Understanding the co-evolutionary relationships between Indigenous cultures and non-native species can inform more effective approaches to conservation: the example of pigs (pua’a; Sus scrofa) in Hawai‘i
Kūpa‘a K. Luat-Hū‘eu A H , Kawika B. Winter
A H , Kawika B. Winter  A B C D , Mehana B. Vaughan A E F , Nicolai Barca G and Melissa R. Price
A B C D , Mehana B. Vaughan A E F , Nicolai Barca G and Melissa R. Price  A
A
A Natural Resources and Environmental Management, University of Hawai‘i at Mānoa, 1910 East-West Road, Sherman Laboratory Room 101, Honolulu, HI 96822, USA.
B Hawai‘i Institute of Marine Biology, 46-007 Lilipuna Road, Kane‘ohe, HI 96744, USA.
C National Tropical Botanical Garden, 3530 Papalina Road, Kalaheo, HI 96714, USA.
D Hawaii Conservation Alliance, 1601 East-West Road, Honolulu, HI 96848-1601, USA.
E University of Hawai‘i Sea Grant College Program, 2525 Correa Road, Honolulu, HI 96822, USA.
F Hui ‘Āina Momona, University of Hawai‘i at Mānoa, 2500 Campus Road, Honolulu, HI 96822, USA.
G The Nature Conservancy Hawai‘i, 923 Nu‘uanu Avenue, Honolulu, HI 96817, USA.
H Corresponding author. Email: kupaalh@hawaii.edu
Pacific Conservation Biology 27(4) 442-450 https://doi.org/10.1071/PC20086
Submitted: 2 November 2020 Accepted: 11 July 2021 Published: 10 August 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Indigenous resource management (IRM) is dynamic and ever evolving, in part because it is based on co-evolutionary relationships between Indigenous cultures and the biodiversity around them. Forms of conservation imposed on Indigenous people and places by settler-colonialism tend to idealise pre-human and human-excluded environments, leading to conflicts between settler-coloniser conservationists and Indigenous communities detrimental to conservation goals. Conservation efforts that align with IRM and acknowledge the co-evolutionary relationships at the foundation of Indigenous culture can lead to more effective conservation efforts. In Hawai‘i, the evolving relationship between Kānaka (Hawaiians) and pua‘a (pigs; Sus scrofa) has been the flash point of conflicts between settler-coloniser conservationists and Hawaiian communities. This paper examines the co-evolving relationships between Hawaiians and pigs in an effort to better balance the conservation efforts aimed at controlling invasive species with the State of Hawai‘i’s obligation to support Indigenous practices and public hunting. We conducted this research by investigating archival Hawaiian language resources, which allowed us to resurrect knowledge lost to time and pinpoint key historical changes over the past 250 years. Our results elucidate this co-evolutionary relationship that shifted from an animal-husbandry relationship to a hunter–prey relationship in the first half of the 19th century. This change in the trajectory of the co-evolutionary relationship was a result of various shifts throughout Hawaiian socio-ecological systems, and therefore necessitates adaptive governance relating to management of and access to pigs. We conclude that Indigenous perspectives offer opportunities to transform conservation biology through multi-objective approaches that address both hunting and conservation goals.
Keywords: biocultural evolution, conservation biology, conservation policy, feral pigs, Hawai‘i, Indigenous culture, Indigenous resource management, wildlife management.
Introduction
Indigenous resource management (IRM) is dynamic and evolving because it is based on the relationships between people and place – including biodiversity – which changes through time (Berkes 2012). These ‘biocultural relationships’ are at the foundation of IRM in Hawai‘i (Winter et al. 2018a, 2020a). Such relationships may be viewed as co-evolutionary, and this co-evolution can often be quantified (Winter and McClatchey 2009). Changes in the relationship may be evaluated to identify evolutionary drivers, as well as determine when novel interactions were invented or introduced, and how they spread within and among geographic regions over time. Biocultural evolution is accelerated when a new practice and/or biological taxa are introduced into a population (Winter and McClatchey 2008; Winter et al. 2020b). When examining eras of the past, inferences can be made about the factors influencing adoption or rejection of novel interactions as traditions, such as those associated with ecology or governance (Winter and McClatchey 2009).
Understanding the dynamics of the interactions between humans and their environment, particularly as interactions change and are adapted over extended periods of time, as with many Indigenous peoples, can help to align management of natural resources with social and ecological values (Salmón 2000; Winter and McClatchey 2008). Human interactions with and management of the environment influence the health and function of socio-ecological systems (SES) and the ability of humans to survive and thrive in socio-ecological systems (Folke et al. 2005). The SES framework is a lens through which we may examine how humans (social subsystems) and the environment (ecological subsystems) are integrated, with the understanding that separating the two will not lead to sustainable management outcomes for either or both (Berkes and Folke 1998). Incorporating Indigenous perspectives to manage SESs has potential to transform our ability to conserve resources effectively (Kendrick 2003; Garibaldi and Turner 2004), particularly given that Indigenous peoples have tenure rights on approximately 25% of the land worldwide and have managed their lands for millennia (Garnett et al. 2018). In many parts of the world, IRM enhances species richness and abundance through the maintenance of habitats (Turner and Berkes 2006; Comberti et al. 2015; Mistry et al. 2016). The same is true in Hawai‘i, where Hawaiians cared for their environment through decentralised IRM practices to maintain states of sustainable resource abundance (‘āina momona) over the course of centuries (Winter et al. 2018b, 2020a).
The purpose of this research was to explore how a more nuanced understanding of biocultural evolution may develop socially-informed policy and practice in the realm of wildlife management and conservation across the Pacific Ocean, as they relate to culturally-valued nonnative species. The 19th century was a time of rapid biocultural evolution due to the new plants and animals that were being introduced to Hawai‘i and incorporated into Hawaiian traditions (Table 1). In this study, we examined the relationship between Kānaka (Hawaiians) and pigs (Sus scrofa) as a biocultural relationship that was also evolving rapidly in this same period of time. Pigs are originally native to Eurasia, but due to their role in many cultures, they have been intentionally introduced around the world, accompanying humans on countless diaspora. As such, pigs now inhabit every continent except Antarctica, and many oceanic islands throughout the globe (Barrios-Garcia and Ballari 2012). Pigs have substantial cultural value to Indigenous Pacific Islanders, including Hawaiians, not only as food, but as symbols of social, economic, and political power (Dening 1980; Schieffelin and Crittenden 1991; Kirch 2014). Pigs have been a component of Hawaiian SESs for nearly 1000 years (Pearson et al. 1971; Kirch 2011; Winter et al. 2018a), and were predominantly domesticated and managed through husbandry practices as in other Pacific Islands. Prior to European arrival, Hawaiians managed domesticated pigs near their kauhale (household complexes) and fed pigs Indigenous crops such as ‘ulu (Artocarpus altilis) and kalo (Colocasia esculenta) (Handy et al. 1972; Maly 1998). Today, the majority of pig populations in Hawai‘i are now recognised as feral – meaning they originated from domestic stock but have reverted from domesticity to become free-living, no longer depending on humans for sustenance or breeding, although there are still farms with domestic pigs across the islands (Pullar 1950; Kruska and Röhrs 1974). The State of Hawai‘i’s Department of Land and Natural Resources (DLNR) currently manages feral pigs through a centralised governance system as both a resource species for game hunting and as an invasive species that threatens native ecosystems and biodiversity. However, the State of Hawai‘i Constitution obligates the DLNR to affirm traditional and customary practices of Native Hawaiians, and conservation planning has often failed to acknowledge the hunting of pigs as a traditional Indigenous practice, leading to conflicts between Hawaiian pig hunters and agency resource managers.
