The status and conservation needs of the Micronesian Megapode (Megapodius laperouse laperouse) across the Mariana archipelago
Paul M. Radley A D * , Richard J. Camp
A D * , Richard J. Camp  B E , Frederick A. Amidon C , Ann P. Marshall C , P. Marcos Gorresen
B E , Frederick A. Amidon C , Ann P. Marshall C , P. Marcos Gorresen  B and Curt Kessler C
B and Curt Kessler C
A
B
C
D Present address:
E Present address:
Abstract
Accurate baseline data for wildlife populations are important to track trends of these populations over time and to identify threats to their long-term persistence.
We aimed to assess the status and distribution of the little studied megapode (Megapodius laperouse laperouse) across the Mariana Islands.
Using passive and call playback facilitated surveys in 2008 through 2010, we employed point–transect distance sampling to assess island-level and archipelago-wide status of this megapode. To assess conservation needs, we defined human presence as the current, recent, or intermittent occurrence of humans on islands.
We recorded 657 megapode detections and estimated an archipelago level abundance of 11,542 individuals (95% CI: 5456–17,623) from 699 sampling points across 10 islands. Three islands supported 86% of the megapode population, but cumulatively comprise only 2% of the archipelago’s land area.
Micronesian Megapodes preferred native forest. Human presence and the availability of native forest may limit their abundance and distribution in the Mariana Islands. Although the probability of detecting megapodes was significantly greater on islands without high human presence, significantly more detections were recorded in forests with dense or closed understory on those islands that supported greater human populations.
Given their status and confined distribution in the Mariana Islands, additional studies investigating megapode incubation sites and movement within and between islands would provide fundamental information on megapode ecology and enhance conservation efforts. Continued and expanded ungulate removal, predator control, and habitat restoration would further enhance the likelihood of megapode persistence in the archipelago.
Keywords: distance sampling, human presence, Mariana archipelago, Mariana Islands, Megapodius laperouse, Micronesian Megapode, native forest, occupancy.
Introduction
Obtaining reliable baseline data for wildlife populations is essential to track population trends over time and to identify future changes in a species’ distribution and abundance (Bibby et al. 2000). Such changes in a species’ occurrence can help to determine the anthropogenic or stochastic factors that may be adversely affecting its persistence (Soulé 1986). This is particularly important for the conservation of terrestrial island taxa, which are by definition range restricted and therefore tend to be disproportionally threatened with extinction compared to taxa on continental landmasses (Simberloff 2000; Şekercioğlu et al. 2004; Tershy et al. 2015). The magnified threats to island taxa can include acute habitat loss or degradation resulting from anthropogenic activities, the introduction of alien predators and competitors, volcanic activity, and the compounding effects of intense weather events and climate change (e.g. Şekercioğlu et al. 2004; Dalsgaard et al. 2007; Jetz et al. 2007; Fordham and Brook 2010; Doherty et al. 2016). Whereas these factors may potentially be detrimental to the Micronesian Megapode (Megapodius laperouse) throughout its range (Radley et al. 2018, 2021a; BirdLife International 2021), like many island taxa, this species is relatively understudied and the effects of such perturbations on its population are not yet well understood.
The megapodes belong to the avian family Megapodiidae, which comprises 22 species distributed throughout the central Indo-Pacific and Australasia (Jones et al. 1995; Harris et al. 2014). Megapodes are strictly ground nesters that, unlike any other families of birds, employ external biological or environmental sources of heat to incubate their eggs as opposed to body warmth (Jones et al. 1995; Sinclair 2002). The Micronesian Megapode currently consists of two subspecies; Megapodius laperouse senex in the Palauan archipelago (hereafter referred to as Palau) and the nominate Megapodius laperouse laperouse, which occurs in the Mariana Islands. Although relatively little is known about their natural history, megapodes in the Mariana Islands (hereafter also referred to as the Marianas) are known to occur primarily in native forest and are generally omnivorous, consuming seeds, small invertebrates, and plant matter (Jones et al. 1995; Radley et al. 2018). Previous unpublished studies (e.g. Glass and Aldan 1988; CG Rice and DW Stinson 1992, unpubl. data) have found that the Mariana subspecies uses heat generated by all three sources commonly employed by this family of bird to incubate its eggs; geothermal, passive solar radiation, and microbial decomposition of organic matter placed in mounds or burrows (Harris et al. 2014; Radley et al. 2018). Breeding activity may increase from the middle to the very latter part of the rainy season (September–February), and nesting has been observed in May, June, and September (Jones et al. 1995). The exact timing of breeding by the Micronesian Megapode in the Mariana Islands, which may vary annually, is not yet determined.
Although the Micronesian Megapode (known as sasangat in Chamorro and sasangal in Carolinian) likely once inhabited all the Mariana Islands (Steadman 2006), the population is now primarily confined to the comparatively young, volcanic islands of the archipelago’s northern arc (Fig. 1). Exceptions to this are the relatively small populations that occur on Saipan Island (hereafter referred to as Saipan) and Aguijan Island (hereafter referred to as Aguiguan), and occasional records of megapodes from Tinian Island (hereafter referred to as Tinian) (Wiles et al. 1987; USFWS 1998; O’Daniel and Krueger 1999; Amidon et al. 2011). The Micronesian Megapode is currently listed as Endangered in the Mariana Islands by both the U.S. Fish and Wildlife Service (USFWS 1970) and Commonwealth of the Northern Mariana Islands (CNMI) (CNMI 2016), and listed as Near Threatened across its range by the International Union for Conservation of Nature (IUCN) (BirdLife International 2021).
Map of the Micronesian Megapode (Megapodius laperouse laperouse) survey study area, Mariana archipelago. All islands except Guam, Rota and Farallon de Medinilla were surveyed from 2008 to 2010. Refer to Supplementary material 4a–e for island-level details of survey areas and forested cover.
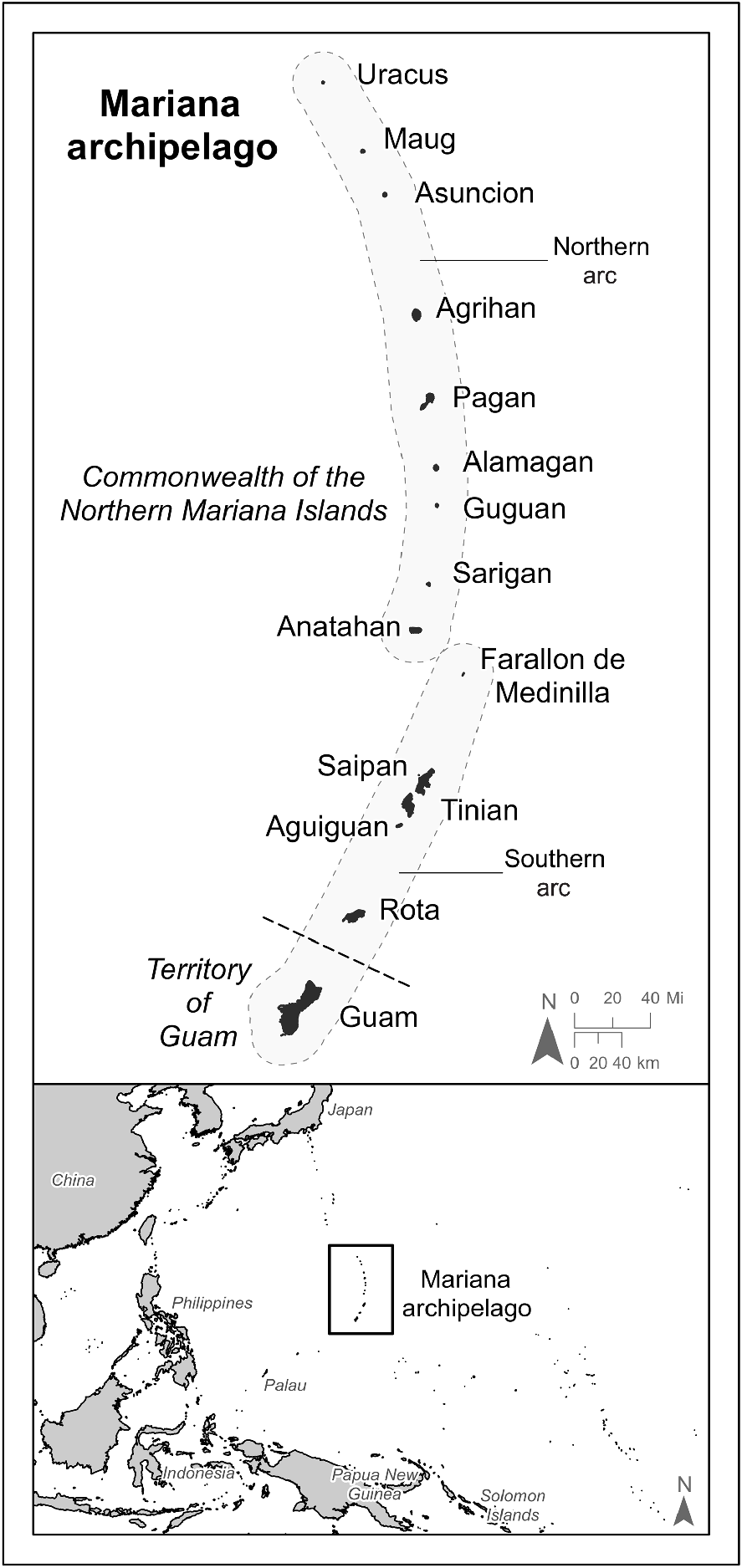
In 2009 and 2010, the U.S. Fish and Wildlife Service (USFWS) undertook the first ever archipelago-wide surveys for Micronesian Megapodes and other endangered taxa in the Mariana Islands as part of a wildlife survey funded by the U.S. Department of Defense (Amidon et al. 2010; USFWS 2010). The purpose of these surveys was to evaluate the effects to flora and fauna of proposed military training in portions of the Northern Mariana Islands. The goals of the megapode surveys were to estimate their populations by island and, if feasible, to determine their numbers by habitat cover type (Amidon et al. 2011). Surveys were conducted via point–transect distance sampling and call playback surveys as described by Buckland et al. (2001) and Amidon et al. (2010). As Micronesian Megapodes were believed to be transient on Tinian and Uracus Island (hereafter referred to as Uracus), only presence/absence surveys were conducted on these islands. Access to Anatahan Island (hereafter referred to as Anatahan) was constrained by safety and logistic issues, so only limited playback surveys were completed in areas of the island still vegetated after relatively large volcanic eruptions in 2003 and 2005. Farallon de Medinilla was surveyed extensively by the U.S. Navy in 2008 (S Vogt 2009, unpubl. data). Owing to restricted access to this island and safety issues presented by unexploded ordinance (a portion of Farallon de Medinilla is a live-fire range), this island was not resurveyed. Both Guam and Rota were excluded from surveys because of a lack of contemporary evidence of extant megapode populations on either island (Steadman 2006).
