulation of apoptosis by heat shock proteins. IUBMB Life 66, 327–338.
| Regulation of apoptosis by heat shock proteins.Crossref | GoogleScholarGoogle Scholar | 24861574PubMed |
Kereliuk, S., Brawerman, G., Dolinsky, V., Kereliuk, S. M., Brawerman, G. M., and Dolinsky, V. W. (2017). Maternal Macronutrient Consumption and the Developmental Origins of Metabolic Disease in the Offspring. Int. J. Mol. Sci. 18, 1451.
| Maternal Macronutrient Consumption and the Developmental Origins of Metabolic Disease in the Offspring.Crossref | GoogleScholarGoogle Scholar |
Khorram, O., Keen-Rinehart, E., Der Chuang, T., Ross, M. G., and Desai, M. (2015). Maternal undernutrition induces premature reproductive senescence in adult female rat offspring. Fertil. Steril. 103, 291–298.
| Maternal undernutrition induces premature reproductive senescence in adult female rat offspring.Crossref | GoogleScholarGoogle Scholar | 25439841PubMed |
Kotoglou, P., Kalaitzakis, A., Vezyraki, P., Tzavaras, T., Michalis, L. K., Dantzer, F., Jung, J. U., and Angelidis, C. (2009). Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones 14, 391–406.
| Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks.Crossref | GoogleScholarGoogle Scholar | 19089598PubMed |
Langley-Evans, S. C. (2015). Nutrition in early life and the programming of adult disease: a review. J. Hum. Nutr. Diet. 28, 1–14.
| Nutrition in early life and the programming of adult disease: a review.Crossref | GoogleScholarGoogle Scholar | 24479490PubMed |
Langley-Evans, S. C., and McMullen, S. (2010). Developmental origins of adult disease. Med. Princ. Pract. 19, 87–98.
| Developmental origins of adult disease.Crossref | GoogleScholarGoogle Scholar | 20134170PubMed |
Léonhardt, M., Lesage, J., Croix, D., Dutriez-Casteloot, I., Beauvillain, J. C., and Dupouy, J. P. (2003). Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol. Reprod. 68, 390–400.
| Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty.Crossref | GoogleScholarGoogle Scholar | 12533401PubMed |
Letai, A., and Kutuk, O. (2008). Regulation of Bcl-2 Family Proteins by Posttranslational Modifications. Curr. Mol. Med. 8, 102–118.
| Regulation of Bcl-2 Family Proteins by Posttranslational Modifications.Crossref | GoogleScholarGoogle Scholar | 18336291PubMed |
Martin, G. B., Blache, D., Miller, D. W., and Vercoe, P. E. (2010). Interactions between nutrition and reproduction in the management of the mature male ruminant. Animal 4, 1214–1226.
| Interactions between nutrition and reproduction in the management of the mature male ruminant.Crossref | GoogleScholarGoogle Scholar | 22444618PubMed |
Meng, X., Lindahl, M., Hyvönen, M. E., Parvinen, M., de Rooij, D. G., Hess, M. W., Raatikainen-Ahokas, A., Sainio, K., Rauvala, H., Lakso, M., Pichel, J. G., Westphal, H., Saarma, M., and Sariola, H. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493.
| Regulation of cell fate decision of undifferentiated spermatogonia by GDNF.Crossref | GoogleScholarGoogle Scholar | 10688798PubMed |
Montero, J., and Letai, A. (2018). Why do BCL-2 inhibitors work and where should we use them in the clinic? Cell Death Differ. 25, 56–64.
| Why do BCL-2 inhibitors work and where should we use them in the clinic?Crossref | GoogleScholarGoogle Scholar | 29077093PubMed |
Murphy, C. J., and Richburg, J. H. (2014). Implications of Sertoli cell induced germ cell apoptosis to testicular pathology. Spermatogenesis 4, e979110.
| Implications of Sertoli cell induced germ cell apoptosis to testicular pathology.Crossref | GoogleScholarGoogle Scholar | 26413394PubMed |
Pedrana, G., Viotti, H., Lombide, P., Cavestany, D., Martin, G. B., Vickers, M. H., and Sloboda, D. M. (2020). Maternal undernutrition during pregnancy and lactation affects testicular morphology, the stages of spermatogenic cycle, and the testicular IGF-I system in adult offspring. J. Dev. Orig. Health Dis. 11, 473–483.
