Animal detections increase by using a wide-angle camera trap model but not by periodically repositioning camera traps within study sites
Anke Seidlitz A E , Kate A. Bryant
A E , Kate A. Bryant  A B , Nicola J. Armstrong
A B , Nicola J. Armstrong  C and Adrian F. Wayne
C and Adrian F. Wayne  D
D
A Environmental and Conservation Sciences, Murdoch University, Murdoch, WA 6150, Australia.
B Centre for Climate-Impacted Terrestrial Ecosystems, Harry Butler Institute, Murdoch University, Murdoch, WA 6150, Australia.
C Mathematics and Statistics, Murdoch University, Murdoch, WA 6150, Australia.
D Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 2, Manjimup, WA 6258, Australia.
E Corresponding author. Email: anke.seidlitz@gmx.net
Pacific Conservation Biology 28(1) 25-35 https://doi.org/10.1071/PC20076
Submitted: 30 September 2020 Accepted: 25 January 2021 Published: 25 February 2021
Journal Compilation © CSIRO 2022 Open Access CC BY
Abstract
When using camera traps for wildlife studies, determining suitable camera models and deployment methods is essential for achieving study objectives. We aimed to determine if camera trap performance can be increased by (1) using cameras with wider detection angles, and (2) by periodically repositioning cameras within sites. We compared three camera trap groups: stationary Reconyx PC900/HC600 (40° detection angle), and paired, periodically-repositioned Reconyx PC900/HC600 and Swift 3C wide-angle camera traps (110° detection angle). Cameras operated simultaneously at 17 sites over 9 weeks within the Upper Warren region, Western Australia. Swift cameras had significantly higher detection rates, leading to better performance, especially for species <1 kg and >10 kg bodyweight. Reconyx cameras missed 54% of known events, with most being animals that moved within the cameras’ detection zones. Stationary and periodically-repositioned Reconyx camera traps performed similarly, although there were notable differences for some species. The better performance of Swift 3C wide-angle camera traps makes them more useful for community-level and species-level studies. The increased sensitivity of the Swift’s passive infrared sensor along with the wider detection zone played an important role in its success. When choosing camera trap models, detection angle and sensor sensitivity should be considered to produce reliable study results. Periodically repositioning cameras within sites is a technique that warrants further investigation as it may reduce camera placement bias, animal avoidance of camera traps, and increase spatial/habitat information when a limited number of cameras are deployed.
Keywords: Bettongia penicillata ogilbyi, camera trapping, comparative study, Dasyurus geoffroii, detection rates, endangered, Myrmecobius fasciatus, passive infrared sensor sensitivity, Pseudocheirus occidentalis, wide angle.
Introduction
Passive infrared (PIR) triggered camera traps are increasingly used in wildlife investigations for a wide range of applications (Rowcliffe and Carbone 2008; Burton et al. 2015; Meek et al. 2015). Before deploying camera traps, researchers need to consider which camera trap model, and deployment techniques (e.g. camera height and position) are most suitable for achieving study objectives. The right choice is important because camera trap model, and deployment technique can affect study results, and therefore the inferences made, for example, on species richness and occurrence (Swan et al. 2014a). As new camera trap models and ideas for different set up techniques emerge, they need to be tested for their applicability in wildlife research. To expand the camera trapping body of knowledge we present here results from a comparative camera trap study that explored if animal detections could be increased (1) by using Swift 3C wide-angle instead of the commonly used Reconyx PC900/HC600 standard-lens camera trap models, and (2) by periodically repositioning camera traps within study sites.
Camera trap detection angle
The size of the detection area of camera traps can greatly affect animal detection rates (Rowcliffe et al. 2011), which should be maximised for reliable animal population estimates of either abundance or occupancy. When unobstructed, the detection area of a camera trap is determined by the width of the PIR sensor’s detection angle, and the distance up to which a PIR sensor can detect animals (specifically, objects that move within the detection zone with a surface temperature that differs from background objects). Detection distance of camera traps is commonly >10 m according to manufacturer’s specifications, although that may depend on animal size. In field conditions, vegetation or landscape features may also constrain detection distance. Rowcliffe et al. (2011) found that Central American agouti (Dasyprocta punctata), a small to medium sized mammal (~3.5 kg bodyweight), were detected mostly within the nearest 4–5 m of Reconyx RC55 camera traps. Therefore, it may be important to maximise the detection angle of camera traps for increased detection zone size. Detection angles of commonly used standard camera traps lie between 40° and 60° but can exceed 100° in wide-angle camera traps (Meek et al. 2012; Trolliet et al. 2014; Wearn and Glover-Kapfer 2017). Even though wide-angle camera traps can perform well when compared with standard camera traps (Swann et al. 2004; Fancourt et al. 2018), they are not widely used for wildlife studies. To our knowledge, animal detection rates of wide-angle camera traps have not yet been compared with standard camera traps in a field setting with a variety of wildlife species of different size classes.
Periodic repositioning of camera traps
During wildlife studies, camera traps are typically stationary within sites for the entire study period (e.g. Jacobs and Ausband 2018; Moore et al. 2020). If animals move through the habitat randomly, without giving preference to any particular features, animal detection rates should not be affected by camera trap location. However, habitats are heterogeneous and contain a mosaic of more or less preferred areas for animals (Barraquand and Benhamou 2008). Therefore, their movements are likely to be non-random. Non-baited, random camera trap placement (often desired to meet assumptions of population statistics) may cause some cameras to be located in areas less preferred by target species. Those cameras may have reduced detection rates (Kolowski and Forrester 2017): an unwanted situation especially for rarely detected species. One way to overcome this problem is to use multiple camera traps per site to increase detections (Kolowski and Forrester 2017; O’Connor et al. 2017; Evans et al. 2019). This increase in detections may result in part from sampling additional areas within the same site, which might be more frequently used by the animals of interest. We asked, could the same be achieved by periodically repositioning single camera traps within study sites?