Here we present the results of the first archipelago-wide survey for the Micronesian Megapode in the Mariana Islands, conducted in 2008, 2009 and 2010. We used data collected during these surveys to assess the status and distribution of the megapode in the Mariana archipelago and compared our results to past survey efforts in the Mariana Islands and those for other species of megapodes elsewhere. We additionally use these data to assess and weigh potential threats and habitat needs for the species in the Marianas and propose conservation management actions that may help to maintain populations of Micronesian Megapodes in the archipelago.
Methods
Study area
The Mariana archipelago (or Mariana Islands) comprises two geologic arcs totalling 15 islands located in the western Pacific Ocean to the south of Japan, east of the Philippines, and north of the islands of New Guinea (Fig. 1). The Mariana Islands extend approximately 750 km north to south, comprise a land area of 1018 km2 (101,769 ha), and politically constitute the U.S. Territory of Guam and the CNMI. The southern six islands of the archipelago (the ‘southern arc’) are composed of uplifted limestone atop ancient volcanic formations, whereas the islands north of Farallon de Medinilla (the ‘northern arc’) are relatively young and active stratovolcanoes (Trusdell 2009). The climate of the Mariana Islands is marine tropical, hot and humid, and characterised by relatively high and uniform yearly temperatures. Although politically separate, Guam and the islands of the CNMI are biologically similar with minor variation in the diversity of local flora and fauna.
Most of the human population of the Mariana Islands reside on Guam, Rota, Tinian and Saipan. Aside from occasional visits by government researchers and local goat hunters, Aguiguan has not been regularly populated by humans since before World War II (Butler 1992). Although most islands north of Saipan are uninhabited, Alamagan Island (hereafter referred to as Alamagan), Pagan Island (hereafter referred to as Pagan), and Agrihan Island (hereafter referred to as Agrihan) are intermittently occupied and/or visited by humans. A small population of people had lived on Anatahan until 2003 when they were evacuated after the island volcanically erupted. Guguan Island, Asuncion Island, Maug Island (hereafter referred to as Guguan, Asuncion, and Maug, respectively) and Uracus are seldom visited by humans and are designated by the CNMI as Wildlife Conservation Areas (CNMI Constitution, Article XIV, Section 2 and CNMI Public Law 14–49) and Asuncion, Maug, and Uracus are further protected as part of the Mariana Trench Marine National Monument (Presidential Documents 2009). Native plants and wildlife on Sarigan Island (hereafter referred to as Sarigan), also rarely visited by humans, are protected by the CNMI under the Northern Mariana Islands Administrative Code (NMIAC § 85-30.1-335 [f] 2016). Farallon de Medinilla is used for live fire and aerial bombardment training by the U.S. Navy and civilian access is not permitted (S Vogt 2009, unpubl. data).
The land area of the islands we surveyed range from 200 to 12,000 ha (Supplementary material 1) and are covered in a mix of habitat cover types. Saipan’s land area is characterised by a mosaic of mixed introduced forest (36%), followed by thickets of introduced Leucaena leucocephala (19%), developed vegetation (13%) and developed land (10%), and mixed grass/herbaceous cover (7%) (Amidon et al. 2017). Tinian’s land area is chiefly characterised by extensive thickets of Leucaena leucocephala (33%), followed by mixed introduced forest (25%) and mixed grass/herbaceous cover (21%) (Amidon et al. 2017). Although native limestone forest covers only 1% and 4% of land on Saipan and Tinian, respectively, this cover type dominates Aguiguan (55%) followed by scrub/shrub cover (26%) and bare/exposed rock (9%) (Amidon et al. 2017). The land area of eight of the remaining islands surveyed north of Saipan (Supplementary material 1) is characterised by mixed grass/herbaceous cover (, range = 25–48%), followed by native volcanic forest (, range = 0–39%), bare soil/gravel (, range = 0–39%), and coconut (Cocos nucifera) forest (, range = 0–25%) (Amidon et al. 2017). Landcover on Uracus consists exclusively of bare rock and bare soil/gravel (78%) with some mixed grass/herbaceous cover (22%) (Amidon et al. 2017).
Point–transect surveys
We conducted point–transect distance sampling surveys on 13 and 14 July 2009 on Aguiguan, from 29 January to 3 February 2010 on Saipan and between 18 May and 7 July 2010 on the seven remaining islands, excluding Anatahan where only call playback surveys were conducted and Tinian and Uracus where only presence/absence surveys were conducted (these alternate surveys are described below). We sampled 590 stations along 66 transects across all islands surveyed (Table 1). Four of the transects on Aguiguan, two on Saipan and five on Sarigan coincided with transects established for previous survey and monitoring efforts by the CNMI Division of Fish and Wildlife (DFW) (Cruz et al. 2000a; Cruz and Williams 2003; Martin et al. 2008). The remaining transects and stations were established as part of our survey effort and were placed specifically in forested cover to maximise the likelihood of megapode detections and provide coverage of all habitat cover types in which megapodes were likely to occur. We determined the starting point for all newly established transects by layering a 150-m grid over each island in ArcMap 9.3.1 (Esri 2009) and randomly selecting numbered points following Zar (2010). Stations were spaced 150 m apart along all transects.
| Island A | No. of Stations | No. of Stns Occup | No. of Detections | Rel Occup | Rel Abund | No. of Samples B | No. of Smpl Occup | Smpl Occup | Smpl Abund | |
|---|---|---|---|---|---|---|---|---|---|---|
| Point-transect | ||||||||||
| Aguiguan | 74 | 14 | 25 | 0.19 | 0.34 | 74 | 14 | 0.19 | 0.34 | |
| Saipan | 80 | 6 | 8 | 0.08 | 0.10 | 159 | 6 | 0.04 | 0.05 | |
| Sarigan | 41 | 33 | 127 | 0.80 | 3.10 | 82 | 54 | 0.66 | 1.55 | |
| Guguan | 32 | 27 | 74 | 0.84 | 2.31 | 61 | 38 | 0.62 | 1.21 | |
| Alamagan | 72 | 6 | 10 | 0.08 | 0.14 | 72 | 6 | 0.08 | 0.14 | |
| Pagan | 144 | 3 | 4 | 0.02 | 0.03 | 153 | 3 | 0.02 | 0.03 | |
| Agrihan C | 67 | 0 | 0 | 0.00 | 0.00 | 134 | 0 | 0.00 | 0.00 | |
| Asuncion | 38 | 34 | 151 | 0.89 | 3.97 | 76 | 55 | 0.72 | 1.99 | |
| Maug | 14 | 12 | 41 | 0.86 | 2.93 | 28 | 20 | 0.71 | 1.46 | |
| Totals | 562 | 135 | 440 | 3.76 | 12.92 | 839 | 196 | 3.04 | 6.77 | |
| Playbacks | ||||||||||
| Aguiguan | 109 | 36 | 58 | 0.33 | 0.53 | 110 | 37 | 0.34 | 0.53 | |
| Saipan | 81 | 17 | 27 | 0.21 | 0.33 | 322 | 23 | 0.07 | 0.08 | |
| Anatahan | 21 | 10 | 23 | 0.48 | 1.10 | 58 | 16 | 0.28 | 0.40 | |
| Sarigan | 41 | 41 | 182 | 1.00 | 4.44 | 160 | 141 | 0.88 | 1.14 | |
| Guguan | 32 | 32 | 115 | 1.00 | 3.59 | 124 | 89 | 0.72 | 0.93 | |
| Alamagan | 72 | 23 | 44 | 0.32 | 0.61 | 288 | 37 | 0.13 | 0.15 | |
| Pagan | 144 | 13 | 28 | 0.09 | 0.19 | 565 | 28 | 0.05 | 0.05 | |
| Agrihan C | 182 | 0 | 0 | 0.00 | 0.00 | 728 | 0 | 0.00 | 0.00 | |
| Asuncion | 38 | 38 | 174 | 1.00 | 4.58 | 152 | 128 | 0.84 | 1.14 | |
| Maug | 14 | 13 | 30 | 0.93 | 2.14 | 21 | 20 | 0.95 | 1.43 | |
| Totals | 734 | 223 | 681 | 5.36 | 17.51 | 2528 | 519 | 4.26 | 5.85 | |
For both the numbers of stations (No. of stations) and numbers of samples (No. of samples) indices of occupancy (in relative terms, Rel Occup; and by sample, Smpl Occup, respectively) and abundance (relative, Rel Abund; and by sample, Smpl Abund, respectively) were calculated for each survey type. A station (No. of Stns Occup) or sample (No. of Smpl Occup) was considered occupied when one or more megapodes were detected during counts. The numbers of megapodes detected (No. of Detections) is presented by survey type and island.