Previous studies of pigs in Hawai‘i have been limited in several key ways. The majority of studies primarily focused on the ecological impacts of feral pigs without accounting for sociocultural values (e.g. Kramer 1971; Diong 1982; Tomich 1986; Nogueira-Filho et al. 2009). Some studies (e.g. Duffy and Lepczyk 2021) have acknowledged the historical relationships between Hawaiians and pigs, and the social dimensions of their management over time, but are lacking perspectives from Hawaiian people. Critically, many of the studies to date rely either disproportionally or exclusively on written accounts that are now recognised as narrow and unreliable colonial perspectives on Hawaiian culture and practices – such as one-off observations from foreign explorers (e.g. Cook 1846) and the musings of writers who dehumanised Hawaiians and devalued Indigenous knowledge systems (e.g. Kuykendall 1938) – but largely ignore the writings of Native Hawaiian historians. To the best of our knowledge, there are no studies that have conducted comprehensive reviews of archival resources (e.g. Hawaiian language newspapers) to understand Indigenous perspectives of this species.
Today, technology has made archival resources increasingly accessible to scholars via digitisation of physical documents (e.g. Ulukau (http://wehewehe.org/gsdl2.85/cgi-bin/hdict?l=en) and Papakilo Database) and translation of Hawaiian language materials into English (e.g. Awaiaulu), which have expanded access to knowledge recorded in the previous centuries. Hawaiian language newspapers are a valuable repository of knowledge that spans over a hundred years, with the first Hawaiian newspaper (Ka Lama Hawaii) published in 1834 and the last Hawaiian newspaper (Ka Hoku o Hawaii) published in 1948 (Nogelmeier 2010). The newspapers were collectively published for 114 years and documented many of the oral traditions of the previous centuries, alongside current events, as well as observations and stories from around the Hawaiian archipelago and the world (Nogelmeier 2010). For this reason, scholars have increasingly incorporated these resources into their work, including scientific studies to identify foundational knowledge and historical baselines in fields such as conservation biology and climatology (e.g. Winter 2012; Businger et al. 2018; Sato et al. 2018; Gon et al. 2021).
In this study, as a means to provide a more holistic and comprehensive understanding regarding pigs in Hawai‘i, we set out to answer two main research questions. (1) What are the drivers of the co-evolutionary relationship between Hawaiians and pigs? (2) How does this co-evolving relationship inform current and future management actions for a species that is simultaneously a culturally-important species, a game species, and a non-native species that impacts other culturally-valued native species?
Materials and methods
We examined Indigenous knowledge that is contained in the Hawaiian language newspapers, published from 1834 to 1948 to elucidate the nature of the biocultural relationships that existed in Hawai‘i both contemporaneously to that period and prior to European contact in 1778 (Gon et al. 2021). The majority of Hawaiian language newspapers are digitised and readily available to the public via online databases, such as the Papakilo Database, which currently contains 410 701 articles (Office of Hawaiian Affairs 2011; accessed 25 May 2021). We used the Papakilo Database to examine primary source, Hawaiian perspectives of the biocultural relationship with pigs in both pre-contact and post-contact periods. Our approach to conducting this research was informed from past studies that also used this database (e.g. Businger et al. 2018; Sato et al. 2018).
To assess whether or not hunting was an existing practice at the point of contact or whether it is an example of the rapid biocultural evolution that was taking place in the 19th century, word searches were done in the Papakilo Database using the terms for hunting (alualu, uhai, hahai) that were applied to pigs (a species existing in Hawai‘i at the point of contact) and goats (the first ungulate species introduced by Europeans in 1793). These word searches were conducted in May 2021. All newspaper search results were reviewed for relevance and substance (two of five authors are fluent in the Hawaiian language).
Utilising the results from our word searches and translations of articles, we reconstructed the historical context and events relating to pigs during this time period to identify when and how major co-evolutionary changes took place. Due to the multi-layered Hawaiian meanings that can get lost in translation we are not providing full translations of all articles here. However, for the convenience of authors, reviewers, and readers who are not literate in the Hawaiian language, we have provided a translation of a subset of articles (see Table S1, available as Supplementary material).
Results
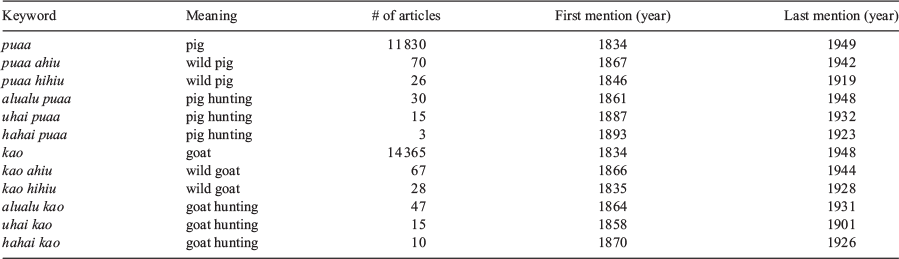
In May 2021, the searches of the Papakilo Database yielded a total of 11 830 articles/announcements with mentions of puaa (pig) between 1834 and 1949, 70 mentions of puaa ahiu (lexiconic variant of ‘wild pig’) between 1867 and 1942, 26 mentions of puaa hihiu (lexiconic variant of ‘wild pig’) between 1846 and 1919, 30 mentions of alualu puaa (lexiconic variant of ‘pig hunting’) between 1861 and 1948, 15 mentions of uhai puaa (lexiconic variant of ‘pig hunting’) between 1887 and 1932, and 3 mentions of hahai puaa (lexiconic variant of ‘pig hunting’) between 1893 and 1923; 14 365 articles/announcements with mentions of kao (goat) between 1834 and 1948, 67 mentions of kao ahiu (lexiconic variant of ‘wild goat’) between 1866 and 1944, 28 mentions of kao hihiu (lexiconic variant of ‘wild goat’) between 1835 and 1928, 47 mentions of alualu kao (lexiconic variant of ‘goat hunting’) between 1864 and 1931, 15 mentions of uhai kao (lexiconic variant of ‘goat hunting’) between 1858 and 1901, and 10 mentions of hahai kao (lexiconic variant of ‘goat hunting’) between 1870 and 1926 (Table 2).