Aims and hypotheses
Our aim was to compare animal detection rates, detection probabilities, and site accumulation rates for individual species, as well as species accumulation rates for three camera trap groups: stationary Reconyx PC900/HC600 (40° detection/lens angle), and paired, periodically-repositioned Reconyx PC900/HC600 and Swift 3C wide-angle camera traps (110° detection- and 100° lens angle). Swift 3C wide-angle camera traps were shown to detect numbats (Myrmecobius fasciatus) more effectively than Reconyx PC900 camera traps in zoo enclosures (Seidlitz et al. 2020). We therefore hypothesised that Swift camera traps would generally perform better in field conditions than the Reconyx camera traps for the above-mentioned metrics. Since periodically repositioning camera traps may allow camera traps to sample a wider range of habitat features, we hypothesised that repositioned Reconyx camera traps would generally perform better than stationary Reconyx camera traps.
Materials and methods
Study area
This study was conducted within the Kingston National Park and adjacent state forest of the Upper Warren region in south-western Australia, 300 km south of Perth (Fig. 1). South‐western Australia is a global biodiversity hotspot (Myers et al. 2000). The region’s publicly managed forests cover an area of more than 140 000 ha, which support several mammalian species classed as threatened under the Western Australian Biodiversity Conservation Act 2016. These species include the numbat (endangered), western ringtail possum Pseudocheirus occidentalis (critically endangered), western quoll Dasyurus geoffroii (vulnerable), and brush-tailed bettong Bettongia penicillata ogilbyi (critically endangered). The region’s forests consist mainly of open sclerophyll forests and woodlands dominated by three tree species: the jarrah (Eucalyptus marginata), marri (Corymbia calophylla) and wandoo (Eucalyptus wandoo) (Yeatman et al. 2016). Forest management activities are carried out by state authorities, and include prescribed fuel-reduction burns, timber harvesting, and feral predator control using 1080-poisoned bait (Wayne et al. 2013). The region has a Mediterranean type climate with an annual average rainfall of approximately 650–900 mm (Zosky et al. 2017).

|
Study period and weather
This study was conducted over 9 weeks from mid-March to mid-May 2018, coinciding with the Australian autumn. During this period, the average temperature was 15.5°C (minimum 8.4°C; maximum 32.2°C), and the average relative humidity was 68.8% (minimum 19.2%; maximum 92.2%). During the study there were 11 rainy days with a total of 24.6 mm precipitation (minimum 0.1 mm/day; maximum 13.5 mm/day). Weather data were obtained online from the Yerramin weather station located approximately 15 km from the study area (https://weather.agric.wa.gov.au/station/YERR).
Study sites
Camera stations were set at 17 existing sites (Fig. 1). These sites are a subset of 50 study sites established in 2015 for the purpose of numbat monitoring (J. Wayne, pers. comm.). This subset was chosen because numbats and other mammal species were frequently detected here (A. Seidlitz, unpubl. data). The average distance between sites was 2.34 km (minimum 1.88 km; maximum 2.73 km). Sites were located adjacent to unsealed roads and tracks. At each of the 17 sites, 10 plots (40 × 100 m) were established with 5 plots on either side of the track (unless the track was bordered by private property in which case all plots were located on the forested side, n = 3). Plots were placed adjacent to each other with the short edge parallel to the track.
Camera trapping
We used 51 camera traps consisting of 17 Swift 3C wide-angle cameras (Outdoor Cameras Australia, Toowoomba, Qld, Australia), and 34 Reconyx cameras (17 of each model: PC900 and HC600; RECONYX, LLP, Holmen, WI, USA). Reconyx PC900 and HC600 models were here treated as equivalent because differences between the two models are predominantly related to software functions. Camera settings used during this study were available in both models; specifications and accessories are detailed in Table 1.

|
At each site, three camera traps were deployed, one of each model. On a central plot, one Reconyx camera trap (PC900 or HC600 model randomly chosen) was attached to a tree for the entire study period (sticks wedged between cameras and tree trunks were used to make fine-scale adjustments to camera positioning). We refer to this camera deployment as ‘stationary’. The second Reconyx, and a Swift 3C wide-angle camera trap were mounted separately to wooden plates that, in turn, were attached side-by-side to a wooden board using small right-angle brackets. The use of brackets and wooden plates allowed small up/down/left/right adjustments to fine-tune individual camera trap positioning. The wooden board with the cameras (left/right position randomly chosen, cameras approximately 1.5 cm apart) was mounted to a metal stake (Fig. 2). This camera set up was repositioned approximately weekly (eight times) to a different plot (randomly chosen) within the same site. We refer to this camera deployment as ‘repositioned’ camera traps. We therefore had three camera trap groups: stationary Reconyx, repositioned Reconyx, and repositioned Swift camera traps. No bait or lures were used at camera trap stations to avoid possible bias associated with attractants. All camera traps were set central within plots with a minimum distance of 30 m to roads/tracks. Cameras were oriented towards south to avoid direct solar interference. To minimise obstruction, camera traps were aimed towards natural clearings. Vegetation was minimally trimmed in front of cameras (within the first 5 m), to reduce unintended camera activation by moving vegetation. Camera traps were set with their PIR motion sensor at approximately 25 cm above ground. To ensure that cameras were aiming parallel to the ground, we placed a square 15 × 15 cm white card at approximately 25 cm above ground at 5 m distance to the cameras. We then attached a laser pointer to the bottom of the camera housings, pointing straight forward. Cameras were adjusted until the laser pointed to an appropriate height on the white card. Additional walk-tests were performed to ensure that cameras were detecting movement in front and beyond the 5 m distance. After adjustments were completed, the white card and laser pointer were removed, test images were retained, and the cameras were activated to operate 24 h/day.