Surveys were conducted by single observers following standard point–transect distance sampling methods first used by J Engbring, FL Ramsey and VJ Wildman (1986, unpubl. data) in the Mariana Islands. Survey counts were 5- or 8-min in duration (5-min counts were conducted on Saipan, all other islands used 8-min counts to compensate for more dense vegetation) during which the horizontal distances to all megapodes heard and/or seen were measured and recorded. We used laser rangefinders to accurately measure distances to the nearest metre and recorded the direction (based on compass bearing) and time of each megapode detection. Sampling conditions recorded at each station included cloud cover (0–100% by 10), rain intensity (none, mist, light, and heavy) and wind strength on the Beaufort scale. Habitat type categories were identified as coconut forest (CF), secondary forest (SF), and native forest (NF). Understory openness was ranked from 1 (closed/dense) to 5 (open/sparse) and canopy height as 1 (short; <3 m), 2 (mid; 3–10 m) and 3 (tall; >10 m). The canopy cover categories we employed were: very scattered (<5% cover), scattered (5–25% cover), open (>25–60% cover), and closed (>60% cover). All sampling condition data were later used as covariates in our density calculations. We conducted surveys only during favourable weather conditions (i.e. low wind and little or no rain), commencing at sunrise and continuing until all points on a transect was completed (typically prior to 11:00 hours).
Call playback surveys
Call playback surveys were conducted on the same dates as point–transect distance sampling surveys on all islands, except for Aguiguan where playback surveys were conducted on 17 and 20 July 2009, and Anatahan where only call playback was employed for surveys from 7 to 9 December 2010. We sampled 652 stations along 77 transects across all islands using call playback surveys (Table 1). Call playback surveys were conducted by a single observer and were 3-min in duration, during which digitally recorded Micronesian Megapode pair duet calls obtained on Sarigan in 2005 (Amidon et al. 2010) were broadcast on an electronic game caller for the first 30 s at each station. During the following 2-min and 30 s of silent observation we measured and recorded the horizontal distances to each megapode heard and/or seen, and if individuals had moved prior to detection (described below). Following protocols for point-transect distance sampling surveys, we used laser rangefinders to measure distances, and direction to (based on compass bearing) and time of each megapode detection were recorded. Weather conditions and habitat cover types were likewise recorded at each station. Counts commenced at sunrise, continued until completed (typically prior to 11:00 hours), and were conducted only during favourable weather conditions (halted during heavy rain or strong winds [>20 kph]).
Estimation of call playback survey correction factor
Preliminary testing of call playback survey technique for Micronesian Megapodes on Aguiguan in 2009 (Amidon et al. 2010) indicated that megapodes may move in response to playbacks. As responsive birds may move toward an observer before being detected, the use of playbacks without correcting for unobserved movement can result in the underestimation of actual observer-to-bird distances and inflate estimated densities (Buckland et al. 2006). To estimate a correction factor for megapode movement we conducted focal bird searches using pairs of observers to determine the distance megapodes moved in response to playback. When a bird was visually detected by one observer, this person noted its exact location with the aid of a GPS (global positioning system) and laser rangefinder, and then radioed the other observer who initiated a playback survey and recorded the distance to the bird (if detected). The difference between the actual and observed distances yielded the ‘unobserved movement’ of the distance the megapode moved prior to detection. Once the correction for unobserved movement was performed densities were calculated as discussed below.
Estimation of available forest area for abundance calculations
When possible, we used landcover estimates from the National Oceanic and Atmospheric Administration (NOAA) Coastal Change Analysis Program (C-CAP) most contemporary with our surveys to estimate available forest cover on each island. When these data did not provide estimated coverage for a specific forest type, we either used an older land cover estimate (if available) or derived forest cover estimates using satellite imagery in ArcGIS 9.3.1 (Esri 2009). Total forested area suitable for megapodes by island is presented in Supplementary material 1 and Table 2.
| Island A | Mean | Percent Coefficient of Variation | S.E. | Lower 95% Confidence Interval B | Upper 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Aguiguan | ||||||
| D | 0.33 | 41.23 | 0.14 | 0.18 | 0.61 | |
| N | 112 | 41.20 | 46 | 61 | 206 | |
| Alamagan C | ||||||
| D | 1.09 | 42.19 | 0.46 | 0.19 | 1.99 | |
| N | 529 | 42.18 | 223 | 92 | 966 | |
| Anatahan | ||||||
| D | 4.19 | 11.69 | 0.49 | 3.25 | 5.21 | |
| N | 703 | 11.66 | 82 | 547 | 875 | |
| Asuncion | ||||||
| D | 18.08 | 25.94 | 4.69 | 9.92 | 27.92 | |
| N | 5714 | 25.94 | 1482 | 3135 | 8821 | |
| Guguan | ||||||
| D | 8.86 | 27.87 | 2.47 | 4.85 | 14.41 | |
| N | 1507 | 27.87 | 420 | 824 | 2449 | |
| Maug | ||||||
| D | 13.60 | 24.94 | 3.39 | 7.69 | 20.84 | |
| N | 544 | 24.94 | 136 | 308 | 834 | |
| Pagan C | ||||||
| D | 0.07 | 57.01 | 0.04 | 0.01 | 0.16 | |
| N | 147 | 57.03 | 84 | 28 | 312 | |
| Saipan C | ||||||
| D | 0.59 | 74.13 | 0.44 | 0.01 | 1.44 | |
| N | 151 | 74.13 | 112 | 27 | 370 | |
| Sarigan | ||||||
| D | 11.93 | 24.56 | 2.93 | 7.05 | 18.16 | |
| N | 2135 | 24.56 | 524 | 1261 | 3250 | |
Density (D; birds/ha) and abundance (N; density × forested area) with variance estimates were calculated using bootstrap procedures (MacKenzie and Bailey 2004). Total abundance equalled 11,542 megapode (95% CI: 5456–17,632).
We used NOAA (National Oceanic and Atmospheric Administration) Office for Coastal Management (2023) forest cover and survey station habitat classification data to estimate the acreage and forest cover type surveyed on Maug (native: 40 ha) and Guguan (native: 170 ha). Estimates for forest cover on Aguiguan (native: 340 ha), Asuncion (native: 260 ha, coconut: 56 ha) and Pagan (native: 237 ha, coconut: 390 ha, and ironwood [Casuarina equisetifolia]: 1344 ha) were based on detailed assessments and estimates from USFWS (2009), Falanruw (1989) and Rogers (2010), respectively. Forest cover estimates for Alamagan and Sarigan were based on NOAA (Office for Coastal Management 2023) land cover estimates. As these estimates included both the native and coconut forest components on the islands, we estimated the area of coconut forest for each (Alamagan: 55 ha, Sarigan: 109 ha) using 2007 and 2006 satellite imagery, respectively, and subtracted this from NOAA’s total forest cover estimate to determine native forest cover (430 and 70 ha, respectively). Estimates for Anatahan (native: 168 ha) were derived from Amidon et al. (2017), which used 2012 imagery for the landcover classification, and only represent forested areas on the eastern and western halves of the island that were surveyed (Supplementary material 4a–e). We did not quantify forest cover for Agrihan because this island was not included in megapode population estimates.
Megapode sampling on Saipan primarily occurred in native (limestone) forest and we used native forest estimates (148 ha) from Falanruw et al. (1989). As our surveys did not achieve even coverage of all native forest on Saipan, we limited our calculations of available cover to only those areas of the island we sampled. The estimate of native forest cover provided by Falanruw et al. (1989) dates to the 1980s and it may have overestimated the amount of limestone forest currently available on Saipan. Unlike other sources (e.g. U.S. Forest Service 2006), however, Falanruw et al. (1989) included in their estimates small patches of native forest on northern Saipan that Micronesian Megapodes are known to use.
Density and abundance calculation
We estimated megapode population densities using Program Distance, ver. 6.0 release 2 (Thomas et al. 2010), as birds per ha by habitat cover types. Densities were calculated from a global detection function for which data were post-stratified by survey and cover type. We calculated abundance (i.e. total number of megapodes) by multiplying megapode densities by the available area of each cover type. To enable model fitting, data were right-truncated when detection probability was approximately 0.1; candidate models included half-normal and hazard-rate detection functions with expansion series of order two (Buckland et al. 2001: 361, 365). We modelled sampling covariates in the multiple covariate distance engine of Program Distance (Thomas et al. 2010) and used the model with the lowest sample size corrected Akaike Information Criterion (AICc) to select the detection function that best approximated our data (Burnham and Anderson 2002). Covariates were employed to generate the global detection function when the best approximating model was improved by four or more AICc units; model diagnostics were assessed to determine adequate fit (Supplementary material 2). We derived variances and confidence intervals (CI) by using bootstrapping methods with 999 iterations (Thomas et al. 2010).
