A review of urchin barrens and the longspined sea urchin (Centrostephanus rodgersii) in New South Wales, Australia
Rachel Przeslawski A * , Rowan C. Chick
A * , Rowan C. Chick  B , Tom Davis
B , Tom Davis  B , Jeremy K. Day C D , Tim M. Glasby B , Nathan Knott
B , Jeremy K. Day C D , Tim M. Glasby B , Nathan Knott  A C and Maria Byrne E
A C and Maria Byrne E
A
B
C
D
E
Abstract
Centrostephanus rodgersii is the most dominant urchin species in south-eastern Australia and the primary macroalgal grazer, thus creating and maintaining barrens habitat. The role, formation and persistence of barrens are common topics of interest among academic, industry, Indigenous, conservation and government groups.
This review focuses on the role of barrens habitat and C. rodgersii in New South Wales (NSW), with an aim to inform debate and guide continued inter-jurisdictional management and research.
Over 30 years of research shows that barrens occur across most of the NSW coastline, but they tend to be larger and more numerous along the southern coast. Centrostephanus rodgersii and barrens have remained stable in shallow NSW waters since the 1960s, but limited earlier data prevent the identification of longer historical patterns. Climate change predictions show that C. rodgersii may disappear from northern NSW and increase along the far NSW southern coast over the next 100 years, although this may be modulated by local thermally acclimated populations.
This review indicates that the presence, persistence and role of barrens and C. rodgersii seem unique in NSW and likely warrant different management strategies as the species shifts its distribution.
Keywords: coralline algae, echinoderm, kelp, macroalgae, marine invertebrate, rocky reef, shifting baseline, south-eastern Australia.
Introduction
Urchin barrens and the complex ecological interactions associated with them continue to be a topic of interest and debate among academic, industry, Indigenous, conservation and government groups (Andrew 2022; Environment and Communications References Committee 2023; Kingsford and Byrne 2023). In Australasia, much of this interest has focussed on the climate change-related range extension of the longspined sea urchin (Centrostephanus rodgersii) into Tasmania where it has driven an ecosystem shift from kelp forests to barrens (Johnson et al. 2005; Ling 2008; Ling and Keane 2024), as well as population increases in Victoria and New Zealand (Gorfine et al. 2012; Balemi and Shears 2023). Centrostephanus rodgersii is native to New South Wales (NSW) (Thomas et al. 2021), and its latitudinal distribution extends from far south Queensland to Wilson Promontory in Victoria (Andrew and Underwood 1989; Andrew 1993; Byrne and Andrew 2020), with an associated wide thermal tolerance (Table 1). There is concern from the public that the area of barrens habitat along the NSW south coast has recently increased whereas kelp cover has decreased, similar to changes observed in Victoria and Tasmania; these concerns were reflected in a 2023 Senate Enquiry (Environment and Communications References Committee 2023).
| Location | Mean minimum (°C) | Mean (°C) | Mean maximum (°C) | Data source | ||
|---|---|---|---|---|---|---|