|
All camera traps were set to high sensitivity with no delay between triggers. Reconyx camera traps were set to take 10 images per trigger in ‘Rapidfire’ mode. This setting was chosen to allow the comparison of results from stationary Reconyx cameras to another, unrelated camera trap study in the Upper Warren region (A. Seidlitz, unpubl. data). Swift 3C camera traps were set to take three images per trigger. From pilot studies, we were aware that Swift 3C camera traps may have high false trigger rates (A. Seidlitz, unpubl. data), caused, for example, by moving vegetation. We therefore chose the three-image-per-trigger setting to conserve battery life and data storage space. We acknowledge that this setting difference may disadvantage Swift 3C camera traps by having a smaller chance to ‘capture’ animals on fewer given images per trigger. Time and date settings were synchronised during set up to allow direct comparison of animal detections from cameras set side-by-side. Sites were visited approximately weekly to reposition camera traps to a new plot. During those visits, batteries and secure digital (SD) cards were checked, and replaced when necessary.
When an animal moves within a camera’s detection zone (defined here as an event), the PIR sensor may detect that animal, trigger the camera and result in one or more images depicting the animal partially or wholly. We defined this as a detection. For our repositioned camera traps, set side-by-side, an event may have resulted in an animal detection for one but not the other camera. We defined this as a missed detection for the camera which did not record the animal. Additional animal detections were counted only when detections of the same species were separated by at least 3 min. A 3 min quiet-time may not warrant independent animal detections that may be important when determining population parameters. As we were evaluating camera trap performance, we kept the quiet-time interval short to maximise detections, yet not too short to avoid excessive re-detection of single animals. For the comparison of paired, repositioned Reconyx and Swift camera traps, we used the white card position seen in test images to categorise detected animals’ distance from the camera as either more or less than 5 m. To determine if missed detections of repositioned Reconyx cameras were in- or outside the camera’s detection zone, we compared animal detection images of paired Swift and Reconyx cameras. Features seen within those images allowed us to approximate the lens angle (field of view) and detection zone of Reconyx camera traps (lens angle and detection zone overlap in Reconyx PC900/HC600 camera traps; Table 1).
At 16 sites, camera traps operated between 63 and 65 days (mean 64 days). At one site, the stationary Reconyx HC600 camera trap operated for 48 days; it failed to record images for 16 days for unknown reasons. Data recorded by repositioned Reconyx and Swift camera traps from the same site and time were excluded from analysis for unbiased comparison of camera trap groups. The remaining data, used for the analysis detailed below, consists of the number of detections from 3216 camera days (1072 camera days for each camera trap group).
Evaluating camera performance and data analysis
We chose to display results using three commonly used metrics (detection rates, detection probabilities, and species/site accumulation plots) to be useful to a wide audience with differing objectives. These metrics may display results differently. For example, detection rates are insensitive to the number of sites where species were detected, whereas detection probabilities relate to re-detections of species at individual sites. For species accumulation, only a single detection of a species is necessary, while site accumulation for individual species provides information on that species’ spatial distribution within the study area. The following species were detected fewer than five times by all camera trap groups and were excluded from analyses: European rabbit (Oryctolagus cuniculus), Rosenberg’s monitor (Varanus rosenbergi), short-beaked echidna (Tachyglossus aculeatus), domestic sheep (Ovis aries), and feral pig (Sus scrofa). We were unable to identify small, mouse-sized mammals (e.g. house mouse, several dunnart species) to species level on some occasions, therefore they were grouped into one category (mouse sized).
All statistical analysis was conducted in R version 3.5.0 (R Core Team 2018). Likelihood-ratio tests in combination with generalised linear models (GLMs) were used to test if the number of animal detections were affected by camera trap group. We used the glm.nb function (negative binomial regression) of the R package MASS 7.3–51.4 (Venables and Ripley 2002) due to overdispersion issues with Poisson models. For this part of the analysis, cats (Felis catus) and foxes (Vulpes vulpes) were grouped as feral predators as they had low detection rates. For the same reason, birds were grouped into two categories, consisting of large birds (>30 cm) and small birds (<30 cm), however, emus (Dromaius novaehollandiae) were listed separately due to their exceptionally large size. Birds and mammals for which species identification was not possible were excluded from analysis.
We first fitted GLMs with the number of animal detections as the response variable, and ‘sites’ (17 study sites) and ‘species’ as explanatory variables. The interaction between ‘sites’ and ‘species’ was also included as species abundance varied between study sites. Prior to assessing camera trap groups, we included the different Reconyx models (Reconyx PC900 and HC600) into GLMs and used likelihood-ratio tests to verify that these models did not statistically differ in their ability to detect animals. Thereafter, data from the two Reconyx models were combined. We then fitted GLMs with ‘camera trap group’ as an additional explanatory variable to determine if there was a difference in camera trap group performance. We first compared repositioned Reconyx and Swift camera traps, and finally stationary Reconyx and repositioned Reconyx camera traps. When exploring differences between animal detection rates from camera trap groups for single species, we used the above described procedure for likelihood-ratio tests except that the covariate ‘species’ became redundant.
To determine the probability of detection of mammal species and bird groups for each camera trap group, we used the single season occupancy modelling framework (specified in MacKenzie et al. 2018). For each species/group, a matrix with detections (1) and non-detections (0) was established from spatial replicates (17 sites) and temporal repeats (camera days). Days on which camera traps did not operate were included in the data matrix as missing observations. Models to estimate detection probabilities were fitted using the RPresence package 2.12.33 (MacKenzie and Hines 2018). The occupancy component of models was kept constant (psi ~1), and the detection probability for each species was determined for each camera trap group. We accessed model fit by estimating c-hat and χ2 goodness of fit tests from 5000 bootstrap iterations and found no issues (data not shown). To graphically display detection probabilities, we used the R package ‘forestplot’ version 1.9 (Gordon and Lumley 2019).
We used the Vegan Community Ecology package version 2.5–5 (Oksanen et al. 2019) to compute the accumulation of species detected, and the accumulation of sites where selected species were detected over time by each camera trap group. For site accumulation, the site which experienced camera trap failure was excluded. The method ‘random’ was applied which finds the mean accumulation curve and its standard deviation from 100 random permutations of the data.