Occupancy surveys
We conducted surveys for megapode occupancy concurrently with the point–transect and call playback surveys described above. Except for those on Anatahan, Agrihan, Maug, and Aguiguan, we sampled stations on all islands four times, distributing sampling across two time periods (i.e. 06:00–11:00 hours and 14:00–18:00 hours). We sampled all stations on Anatahan a minimum of two times (one morning and one afternoon) whereas eight stations along two transects were sampled four times (two mornings and two afternoons). Owing to time constraints on Agrihan, we sampled stations along Transect 9 only one or two times over the two time periods noted above; the remaining stations on the island were sampled four times. We sampled all stations on Maug only one or two times because of equipment problems and time constraints. Occupancy surveys were conducted on Aguiguan between 13 and 16 July 2009. A total of 35 stations, along six transects, were sampled on this island between 2 and 11 times with sampling distributed across three time periods (06:00–10:00 hours, 10:00–14:00 hours, 14:00–18:00 hours). All surveys for occupancy were conducted by one observer following the call playback methods described above. Given the methods employed for presence/absence surveys on Tinian and Uracus, occupancy data were not collected on either of these islands.
Occupancy calculations
For our analysis, we defined human presence as the current or routinely intermittent occurrence of humans or human populations on islands. Occupancy models included the following site covariates for occupancy probability (ψ) and occurrence probability (p): cover type (ψ), understory closure/openness (ψ), canopy height (ψ), canopy cover (ψ), and time of day (morning or afternoon; p) (Supplementary material 3). On Aguiguan, site covariates for occupancy probability were cover type, exposure to prevailing weather, understory closure/openness, distance to the coast, and elevation. A covariate for island identity was not used because of the low number of observations on several islands. We first ranked occupancy models according to AICc. Model goodness-of-fit statistics (ⓒ) were assessed with a parametric bootstrap procedure (MacKenzie and Bailey 2004) in which a Pearson chi-square test statistic with P-value >0.05 indicated an adequate model fit. The global model (i.e. one which includes all covariates) describing occupancy on ungulate-free islands had a ⓒ > 1. AICc values were therefore subsequently adjusted by the model fit statistic to derive quasi-AICc values (i.e. QAICc) and re-ranked accordingly. Inference was made only for the top-ranked models.
Presence/absence surveys
Owing to the low likelihood of obtaining sufficient detections of megapodes on Tinian and Uracus to estimate density and abundance (e.g. USFWS 1998), we conducted presence/absence surveys in areas of each island where megapodes were previously reported (Supplementary material 4a and c). On Tinian we used a modified version of the playback surveys described above, at 21 stations along three transects in the island’s Lasu and Maga regions between 13 and 18 August 2008. Surveys were 5-min in duration, during which digitally recorded Micronesian Megapode pair duet calls obtained on Sarigan in 2005 (Amidon et al. 2010) were broadcast with an electronic game caller for the first minute of surveys at each station. During the following 4-min of silent observation we measured and recorded the horizontal distances to each megapode heard and/or seen. On Uracus we used a walking survey technique (Forsman et al. 1977) to conduct a presence/absence survey on the island between 10:00 and 17:00 hours on 17 July 2010. Pair duet calls were broadcast on an electronic game caller at approximately 100 m intervals, followed by a period of silent observation, along a walking route by one observer (Supplementary material 4a–e). The number of megapodes that responded to playback were recorded along with any incidental observations outside the survey.
Following Reynolds and Snetsinger (2001), we calculated detection probabilities to estimate the likelihood of extinction (extirpation) of megapodes on Tinian and Uracus. Scott et al. (1986) calculated the probability (p) of detecting one bird from a randomly distributed population of n individuals as:
The effective search area (a) for megapodes on Tinian and Uracus was approximated 4 and 9.4 ha, respectively, by calculating the area for the effective detection radius (EDR) (24.7 m and 19.3 m, respectively; derived from islands with and without ungulates for this study) using ArcGIS Pro 2.9 (Esri 2021). The range of megapodes on Tinian and Uracus () was estimated as 80 and 48.6 ha, respectively, based on the total available potential habitat (native dominated limestone forest on Tinian and mixed grass and herbaceous cover on Uracus) on the islands (Amidon et al. 2017). We set the hypothetical population size (n) at 1 for both islands as this was the last estimated number of birds on either (Falanruw 1975).
Using Reed’s (1996) modification of statistical methods by Guyann et al. (1985) to infer extinction, we also calculated the minimum number of visits, Nmin needed for 95% (α = 0.05) and 99% (α = 0.01) probability of detection as:
where n is the number of independent visits. We defined one visit as 5 h of search effort.
Results
Population density and size
We recorded 440 Micronesian Megapode detections during point–transect distance sampling surveys, and 657 megapode detections during call playback surveys (Table 1). Megapodes were detected on all islands surveyed except Tinian, Uracus and Agrihan. On Agrihan, however, one bird was observed outside the counting period on the north end of the island. Mean densities on islands where megapodes were detected during surveys ranged from a low of 0.07 birds per ha on Pagan to a high of 18.08 birds per ha on Asuncion (Table 2). The total abundance of megapodes on all islands surveyed was 11,542 individuals (95% CI: 5456–17,623). Micronesian Megapode detections sufficient to estimate density by cover type were made only on Asuncion and Sarigan. Although megapodes were on average more abundant in coconut forest than native forest on both islands, the differences were not statistically significant (i.e. all 95% CI bracketed the calculated means; Table 3).
| Island-Habitat | Density | Percent Coefficient of Variation | S.E. | Lower 95% Confidence Interval | Upper 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Asuncion-CF | 26.60 | 26.83 | 7.14 | 14.79 | 43.64 | |
| Asuncion-NF | 15.13 | 29.19 | 4.42 | 9.14 | 25.24 | |
| Sarigan-CF | 13.08 | 26.33 | 3.44 | 7.43 | 20.54 | |
| Sarigan-NF | 9.19 | 32.90 | 3.02 | 4.45 | 15.88 |
Density (birds/ha) with variance estimates were calculated using bootstrap procedures (MacKenzie and Bailey 2004). Estimates were produced from the global model using post-stratification procedures with strata of island and habitat type.
Occupancy
Preliminary models collectively describing Micronesian Megapode occupancy on islands surveyed did not converge; separate models, therefore, were developed for islands with or without regular human presence (i.e. islands with current or recent human populations or that experience regular visitation). The top-ranked models for islands with high human presence (i.e. Saipan, Alamagan and Pagan; Agrihan was excluded from analysis for lack of data) included the site covariates understory closure/openness and habitat cover type (Supplementary material 5, Panel a). Models that included the time of day during which surveys were conducted also emerged as highly ranked candidates.
Top-ranked models for islands with low human presence (i.e. conservation islands Sarigan, Guguan, Asuncion and Maug) only included a model with the covariate for understory closure/openness and a null model without covariates (i.e. ψ(·)p(·) where occupancy and detection probabilities are both treated as constants; Supplementary material 5, Panel b). Other site covariates (i.e. habitat cover type), therefore, did not appear to be strongly associated with megapode occurrence.
The probability of detecting megapodes was markedly different among islands with and without high human presence. On islands where human presence was high, detection probability (p) averaged 0.37 (95% CI = 0.29–0.45) for the top-ranked model ψ(understory)p(.) (Table 4, Panel a). On islands with low human presence the same model yielded a mean p of 0.84 (95% CI = 0.80–0.87; Table 4, Panel b). Therefore, on any one visit there was an 84% chance of detecting megapodes on islands with low human presence, but less than half that likelihood (37%) on islands with high human presence.
| Model | p | 95% CI Lower | 95% CI Upper | Sampling Covariate | |
|---|---|---|---|---|---|
| Panel a. Occupancy probability (p) for islands with high human presence (i.e. Saipan, Alamagan, and Pagan). | |||||
| ψ(understory)p(.) | 0.37 | 0.29 | 0.45 | None | |
| ψ(understory)p(time) | 0.40 | 0.30 | 0.50 | Morning | |
| 0.34 | 0.25 | 0.44 | Afternoon | ||
| ψ(understory, vegetation)p(.) | 0.37 | 0.29 | 0.45 | None | |
| ψ(vegetation)p(.) | 0.37 | 0.29 | 0.45 | None | |
| ψ(understory, vegetation)p(time) | 0.40 | 0.30 | 0.50 | Morning | |
| 0.34 | 0.25 | 0.44 | Afternoon | ||
| Panel b. Occupancy probability (p) for islands with low human presence (i.e. Sarigan, Guguan, Asuncion and Maug). | |||||
| ψ(understory)p(.) | 0.84 | 0.80 | 0.87 | None | |
| ψ(.)p(.) | 0.84 | 0.80 | 0.87 | None | |
Results for islands with high or low human presence are shown separately (Panels a and b, respectively). Results are shown only for the top-ranked subset of models as indicated by shading in Supplementary material 5.