| Caloundra (warm range edge) | 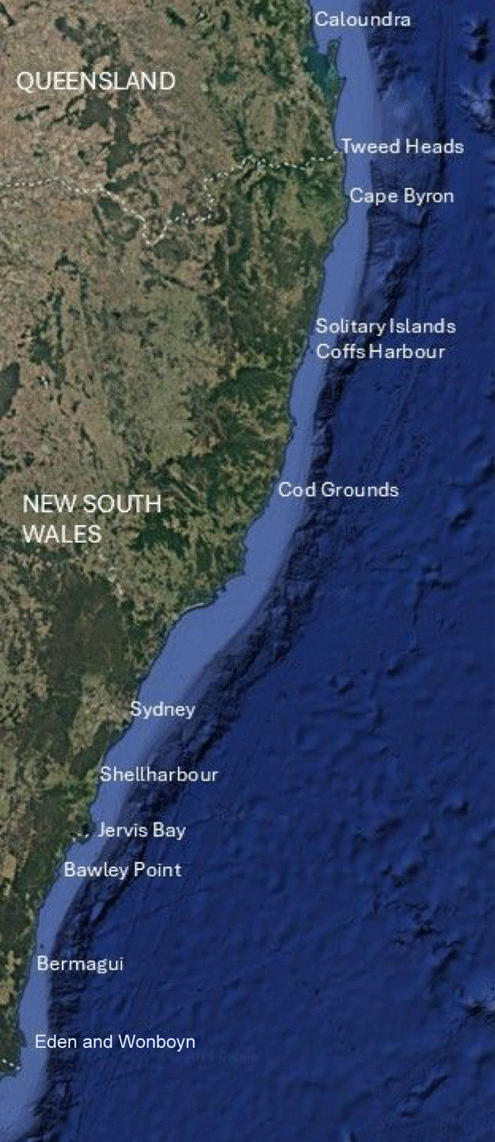 | 19.6 ± 0.01 (n = 10,151) | 23.1 ± 0.01 (n = 112,700) | 26.5 ± 0.01 (n = 7843) | Caloundra: 2013–21 (see https://www.data.qld.gov.au/dataset/coastal-data-system-waves-caloundra) | |
| Solitary Islands and Coffs Harbour (warm range edge main distribution) | 19.2 ± 0.01 (n = 11,904) | 21.6 ± 0.01 (n = 129,409) | 24.3 ± 0.01 (n = 10,417) | 2001–10 Australian Institute of Marine Science (AIMS). In situ logger data, South Solitary Island, NSW, Jan 2001–Mar 2011 (see https://apps.aims.gov.au/metadata/view/a8149e3d-21eb-40ba-9d4c-8d19fba84beb, accessed 28 June 2021) | ||
| Jervis Bay (mid-range) | 14.6 ± 0.01 (n = 8575) | 18.4 ± 0.003 (n = 101,678) | 22.4 ± 0.01 (n = 8064) | Jervis Bay in situ loggers 16 Sep–18 Nov (NSW DPI Fisheries, unpubl. data) | ||
| South-eastern Tasmania (cool range edge and edge main distribution, C. rodgersii) | 11.8 ± 0.24 | 14.4 ± 1.3 | 17.6 ± 0.39 | Modis-Aqua (see https://oceancolor.gsfc.nasa.gov/about/missions/aqua/) |
Jervis Bay NSW approximates the mid-range of C. rodgersii, and its southern limit is south-eastern Tasmania (Byrne et al. 2022). Map indicates locations referred to throughout this review, with underlay from Google Earth.
Barrens associated with C. rodgersii are recognised as a typical and distinctive habitat of NSW rocky reef ecosystems (Underwood et al. 1991; Wright et al. 1997). Barrens are rocky reef areas covered by crustose coralline algae that can support distinct fauna (sponges, ascidians, urchins, limpets, fishes) (Andrew and Underwood 1989; Curley et al. 2002). Sea urchins are conspicuous in barrens and, in NSW, species include Centrostephanus rodgersii, Heliocidaris erythrogramma, Heliocidaris tuberculata, Pseudoboletia Indiana, Tripneustes australiae and Phyllacanthus parvispinus. In NSW, C. rodgersii is the dominant urchin species in barrens (Andrew and Underwood 1989; Connell and Irving 2008) and the main species responsible for grazing kelp, thus creating and maintaining this habitat (Fletcher 1987; Andrew 1993).
Subtidal rocky reefs in NSW are characterised by mosaics of barrens, turfing algae and macroalgae beds (Underwood et al. 1991) (Fig. 1), and urchin density is positively correlated with area of barrens (Davis et al. 2020). Outside NSW, barrens have been associated with low macro-biodiversity, low primary productivity (Ling 2008) and loss of blue carbon (Carnell et al. 2020). In these areas, barrens are often considered an undesired alternate state of kelp ecosystems (Filbee-Dexter and Scheibling 2014; Ling et al. 2015; Young et al. 2023) and, at a global scale, they have been identified lower-ranked than kelp forests in 11 of 15 ecosystem properties (Eger et al. 2024). By contrast, studies in NSW show high fish biodiversity in barrens (Curley et al. 2002) and comparable microscopic biodiversity in both systems (Coleman and Kennelly 2019), similar to what has been found in the Mediterranean with high abundance and biodiversity of benthic invertebrates in barrens (Agnetta et al. 2024).
Rocky reef at North Rock, Broughton Island, NSW, Australia (aerial image: 23/09/2014) with associated habitats: (a) barrens, (b) macroalgae, (c) sand and (d) turfing algae.