| Maternal undernutrition during pregnancy and lactation affects testicular morphology, the stages of spermatogenic cycle, and the testicular IGF-I system in adult offspring.Crossref | GoogleScholarGoogle Scholar | 32340648PubMed |
Prokhorova, E. A., Kopeina, G. S., Lavrik, I. N., and Zhivotovsky, B. (2018). Apoptosis regulation by subcellular relocation of caspases. Sci. Rep. 8, 12199.
| Apoptosis regulation by subcellular relocation of caspases.Crossref | GoogleScholarGoogle Scholar | 30111833PubMed |
Puli, O. R., Danysh, B. P., McBeath, E., Sinha, D. K., Hoang, N. M., Powell, R. T., Danysh, H. E., Cabanillas, M. E., Cote, G. J., and Hofmann, M. C. (2018). The Transcription Factor ETV5 Mediates BRAFV600E-Induced Proliferation and TWIST1 Expression in Papillary Thyroid Cancer Cells. Neoplasia 20, 1121–1134.
| The Transcription Factor ETV5 Mediates BRAFV600E-Induced Proliferation and TWIST1 Expression in Papillary Thyroid Cancer Cells.Crossref | GoogleScholarGoogle Scholar | 30265861PubMed |
Rossi, P., and Dolci, S. (2013). Paracrine Mechanisms Involved in the Control of Early Stages of Mammalian Spermatogenesis. Front. Endocrinol. 4, 181.
| Paracrine Mechanisms Involved in the Control of Early Stages of Mammalian Spermatogenesis.Crossref | GoogleScholarGoogle Scholar |
Russell, L. D., Chiarini-garcia, H., Korsmeyer, S. J., and Knudson, C. M. (2002). Bax-Dependent Spermatogonia Apoptosis Is Required for Testicular Development. Biol. Reprod. 66, 950–958.
| Bax-Dependent Spermatogonia Apoptosis Is Required for Testicular Development.Crossref | GoogleScholarGoogle Scholar | 11906913PubMed |
Sagare-Patil, V., Bhilawadikar, R., Galvankar, M., Zaveri, K., Hinduja, I., and Modi, D. (2017). Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction. J. Assist. Reprod. Genet. 34, 495–503.
| Progesterone requires heat shock protein 90 (HSP90) in human sperm to regulate motility and acrosome reaction.Crossref | GoogleScholarGoogle Scholar | 28236106PubMed |
Saradha, B., Vaithinathan, S., and Mathur, P. P. (2008). Lindane alters the levels of HSP70 and clusterin in adult rat testis. Toxicology 243, 116–123.
| Lindane alters the levels of HSP70 and clusterin in adult rat testis.Crossref | GoogleScholarGoogle Scholar | 17997001PubMed |
Sarge, K. D., and Cullen, K. E. (1997). Regulation of hsp expression during rodent spermatogenesis. Cell. Mol. Life Sci. 53, 191–197.
| Regulation of hsp expression during rodent spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 9118007PubMed |
Schlesser, H. N., Simon, L., Hofmann, M. C., Murphy, K. M., Murphy, T., Hess, R. A., and Cooke, P. S. (2008). Effects of ETV5 (Ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biol. Reprod. 78, 483–489.
| Effects of ETV5 (Ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice.Crossref | GoogleScholarGoogle Scholar | 18032421PubMed |
Schmidt, J. A., Avarbock, M. R., Tobias, J. W., and Brinster, R. L. (2009). Identification of glial cell line-derived neurotrophic factor-regulated genes important for spermatogonial stem cell self-renewal in the rat. Biol. Reprod. 81, 56–66.
| Identification of glial cell line-derived neurotrophic factor-regulated genes important for spermatogonial stem cell self-renewal in the rat.Crossref | GoogleScholarGoogle Scholar | 19339709PubMed |
Scieglinska, D., and Krawczyk, Z. (2015). Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaperones 20, 221–235.
| Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans.Crossref | GoogleScholarGoogle Scholar | 25344376PubMed |
Selim, M. E., Rashed, E. H., Aleisa, N., and Daghestani, M. H. (2012). The protection role of heat shock protein 70 (HSP-70) in the testes of cadmium-exposed rats. Bioinformation 8, 58–64.
| The protection role of heat shock protein 70 (HSP-70) in the testes of cadmium-exposed rats.Crossref | GoogleScholarGoogle Scholar | 22359436PubMed |
Shaha, C., Tripathi, R., and Prasad Mishra, D. (2010). Male germ cell apoptosis: Regulation and biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1501–1515.