Results
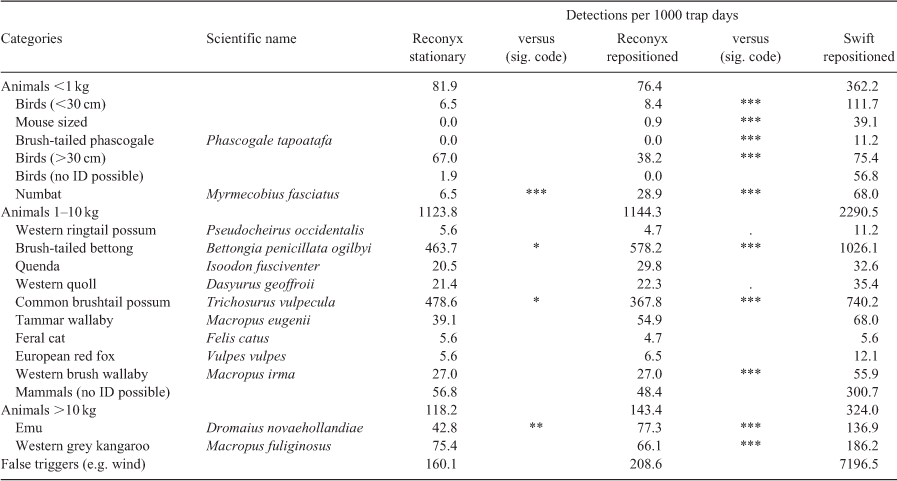
Across all camera deployments, there were 6095 animal detections (repositioned Reconyx = 1468, repositioned Swift = 3201, stationary Reconyx = 1426). We identified 16 different mammals, one reptile, and 17 bird taxa (grouped as birds <30 cm and >30 cm). Animal groups, species and respective detection rates per 1000 camera trap days are listed in Table 2. From repositioned Reconyx and Swift camera traps set side-by-side, we determined that there were 3218 known events of which Swift cameras did not detect 17 (0.5%), and Reconyx cameras did not detect 1750 (54%). Of the events missed by Reconyx cameras, most (76%) lay within the detection zone of the cameras (Fig. 3). A total of 436 unidentifiable mammals were detected, with repositioned Swift cameras recording 323 (248 at night at >5 m distance), repositioned Reconyx cameras 52 (48 at night at <5 m distance), and stationary Reconyx cameras 61 (58 at night at <5 m distance). There were 8125 cases where camera traps triggered for unknown reasons, including suspected triggers due to moving vegetation (repositioned Reconyx = 224, repositioned Swift = 7729, stationary Reconyx = 172).

|
Detection rates
Using overall animal detections, the likelihood-ratio test to determine if including camera model (Reconyx HC600 and PC900) as a factor resulted in an improved model was not significant (χ2 = 0.477, d.f. = 1, P = 0.490). Conversely, when periodically-repositioned Reconyx and Swift camera traps were included, the likelihood-ratio test indicated a significant difference between the camera trap groups’ ability to detect animals (χ2 = 239.486, d.f. = 1, P = <0.001). There was no improvement to model fit when repositioned and stationary Reconyx camera traps were included in models (χ2 = 0.610, d.f. = 1, P = 0.435). Significance codes are displayed in Table 2 indicating differences between camera groups in their ability to detect single species. When compared with Reconyx camera traps, detections from Swift 3C cameras were significantly higher for 3 out of 9 species of the 1–10 kg group, and for all species of the <1 kg and >10 kg groups. In no instance did Reconyx camera traps have higher detection rates than Swift camera traps. There were four significant differences between detections from stationary and repositioned Reconyx camera traps with repositioned cameras having higher detections for numbats, brush-tailed bettongs and emus, and stationary cameras for common brushtail possums.
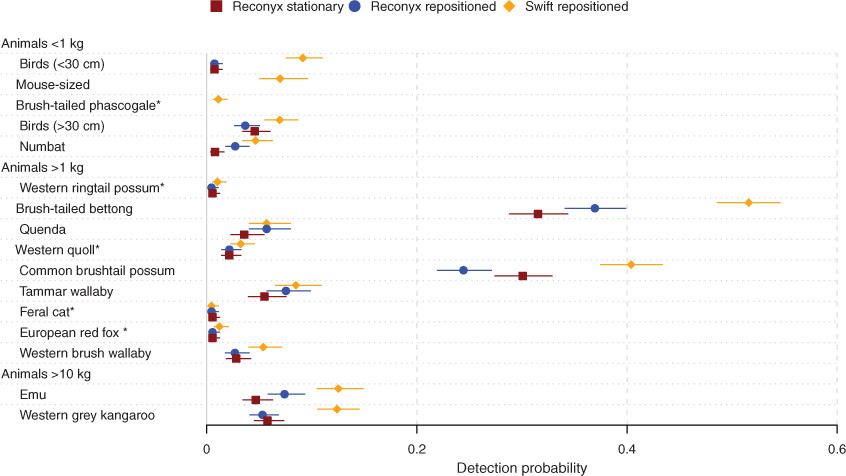
Detection probabilities
Swift 3C wide-angle camera traps had the highest detection probabilities for all species <1 kg and >10 kg bodyweight, with confidence intervals noticeably overlapping only for numbats and birds >30 cm (Fig. 4). Swift cameras detected mouse-sized mammals and brush-tailed phascogales at eight and nine sites respectively (data not shown). For those species, detection probabilities could not be computed for Reconyx camera traps as there was only one detection of mouse-sized mammals and none of brush-tailed phascogales. For numbats, brush-tailed bettongs, and emus, repositioned camera traps achieved higher detection probabilities than stationary camera traps. Differences between detection probabilities of camera trap groups were less marked for animals with a bodyweight between 1 and 10 kg and most confidence intervals overlapped (Fig. 4). However, for most species in this size group, Swift camera traps tended to have slightly, but insignificantly, higher detection probabilities than Reconyx camera traps. For the brush-tailed bettong and common brushtail possum, Swift detection probabilities were clearly higher; however, for the quenda (Isoodon fusciventer) and feral cat there was no discernible difference.