Centrostephanus rodgersii is a conspicuous species along the NSW coast, homing to crevices in rocky reefs during the day (Andrew 1993; Flukes et al. 2012) and moving out to feed at night (Jones and Andrew 1990; Smith et al. 2024). Centrostephanus rodgersii individuals seem unable to directionally sense macroalgal food and instead forage in a generalised pattern around their nocturnal home crevices (Flukes et al. 2012). Gut content analyses on NSW urchins shows a diverse diet of predominantly molluscs, crustaceans and potentially drift algae (Day et al. 2024a), and the species can also graze kelp gametophytes (Veenhof et al. 2023). Spawning is highly synchronous and occurs in winter (King et al. 1994), with southern populations having a longer spawning period than northern populations (Byrne et al. 1998). Although booms and busts in populations are recorded (Andrew 1991), most NSW populations have been shown to be stable over multiple years (Andrew and Underwood 1989; Glasby and Gibson 2020; McLaren et al. 2024). The oldest NSW individual was 27 years old on the basis of sclerochronological aging estimates from jaw growth ridges (Blount et al. 2024a), whereas in Tasmania the oldest individuals were estimated to be more than 50 years old on the basis of jaw growth (Ling and Johnson 2009). It is not clear if the different methods used in these studies are comparable. Size frequencies among sites are variable (Andrew and O’Neill 2000), although C. rodgersii individuals are smaller in barrens than nearshore mosaic habitats, and size structure shifts to smaller individuals from north to south (Blount et al. 2024b). Genetic diversity among populations is associated with sea surface temperature (SST) and geography rather than spatial proximity (Banks et al. 2007), likely reflecting the long larval duration (1–4 months depending on temperature) and the high dispersal potential of this species (Huggett et al. 2005; Mos et al. 2020).
Increases in urchin abundance have been linked to a reduction in the size of predators, possibly driven by fishing pressure (Andrew and Byrne 2006; Ling et al. 2009). The main predators of C. rodgersii in NSW are uncertain, but are likely to include the eastern rock lobster (Sagmariasus verreauxi) (Day et al. 2021), a generalist that can eat up to three urchins per day in captivity (Provost et al. 2017), and larger individuals of species such as snapper (Pagrus auratus) and eastern blue groper (Achoerodus viridis) (Gillanders 1995; Byrne and Andrew 2020). Previous and recent observations point to the horned sharks as potential predators of C. rodgersii in NSW (McLaughlin and O’Gower 1971; Day et al. 2024b). Abiotic stressors may also decrease urchin abundance, including ocean acidification, which could result in smaller larvae (Foo et al. 2012) and flooding events that can result in mass mortality (Andrew 1991; Webb and Byrne 2025). The larvae of C. rodgersii are broadly thermotolerant and may be comparatively resilient to concurrent warming and acidification because of the presence of tolerant genotypes (Foo et al. 2012; Byrne et al. 2022).
Centrostephanus rodgersii is the main species of urchin harvested in NSW, with annual commercial catches generally increasing over the past 20 years, ranging from 24 to 250 tonnes (Mg) per year (Chick 2020, 2023). Most harvest occurs from March to early May, when the gonads (roe) are at their maximum weight and suitable for commercial harvest (Byrne et al. 1998). Commercial harvest in NSW is managed through the NSW Sea Urchin and Turban Shell (SUTS) restricted fishery. The greatest yield of high-quality roe is collected during summer and autumn from moderately sized urchins (Andrew et al. 1998; Blount and Worthington 2002).
Previous reviews and data syntheses on the ecology of barrens and associated urchin densities have adopted a global scale (Filbee-Dexter and Scheibling 2014; Ling et al. 2015) or within regions outside the main distribution centre of C. rodgersii (Ling and Keane 2024). The aim of this review is to summarise the spatiotemporal patterns and potential impacts of C. rodgersii and barrens on the environment and fisheries of NSW, the centre of historic distribution of this species. In addition, we identify key knowledge gaps and provide suggestions for future research. This paper is framed according to questions commonly asked by stakeholders, including recreational, commercial and cultural fishers (Przeslawski et al. 2023). We anticipate that this review will inform more accurate, broader discussions about urchin barrens in eastern Australia and contribute to improved further inter-jurisdictional management and research.