There are two lexiconic variants of ‘wild pig’ – puaa hihiu and puaa ahiu – with the latter being the dominant term in Hawaiian language newspapers as measured by the number of mentions, the duration of its usage, and the geographic extent of its usage. The earliest mention of a term for ‘wild pig’ in the Hawaiian language newspapers used the lexiconic variant puaa hihiu in 1846; however, that article was about political events in Oregon, and mentioned that the King of France died while hunting wild pigs in the forest of France with his wife and other nobles. The first mention of a term for ‘wild pig’ associated with a location in Hawai‘i also used the lexiconic variant puaa hihiu in an 1853 article. This article listed a petition of requests submitted to the government, with one of the requests being to lift a taboo from goats and wild pigs in the mountain and forests. The article did not describe the type of taboo; however, it was most likely a taboo that banned people from harvesting wild pigs and goats. The last time puaa hihiu was used in a Hawai‘i-focused article was in 1865 (Booth 1865). The lexiconic variant puaa ahiu was first mentioned in 1867, 21 years after puaa hihiu was first mentioned and 2 years after it was last mentioned (Table 2). Puaa ahiu was last used in 1932. As for geographic distribution of the lexiconic variants, puaa hihiu was only mentioned in articles referring to O‘ahu and other places outside of the Hawaiian Islands, whereas puaa ahiu was mentioned in articles referring to O‘ahu Hawai‘i Island, and Maui. Both lexiconic variants for ‘wild pig’ are only used in articles and public announcements in reference to contemporaneous events, and neither is mentioned in the documented oral histories (mo‘olelo, ka‘ao) corresponding to the pre-contact era.
There are three lexiconic variants for ‘pig hunting’ – uhai puaa, hahai puaa, and alualu puaa – with the latter being dominant in Hawaiian language newspapers as measured by the number of mentions, the duration of its usage, and the geographic extent of its usage. Although pig hunting was first alluded to in an 1858 article titled, ‘He Puaa hihiu nui loa’, that wrote of a hunting trip in Niu Valley, on the island of O‘ahu; the first appearance of a lexiconic variant of ‘pig hunting’ (alualu puaa) was in an article in 1861 (Parke 1861). All three lexiconic variants for ‘pig hunting’ are only used in articles and public announcements in reference to contemporaneous events, but none were mentioned in the documented oral histories (mo‘olelo, ka‘ao) corresponding to the pre-contact era.
The Polynesian-introduced pig and the European-introduced goat are both mentioned in the first issue of the first Hawaiian language newspaper in 1834, suggesting that both species were culturally important at that point. The first appearance of a lexiconic variant of ‘wild goat’ (kao hihiu) appeared the following year in 1835, which preceded the first appearance of a lexiconic variant of ‘wild pig’ (puaa hihiu) in 1846. The first appearance of a lexiconic variant for ‘goat hunting’ (uhai kao) appeared in 1858, which preceded the first appearance of a lexiconic variant for ‘pig hunting’ (alualu puaa) in 1861.
Discussion
Cultures display resilience by evolving over time, responding to external and internal pressures, to maintain functioning SES (Crane 2010). In this study, we characterised the resilience of Hawaiian communities through an evolving relationship with pigs over time, using Indigenous and local writings from 1834 to 1948, to identify the ways in which biocultural practices evolved over time. Evolution, or the process by which the proportion of traits in a population changes over time, requires inheritance, variation, and selection (Campbell 1965; Kallis and Norgaard 2010). Co-evolution is based on similar concepts, but involves two systems that evolve together and causally influence respective change (Kallis 2007). Similar to a biologically evolving unit, such as a gene, there are units of measure for the co-evolution of biocultural relationships (Winter and McClatchey 2008). In this study, the biocultural unit of co-evolution was defined as the set of biocultural practices utilised by Hawaiians in association with pigs.
Biocultural evolution: shifts in the frequency of biocultural practices over time
Similar to other societies around the world, Hawaiians historically bred domestic pigs for valuable traits including higher reproduction rates, shorter time needed to reach maturity, larger litters, and bigger body sizes (Krauss 1923; Lega et al. 2016). Domestic meat, fattened with Indigenous agricultural by-products and surplus, was likely preferred by Hawaiians prior to European arrival, and complemented their cultural practices such as religious offerings (Malo 1951; Lockwood 2009; Hommon 2013). Some variation existed among regions in the abundance of domesticated pigs and associated cultural practices, likely because some areas lacked the environmental parameters and agricultural productivity, such as presence of water and Indigenous crops, necessary for domesticating pigs (Kirch 2014), but the method by which pigs were obtained (e.g. husbandry), does not appear to have varied across regions to include hunting. The absence of either of the lexiconic variants for ‘wild pigs’ (puaa hihiu, puaa ahiu) in the pre-contact oral histories (mo‘olelo, ka‘ao) documented in the Hawaiian language newspapers is a clear indication that pigs were managed exclusively through animal husbandry rather than as a wild food resource in the pre-contact era.
Terms for wild ungulates and the hunting of wild ungulates do not appear until the first half of the 19th century, and they are applied first to goats – a species introduced by Europeans. This suggests that hunting is a practice that emerged in this time period in response to the appearance of feral ungulate populations, and our data suggest that the first of these two species to develop wild populations was the goat. The existence of multiple variant words for both hunting (alualu, uhai, hahai) and wild (‘āhiu, hihiu) applied to ungulates throughout the archipelago in the 19th century is linguistic evidence that suggests the appearance of feral ungulate populations and the subsequent emergence of hunting as a response to a new food source happened simultaneously, or nearly so, on multiple islands, rather than being a longstanding practice that was universal throughout Hawaiian culture at the time. In contrast, there is only a single word for fishing (lawai‘a), farming (mahi ‘ai), and tame – in reference to an animal that is tended through husbandry – (laka); which speaks to the universality of those practices in the same period.
The eventual dominance of the Hawai‘i island terms for hunting (alualu) and for wild (‘āhiu) are reflective of the socio-political dynamics of the 19th century following the unification of the islands under the Kamehameha dynasty. The Kamehameha dynasty originated on the island of Hawai‘i, and following Kamehameha I’s conquest of the entire archipelago and the consolidation of power under a single sovereign, the archipelago was collectively re-named, “Hawai‘i,” as an homage to his island of origin. The eventual dominance of the terms puaa ahiu and alualu puaa are examples of the trend toward dominance of the lexicon of the island of Hawai‘i over the rest of the archipelago.
Drivers of biocultural evolution
For a system to evolve, there must be genetic variation, inheritance, and selection, along with differential survival based on suitability of genetic variants in a given environment (Kallis and Norgaard 2010). For biocultural units to evolve, there must similarly be variation in cultural practices associated with a biotic element (i.e. plant, animal), and changes in those practices over time in response to social and environmental variation (Winter and McClatchey 2008, 2009). In the case of this study, three factors likely caused a shift in frequency of the biocultural practices in regards to pigs from domestic husbandry to hunting: (1) shifts in resource abundance and land use practices; (2) shifts in governance and land tenure; and (3) the introduction of novel tools and practices.