| Male germ cell apoptosis: Regulation and biology.Crossref | GoogleScholarGoogle Scholar | 20403866PubMed |
Shamas-Din, A., Kale, J., Leber, B., and Andrews, D. W. (2013). Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 5, a008714.
| Mechanisms of action of Bcl-2 family proteins.Crossref | GoogleScholarGoogle Scholar | 23545417PubMed |
Simon, L., Ekman, G. C., Tyagi, G., Hess, R. A., Murphy, K. M., and Cooke, P. S. (2007). Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp. Cell Res. 313, 3090–3099.
| Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance.Crossref | GoogleScholarGoogle Scholar | 17574550PubMed |
Sinclair, K. D., Rutherford, K. M. D., Wallace, J. M., Brameld, J. M., Stöger, R, Alberio, R, Sweetman, D, Gardner, D. S., Perry, V. E. A., Adam, C. L., Ashworth, C. J., Robinson, J. E., and Dwyer, C. M. (2016). Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod., Fertil. Dev. 28, 1443–1478.
| Epigenetics and developmental programming of welfare and production traits in farm animals.Crossref | GoogleScholarGoogle Scholar |
Singh, D., Paduch, D. A., Schlegel, P. N., Orwig, K. E., Mielnik, A., Bolyakov, A., and Wright, W. W. (2017). The production of glial cell line-derived neurotrophic factor by human sertoli cells is substantially reduced in sertoli cell-only testes. Hum. Reprod. 32, 1108–1117.
| The production of glial cell line-derived neurotrophic factor by human sertoli cells is substantially reduced in sertoli cell-only testes.Crossref | GoogleScholarGoogle Scholar | 28369535PubMed |
Tadokoro, Y., Yomogida, K., Ohta, H., Tohda, A., and Nishimune, Y. (2002). Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech. Dev. 113, 29–39.
| Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway.Crossref | GoogleScholarGoogle Scholar | 11900972PubMed |
Takashima, S., Kanatsu-Shinohara, M., Tanaka, T., Morimoto, H., Inoue, K., Ogonuki, N., Jijiwa, M., Takahashi, M., Ogura, A., and Shinohara, T. (2015). Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Reports 4, 489–502.
| Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal.Crossref | GoogleScholarGoogle Scholar | 25684228PubMed |
Toledo, F. C., Perobelli, J. E., Pedrosa, F. P. C., Anselmo-Franci, J. A., and Kempinas, W. D. G. (2011). In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod. Biol. Endocrinol. 9, 94.
| In utero protein restriction causes growth delay and alters sperm parameters in adult male rats.Crossref | GoogleScholarGoogle Scholar | 21702915PubMed |
Tyagi, G., Carnes, K., Morrow, C., Kostereva, N. V., Ekman, G. C., Meling, D. D., Hostetler, C., Griswold, M., Murphy, K. M., Hess, R. A., Hofmann, M. C., and Cooke, P. S. (2009). Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol. Reprod. 81, 258–266.
| Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 19369650PubMed |
Verba, K. A., and Agard, D. A. (2017). How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches. Trends Biochem. Sci. 42, 799–811.
| How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches.Crossref | GoogleScholarGoogle Scholar | 28784328PubMed |
Wadhwa, P. D., Buss, C., Entringer, S., and Swanson, J. M. (2009). Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 27, 358–368.
| Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms.Crossref | GoogleScholarGoogle Scholar | 19711246PubMed |
Wright, A., Reiley, W. W., Chang, M., Jin, W., Lee, A. J., Zhang, M., and Sun, S. C. (2007). Regulation of Early Wave of Germ Cell Apoptosis and Spermatogenesis by Deubiquitinating Enzyme CYLD. Dev. Cell 13, 705–716.
| Regulation of Early Wave of Germ Cell Apoptosis and Spermatogenesis by Deubiquitinating Enzyme CYLD.Crossref | GoogleScholarGoogle Scholar | 17981138PubMed |
Yamamoto, C. M., Hikim, P., Lue, Y., Portugal, M., Guo, T. B., Hsu, S. Y., Salameh, W., Wang, C., Hsueh, J., and Swerdloff, R. S. (2001). Impairment of spermatogenesis in transgenic mice with selective overexpression of Bcl-2 in the somatic cells of the testis. J. Androl. 22, 981–991.
| Impairment of spermatogenesis in transgenic mice with selective overexpression of Bcl-2 in the somatic cells of the testis.Crossref | GoogleScholarGoogle Scholar | 11700863PubMed |