The time of day during which a survey was conducted appeared to have a moderate effect on megapode detectability. Although confidence intervals overlap, the model ψ(understory)p(time) showed a slightly higher mean in the morning (P = 0.40; 95% CI = 0.30–0.50) than in the afternoon (P = 0.34; 95% CI = 0.25–0.44; Table 4). Time of day was not a top-ranked model on islands without ungulates, which may indicate that bird response is high throughout the day on islands with an intact and dense understory that has not been disturbed by ungulates.
Micronesian Megapode occupancy on islands with high human presence was relatively low overall, ranging from a mean of 0.04 (95% CI = 0.01–0.13) where the understory was open, up to 0.36 (95% CI = 0.26–0.48) where the understory was closed and dense. In contrast, occupancy was 1.00 (i.e. 100%) on islands with low human presence regardless of vegetation composition at survey stations. Megapodes were detected, in other words, at every station sampled on Sarigan, Guguan, Asuncion, and at all but one station on Maug (Table 1).
Compared to islands with low human presence, vegetation within habitat cover types emerged as an important variable on islands with high human presence. Cover dominated by coconut or ironwood had low levels of megapode occurrence, which averaged about 0.10 (i.e. 10% occupancy; Supplementary material 6, Panel a). Sites classified as native forest cover exhibited approximately three times the occupancy of areas dominated by coconut or ironwood and about twice that in secondary forest. Vegetation type interacted with understory such that more closed and dense sites supported higher levels of megapode occurrence.
For Aguiguan, the top-ranked model was the null model ψ(·)p(·) (Supplementary material 5 Panel b). Models that included understory, elevation or both covariates also emerged as highly ranked candidate models. The detection probability (p) for the null model averaged 0.23 (95% CI = 0.16–0.31), and mean occupancy was estimated at 0.69 (95% CI = 0.45–0.86). Occupancy for the next three top-ranked models ranged from 0.35 (95% CI = 0.09–0.74) to 0.93 (95% CI = 0.01–1.00; Supplementary material 6, Panel b).
Presence/absence surveys
We detected no Micronesian Megapodes during 5 h of surveying the eastern slope of Uracus and during 11 h of surveying the Lasu and Maga regions of Tinian. The likelihood of detecting one megapode on Uracus ranged from slightly better than 19% to almost 98%, depending on the survey and the starting population (Supplementary material 7). The likelihood of detecting one megapode on Tinian ranged from slightly better than 6–69%. If a population of one individual persisted on Tinian and Uracus, an additional 51 and 14 searches, respectively, would be required to achieve a 95% likelihood of detecting an individual megapode. We therefore cannot definitively infer extirpation of a resident population of megapodes on either Tinian or Uracus based on surveys of those islands in 2008 and 2010, respectively.
Discussion
From the combined findings of our surveys and extensive palaeontological work completed in the southern arc of the Mariana archipelago (Steadman 1999, 2006; Pregill and Steadman 2009), it can be inferred that Micronesian Megapodes once occurred across all the Mariana Islands. Although it still occurs on most islands in the northern arc, the species was extirpated from most of the Marianas’ southern arc by human activities (Steadman 1999, 2006). At the time of our surveys in 2010, megapodes occupied 11 (73%) of the 15 Mariana Islands. Relatively recent records of Micronesian Megapodes exist for Tinian (e.g. Wiles et al. 1987; O’Daniel and Krueger 1999; NAVFAC Marianas 2014), but we exclude the island from our surveys because these records are regarded as pertaining to birds commuting or dispersing from either nearby Aguiguan or Saipan and there is no evidence of an extant population on the island. Extensive terrestrial surveys of Tinian in 2008, for which megapodes were not a focal species, yielded no incidental detections (USFWS 2009).
The majority (86%; n = 9386) of our estimated Micronesian Megapode population occurred on only three of the 15 Mariana Islands, Asuncion, Guguan, and Sarigan; Asuncion alone supported 53% of megapodes across islands surveyed. These three islands cumulatively comprise only 2% (1659 ha) of the total land area of the Mariana Islands and were uninhabited during the time of our surveys. Previous surveys of these islands yielded estimated abundances for megapodes ranging from 25 to 1757 individuals on Asuncion (USFWS 1998; Radley 2009), 305 to 2200 on Guguan (Glass and Villagomez 1986; Cruz et al. 2000b), and 360 to 1772 on Sarigan (Fancy et al. 1999; Cruz et al. 2000a; Martin et al. 2008; Craig 2021).
Sarigan and Asuncion were previously surveyed by the CNMI DFW only 4 and 2 years, respectively, prior to our surveys in 2010 (Martin et al. 2008; Radley 2009). Martin et al. (2008) counted megapodes as part of broader bird surveys on Sarigan in mid-April 2006. These passive surveys (i.e. unaided by call playback) were conducted from 61 stations, detected 92 individual megapodes from 41 stations, and calculated densities of 5.32 (s.e. = 1.05) megapodes per ha; we calculated 11.93 (s.e. = 2.93) birds per ha with call playback (Table 2). Our survey of Sarigan in mid-May of 2010 yielded 363 more megapodes than Martin et al. (2008), and abundances for the two surveys differed significantly (Z-test; difference in density = 6.61, s.e. = 3.11, Z-statistic = −2.12, P = 0.03). Our survey of Asuncion in late June and early July of 2010, however, yielded a calculated abundance of 3957 more megapodes than Radley (2009) in mid-May of 2008. This difference in abundance was also significant (Z-test; difference in density = 12.52, s.e. = 4.79, Z-statistic = −2.61, P = 0.01) and may in part be related to a difference in methods and effort between the two surveys. Radley (2009) conducted passive surveys from 20 stations, detected 35 birds from 16 stations and calculated a density of 5.56 (s.e. = 0.99) megapodes per ha; we calculated 18.08 (s.e. = 4.69) megapodes per ha with call playback (Table 2). Whereas our passive surveys on Sarigan detected 28% more megapodes than Martin et al. (2008), we passively detected 77% more megapodes (n = 151) on Asuncion than Radley (2009). As we surveyed Asuncion more than 2 months later than the previous effort, this disparity may be the result of seasonal differences in behaviour related to breeding, which for many species of megapodes can be influenced by rainfall regimes (Jones et al. 1995). However, we have no historical rainfall data for Asuncion to validate a hypothesis related to breeding.
Our findings indicate that human presence and the availability of suitable habitat may ultimately limit island-level abundance and the overall distribution of Micronesian Megapodes across the Mariana Islands. By extension, human presence can also imply the alteration and degradation of habitat and/or the presence of feral ungulates and introduced predators resulting from current or historical human occupation of islands. Megapodes in our study occurred in notably greatest abundance on the island of Asuncion, followed by Sarigan and Guguan. Like other islands of the Marianas’ northern arc, these three are geothermally active and support areas of native forest suitable for megapodes. These three islands also experience relatively little human presence or visitation and there were no feral ungulates observed during the time of our surveys. Of these islands, Sarigan (on which humans have not resided since the mid-1940s) previously supported feral goats (Capra hircus) and pigs (Sus scrofa scrofa), which were eradicated by 2000 (Kessler 2002). Feral ungulates affect island habitat by over-browsing vegetation, inhibiting native plant growth and regeneration, causing erosion, and facilitating the establishment of invasive plants that can further impede forest regeneration by displacing or smothering native plants (e.g. Perry and Morton 1999; Loope et al. 2001; Wiles 2005). These results can in turn negatively affect the availability of foraging, breeding, and roosting cover or resources for avian species, including the Micronesian Megapode. This is demonstrated by the relatively low abundances of megapodes reported by Fancy et al. (1999), Cruz et al. (2000a) and Craig (2021) for Sarigan pre-ungulate eradication compared to those reported post-eradication by Martin et al. (2008) and our study.
Megapodes are scratch feeders that forage exclusively on the ground, and understory browsing by ungulates may affect the microclimate of terrestrial invertebrates and lessen the availability of forage or food items (Sitters and Andriuzzi 2019). Dense understory vegetation may also provide cover and concealment for megapodes from predators such as feral cats and dogs, monitor lizards (Varanus tsukamotoi), and perhaps humans (GM Ludwig 1979, unpubl. data; Dekker et al. 2000). Although there are no published data regarding the benefits of dense understory for adult megapodes, Australian Brush-turkey (Alectura lathami) chicks in Queensland exhibited significantly greater survival and dispersed significantly shorter distances (exposing themselves less-so to predators) in rain forest with greater available understory vegetation (Göth and Vogel 2002, 2003). Other species of ground-dwelling Galliformes (e.g. Black Francolin [Francolinus francolinus] and Red-winged Francolin [Scleroptila levaillantii]) often prefer dense shrub or brush habitat, frequently as a component of open canopy forest, and may occur in greater numbers in such protective cover when the presence or density of potential predators is higher (Jansen et al. 2000, 2001; Kumar et al. 2020).
The surveys of Sarigan conducted by Fancy et al. (1999) prior to the ungulate eradication by Kessler (2002) yielded 65% fewer megapodes per hectare (n = 4.17) than our surveys in 2010 (n = 11.93). This result may be related to the observed increase in forest understory as a consequence of ungulate eradication (Martin et al. 2008), which subsequently provided enhanced forage and cover for megapodes. The removal of pigs, the primary ungulate eradicated from Sarigan, may also have been beneficial. Although there is currently no direct evidence regarding megapodes in the Mariana Islands, feral pigs are a known egg predator of ground-nesting birds (e.g. Sanders et al. 2020; Mori et al. 2021) and may further limit Micronesian Megapode populations.