Shifts in resource abundance and land use practices
Pigs were already a component of the Indigenous food system in Hawai‘i at the point when foreign varieties of domestic pigs were introduced following the arrival of Europeans. Foreign pigs crossbred with the Polynesian pig, which resulted in an increase in physical size and fecundity of pig populations (Tomich 1986; Linderholm et al. 2016). Shortly thereafter, fruiting trees (e.g. guava, Psidium spp. and roseapple, Syzygium jambos) were introduced (Smith 1985; Staples et al. 2000), became naturalised, and spread rapidly through low and mid-elevation forests, which increased food availability for feral pig populations (Jacobi and Warshauer 1992). These new food sources also facilitated free-range farming practices, which sometimes sparked expansion of feral populations to new areas (Diong 1982; Tomich 1986). Although pigs have a generalist diet (plants, invertebrates, and vertebrates), the introduction and subsequent naturalisation of carbohydrate-rich fruiting plants into forested areas that previously had fewer food sources to sustain wild pig populations likely led to an increase in the carrying capacity of the environment for feral pigs (Smith and Diong 1977; Giffin 1978; Stone 1985). The environmental carrying capacity for feral pigs was further increased through the introduction of earthworms to the Hawaiian Islands, which provided a readily-available protein source for pigs, and subsequently increased the ecological impacts of pigs by encouraging soil disturbance through rooting (Barrett and Stone 1983; Anderson 1994). The spread of invasive plants and the growth and expansion of feral pig populations interacted in a positive feedback loop, and facilitated the transition of ecosystems from native-dominated to invasive-dominated states (Wehr et al. 2018). As such, the increased availability and diversity of foraging resources for feral pig populations has been an important driver of their establishment and range expansion.
Although pig hunting did not exist as an Indigenous practice prior to the series of ecological shifts that allowed for the environment to sustain feral pig populations, the addition of the practice of pig hunting allowed Hawaiians to increase food security and maintain associated cultural practices as domestic husbandry of pigs declined. As such, hunting of wild pigs was an important adaptation in the face of shifts in resource availability noted from that period – such as collapses in nearshore fisheries (Jokiel et al. 2011; Kittinger et al. 2011), along with concurrent changes in land tenure (Kame‘eleihiwa 1992) and the destruction or abandonment of Indigenous aquaculture systems (Chimner et al. 2006). Other Indigenous groups across Oceania have faced similarly complex issues pertaining to non-native species, such as West Papua, where introduced deer have become an important food source for local tribes (Hardjanti and Zainal 2003; Pfeiffer and Voeks 2008). Since its introduction to Aotearoa (New Zealand) in 1769, feral pigs and feral pig hunting have since become an important food source and activity for Maori people and local residents (Dunmore 1969; Nugent et al. 1996).
Shifts in governance and land tenure
In the context of Indigenous governance and resource management, ali‘i (ruling class) and konohiki (head resource managers) served as the land managers of their ahupua‘a (bounded communities) (Kamakau et al. 1964), using place-based knowledge and collaborating with the maka‘āinana (fishers and farmers) to authorise access to resources, and to maintain a state of ‘āina momona, or sustainable abundance (Andrade 2008). In order to sustainably exist in resource-limited island systems, various conservation practices existed in this form of resource management. These practices included the designation of sacred forest zones (wao akua) to maintain watershed function, habitat, and native biodiversity; and the either occasional or regular imposition of harvest restrictions to manage the population dynamics and abundance of key resource species (Winter et al. 2018b). Access to sensitive habitats was limited to maintain abundance and respect for akua (deities). Native biodiversity was considered the physical manifestation of the pantheon of deities in Hawaiian religion, and one of the objectives in Hawaiian resource management was/is the maintenance of pono (balance) between humans (kānaka) and the divine (akua) (Gon et al. 2021). Sociocultural institutions instilled philosophies and ethics – such as a sense of kūleana (responsibility) for stewardship, which ultimately maintained the productivity of ‘āina (loosely translating to ‘that which feeds’, or land) and their communities (Vaughan 2018; Winter et al. 2020a).
Major changes occurred following the arrival of Europeans in 1778 that affected governance and resource management including the unification of Hawai‘i under one ruler (Kamehameha I) in 1810, the abolishment of the Hawaiian religion in 1819, and the privatisation of land in 1848 (commonly known as the Great Māhele) enacted by Kauikeaouli (Kame‘eleihiwa 1992). The Great Māhele was a substantial change from the previous systems where land was a commonly-held resource and responsibility, with the exception of ali‘i (Chinen 1958; Andrade 2008). This allowed people more autonomy to regulate and manage lands on their own terms. This period of time also included major cultural shifts, such as the shift to allow women to eat pigs, a practice which was previously prohibited under the Hawaiian religion (Kamakau 1961; Kame‘eleihiwa 1992).
Rights related to access, as well as responsibility over land-based resources, which were all held in common by ahupua‘a tenants (hoa‘āina) in pre-contact forms of governance and resource management, shifted to the domain of the landowner after the Great Māhele (Andrade 2008), which is itself an example of the strong colonising pressures exerted on Hawaiian religion and governance (Sai 2008). As evident from the Hawaiian language newspapers at the time, landowners began to self-govern their properties for their self-interests at the expense of the community’s interests. Examples of this can be seen in the access restrictions that were imposed to prevent the hunting of feral ungulates. In the 1860s certain landowners from Hawai‘i Island engaged in efforts to prohibit people from hunting pigs on their properties without permission, which likely allowed for the growth of wild pig populations (Parke 1861; Spencer 1869).
In the contemporary era, governance and private land ownership currently limits access and regulates interactions between people and pigs in Hawai‘i. Due to pig populations shifting from domestic Polynesian pigs to mainly wild pigs, people primarily access pig meat through supermarkets, but also through other means such as hunting, customary sharing (māhele), or trade/purchase from hunters. Hunting in the State-designated public hunting areas of Hawai‘i (owned or leased by the State of Hawai‘i) is regulated by the DLNR. Mechanisms of regulation primarily include delineating public hunting unit boundaries, hunting regulations (e.g. closed seasons, bag limits, hunting days out of the week), (HAR 13–123), special permitting (e.g. tags, control hunts), regulating access, enforcement, and ungulate fencing. Hunting on undeveloped private lands is also a common means for people to access feral pigs, which continues to be a point of conflict between some land owners and pig hunters. In contrast to management of game species in North America, game animals on private lands have been considered the property of the landowner, not the property of the State. Although some state hunting regulations still apply when hunting on private lands such as the requirement for permission from landowners – that may include formal cooperative agreements to cover landowner and hunter liability – hunting on these lands by Hawaiians is now protected by case law. The State vs Palama court case in 2015 established the first definitive ruling on the status of pig hunting as a traditional and customary right of Native Hawaiians, thus declaring it a right protected under the State Constitution (State vs Palama 2015). This case has limited DLNR’s ability to enforce access to forested areas by Hawaiian pig hunters, and on undeveloped private lands where rights to enter are difficult to delineate and many other activities are protected as traditional and customary rights under the Hawai‘i State Constitution, Article XII Section 7.