Species and site accumulation rates
Repositioned Swift 3C camera traps detected animal species at a faster and higher rate than stationary, and repositioned Reconyx camera traps (Fig. 5a). This difference mainly derived from animals with a bodyweight of <1 kg (Fig. 5b, c), which included 17 bird species (Supplementary Table S1). Site accumulation rates for mammal species were never lower for Swift 3C wide-angle camera traps than for stationary and repositioned Reconyx camera traps. Examples of site accumulation curves are given in Fig. 6a–f. Site accumulation for quenda and brush-tailed bettong was similar for all camera trap groups (Fig. 6a, b), whereas western grey kangaroos were detected at a faster rate and at more sites by Swift 3C wide-angle camera traps. Site accumulation rates for numbats differed between all camera trap groups, with Swift 3C wide-angle cameras traps showing markedly higher accumulation at faster rates than the others (Fig. 6d). Numbats, western quolls and tammar wallabies were detected at more sites by repositioned Reconyx and Swift camera traps compared with stationary Reconyx cameras (Fig. 6d–f).

|
Discussion
This study compared the performance of stationary Reconyx PC900/HC600, and paired, periodically-repositioned Reconyx PC900/HC600 and Swift 3C wide-angle camera traps. Compared with Reconyx PC900/HC600 camera traps, Swift 3C wide-angle camera traps had higher species accumulation rates. Swift camera traps had also significantly higher animal detection rates that lead to higher detection probabilities and site accumulation for many species, particularly within the <1 kg and >10 kg categories. Reconyx camera traps missed 54% of detections recorded by paired Swift camera traps. Of those, only 24% were caused by the smaller detection angle of Reconyx cameras. Contrary to our expectations, stationary and periodically-repositioned Reconyx PC900/HC600 camera traps performed similarly except for some species. This study shows that some camera traps (here set without lures or baits) may miss a high percentage of detectable animal movements, and highlights the importance of selecting an appropriate camera trap model for wildlife detection studies.
Camera trap detection angle
Detection angle size was hypothesised to be an important determinant for higher detection rates of rabbits (Oryctolagus cuniculus) from Ltl Acorn Ltl-5310A camera traps when compared with Reconyx PC900 cameras (Fancourt et al. 2018). However, the authors did not report if missed detections derived from rabbit movement within or outside the detection zone of Reconyx cameras, so it remains unclear if missed detections were caused by a smaller detection angle or other differences between the camera models. When comparing Swift 3C camera trap models (wide-angle versus standard), wide-angle cameras were shown to have higher numbat detection rates in a trial conducted in zoo enclosures (Seidlitz et al. 2020). Yet the increase in detections may have been amplified by non-random movements of numbats in zoo enclosures (Seidlitz et al. 2020). During this study, the smaller detection angle of Reconyx PC900/HC600 caused missed detections; however, it was not the main determinant of the cameras’ lower detection rates. The majority of missed detections from Reconyx cameras derived from animal movement events within the cameras’ detection zone. Therefore, other model differences must be considered. Faster trigger speed did not cause higher detection rates from Swift camera traps as they have slower trigger speeds than Reconyx PC900/HC600 camera traps. Other factors possibly causing performance differences between Swift 3C wide-angle and Reconyx PC900/HC600 camera traps are the temperature differential threshold of PIR sensors, and the number and characteristics of Fresnel lenses that condense infrared radiation onto the sensor (see Welbourne et al. 2016 for information on camera trap functionality). Both affect the sensitivity of camera traps. High camera trap sensor sensitivity was also found to improve animal detection rates in a study comparing customised high-sensitivity Reconyx PC850 models to their unmodified counterparts (Heiniger and Gillespie 2018). One disadvantage of higher PIR sensitivity is the increased occurrence of false triggers caused, for example, by moving vegetation. During this study, the number of false triggers from Swift camera traps was manageable, and the importance of improved data accuracy outweighed this disadvantage. Artificial intelligence technologies may also render false triggers easily excluded (e.g. Yu et al. 2013; Gomez Villa et al. 2017; Falzon et al. 2020).
Animal size
With increasing body size, animals are more easily detected by camera traps (Wearn and Glover-Kapfer 2017). During comparative studies, it was found that differences between camera trap model performance typically reduced with increasing animal size (Swan et al. 2014a; Urlus et al. 2014; but see Damm et al. 2010). During this study, differences between Swift and Reconyx camera traps were more pronounced for animals <1 kg and >10 kg, and this was evident from detection rates, detection probabilities and accumulation curves. The PIR sensor of Reconyx PC900/HC600 models may potentially be less sensitive than that of Swift 3C camera traps, causing the reduced detection of animals <1 kg. Reduced detection of this weight class may not occur with a targeted camera trap set up using bait (see, for example, Meek and Vernes 2016). However, this finding is still important, as some small animals, such as the numbat, can’t be attracted by bait (Burrows and Christensen 2002), and a targeted camera trap set up may not be suitable when exploring multiple species of different size classes. The performance difference between Reconyx and Swift camera models for animals >10 kg weight may derive from the Swift camera trap’s ability to detect large animals at a greater distance than the tested Reconyx models. Although present, differences in detection rates between the tested camera models were not as pronounced for animals between 1 and 10 kg bodyweight, and significantly greater detection by Swift cameras appeared to be restricted to a few species only. Without further, targeted studies it is difficult to speculate on why some species in this size class were detected differently by the camera models and others were not.
Periodic repositioning of camera traps
Contrary to our expectations, periodically repositioning of camera traps within sites did not increase overall animal detection rates. This may be due to the choice of always aiming camera traps at areas of natural clearings to reduce false triggers from moving vegetation. Sampling more randomly across more heterogenic habitat features may increase the detection of some species (Swan et al. 2014b; Kolowski and Forrester 2017; Hofmeester et al. 2019). Therefore, to truly avoid camera trap placement bias, cameras need to be genuinely placed randomly, even at the micro-habitat scale to include features such as logs, dense vegetation, and water bodies. Species which may have occurred (Wayne et al. (2017) for list of species) but were not detected during this study were the rakali or water rat (Hydromys chrysogaster), southern bush rat (Rattus fuscipes), and introduced black rat (Rattus rattus). Mammals smaller than rats were detected (grouped as ‘mouse sized’) but since it was not possible to identify these species, we are unsure if species such as the western pygmy possum (Cercartetus concinnus) and mardo (Antechinus flavipes) were detected. To improve small mammal species identification, a more targeted camera trap placement with bait may be required (see, for example, Gray et al. 2017; Gracanin et al. 2019).