Asuncion and Guguan are both protected by the CNMI as conservation areas that do not support feral ungulates. Asuncion is additionally protected as part of the federal Mariana Trench Marine National Monument (Presidential Documents 2009), which helps to deter human visitation, and by its nearly 500-km distance from Saipan, the nearest centre of human population. Maug, which is composed of three separate islands that are the remains of an ancient volcano, is 39.3 km northeast of Asuncion and seldom visited. This island assemblage is also protected both by the CNMI and as part of the federal Mariana Trench Marine National Monument and does not support feral ungulates; because of logistical constraints, we sampled only Maug’s East Island. Here megapodes occurred at the second highest density (13.60 birds/ha) of all islands surveyed after Asuncion (18.08 birds/ha), but in relatively low abundance owing to the islet’s small land area and correspondingly limited area of suitable habitat (Table 2)
By comparison, all other islands we surveyed yielded lower abundances and densities of Micronesian Megapodes (Table 2). These islands tended to experience relatively high levels of present and/or historic human visitation or supported significant human populations (e.g. Saipan) and exhibited varying degrees of anthropogenic habitat alteration and degradation. Much of the native forest cover that once existed on Saipan and Aguiguan, for example, had been converted to sugar cane plantations by the Japanese prior to World War II (Butler 1992; Mori 2019). Although thought to have been extirpated from Saipan sometime in the 1930s (Pratt and Bruner 1978), evidence and oral accounts suggest that the small population of megapodes on the island was the result of the local human populace reintroducing the species from islands in the northern portion of the Mariana archipelago (Glass and Aldan 1988).
Also present on islands yielding low abundances and densities of Micronesian Megapodes were extant populations of introduced feral ungulates and generally large numbers of introduced predators (i.e. cats and dogs). Regardless of the presence of feral pigs, the relatively few megapodes documented during our surveys on Pagan were all detected on the southern portion of the island, where small areas of suitable habitat still existed. Habitat on the northern half of the island had been historically altered by volcanic activity while the effects of past human presence has been continually maintained by large numbers of feral cattle (Bos taurus), goats and pigs. We detected megapodes on neighbouring Alamagan in forested ravines across the island where ungulate browsing was likely reduced because of limited or more challenging access.
Our results indicate that Micronesian Megapodes in the Mariana Islands favour native forest cover types. Although the species was numerically more abundant in coconut forest than native forest on both Asuncion and Sarigan (Table 3), these differences were not significant and were not supported by the results of occupancy analysis. This is the result of imprecise density estimates as indicated by relatively large Coefficients of Variation and 95% CI that bracket the calculated means (Table 3). The probability of detecting megapodes across islands surveyed was nearly three times greater in native forest cover than either coconut or ironwood forest. Although we additionally found that the probability of detecting megapodes was substantially greater on islands without high human presence, substantially more megapodes were detected in forested cover with dense or closed understory vegetation on those islands that supported greater human populations (examples of the protective benefits provided by dense understory for Australian Brush-turkeys and other Galliformes are discussed above).
These findings are broadly similar to those for Micronesian Megapodes in Rock Islands Southern Lagoon conservation area of Palau where Radley et al. (2021b) found that birds did not apparently favour or select any specific habitat variable while foraging. Like other species of megapodes, the Micronesian Megapode is omnivorous (Jones et al. 1995), and the species’ generalist feeding behaviour might be reflected in its non-specific use of foraging habitat (Radley et al. 2021b). For breeding, however, megapodes in littoral forest of the Rock Islands preferentially selected large individual ironwood trees against which to construct their incubation mounds and avoided areas of coconut forest (Radley et al. 2021b). Interestingly, and in contrast to this, one of only a few recorded megapode breeding areas in the Mariana Islands was found in coconut forest on the slopes of Agrihan Island (GM Ludwig 1979, unpubl. data). Similar to our study, Radley et al. (2021a) also found that the probability of detecting megapodes was substantially greater on islands in the Rock Islands Southern Lagoon of Palau that experienced lower human visitation.
Aside from the lower presence of humans, another probable reason for greater occurrence and abundances of Micronesian Megapodes on islands of the Marianas’ northern arc is their active volcanic nature. These geologically young islands are estimated to be no more than 3-million years old (Trusdell 2009) and provide readily accessible sources of geothermal heat that other species of megapodes locally exploit throughout their ranges to incubate their eggs (e.g. Jones et al. 1995; Bowen 1996; Göth and Vogel 1999). Although GM Ludwig (1979, unpubl. data) documented Micronesian Megapodes incubating their eggs colonially in low incubation mounds on Agrihan, he reported being informed by locals that the species tended to breed in areas of geothermally heated soil on the island. In the early 1990s CG Rice and DW Stinson (1992, unpubl. data) did not document any megapodes on Agrihan during a 3-day visit confined to its north end, and our more extensive coverage likewise found megapodes almost entirely absent from this island. Like GM Ludwig (1979, unpubl. data), however, we observed feral dogs and cats during our surveys on Agrihan, both of which are known to severely impact populations of other megapode species (Jones et al. 1995; Dekker et al. 2000) and both of which the IUCN (BirdLife International 2021) considers a threat to the Micronesian Megapode. The presence of these introduced predators, along with the pigs we observed, may very well have suppressed megapodes on the island.
The only island known to support Micronesian Megapodes that we did not survey is Farallon de Medinilla. This island is an upraised limestone plateau with a maximum elevation of 82 m that covers 73 ha of area (Lusk et al. 2000). Approximately 11% of the island is considered forested (Office for Coastal Management 2023), primarily by low stature Pisonia grandis, and there are no records of human habitation or presence of feral ungulates (Lusk et al. 2000). Megapodes were first reported on the island in 1996 (Lusk et al. 2000) when fewer than 10 birds were documented; to what extent the island was previously surveyed by naturalists is unknown (USFWS 1998). S Vogt (2009, unpubl. data) used call playback to survey Farallon de Medinilla in August 2008 and documented 12 pairs of Micronesian Megapodes and approximately four unpaired individuals, one of which was a chick. These observations, the majority of which were documented in the ‘no-fire zone’ on the northern portion of the island, indicate breeding by the species on Farallon de Medinilla.
Future research and conservation implications
The megapodes are an understudied avian family, particularly in issues regarding their ecology (Jones 1999; Radley et al. 2018). Although perhaps better understood than many of its congeners, in-depth ecological research is warranted for the Micronesian Megapode to better facilitate its long-term conservation in the Mariana Islands. The species would benefit from research that includes a focus on its breeding in the Mariana Islands, including locating and monitoring potential breeding sites, the degree and distance of inter-island dispersal movements and commuting by juveniles and adults, and associated study of inter-archipelago gene flow to better inform population level protection for Micronesian Megapodes (Ando 2019; Mapel et al. 2021).
Searching for Micronesian Megapode incubation sites was not a component of our study, and we did not incidentally document or confirm nesting by the species during surveys. However, identifying megapode incubation sites across the Mariana Islands is of key importance to protecting the species. Aside from observations by GM Ludwig (1979, unpubl. data) on Agrihan, megapodes in the Marianas have historically been reported employing incubation mounds (e.g. Baker 1951; GM Ludwig 1979, unpubl. data; Stinson 1992), a strategy that is used exclusively by Micronesian Megapodes in Palau (Olsen et al. 2016). Megapodes in the Mariana Islands’ volcanic northern arc have also been documented using geothermal sources of heat to incubate their eggs (GM Ludwig 1979, unpubl. data) and are thought likely to utilise heat from passive solar radiation (Harris et al. 2014; Radley et al. 2018). As potential evidence of this, during our surveys we encountered a sun-exposed field of loose pumice or volcanic cinder on Guguan that exhibited numerous shallow cone-shaped divots (P.M. Radley and F.A. Amidon, pers. obs.) indicative of locations where megapodes may have excavated to bury their eggs. This area coincides with a communal nesting area originally mapped and documented as active by Glass and Aldan (1988) in 1987. CG Rice and DW Stinson (1992, unpubl. data) later confirmed this site’s continued active status, mapped an additional portion of it on Guguan’s west side and documented apparent megapode nesting areas on southern Pagan. Future research and management activities that could further conservation of the species include (a) relocating and documenting the active status of known, historical breeding sites, (b) searching for other potential suitable breeding locations via geographic information system followed by on-site ground searches, and (c) monitoring breeding activity at historical or newly found breeding sites via direct observation or trail cameras.
The relatively low numbers of megapodes found by our study on larger islands in the Marianas are also of particular concern. Our results show that the vast majority of the megapode population in the Marianas occurs on relatively small islands (Table 1) that, owing to their diminutive size, are more susceptible to catastrophic stochastic events (Whittaker and Fernández-Palacios 2006). The larger islands of the Marianas could potentially support more robust megapode populations and provide larger expanses of more varied topographies, which could potentially reduce the effects of catastrophic events. These larger islands also serve to provide important connectivity between the smaller islands, which is important for gene flow to maintain diversity across the Mariana archipelago (Heaney 2007). Many of these larger islands (i.e. Agrihan, Pagan and Alamagan), however, that span considerable distances of ocean between megapode population centres (i.e. Asuncion, Guguan and Sarigan) display signs of current or recent human presence, support moderate to large numbers of feral ungulates and introduced predators, and as a result exhibit greater levels of habitat disturbance and alteration. Increased priority and emphasis on management of these large islands could enhance and improve the status of the Micronesian Megapode population in the Marianas.