Access management can be conceptualised broadly to include mechanisms such as access, technologies, knowledge, authority, social identity, and labour that provide the ability to benefit from a resource (Ribot and Peluso 2003). Globally, governments and landowners have managed human and animal access to resources (e.g. land) by fences, gates, and roads (Peluso 1992; Rose 1994). In North America, access to public and private lands for hunting has been driven by factors such as the commercialisation of wildlife, public perceptions of hunting, land ownership tenure, land management priorities, and property size (Miller 2002; Eliason 2016; Burke et al. 2019). The trajectory of change in Hawai‘i parallels the plights of Indigenous people both globally and in North America, where government has enacted policies to limit and/or prevent access of Indigenous people to their ancestral lands and resources, severing deep-rooted interactions between humans and the environment (Sobrevila 2008).
Introduction of novel tools and practices
For centuries, Hawaiians sustained themselves and their communities under a complex system of IRM, including various agro-ecology and aquaculture technologies (Winter et al. 2018a, 2020a). New tools and practices introduced by Europeans and other foreigners to Hawai‘i facilitated a shift in biocultural practices and ultimately precipitated biocultural evolution. One tool of particular relevance to this study was the introduction of firearms to Hawai‘i in 1778 by Captain Cook (Kamakau et al. 1964). Muskets and other types of firearms contributed to the Kamehameha dynasty’s conquest of the Hawaiian archipelago (Kamakau et al. 1964), and they would later provide a means of hunting wild pigs. The first mention of using firearms to hunt pigs in the newspapers was in 1861, where a landowner on Hawai‘i Island prohibited people from shooting guns and hunting pigs (Parke 1861). Another article in 1863 mentioned the use of firearms, but in a different context as an announcement to promote the killing of pigs at Malaekahana on the island of O‘ahu by shooting them with guns (Moffitt and Hopkins 1863).
Another contemporary method for hunting pigs in Hawai‘i is the use of dogs, a practice utilised by other Indigenous islanders across Oceania, including the Phillipines and Papua New Guinea (Ōtsuka 1983; Goodman et al. 1985). Hawai‘i’s native wet forests are extremely dense with vegetation, which makes it difficult to hunt solely with firearms due to limited visibility. Dogs were first brought to Hawai‘i alongside pigs during early Polynesian settlement and were predominantly domesticated as a highly prised food source (Titcomb and Pukui 1969). Macrae described Hawaiian dogs as ‘small, with long bodies and ears, sharp pricked noses and short feet’ (Macrae 1922). Thus, due to their relatively small size, the Polynesian dogs were likely unable to assist with hunting pigs once wild pigs hybridised with larger varieties. As foreigners began to immigrate to Hawai‘i for plantation and ranching purposes, they brought other breeds of dogs with them, some larger and more suitable for hunting. The first mention of dogs for hunting pig in our newspaper searches was in an 1858 article that described dogs of hunters grabbing a large pig and holding onto it until one of the hunters could dispatch the pig. This points to the mid-19th century as the era in which pig hunting with dogs emerged as a Hawaiian practice, and subsequently became a tradition. As nearshore fisheries collapsed, Hawaiian aquaculture systems were destroyed or fell into disrepair, changes in land tenure led to a decline in the farming of domesticated pigs, and feral pigs became more abundant across the landscape, novel tools (e.g. guns) and practices (hunting) enabled Hawaiians to maintain their biocultural relationships with pigs, albeit in an evolved form.
Conclusions
For Indigenous communities, the hunting of introduced and invasive species reflects an adaptation to shifts in SES that provides a means to perpetuate cultural practices while maintaining independence through food self-sufficiency and informal non-market sharing. It is in this context that the hunting of ungulates emerged as an Indigenous practice in Hawai‘i in the first half of the 19th century as a response to the appearance of feral ungulate populations that had not existed previously. The evidence presented in this study suggests the practice of hunting was first applied to goats (a European introduction), and then applied to pigs as ecological shifts in the early 19th century allowed for populations of that species to become feral. The data from this research is evidence to support the notion that biocultural evolution happens rapidly when taxa of use to humans are introduced to and spread within Indigenous SES. In this case, the emergence of a new practice of hunting and the subsequently incorporation of that practice into Hawaiian culture as an Indigenous tradition is an example of the rapid biocultural evolution that was happening in Hawai‘i in the 19th century.
A rapid biocultural evolution of the relationship between Hawaiians and pigs was driven by changes in pig ecology, abundance and distribution, as well as the introduction of new working animals such as hunting dogs, and the introduction of novel tools such as rifles and metal knives, changes in land tenure, and a shift from a communally-managed resource system to one of individual land and resource ownership. The cultural shift toward hunting feral pigs allowed Hawaiians to maintain their Indigenous food systems in the face of catostrophic ecological shifts (e.g. collapsing fisheries) noted for the 19th century while maintaining other deeply rooted biocultural relationships with pigs such as various culinary traditions associated with large social gatherings (e.g. weddings) and for ritual offerings. Hunting is also a cultural expression of self-sufficiency that might otherwise have been lost with the decrease in traditional food sources. The manner in which governance and policy have responded to these shifts in ecology and evolution of Indigenous culture and practice, however, has often led to conflicts between Indigenous people, land owners, and resource managers.
The philosophies and ethics of IRM in Hawai‘i, which value native biodiversity and the integrity of native-dominated landscapes, does not support the abundance of a single resource species at the expense of hundreds of others. The shifts to nonnative-dominated landscapes, which excessive levels of ungulates can facilitate, threaten the foundation of Indigenous spirituality and practice, as well as other key ecosystem services and processes; yet the hunting of ungulates to maintain food sovereignty is by now a long-standing Indigenous tradition. Policies need to balance the conservation of native biodiversity and habitats with the constitutional obligation to support Indigenous practices. We recommend that government agencies work in greater collaboration with Indigenous peoples and local communities, particularly hunters, using multi-objective approaches to manage culturally-valued nonnative species like feral pigs, alongside other culturally-valued native species, to reduce conflicts among stakeholders and support the perpetuation of all cultural practices.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the University of Hawai‘i’s Hamilton Library Pacific Collection librarians who provided guidance on where to access literature. We also thank the people involved in the Hawai‘i Wildlife Ecology Lab and Vaughan Piko for their support and feedback throughout the process of this research. This research was funded by the College of Tropical Agriculture & Human Resources at the University of Hawai‘i at Mānoa and the United States Department of Agriculture. This paper reports work from the lead author’s unpublished masters dissertation (Luat-Hū‘eu, 2020 ‘Finding Pathways Toward Co-Management of Hawai‘i’s Feral Pigs (Pua‘a; Sus scrofa): A Historical Review of Biocultural Coevolution of Relationships Between Hawaiians and Pigs and Semi-Structured Interviews with Local Pig Hunters’) at the University of Hawai‘i at Mānoa. The data that support this study are available in the article and accompanying online Supplementary material.
References
Anderson, S. P. (1994). Some environmental indicators related to feral pig activity in a Hawaiian rain forest. MSc thesis. Department of Natural Resources and Environmental Management, University of Hawai‘i at Manoa.Andrade, C. (2008). ‘Ha‘ena: through the eyes of the ancestors.’ (University of Hawai‘i Press: Honolulu, HI, USA)
Barrett, R. H., and Stone, C. P. (1983). Managing wild pigs in Hawaii Volcanoes National Park. Unpublished Report for Resources Management, Hawaii Volcanoes National Park, 21 November 1983. Research Center, Hawaii Volcanoes National Park, Hawaii.