Ecological importance: why do barrens and C. rodgersii matter?
It has been suggested that barrens support lower biodiversity than do kelp beds and other rocky reef habitat (Filbee-Dexter and Scheibling 2014; Medrano et al. 2020). However, this has not been quantitatively assessed in NSW except for abalone (Andrew et al. 1998). Indeed, barrens around Terrigal and Sydney in NSW typically support a greater number of fish species than do kelp beds and a similar number of species to sponge habitat (Curley et al. 2002). Densities of C. rodgersii can be negatively correlated with abalone densities (Andrew and Underwood 1992), thereby decreasing local productivity (Andrew and O’Neill 2000).
Barrens have also been linked to negative commercial impacts, including challenges harvesting abalone (Strain et al. 2013) and the quantity and quality of commercial urchin roe (Blount and Worthington 2002). The reproductive output of C. rodgersii in barrens can be lower than those living on the fringe of macroalgal beds, and urchins from barrens are generally not considered worth harvesting (Byrne et al. 1998). Urchins transplanted to habitat with an abundance of macro-algae showed significant improvements in colour and yield of roe after 6 weeks, with the magnitude of change greatest at low urchin densities and between October and January (Blount et al. 2017). Furthermore, significant improvements in both roe colour and yield from urchins in barrens occurred after urchin density was reduced by 66% over 3 months, with greater improvement after 2 years (Blount et al. 2017). However, smaller-scale experiments along similar sections of coastline found that indices of urchin gonad quality were similar between macroalgae and barrens habitat at four of five locations along the southern NSW coast (Day et al. 2024a). These results indicate strong spatiotemporal variation in roe quality of urchin within and among different habitats, including nearshore barrens. Urchin density and food availability are important drivers, and food subsidies provided by drift macroalgae also need to be considered.
Centrostephanus rodgersii is an ecologically important species along the NSW coast, playing a key role in the maintenance of the habitat mosaics of macroalgae, turfing algae and barrens that comprise NSW shallow rocky reefs (Andrew 1993) (Fig. 1). The species also acts as a biogenic habitat, with 80 taxa recorded from underneath 180 urchins along the central and southern NSW coast, predominantly gastropods, malacostracans and chitons (Davis 2002). Similarly, Montgomery (2023) found C. rodgersii was associated with a diverse guild of associates including amphipods, reef fish (Lepidoblennius haplodactylos, Aspasmogaster costata), decapods (Rhynchocinetes serratus, Pagurus lacertosus), gastropods (Astraea tentoriiformis, Turbo torquatus) and other echinoderms (Clarkcoma pulchra, Phyllacanthus parvispinus, Ptilometa australis). Although the abundance of some of these groups differ between macroalgal habitats and barrens, total richness and abundance does not (Davis 2002).
Spatial patterns: where do barrens and C. rodgersii occur in NSW?
There are 10 published NSW statewide or large-scale assessments of barrens or associated urchin densities, including 3 that used aerial imagery informed by ground truthing (Andrew and O’Neill 2000; Glasby et al. 2017; Glasby and Gibson 2020), 4 that used underwater imagery (Jordan et al. 2010; Perkins et al. 2015; Davis et al. 2020; Williams et al. 2020) and 3 that used diver transects (Underwood et al. 1991; Connell and Irving 2008; Blount et al. 2024b) (Table S1 of the Supplementary material). In addition, Reef Life Survey maintains an extensive database on urchin density, extending along the entire NSW coast (see reeflifesurvey.com/explorer/map). These data can be used to identify the distribution of urchins and how barrens may change over time.
Barrens occur across much of the NSW coastline where there are rocky reefs. There is a general trend for the extent of barrens in NSW to increase from north towards the south, although they are absent or cover small areas at various locations throughout the state (Underwood et al. 1991; Andrew and O’Neill 2000; Davis et al. 2020; Glasby and Gibson 2020). The largest patches of barrens occur on the NSW southern coast (Connell and Irving 2008) where they are estimated to cover >50% of nearshore rocky reefs (Underwood et al. 1991; Andrew and O’Neill 2000; Davis et al. 2020), whereas in northern NSW barrens generally cover <20% of rocky reefs (Underwood et al. 1991; Davis et al. 2020). Barrens do not often occur on rocky reefs that are affected by sand movement, such as the narrow sloped reefs in northern NSW (north of Port Stephens) and in the southern-most part of the state (south of Wonboyn) (Andrew and O’Neill 2000; Glasby and Gibson 2020) (Table 1), possibly because of scouring effects on urchin larvae or food sources.