To highlight the importance of inter-island connectivity, earlier surveys indicated that Anatahan supported between 300 and 423 megapodes (JD Reichel and PO Glass 1988, unpubl. data; Cruz et al. 2000c). Volcanic eruptions that started in 2003 covered the island and all available megapode habitat in ash, and no incidental observations of the species were previously noted on Anatahan after 2005 despite numerous trips to the island for ungulate control (C. Kessler, pers. obs.). Megapodes were detected on the island through call playback surveys employed during our study (Table 1); we estimated a moderate population of 703 birds to be present (Table 2). If the species had indeed been extirpated from Anatahan because of increased volcanic activity, it is plausible that the island was recolonised by megapodes from the neighbouring islands of Sarigan and/or Farallon de Medinilla. The possibility of such inter-island movements by megapodes in the Marianas has also been suggested by others (e.g. CG Rice and DW Stinson 1992, unpubl. data) and may help to explain the differences between our passive survey detections on Asuncion and those reported by Radley (2009) more than 2 years earlier. Such inter-island movement or commuting has been suggested as likely or possible for Micronesian Megapodes in Palau (Wiles and Conry 1990, 2001; Olsen et al. 2016) and is reported to occur in several other Megapodius species (Jones et al. 1995; R. Dekker, oral comm. 2013). Maintaining suitable habitat for Micronesian Megapodes across all islands in the Mariana’s northern arc is therefore important to facilitate inter-island movement and limiting or reducing potential population level effects of catastrophic stochastic events such as volcanic eruptions and typhoons.
Amongst the leading threats to megapodes across their global ranges are habitat loss and degradation, and introduced predators such as cats and dogs (Dekker et al. 2000). As discussed earlier, feral pigs may also predate eggs at megapode incubation sites (e.g. Sanders et al. 2020; Mori et al. 2021). Although introduced predators are likely a serious threat to Micronesian Megapodes in the Mariana Islands (USFWS 1998; Dekker et al. 2000), the primary threat to this population continues to be the loss and degradation of habitat by feral ungulates and clearing by humans, both of which may affect the quantity and diversity of available forage and cover for megapodes (USFWS 1998). Feral ungulates such as pigs may further mechanically disturb or destroy megapode breeding areas by rooting and wallowing, rendering these areas unusable by breeding megapodes, as documented by CG Rice and Stinson (1992, unpubl. data) on south Pagan. As our results imply, improving the quality and quantity of forest cover within the existing range of the Micronesian Megapode in the Mariana Islands will benefit their long-term protection and resilience. Removing feral ungulates and predators from Agrihan, Pagan, Alamagan and Aguiguan may decrease habitat degradation and fragmentation, reduce disturbances to Micronesian Megapodes and declines in their populations, and aid their recovery.
Data availability
Data analysed in this study are available on written request from the U.S. Fish and Wildlife Service, Pacific Islands Fish and Wildlife Office, 300 Ala Moana Boulevard, Room 3-122. Honolulu, Hawaii 96850, USA or by emailing Frederick Amidon (fred_amidon@fws.gov).
Declaration of funding
The 2008 Aguiguan and Tinian surveys were funded by the U.S. Navy and the U.S. Fish and Wildlife Service (USFWS). In 2009 and 2010, the USFWS coordinated Micronesian Megapode surveys in the CNMI as part of the ‘Marianas Expedition Wildlife Survey 2010’, funded through a contract with the Department of Defense (Naval Facilities Engineering Command Pacific), Honolulu, Hawaii. Analyses were conducted by the U.S. Geological Survey (USGS) – Pacific Islands Ecosystem Research Centre under Cooperative Agreement No.: 03WRAG0036.
Author contributions
Conceptualisation, F. A. A., R. J. C., P. M. G, C. K., A. P. M., and P. M. R.; methodology, F. A. A., R. J. C., P. M. G, and A. P. M.; data acquisition, F. A. A., R. J. C., P. M. G, C. K., A. P. M., and P. M. R.; formal analysis, R. J. C. and P. M. G; visualisation, F. A. A. and R. J. C.; funding acquisition, C. K.; data curation, F. A. A. and R. J. C.; writing – original draft, P. M. R., writing – review and editing, F. A. A., R. J. C., P. M. G, C. K., A. P. M., and P. M. R. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the USFWS. We thank Major Brad Adams, Angela Anders, James Bradley, Antonio Castro, Lenny Corin, Cari Eggleston, Chris Eggleston, Howard Ferguson, Joshua Fisher, Samantha Hudson, Jiny Kim, Shelly Kremer, Jaan Lepson, Michael Lusk, Gayle Martin, Theresa Mathis, Tim Robert, Christina Rockwell, Rachel Rounds, Rick Spaulding, and Laura Williams for data collection. Paul Wagner, Tom McCall, Benny Myhre, Ben and Greg Camacho, Gus Castro, Sandy Castro, Jess Omar, Jon Manygoats, Lerins Stole, Ben Mettao, Frank Kaipat, Elvin Masga Craig Clark, Howard Ferguson, and Curt Kessler provided logistical and other support. We also thank Americopters, Saipan Crewboats, the U.S. Navy, particularly Vanessa Pepi and Scott Vogt, the USGS, the U.S. Coast Guard, the Northern Islands Mayor’s Office, the Tinian Mayor’s office, Dr. Ignacio T. dela Cruz, and Sylvan Igisomar. At USFWS we thank Karen McCurry, Pam Kringel, Roxanne Anderson, Jimmy Breedan, Josh Fisher, Dean Mark, Paulette Reyes, Dave Castro, Chanse Aoki, and Elaine McHale, Earl Campbell, Marilet Zablan, Gina Shultz, and Loyal Mehrhoff. Lainie Berry provided comments on an earlier draft of this paper.
References
Amidon F, Camp R, Marshall A, Kessler C, Radley P, Buermeyer K, Gorresen M (2010) Micronesian Megapode survey assessment: Aguiguan 2009. In ‘Status of the Micronesian Megapode in the Commonwealth of the Northern Mariana Islands’. (Eds FA Amidon, AP Marshall, CC Kessler) p. 32. Appendix 4. (U.S. Fish and Wildlife Service, Marianas Expedition Wildlife Surveys 2010: Honolulu, Hawaii)
Ando H (2019) Genetic and ecological conservation issues for oceanic island birds, revealed by a combination of the latest molecular techniques and conventional field work. Ecological Research 34, 255-264.
| Crossref | Google Scholar |
Baker RH (1951) The avifauna of Micronesia, its origin, evolution, and distribution. University of Kansas Publications, Museum of Natural History 3, 1-359.
| Google Scholar |
BirdLife International (2021) Megapodius laperouse (errata version published 2022). The IUCN red list of threatened species 2021. Available at https://dx.doi.org/10.2305/IUCN.UK.2021-3.RLTS.T22678620A191672833.en. [accessed 23 April 2024]
Bowen J (1996) Notes on the Vanuatu Megapode Megapodius layardi on Ambrym, Vanuatu. Bird Conservation International 6, 401-408.
| Crossref | Google Scholar |
Buckland ST, Summers RW, Borchers DL, Thomas L (2006) Point transect sampling with traps or lures. Journal of Applied Ecology 43, 377-384.
| Crossref | Google Scholar |
Craig RJ (2021) The structure and dynamics of endangered forest bird communities in the Mariana Islands. Pacific Science 75, 543-559.
| Crossref | Google Scholar |
Dalsgaard B, Hilton GM, Gray GAL, Aymer L, Boatswain J, Daley J, Fenton C, Martin J, Martin L, Murrain P, Arendt WJ, Gibbons DW, Olesen JM (2007) Impacts of a volcanic eruption on the forest bird community of Montserrat, Lesser Antilles. Ibis 149, 298-312.
| Crossref | Google Scholar |
Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR (2016) Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences 113, 11261-11265.
| Crossref | Google Scholar |
Falanruw MVC (1975) Distribution of the Micronesian Megapode Megapodius laperouse in the Northern Mariana Islands. Micronesica 11, 149-150.
| Google Scholar |
Fancy SG, Craig RJ, Kessler CT (1999) Forest bird and fruit bat populations on Sarigan, Mariana Islands. Micronesica 31, 247-254.
| Google Scholar |
Fordham DA, Brook BW (2010) Why tropical island endemics are acutely susceptible to global change. Biodiversity and Conservation 19, 329-342.
| Crossref | Google Scholar |
Forsman ED, Meslow EC, Strub MJ (1977) Spotted owl abundance in young versus old-growth forests, Oregon. Wildlife Society Bulletin 5, 43-47.
| Google Scholar |
Göth A, Vogel U (1999) Notes on breeding and conservation of birds on Niuafo’ou Island, Kingdom of Tonga. Pacific Conservation Biology 5, 103-114.
| Crossref | Google Scholar |
Göth A, Vogel U (2002) Chick survival in the megapode Alectura lathami (Australian Brush-turkey). Wildlife Research 29, 503-511.