Barrios-Garcia, M. N., and Ballari, S. A. (2012). Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biological Invasions 14, 2283–2300.
| Impact of wild boar (Sus scrofa) in its introduced and native range: a review.Crossref | GoogleScholarGoogle Scholar |
Berkes, F. (2012). ‘Sacred ecology.’ (Routledge: New York, NY, USA.)
Berkes, F., Folke, C. (Eds) (1998). ‘Linking social and ecological systems: management practices and social mechanisms for building resilience.’ (Cambridge University Press: Cambridge, UK.)
Booth, J. (1865). ‘Olelo Hoolaha.’ Ke Au Okoa, 18 September, p. 3.
Burke, C. R., Peterson, M. N., Sawyer, D. T., Moorman, C. E., Serenari, C., Meentemeyer, R. K., and DePerno, C. S. (2019). Predicting private landowner hunting access decisions and hunter density. Human Dimensions of Wildlife 24, 99–115.
| Predicting private landowner hunting access decisions and hunter density.Crossref | GoogleScholarGoogle Scholar |
Businger, S., Nogelmeier, M. P., Chinn, P. W., and Schroeder, T. (2018). Hurricane with a history: Hawaiian newspapers illuminate an 1871 storm. Bulletin of the American Meteorological Society 99, 137–147.
| Hurricane with a history: Hawaiian newspapers illuminate an 1871 storm.Crossref | GoogleScholarGoogle Scholar |
Campbell, D. T. (1965). Variation and selective retention in socio-cultural evolution. In Social change in developing areas: a reinterpretation of evolutionary theory, 19–49.
Chimner, R. A., Fry, B., Kaneshiro, M. Y., and Cormier, N. (2006). Current extent and historical expansion of introduced mangroves on O’ahu, Hawai‘i’. Pacific Science 60, 377–383.
| Current extent and historical expansion of introduced mangroves on O’ahu, Hawai‘i’.Crossref | GoogleScholarGoogle Scholar |
Chinen, J. J. (1958). ‘The great Mahele: Hawaii’s land division of 1848. Vol. 1, No. 1.’ (University of Hawaii Press: Honolulu, HI, USA.)
Comberti, C., Thornton, T. F., de Echeverria, V. W., and Patterson, T. (2015). Ecosystem services or services to ecosystems? Valuing cultivation and reciprocal relationships between humans and ecosystems. Global Environmental Change 34, 247–262.
| Ecosystem services or services to ecosystems? Valuing cultivation and reciprocal relationships between humans and ecosystems.Crossref | GoogleScholarGoogle Scholar |
Cook, J. (1846). ‘The Voyages of Captain James Cook (Issue v. 2).’ (W. Smith: London, United Kingdom.)
Crane, T. A. (2010). Of models and meanings: cultural resilience in social–ecological. Ecology and Society 15, 4.
| Of models and meanings: cultural resilience in social–ecological.Crossref | GoogleScholarGoogle Scholar |
Dening, G. (1980). ‘Islands and beaches: discourse on a silent land: Marquesas, 1774–1880.’ (University Press of Hawaii: Honolulu, HI, USA.)
Diong, C. H. (1982). ‘Population biology and management of the feral pig (Sus scrofa) in Kipahulu Valley, Maui.’ (University of Hawai‘i: Ma¨noa, Honolulu, HI, USA.)
Duffy, D. J., and Lepczyk, C. A. (2021). The historical ecology of game species introductions in Hawai‘i. Pacific Science 75, 1–41.
| The historical ecology of game species introductions in Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Dunmore, J. (1969). ‘The fateful voyage of the St Jean Baptiste: a true account of M. de Surville’s expedition to New Zealand and the unknown south seas in the years 1769-70.’ (Pegasus: New Zealand.)
Eliason, S. L. (2016). Access to public resources on private property: resident hunterperceptions of the commercialization of wildlife in Montana. Journal of Outdoor Recreation and Tourism 16, 37–43.
| Access to public resources on private property: resident hunterperceptions of the commercialization of wildlife in Montana.Crossref | GoogleScholarGoogle Scholar |
Folke, C., Hahn, T., Olsson, P., and Norberg, J. (2005). Adaptive governance of social-ecological systems. Annual Review of Environment and Resources 30, 441–473.
| Adaptive governance of social-ecological systems.Crossref | GoogleScholarGoogle Scholar |
Garibaldi, A., and Turner, N. (2004). Cultural keystone species: implications for ecological conservation and restoration. Ecology and Society 9, 1.
| Cultural keystone species: implications for ecological conservation and restoration.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., Burgess, N. D., Fa, J. E., et al. (2018). A spatial overview of the global importance of Indigenous lands for conservation. Nature Sustainability 1, 369.
| A spatial overview of the global importance of Indigenous lands for conservation.Crossref | GoogleScholarGoogle Scholar |
Giffin, J. G. (1978). ‘Ecology of the feral pig on the island of Hawaii.’ Honolulu, HI, USA.
Gon, Samuel M., Winter, K. B., and Demotta, M. (2021). KUA–LAKO–MO ‘O: a methodology for exploring Indigenous conceptualisations of nature and conservation in Hawai‘i. Pacific Conservation Biology , .
| KUA–LAKO–MO ‘O: a methodology for exploring Indigenous conceptualisations of nature and conservation in Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Goodman, M. J., Griffin, P. B., Estioko-Griffin, A. A., and Grove, J. S. (1985). The compatibility of hunting and mothering among the Agta hunter-gatherers of the Philippines. Sex Roles 12, 1199–1209.
| The compatibility of hunting and mothering among the Agta hunter-gatherers of the Philippines.Crossref | GoogleScholarGoogle Scholar |
Handy, E. S., Handy, E. G., and Pukui, M. K. (1972). ‘Native planters in old Hawaii: their life, lore, and environment. Vol. 233.’ (Bishop Museum: Honolulu, HI, USA.)
Hardjanti, F., and Zainal, I. (2003). Indonesia report. Invasive alien species in south-southeast Asia. National Reports and Directory of Resources , 30–33.
Hommon, R. J. (2013). ‘The ancient Hawaiian state: origins of a political society.’ (Oxford University Press: New York, NY, USA.)
Jacobi, J. D., and Warshauer, F. R. (1992). Distribution of six alien plant species in upland habitats on the island of Hawaii. In ‘Alien plant invasions in native ecosystems of Hawai‘i’. pp. 155–188. (University of Hawai‘i Press: Honolulu, HI, USA.)