The distribution of urchins and associated barrens in NSW is depth-related, with barrens restricted to waters >2 m, likely because of strong wave action in the surge zone that limits foraging of C. rodgersii (Davis 2002). Barrens occur most frequently at depths of <20 m (Jordan et al. 2010), but at some sites can be common in depths up to 30 m (e.g. cod grounds in Jordan et al. 2010). Interestingly, barrens do not seem to extend much deeper than 30 m, with Perkins et al. (2015) finding a lower depth limit of 27 m for NSW barrens despite surveying rocky reef down to 50 m.
The distribution and abundance of C. rodgersii in NSW does not have consistent spatial patterns among localities (Andrew and Underwood 1992), although the far-northern NSW has lower urchin densities reflecting the distribution limits of this species (Connell and Irving 2008; Davis et al. 2023). Densities of C. rodgersii in NSW show high spatio-temporal variation at some sites across seasons (Underwood et al. 1991), and average urchin densities range from <0.01 urchins per square metre around Tweed Heads and Cape Byron in northern NSW to 1.2 urchins per square metre around Eden along the far southern coast (Davis et al. 2020) (Table 1). The highest published densities of C. rodgersii recorded in New South Wales are ~24 urchins per square metre (fringing habitat in summer at Guerilla Point in 1988, Underwood et al. 1991; unspecified rocky reef habitat in autumn at Jervis Bay in 2009, Davis et al. 2023). This is more than the maximum density of C. rodgersii recorded in Tasmania at 5.4 urchins per square metre (fringing habitat in southern Tasmania in 2021) (John Keane, pers. comm.).
Temporal patterns: are the area of barrens or densities of C. rodgersii increasing in NSW?
The shallow subtidal reefs of NSW, including barrens, have been characterised and monitored since the 1980s, and aerial imagery is available in some locations since the 1960s (Andrew and Underwood 1989; Andrew and O’Neill 2000; Glasby and Gibson 2020) (Supplementary Table S1). In general, data over a 50-year time period indicate that barrens and C. rodgersii are a dominant yet stable feature of NSW shallow subtidal ecosystems (Hill et al. 2003; Glasby and Gibson 2020; McLaren et al. 2024).
There is evidence of natural variation (increases and decreases) in the area of barrens at large spatial scales (one to tens of kilometres) from the 1980s to 2010s, with most sites between Newcastle and Eden fluctuating by ±10% in area (Glasby and Gibson 2020). The greatest increase in area of barrens has occurred in the Sydney region, in which three sites had increases in barrens of 20–35% (Glasby and Gibson 2020). Small increases in the area of barrens of 10–15% occurred at Shellharbour and Bawley Point and ~20% at Bermagui, whereas other areas showed less than 5% decreases in barrens (Glasby and Gibson 2020). Averaged across the 21 sites that were sampled, the extent of barrens in NSW has increased at a rate of 0.2% per year between the 1980s and 2010s (Glasby and Gibson 2020). At a local scale, monitoring in the Batemans Marine Park on the southern coast indicates that barrens have remained stable or even decreased in their coverage from 2008 to 2012 (Coleman et al. 2013). However, there is anecdotal evidence that kelp patches at particular sites in southern NSW have been replaced by barrens in the 2000s (Connell and Irving 2008). It is possible that different perceptions about changes in barrens in NSW could relate to the spatial scale of observation. Many of the aforementioned studies used coarse resolution (tens to hundreds of square metres) to track changes in the aerial extent of barrens in shallow waters, the size of which can be considerable (Connell and Irving 2008). Apparent stability of barrens at large scales (e.g. over hundreds of square metres) can mask fluctuations in kelp patches at smaller scales, noting that these could be either permanent or short-term losses.