Periodic repositioning of camera traps improved detection rates for numbats, brush-tailed bettongs, and emus. Furthermore, the repositioning of camera traps improved detection probabilities for numbats, brush-tailed bettong and emus, and site accumulation for numbats, western quoll, and tammar wallabies. The sampling of a wider range of habitat features may have caused this increase. However, animal behaviour could also have been a reason for this increase (or decrease in the detections of common brushtail possums, and other taxa). Some animals are known to be repelled or attracted by camera traps (Séquin et al. 2003; Meek et al. 2016). Animals repelled by camera traps may, after detecting the device (which does not necessitate the detection of the animal by the camera), avoid the camera station area. Further investigation of periodically-repositioned camera traps could reveal if detection rates of camera trap-shy animals can be increased. If animals actively avoid/seek camera traps, assumptions of animal detections being random and independent may be violated: a concern raised by Meek et al. (2016) and Larrucea et al. (2007).
Although not directly investigated in this study, our results show that, for most species, there is no disadvantage to periodically relocating cameras. By using this technique, additional spatial and micro-habitat information can be obtained. Therefore, for studies where this additional information may be important (e.g. spatial capture-recapture and habitat use studies), periodically moving cameras may be advantageous and worth further investigation.
Camera trap model choice
This study did not investigate the durability or longevity of camera trap models. It is our impression that Swift 3C wide-angle camera traps are not as robustly built as Reconyx PC900/HC600 camera traps. However, Swift 3C camera traps operated reliably during this study (9-week deployment) without failure. Which camera trap model is chosen for a project depends on many factors such as research objectives, camera trap detection efficiencies, occurrence of false triggers, model durability and longevity, camera purchase cost, and operating and servicing times.
Conclusion
Swift 3C wide-angle camera traps detected animals of differing sizes, particularly within the <1 kg and >10 kg categories, more successfully than Reconyx PC900/HC600 camera traps. This led to higher species accumulation rates, improved detection probabilities and site accumulation rates for many species. It is the increased sensitivity of the Swift 3C PIR sensor that plays an important role in its success, along with the wide-angle lens and detection zone. These are important outcomes for studies with a focus on species-level as well as community-level questions. Repositioning camera traps periodically within sites did not increase overall detection rates; however, for some species this technique appeared to be beneficial. When choosing camera trap models for wildlife detection, detection angle and PIR sensor sensitivity need to be considered to produce reliable study results. Periodically repositioning cameras within sites is a technique that needs further investigation as it may reduce camera placement bias, animal avoidance of camera traps, and increase spatial/habitat information when a limited number of cameras are deployed.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge the Nyoongar people as the Traditional Custodians of the lands and animals on which this study was conducted. This study was conducted using permit O2968/17 from the Murdoch University Animal Ethics Committee. We thank all volunteers and co-workers for their invaluable help with this project. We are also thankful to the Department of Biodiversity, Conservation and Attractions for providing camera traps, accessories, shapefiles for area maps, and other in-kind contributions. Furthermore, we would like to thank WWF-Australia for obtaining and managing project funds. We are grateful to two anonymous reviewers who kindly reviewed and helped to improve the manuscript. This project has received funding from the Western Australian Government’s State NRM Program (A16002), Murdoch University, and Coles Supermarkets Australia Pty Ltd. Many thanks also to Leeanne and Neil Chapman for providing a home away from home to the field team.
References
Barraquand, F., and Benhamou, S. (2008). Animal movements in heterogeneous landscapes: Identifying profitable places and homogeneous movement bouts. Ecology 89, 3336–3348.| Animal movements in heterogeneous landscapes: Identifying profitable places and homogeneous movement bouts.Crossref | GoogleScholarGoogle Scholar | 19137941PubMed |
Burrows, N. D., and Christensen, P. E. S. (2002). Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia. Australian Forestry 65, 211–219.
| Long-term trends in native mammal capture rates in a jarrah forest in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Burton, A. C., Neilson, E., Moreira, D., Ladle, A., Steenweg, R., Fisher, J. T., Bayne, E., and Boutin, S. (2015). Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes. Journal of Applied Ecology 52, 675–685.
| Wildlife camera trapping: a review and recommendations for linking surveys to ecological processes.Crossref | GoogleScholarGoogle Scholar |
Damm, P. E., Grand, J. B., and Barnett, S. W. (2010). Variation in detection among passive infrared triggered-cameras used in wildlife research. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 64, 125–130.
Evans, B. E., Mosby, C. E., and Mortelliti, A. (2019). Assessing arrays of multiple trail cameras to detect North American mammals. PLoS One 14, e0217543.
| Assessing arrays of multiple trail cameras to detect North American mammals.Crossref | GoogleScholarGoogle Scholar | 31206527PubMed |
Falzon, G., Lawson, C., Cheung, K.-W., Vernes, K., Ballard, G. A., Fleming, P. J. S., Glen, A. S., Milne, H., Mather-Zardain, A., and Meek, P. D. (2020). ClassifyMe: A Field-Scouting Software for the Identification of Wildlife in Camera Trap Images. Animals 10, 58.
| ClassifyMe: A Field-Scouting Software for the Identification of Wildlife in Camera Trap Images.Crossref | GoogleScholarGoogle Scholar |
Fancourt, B. A., Sweaney, M., and Fletcher, D. B. (2018). More haste, less speed: pilot study suggests camera trap detection zone could be more important than trigger speed to maximise species detections. Australian Mammalogy 40, 118–121.
| More haste, less speed: pilot study suggests camera trap detection zone could be more important than trigger speed to maximise species detections.Crossref | GoogleScholarGoogle Scholar |
Gomez Villa, A., Salazar, A., and Vargas, F. (2017). Towards automatic wild animal monitoring: Identification of animal species in camera-trap images using very deep convolutional neural networks. Ecological Informatics 41, 24–32.
| Towards automatic wild animal monitoring: Identification of animal species in camera-trap images using very deep convolutional neural networks.Crossref | GoogleScholarGoogle Scholar |
Gordon M., and Lumley T. (2019 ). ‘Forestplot: Advanced Forest Plot Using ‘grid’ Graphics, R package’. Available at https://CRAN.R-project.org/package=forestplot [accessed 15 July 2020].