Jokiel, P. L., Rodgers, K. S., Walsh, W. J., Polhemus, D. A., and Wilhelm, T. A. (2011). Marine resource management in the Hawaiian Archipelago: the traditional Hawaiian system in relation to the Western approach. Journal of Marine Biology , .
| Marine resource management in the Hawaiian Archipelago: the traditional Hawaiian system in relation to the Western approach.Crossref | GoogleScholarGoogle Scholar |
Kallis, G. (2007). Socio-environmental co-evolution: some ideas for an analytical approach. The International Journal of Sustainable Development & World Ecology 14, 4–13.
| Socio-environmental co-evolution: some ideas for an analytical approach.Crossref | GoogleScholarGoogle Scholar |
Kallis, G., and Norgaard, R. B. (2010). Coevolutionary ecological economics. Ecological Economics 69, 690–699.
| Coevolutionary ecological economics.Crossref | GoogleScholarGoogle Scholar |
Kamakau, S. M. (1961). ‘Ruling chiefs of Hawaii.’ (Kamehameha Schools Press: Honolulu, HI, USA.)
Kamakau, S. M., Barrère, D. B., and Pukui, M. K. (1964). ‘Ka Po‘e Kahiko: the people of old.’ (Bishop Museum Press: Honolulu, HI, USA.)
Kame‘eleihiwa, L. (1992). ‘Native land and foreign desires.’ (Bishop Museum Press: Honolulu, HI, USA.)
Kendrick, A. (2003). 10-Caribou co-management in northern Canada: fostering multiple ways of knowing. Navigating social–ecological systems. Building resilience for complexity and change , 241–268.
| 10-Caribou co-management in northern Canada: fostering multiple ways of knowing.Crossref | GoogleScholarGoogle Scholar |
Kirch, P. V. (2011). When did the Polynesians settle Hawaii? A review of 150 years of scholarly inquiry and a tentative answer. Hawaiian Archaeology 12, 3–26.
Kirch, P. V. (2014). ‘Kua'āina Kahiko: Life and Land in Ancient Kahikinui, Maui.’ (University of Hawai‘i Press: Honolulu, HI, USA.)
Kittinger, J. N., Pandolfi, J. M., Blodgett, J. H., et al. (2011). Historical reconstruction reveals recovery in Hawaiian coral reefs. PloS One 6, e25460.
| Historical reconstruction reveals recovery in Hawaiian coral reefs.Crossref | GoogleScholarGoogle Scholar | 21991311PubMed |
Kramer, R. J. (1971). ‘Hawaiian land mammals.’ C.E. Tuttle Company, North Clarendon, VT, USA
Krauss, F. G. (1923). ‘Swine raising in Hawaii.’ Washington, DC, USA.
Kruska, D., and Röhrs, M. (1974). Comparative-quantitative investigations on brains of feral pigs from the Galapagos Islands and of European domestic pigs. Zeitschrift für Anatomie und Entwicklungsgeschichte 144, 61–73.
| Comparative-quantitative investigations on brains of feral pigs from the Galapagos Islands and of European domestic pigs.Crossref | GoogleScholarGoogle Scholar | 4851103PubMed |
Kuykendall, R. S. (1938). ‘The Hawaiian kingdom. Vol. 1.’ (University of Hawaii Press: Honolulu, HI, USA.)
Lega, C., Raia, P., Rook, L., and Fulgione, D. (2016). Size matters: a comparative analysis of pig domestication. The Holocene 26, 327–332.
| Size matters: a comparative analysis of pig domestication.Crossref | GoogleScholarGoogle Scholar |
Linderholm, A., Spencer, D., Battista, V., et al. (2016). A novel MC1R allele for black coat colour reveals the Polynesian ancestry and hybridization patterns of Hawaiian feral pigs. Royal Society Open Science 3, 160304.
| A novel MC1R allele for black coat colour reveals the Polynesian ancestry and hybridization patterns of Hawaiian feral pigs.Crossref | GoogleScholarGoogle Scholar | 27703696PubMed |
Lockwood, C. (2009). Pigs, dryland agriculture and social complexity in precontact Hawai‘i: assessing surplus production through landscape geochemistry. PhD thesis, University of Washington, Seattle, WA, USA.
Macrae, J. (1922). ‘With Lord Byron at the Sandwich Islands in 1825: being extracts from the MS diary of James Macrae, Scottish botanist.’ Honolulu, HI, USA
Malo, D. (1951). ‘Hawaiian antiquities (Moolelo Hawaii), translated Nathaniel Emerson. Bernice P.’ (Bishop Museum: Honolulu, HI, USA.)
Maly, K. (1998). ‘Na¯ Ulu La¯‘au Hawai‘i (Hawaiian Forests).’ (Kumu Pono Associates: Hilo, HI, USA.)
Miller, C. A. (2002). Big bucks: balancing resident and nonresident bowhunter demand inIllinois. In ‘Proceedings of the 1st National Bowhunting Conference’. (pp. 125–133). Bethesda, MD, USA.
Mistry, J., Bilbao, B. A., and Berardi, A. (2016). Community owned solutions for fire management in tropical ecosystems: case studies from Indigenous communities of South America. Philosophical Transactions of the Royal Society B: Biological Sciences 371, 20150174.
| Community owned solutions for fire management in tropical ecosystems: case studies from Indigenous communities of South America.Crossref | GoogleScholarGoogle Scholar |
Moffitt, R., and Hopkins, G. (1863). ‘Malaekahana.’ Ka Hoku o Ka Pakipika, 16 April, p. 3.
Nogelmeier, P. (2010). ‘Mai Pa’a i Ka Leo: historical voice in Hawaiian primary materials; looking forward and listening back.’ (Bishop Museum Press: Honolulu, HI, USA.)
Nogueira-Filho, S. L., Nogueira, S. S., and Fragoso, J. M. (2009). Ecological impacts of feral pigs in the Hawaiian Islands. Biodiversity and Conservation 18, 3677.
| Ecological impacts of feral pigs in the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Nugent, G., Parkes, J. P., Dawson, N., and Caley, P. (1996). Feral pigs in New Zealand as conservation pests and as potential hosts of bovine tuberculosis. Unpublished Landcare Research Contract Report LC9596/54.
Office of Hawaiian Affairs (2011). Papakilo Database. Available at https://www.papakilodatabase.com/pdnupepa/cgi-bin/pdnupepa?a=p&p=home [Accessed 5 May 2021]
Ōtsuka, R. (1983). ‘Oriomo Papuans: ecology of sago-eaters in lowland Papua.’ (University of Tokyo Press: Bunkyō, Tokyo, Japan.)
Parke, J. (1861). ‘Olelo Hoolaha.’ Ka Hae Hawaii, 8 May, p. 23.
Pearson, R. J., Kirch, P. V., and Pietrusewsky, M. (1971). An early prehistoric site at Bellows Beach, Waimanalo, Oahu, Hawaiian Islands. Archaeology and Physical Anthropology in Oceania 6, 204–234.