Examples of longer-term persistence of extensive barrens are rare because underwater surveys began only in about the 1980s, but some large barrens in NSW have persisted for over 30 years and, in one case where longer-term data were available, up to 68 years (Glasby and Gibson 2020). Quantitative data are not available to assess whether the abundances of urchins and the extent of barrens areas have been increasing over long temporal scales (>50 years). However, recent analysis of yarning circles with Yuin Traditional Owners along the NSW southern coast suggests that barrens may have increased since European colonisation, which knowledge holders attribute to fewer abalone and lobsters (Chewying et al., in press). Thus, the comparatively recent research on NSW barrens may reflect shifting baselines, in which people perceive the state of environment as normal at a given recent time, thereby making it harder to detect environmental degradation across intergenerational timeframes (e.g. tens or hundreds of years) (Atmore et al. 2021). Importantly, large-scale ecological changes since colonisation have occurred across many Australian marine ecosystems (Daley 2014; Gillies et al. 2018; Statton et al. 2018), and a return to pre-colonisation conditions is almost certainly impossible, especially with a changing climate.
It is well documented that C. rodgersii has extended its range south to Tasmania since the turn of the century (Johnson et al. 2005; Ling and Keane 2024), and there is evidence suggesting that abundance may be increasing along the far-southern NSW coast (Davis et al. 2023) (Fig. 2a, b). In contrast to most of the state, the far-southern coast of NSW may be experiencing increases in urchin densities (Davis et al. 2023), and this may result in larger and more numerous barrens (Davis et al. 2020). Conversely, densities of C. rodgersii may have already declined at the distribution limits in northern New South Wales (Fig. 2a, b). However, where C. rodgersii co-occurs with the subtropical endemic coral Pocillopora aliciae in northern NSW, urchin densities have been stable over a 10-year period, including periods of significant heatwaves (McLaren et al. 2024). Through its grazing activity and associated reduction in coral competition with algae, C. rodgersii may be facilitating coral populations in the region, as was found for this urchin where it now co-occurs with corals in its extended range (Ling et al. 2018).
Centrostephanus rodgersii urchin density predictions and projections made using the optimal explanatory model developed for Australia. (a) Predictions for average densities from 1990 to 2000, (b) predictions for average densities from 2010 to 2020, (c) projections for average densities under climate change scenario RCP 8.5 for the period 2090–2100. Contours show average summer maximum (February) temperatures for the reference period. Adapted from Davis et al. (2023).

Climate change should be considered when considering local fluctuations in the extent of barrens and densities of urchins. Climate-related extreme events such as the eastern coast lows (extratropical cyclonic conditions in Dowdy et al. 2019) are a feature of the NSW coast. These storms can dislodge thousands of individuals weakened by low salinity (Byrne and Andrew 2020) and drive periodic mass mortality of C. rodgersii such as that observed in Botany Bay in 1986 and 1988 (Andrew 1991) and Botany Bay and Sydney Harbour in 2022 (Fig. 3). Although extreme storms and low salinity are also deleterious for kelp (Ebeling et al. 1985), it appears to recover quickly in NSW from flooding events (Davis et al. 2022a). Climate change will also involve warming waters, and predictions based on the business-as-usual emissions scenario (RCP 8.5) (Intergovernmental panel on Climate Change 2021) show continued range shifts of C. rodgersii such that populations are considerably reduced in all but the far southern coast of NSW (Davis et al. 2023) (Fig. 2c). These observations stress the importance of considering the impacts of changing climate and associated multiple stressors on the dynamics of NSW barrens and kelp habitats, including the response of thermally acclimated local populations of C. rodgersii in northern NSW and along the NSW latitudinal thermal gradient.
Mass mortalities of C. rodgersii and other urchins along Fairlight Beach in July 2022 after severe storms related to an eastern coast low. Image: Claire Reymond.

When considering temporal dynamics of barrens, kelp ecosystems are also important to understand as a potential alternate stable state (Filbee-Dexter and Scheibling 2014; Kriegisch et al. 2016). In NSW, kelp may be more vulnerable to stressors other than urchin herbivory. Mass mortalities and range contractions of Ecklonia radiata have been attributed to marine heat waves (Bosch et al. 2022), storms (Ettinger-Epstein and Kingsford 2008), disease (Valentine and Johnson 2004), poor water quality (Gorman et al. 2009) and flooding (Davis et al. 2022a). Similarly, declines in Phyllospora comosa have been linked to poor water quality (Coleman et al. 2008), warming and disease (Valentine and Johnson 2004). Although loss of kelp forests can occur quickly, natural recovery can take decades depending on the scale and previous history of the ecosystem. Furthermore, herbivorous fish can increase mortality and hinder recovery of kelp in both barrens and vegetated habitats (Andrew 1994), and herbivory is increasing in low latitudes where warmer waters are increasing herbivorous fish abundance (Vergés et al. 2016; Bosch et al. 2022).