Gracanin, A., Gracanin, V., and Mikac, K. M. (2019). The selfie trap: A novel camera trap design for accurate small mammal identification. Ecological Management & Restoration 20, 156–158.
| The selfie trap: A novel camera trap design for accurate small mammal identification.Crossref | GoogleScholarGoogle Scholar |
Gray, E. L., Dennis, T. E., and Baker, A. M. (2017). Can remote infrared cameras be used to differentiate small, sympatric mammal species? A case study of the black-tailed dusky antechinus, Antechinus arktos and co-occurring small mammals in southeast Queensland, Australia. PLoS One 12, e0181592.
| Can remote infrared cameras be used to differentiate small, sympatric mammal species? A case study of the black-tailed dusky antechinus, Antechinus arktos and co-occurring small mammals in southeast Queensland, Australia.Crossref | GoogleScholarGoogle Scholar | 28792958PubMed |
Heiniger, J., and Gillespie, G. (2018). High variation in camera trap-model sensitivity for surveying mammal species in northern Australia. Wildlife Research 45, 578–585.
| High variation in camera trap-model sensitivity for surveying mammal species in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Hofmeester, T. R., Cromsigt, J. P. G. M., Odden, J., Andrén, H., Kindberg, J., and Linnell, J. D. C. (2019). Framing pictures: A conceptual framework to identify and correct for biases in detection probability of camera traps enabling multi-species comparison. Ecology and Evolution 9, 2320–2336.
| Framing pictures: A conceptual framework to identify and correct for biases in detection probability of camera traps enabling multi-species comparison.Crossref | GoogleScholarGoogle Scholar | 30847112PubMed |
Jacobs, C. E., and Ausband, D. E. (2018). An evaluation of camera trap performance – What are we missing and does deployment height matter? Remote Sensing in Ecology and Conservation 4, 352–360.
| An evaluation of camera trap performance – What are we missing and does deployment height matter?Crossref | GoogleScholarGoogle Scholar |
Kolowski, J. M., and Forrester, T. D. (2017). Camera trap placement and the potential for bias due to trails and other features. PLoS One 12, e0186679.
| Camera trap placement and the potential for bias due to trails and other features.Crossref | GoogleScholarGoogle Scholar | 29045478PubMed |
Larrucea, E. S., Brussard, P. F., Jaeger, M. M., and Barrett, R. H. (2007). Cameras, coyotes, and the assumption of equal detectability. Journal of Wildlife Management 71, 1682–1689.
| Cameras, coyotes, and the assumption of equal detectability.Crossref | GoogleScholarGoogle Scholar |
MacKenzie D., and Hines J. (2018 ). ‘RPresence: R Interface for Program PRESENCE’. Available at https://www.usgs.gov/software/presence [accessed 05 April 2019].
MacKenzie, D. I., Nichols, J. D., Royle, J. A., Pollock, K. H., Bailey, L. L., and Hines, J. E. (2018). ‘Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence.’ 2nd edn. (Academic Press: London.)
Meek, P. D., and Vernes, K. (2016). Can camera trapping be used to accurately survey and monitor the Hastings River mouse (Pseudomys oralis)? Australian Mammalogy 38, 44–51.
| Can camera trapping be used to accurately survey and monitor the Hastings River mouse (Pseudomys oralis)?Crossref | GoogleScholarGoogle Scholar |
Meek, P. D., Ballard, G., and Fleming, P. (2012). ‘An Introduction to Camera Trapping for Wildlife Surveys in Australia. PestSmart Toolkit.’ (Invasive Animals Cooperative Research Centre: Canberra, Australia.)
Meek, P. D., Ballard, G.-A., Vernes, K., and Fleming, P. J. S. (2015). The history of wildlife camera trapping as a survey tool in Australia. Australian Mammalogy 37, 1–12.
| The history of wildlife camera trapping as a survey tool in Australia.Crossref | GoogleScholarGoogle Scholar |
Meek, P., Ballard, G., Fleming, P., and Falzon, G. (2016). Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecology and Evolution 6, 3216–3225.
| Are we getting the full picture? Animal responses to camera traps and implications for predator studies.Crossref | GoogleScholarGoogle Scholar | 27096080PubMed |
Moore, H. A., Valentine, L. E., Dunlop, J. A., and Nimmo, D. G. (2020). The effect of camera orientation on the detectability of wildlife: a case study from north-western Australia. Remote Sensing in Ecology and Conservation 6, 546–555.
| The effect of camera orientation on the detectability of wildlife: a case study from north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
| Biodiversity hotspots for conservation priorities.Crossref | GoogleScholarGoogle Scholar | 10706275PubMed |
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P. R., O’Hara R. B., Simpson G. L., Solymos P., Stevens M. H. H., Szoecs E., and Wagner H. (2019 ). ‘Vegan: Community Ecology Package, R package’. Available at https://CRAN.R-project.org/package=vegan [accessed 20 December 2019].
O’Connor, K. M., Nathan, L. R., Liberati, M. R., Tingley, M. W., Vokoun, J. C., and Rittenhouse, T. A. G. (2017). Camera trap arrays improve detection probability of wildlife: Investigating study design considerations using an empirical dataset. PLoS One 12, e0175684.
| Camera trap arrays improve detection probability of wildlife: Investigating study design considerations using an empirical dataset.Crossref | GoogleScholarGoogle Scholar | 28422973PubMed |
R Core Team (2018). ‘R: A Language and Environment for Statistical Computing’. (R foundation for Statistical Computing: Vienna, Austria.) Available at http://www.r-project.org [accessed 20 December 2019].