| Crossref | Google Scholar |
Göth A, Vogel U (2003) Juvenile dispersal and habitat selectivity in the megapode Alectura lathami (Australian Brush-turkey). Wildlife Research 30, 69-74.
| Crossref | Google Scholar |
Guyann DC, Jr, Downing RL, Askew GR (1985) Estimating the probability of non-detection of low density population. Cryptozoology 4, 55-60.
| Google Scholar |
Harris RB, Birks SM, Leaché AD (2014) Incubator birds: biogeographical origins and evolution of underground nesting in megapodes (Galliformes: Megapodiidae). Journal of Biogeography 41, 2045-2056.
| Crossref | Google Scholar |
Heaney LR (2007) Is a new paradigm emerging for oceanic island biogeography? Journal of Biogeography 34, 753-757.
| Crossref | Google Scholar |
Jansen R, Little RM, Crowe TM (2000) Habitat utilization and home range of the redwing francolin, Francolinus levaillantii, in highland grasslands, Mpumalanga province, South Africa. African Journal of Ecology 38, 329-338.
| Crossref | Google Scholar |
Jansen R, Robinson ER, Little RM, Crowe TM (2001) Habitat constraints limit the distribution and population density of redwing francolin, Francolinus levaillantii, in the highland grasslands of Mpumalanga province, South Africa. African Journal of Ecology 39, 146-155.
| Crossref | Google Scholar |
Jetz W, Wilcove DS, Dobson AP (2007) Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biology 5, e157.
| Crossref | Google Scholar | PubMed |
Jones DN (1999) What we don’t know about megapodes. Zoologische Verhandelingen 327, 159-168.
| Google Scholar |
Kessler CC (2002) Eradication of feral goats and pigs and consequences for other biota on Sarigan Island, Commonwealth of the Northern Mariana Islands. In ‘Turning the tide: the eradication of invasive species’. (Eds CR Veitch, MN Clout) pp. 132–140. (IUCN SSC Invasive Species Specialist Group: Gland, Switzerland and Cambridge: U.K.)
Kumar A, Sharma DK, Lochan R, Dewan S, Negi S (2020) Relative abundance, habitat preference, and breeding ecology of Asian Black francolin, Francolinus francolinus asiae (Bonaparte, 1856) (Galliformes: Phasianidae) from North-Western Himalaya. Journal of Asia-Pacific Biodiversity 13, 162-168.
| Crossref | Google Scholar |
Loope LL, Howarth FG, Kraus F, Pratt TK (2001) Newly emergent and future threats of alien species to Pacific landbirds and ecosystems. Studies in Avian Biology 22, 291-304.
| Google Scholar |
Lusk MR, Bruner P, Kessler C (2000) The avifauna of Farallon de Medinilla, Mariana Islands. Journal of Field Ornithology 71, 22-33.
| Crossref | Google Scholar |
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. Journal of Agricultural, Biological, and Environmental Statistics 9, 300-318.
| Crossref | Google Scholar |
Mapel XM, Gyllenhaal EF, Modak TH, DeCicco LH, Naikatini A, Utzurrum RB, Seamon JO, Cibois A, Thibault J-C, Sorenson MD, Moyle RG, Barrow LN, Andersen MJ (2021) Inter- and intra-archipelago dynamics of population structure and gene flow in a Polynesian bird. Molecular Phylogenetics and Evolution 156, 107034.
| Crossref | Google Scholar | PubMed |
Mori A (2019) A history of the excluded: rethinking the sugar industry in the Northern Mariana Islands under Japanese Rule. Historische Anthropologie 27, 410-434.
| Crossref | Google Scholar |
Mori E, Lazzeri L, Ferretti F, Gordigiani L, Rubolini D (2021) The wild boar Sus scrofa as a threat to ground-nesting bird species: an artificial nest experiment. Journal of Zoology 314, 311-320.
| Crossref | Google Scholar |
Office for Coastal Management (2023) C-CAP Regional Land Cover. NOAA National Centers for Environmental Information. Available at https://www.fisheries.noaa.gov/inport/item/48256
Olsen AR, Eberdong M, Ketebengang H, Blailes P, Chen P-H (2016) Survey of megapode nesting mounds in Palau, Micronesia. Western Birds 47, 27-37.
| Google Scholar |
O’Daniel D, Krueger S (1999) Recent sightings of the Micronesian Megapode on Tinian, Mariana Islands. Micronesica 31, 301-307.
| Google Scholar |
Perry G, Morton JM (1999) Regeneration rates of the wood vegetation of Guam’s Northwest Field following major disturbance: land use patterns, feral ungulates, and cascading effects of the brown treesnake. Micronesica 31, 125-142.
| Google Scholar |
Pratt HD, Bruner PL (1978) Micronesian Megapode rediscovered on Saipan. Elepaio 39, 57-59.
| Google Scholar |
Pregill GK, Steadman DW (2009) The prehistory and biogeography of terrestrial vertebrates on Guam, Mariana Islands. Diversity and Distributions 15, 983-996.
| Crossref | Google Scholar |
Presidential Documents (2009) Proclamation No. 8335: establishment of the marianas trench marine national monument. Federal Register 74, 1557-1563.
| Google Scholar |
Radley PM, Davis RA, Dekker RWRJ, Molloy SW, Blake D, Heinsohn R (2018) The vulnerability of Megapodes (Megapodiidae, Aves) to climate change and related threats. Environmental Conservation 45, 396-406.
| Crossref | Google Scholar |
Radley PM, Davis RA, Doherty TS (2021a) Impacts of invasive rats and tourism on a threatened island bird: the Palau Micronesian Scrubfowl. Bird Conservation International 31, 206-218.
| Crossref | Google Scholar |
Radley PM, van Etten EJB, Blake D, Davis RA (2021b) Breeding and feeding habitat selection by an island endemic bird may increase its vulnerability to climate change. Biotropica 53, 422-432.
| Crossref | Google Scholar |
Reed JM (1996) Using statistical probability to increase confidence of inferring species extinction. Conservation Biology 10, 1283-1285.
| Crossref | Google Scholar |
Reynolds MH, Snetsinger TJ (2001) The Hawaii rare bird search 1994–1996. Studies in Avian Biology 22, 133-143.
| Google Scholar |
Sanders HN, Hewitt DG, Perotto-Baldivieso HL, Vercauteren KC, Snow NP (2020) Opportunistic predation of Wild Turkey nests by wild pigs. The Journal of Wildlife Management 84, 293-300.
| Crossref | Google Scholar |
Scott JM, Mountainspring S, Ramsey FL, Kepler CB (1986) Forest bird communities of the Hawaiian Islands: their dynamics, ecology, and conservation. Studies in Avian Biology 9, 1-431.
| Google Scholar |
Şekercioğlu CH, Daily GC, Ehrlich PR (2004) Ecosystem consequences of bird declines. Proceedings of the National Academy of Sciences 101, 18042–18047. 10.1073/pnas.0408049101
Simberloff D (2000) Extinction-proneness of island species – causes and management implications. Raffles Bulletin of Zoology 48, 1-9.
| Google Scholar |
Sinclair JR (2002) Selection of incubation mound sites by three sympatric megapodes in Papua New Guinea. The Condor 104, 395-406.
| Crossref | Google Scholar |
Sitters J, Andriuzzi WS (2019) Impacts of browsing and grazing ungulates on soil biota and nutrient dynamics. In ‘The ecology of browsing and grazing II, vol. 239’. Ecological Studies. (Eds I Gordon, H Prins) pp. 215–236. doi:10.1007/978-3-030-25865-8_9
Steadman DW (1999) The prehistory of vertebrates, especially birds, on Tinian, Aguiguan, and Rota, Northern Mariana Islands. Micronesica 31, 319-345.
| Google Scholar |
Tershy BR, Shen K-W, Newton KM, Holmes ND, Croll DA (2015) The importance of islands for the protection of biological and linguistic diversity. BioScience 65, 592-597.
| Crossref | Google Scholar |
Thomas L, Buckland ST, Rexstad EA, Laake JL, Strindberg S, Hedley SL, Bishop JRB, Marques TA, Burnham KP (2010) Distance software: design and analysis of distance sampling surveys for estimating population size. Journal of Applied Ecology 47, 5-14.
| Crossref | Google Scholar | PubMed |
U.S. Forest Service (2006) Saipan/Rota/Tinian_Release. 2nd edn. U.S. Department of Agriculture Forest Service, Region 5 State and Private Forestry, Forest Health Protection. Available at https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fsbdev3_045361.zip
USFWS (1970) Conservation of endangered species and other fish and wildlife. Federal Register 35, 8491-8498.
| Google Scholar |
Wiles GJ (2005) Decline of a population of wild seeded breadfruit (Artocarpus mariannensis) on Guam, Mariana Islands. Pacific Science 59, 509-522.
| Crossref | Google Scholar |
Wiles GJ, Conry PJ (1990) Terrestrial vertebrates of the Ngerukewid Islands Wildlife Preserve, Palau islands. Micronesica 23, 41-66.
| Google Scholar |
Wiles GJ, Conry PJ (2001) Characteristics of nest mounds of Micronesian Megapodes in Palau. Journal of Field Ornithology 72, 267-275.
| Crossref | Google Scholar |
Wiles GJ, Beck RE, Amerson AB (1987) The Micronesian Megapode on Tinian, Mariana Islands. Elepaio 47, 1-3.
| Google Scholar |