Peluso, N. L. (1992). The ironwood problem: (mis) management and development of an extractive rainforest product. Conservation Biology 6, 210–219.
| The ironwood problem: (mis) management and development of an extractive rainforest product.Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., and Voeks, R. A. (2008). Biological invasions and biocultural diversity: linking ecological and cultural systems. Environmental Conservation 35, 281–293.
| Biological invasions and biocultural diversity: linking ecological and cultural systems.Crossref | GoogleScholarGoogle Scholar |
Pullar, E. M. (1950). The wild (feral) pigs of Australia and their role in the spread of infectious diseases. Australian Veterinary Journal 26, 99–110.
| The wild (feral) pigs of Australia and their role in the spread of infectious diseases.Crossref | GoogleScholarGoogle Scholar | 14772180PubMed |
Ribot, J. C., and Peluso, N. L. (2003). A theory of access. Rural Sociology 68, 153–181.
| A theory of access.Crossref | GoogleScholarGoogle Scholar |
Rose, C. (1994). ‘Property and persuasion: essays on the history, theory, and rhetoric of ownership.’ (Boulder, CO, USA.)
Sai, D. K. (2008). The American occupation of the Hawaiian Kingdom: beginning the transition from occupied to restored state. PhD thesis, University of Hawaii, Manoa.
Salmón, E. (2000). Kincentric ecology: indigenous perceptions of the human–nature relationship. Ecological Applications 10, 1327–1332.
| Kincentric ecology: indigenous perceptions of the human–nature relationship.Crossref | GoogleScholarGoogle Scholar |
Sato, A. Y., Price, M. R., and Vaughan, M. B. (2018). Kāhuli: uncovering Indigenous Ecological knowledge to conserve endangered Hawaiian land snails. Society & Natural Resources 31, 320–334.
| Kāhuli: uncovering Indigenous Ecological knowledge to conserve endangered Hawaiian land snails.Crossref | GoogleScholarGoogle Scholar |
Schieffelin, E. L., and Crittenden, R. (1991). ‘Like people you see in a dream: first contact in six Papuan societies.’ (Stanford University Press: Redwood City, CA, USA.)
Smith, C. W. (1985). Impact of alien plants on Hawaii’s native biota. Hawai‘i’s Terrestrial Ecosystems: Preservation and Management, 180–250.
Smith, C. W., and Diong, C. H. (1977). ‘Proposal to study feral pigs in Kipahulu Valley, Haleakala National Park.’ Honolulu, HI, USA.
Sobrevila, C. (2008). The role of indigenous peoples in biodiversity conservation: the natural but often forgotten partners. In ‘No. 44300’. pp. 1–102. (The World Bank: Washington, DC, USA.)
Spencer, A. (1869). ‘Olelo Hoolaha. Ke Au Okoa.’ Vol. 5, No. 32.
Staples, G. W., Herbst, D. R., and Imada, C. T. (2000). Survey of invasive or potentially invasive cultivated plants in Hawaii. Bishop Museum Occasional Papers: Honolulu, HI, USA.
State of Hawai‘i v. Kui Palama. (2015). ‘Intermediate Court of Appeals of the State of Hawai‘i.’ Honolulu, HI, USA.
Stone, C. P. (1985). Alien animals in Hawaii’s native ecosystems: toward controlling the adverse effects of introduced vertebrates. Hawai‘i’s Terrestrial Ecosystems: Preservation and Management , 251–297.
Titcomb, M., and Pukui, M. K. (1969). ‘Dog and man in the ancient Pacific, with special attention to Hawaii.’ (Star-Bulletin Print Co: Honolulu, HI, USA.)
Tomich, P. (1986). ‘Mammals in Hawaii: a synopsis and national bibliography.’ (Bishop Museum Press: Honolulu, HI, USA.)
Turner, N. J., and Berkes, F. (2006). Coming to understanding: developing conservation through incremental learning in the Pacific Northwest. Human Ecology 34, 495–513.
| Coming to understanding: developing conservation through incremental learning in the Pacific Northwest.Crossref | GoogleScholarGoogle Scholar |
Vaughan, M. B. (2018). ‘Kaiaulu: Gathering Tides.’ (Oregon State University Press: Corvallis, OR, USA.)
Wehr, N. H., Hess, S. C., and Litton, C. M. (2018). Biology and impacts of Pacific islands invasive species. 14. Sus scrofa, the feral pig (Artiodactyla: Suidae) 1. Pacific Science 72, 177–198.
| Biology and impacts of Pacific islands invasive species. 14. Sus scrofa, the feral pig (Artiodactyla: Suidae) 1.Crossref | GoogleScholarGoogle Scholar |
Winter, K. B. (2012). Kalo (Hawaiian taro, Colocasia esculenta (L.) Schott) varieties: an assessment of nomenclatural synonymy and biodiversity. Ethnobotany Research and Applications 10, 423–447.
Winter, K., and McClatchey, W. (2008). Quantifying evolution of cultural interactions with plants: implications for managing diversity for resilience in social-ecological systems. Functional Ecosystems and Communities 2, 1–10.
Winter, K., and McClatchey, W. (2009). The quantum co-evolution unit: an example of ‘Awa (Kava—Piper methysticum G. Foster) in Hawaiian culture. Economic Botany 63, 353.
| The quantum co-evolution unit: an example of ‘Awa (Kava—Piper methysticum G. Foster) in Hawaiian culture.Crossref | GoogleScholarGoogle Scholar |
Winter, K. B., Lincoln, N. K., and Berkes, F. (2018a). The social-ecological keystone concept: a quantifiable metaphor for understanding the structure, function, and resilience of a biocultural system. Sustainability 10, 3294.
| The social-ecological keystone concept: a quantifiable metaphor for understanding the structure, function, and resilience of a biocultural system.Crossref | GoogleScholarGoogle Scholar |
Winter, K. B., Beamer, K., Vaughan, M. B., et al. (2018b). The Moku system: managing biocultural resources for abundance within social-ecological regions in Hawai‘i. Sustainability 10, 3554.
| The Moku system: managing biocultural resources for abundance within social-ecological regions in Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Winter, K. B., Lincoln, N. K., Berkes, F., Alegado, R. A., Kurashima, N., Frank, K., Pascua, P., Rii, Y. M., Reppun, F., Knapp, I. S. S., McClatchey, W., Ticktin, T., Smith, C., Franklin, E. C., Oleson, K., Price, M. R., McManus, M. A., Donahue, M. J., Rodgers, K., Bowen, B. W., Nelson, C. E., Thomas, B., Leong, J. A., Madin, E., Rivera, M. A. J., Falinski, K. A., Bremer, L. L., Deenik, J. L., Gon, S. M., Neilson, B., Okano, R., Olegario, A., Kawelo, A. H., Kotubetey, K., Kukea-Shultz, J. K., and Toonen, R. J. (2020a). Ecomimicry in Indigenous resource management: optimizing ecosystem services to achieve resource abundance with examples from Hawai‘i. Ecology and Society 25, 26.
| Ecomimicry in Indigenous resource management: optimizing ecosystem services to achieve resource abundance with examples from Hawai‘i.Crossref | GoogleScholarGoogle Scholar |
Winter, K. B., Ticktin, T., and Quazi, S. (2020b). Biocultural restoration in Hawai‘i also achieves core conservation goals. Ecology and Society 25, 26.
| Biocultural restoration in Hawai‘i also achieves core conservation goals.Crossref | GoogleScholarGoogle Scholar |