Rowcliffe, J. M., and Carbone, C. (2008). Surveys using camera traps: are we looking to a brighter future? Animal Conservation 11, 185–186.
| Surveys using camera traps: are we looking to a brighter future?Crossref | GoogleScholarGoogle Scholar |
Rowcliffe, J. M., Carbone, C., Jansen, P. A., Kays, R., and Kranstauber, B. (2011). Quantifying the sensitivity of camera traps: an adapted distance sampling approach. Methods in Ecology and Evolution 2, 464–476.
| Quantifying the sensitivity of camera traps: an adapted distance sampling approach.Crossref | GoogleScholarGoogle Scholar |
Seidlitz, A., Bryant, K. A., Armstrong, N. J., Calver, M., and Wayne, A. F. (2020). Optimising camera trap height and model increases detection and individual identification rates for a small mammal, the numbat (Myrmecobius fasciatus). Australian Mammalogy , .
| Optimising camera trap height and model increases detection and individual identification rates for a small mammal, the numbat (Myrmecobius fasciatus).Crossref | GoogleScholarGoogle Scholar |
Swan, M., Di Stefano, J., and Christie, F. (2014a). Comparing the effectiveness of two types of camera trap for surveying ground-dwelling mammals. In ‘Camera Trapping: Wildlife Management and Research’. (Eds P. Meek, P. Fleming, G. Ballard, P. Banks, A. Claridge, J. Sanderson, and D. Swann.) pp. 166–175. (CSIRO Publishing: Melbourne, Vic.)
Swan, M., Di Stefano, J., Christie, F., Steel, E., and York, A. (2014b). Detecting mammals in heterogeneous landscapes: implications for biodiversity monitoring and management. Biodiversity and Conservation 23, 343–355.
| Detecting mammals in heterogeneous landscapes: implications for biodiversity monitoring and management.Crossref | GoogleScholarGoogle Scholar |
Swann, D. E., Hass, C. C., Dalton, D. C., and Wolf, S. A. (2004). Infrared-triggered cameras for detecting wildlife: An evaluation and review. Wildlife Society Bulletin 32, 357–365.
| Infrared-triggered cameras for detecting wildlife: An evaluation and review.Crossref | GoogleScholarGoogle Scholar |
Séquin, E. S., Jaeger, M. M., Brussard, P. F., and Barrett, R. H. (2003). Wariness of coyotes to camera traps relative to social status and territory boundaries. Canadian Journal of Zoology 81, 2015–2025.
| Wariness of coyotes to camera traps relative to social status and territory boundaries.Crossref | GoogleScholarGoogle Scholar |
Trolliet, F., Huynen, M.-C., Vermeulen, C., and Hambuckers, A. (2014). Use of camera traps for wildlife studies. A review. Biotechnologie, Agronomie, Société et Environnement 18, 446–454.
Urlus, J., McCutcheon, C., Gilmore, D., and McMahon, J. (2014). The effect of camera trap type on the probability of detecting different size classes of Australian mammals. In ‘Camera Trapping: Wildlife Management and Research’. (Eds P. Meek, P. Fleming, G. Ballard, P. Banks, A. Claridge, J. Sanderson, and D. Swann.) pp. 153–165. (CSIRO Publishing: Melbourne, Vic.)
Venables, W. N. and Ripley, B. D. (2002). ‘Modern Applied Statistics with S’, 4th edn. (Springer: New York.)
Wayne, A. F., Maxwell, M. A., Ward, C. G., Vellios, C. V., Ward, B. G., Liddelow, G. L., Wilson, I., Wayne, J. C., and Williams, M. R. (2013). Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata. Wildlife Research 40, 169–183.
| Importance of getting the numbers right: quantifying the rapid and substantial decline of an abundant marsupial, Bettongia penicillata.Crossref | GoogleScholarGoogle Scholar |
Wayne, A. F., Maxwell, M. A., Ward, C. G., Wayne, J. C., Vellios, C. V., and Wilson, I. J. (2017). Recoveries and cascading declines of native mammals associated with control of an introduced predator. Journal of Mammalogy 98, 489–501.
| Recoveries and cascading declines of native mammals associated with control of an introduced predator.Crossref | GoogleScholarGoogle Scholar |
Wearn, O. R., and Glover-Kapfer, P. (2017). Camera-trapping for conservation: a guide to best-practices. WWF Conservation Technology, Number Series 1, Woking, United Kingdom.
Welbourne, D. J., Claridge, A. W., Paull, D. J., and Lambert, A. (2016). How do passive infrared triggered camera traps operate and why does it matter? Breaking down common misconceptions. Remote Sensing in Ecology and Conservation 2, 77–83.
| How do passive infrared triggered camera traps operate and why does it matter? Breaking down common misconceptions.Crossref | GoogleScholarGoogle Scholar |
Yeatman, G. J., Wayne, A. F., Mills, H. R., and Prince, J. (2016). Temporal patterns in the abundance of a critically endangered marsupial relates to disturbance by roads and agriculture. PLoS One 11, e0160790.
| Temporal patterns in the abundance of a critically endangered marsupial relates to disturbance by roads and agriculture.Crossref | GoogleScholarGoogle Scholar | 27501320PubMed |
Yu, X., Wang, J., Kays, R., Jansen, P. A., Wang, T., and Huang, T. (2013). Automated identification of animal species in camera trap images. EURASIP Journal on Image and Video Processing 2013, 52.
| Automated identification of animal species in camera trap images.Crossref | GoogleScholarGoogle Scholar |
Zosky, K. L., Wayne, A. F., Bryant, K. A., Calver, M. C., and Scarff, F. R. (2017). Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in south-western Australia. Australian Journal of Zoology 65, 302–312.
| Diet of the critically endangered woylie (Bettongia penicillata ogilbyi) in south-western Australia.Crossref | GoogleScholarGoogle Scholar |