Overall, there is a lack of evidence that C. rodgersii abundance or barrens area have increased along the NSW coast since the 1960s, but limited data preceding this time period make the identification of historical patterns challenging. It remains unclear whether the extent of barrens in NSW is a long-term natural phenomenon or a result of anthropogenic impacts (e.g. climate change, overfishing) that precede the ecological research cited here. Predictions based on the worst-case climate change scenario show that C. rodgersii may decrease in northern NSW and increase along the far NSW coast over the next 100 years (Davis et al. 2023), although local populations may be more resilient to warming waters (McLaren et al. 2024).
National and global context: how do NSW barrens compare to barrens elsewhere?
In NSW, scientists have not yet documented broadscale increases in barrens or obvious causes of barrens increase in local areas. This differs from our understanding of barrens ecosystems investigated elsewhere in the world. In many regions, barrens are the result of disturbances to rocky reef ecosystems (Ebeling et al. 1985; Eger et al. 2022). In the USA and Mediterranean, reductions in urchin predators through commercial harvest have enabled urchins to reach high densities, and they have overgrazed foliose algae on rocky reefs and substantially altered the rocky reef biodiversity (Guidetti and Dulčić 2007; Eurich et al. 2014). Importantly, the cyclical changes from kelp forests to barrens and the return to kelp forests on both sides of the North American continent are driven by sea urchin disease, periodic warming and mass mortality of predator species (seastars, sea otters) (Scheibling and Lauzon-Guay 2010; Rogers-Bennett and Catton 2019; McPherson et al. 2021; Smith et al. 2022a; Galloway et al. 2023). Such large-scale events have not yet been observed in Australia, and the drivers of barrens appear to differ from elsewhere in the world, possibly because of species-specific urchin behaviour, habitat complexity, geomorphology and oceanography (see discussion in Glasby and Gibson 2020).
Within Australia, unexplained short-term population booms of urchins periodically occur, along with associated increases in barrens, for example, Heliocidaris erythrogramma in NSW (Wright et al. 2005) and Victoria (Carnell and Keough 2019) and Tripneustes australiae at Lord Howe Island (Valentine and Edgar 2010; McLaren et al. 2023) and along the NSW coast (McLaren et al. 2024). These population booms may have contributed to the rapid increase of C. rodgersii in Tasmania after its initial range extension. This range extension and warming has threatened kelp (Ling 2008; Mabin et al. 2019) and is predicted to continue and increase the extent of barrens in Tasmania (Perkins et al. 2020). Recent estimates in local-scale studies (5–40-m depth) range from 0.018% barrens cover in southern Tasmania to 2.10% barrens cover in eastern Tasmania (Sward et al. 2022). Statewide estimates show over 40% continuous barrens at the reef at St Helens in north-eastern Tasmania and 15% averaged across the eastern coast (4–40 m) (Ling and Keane 2024). Barrens occur in significantly deeper waters in Tasmania (16–58 m) than in NSW (7–27 m) (Perkins et al. 2015). The incursion of warm low-nutrient EAC water, which will continue as a result of climate change, is a major threat to kelp in Tasmania (Mabin et al. 2019).
Management: how can barrens be managed in NSW?
Community and Indigenous groups are concerned by the presence of extensive barrens and large numbers of urchins on NSW rocky reefs (Chewying et al., in press), and there is growing interest in the idea of transforming barrens to kelp forests by harvesting or culling urchins (Blount 2022). The removal of all visible C. rodgersii individuals causes an ecosystem shift from barrens to habitats dominated by foliose algae if the abundance of urchins is kept very low for an extended period of time (Fletcher 1987; Andrew et al. 1998). Partial removal of even up to 66% of C. rodgersii in NSW barrens does not allow kelp and other foliose algae to successfully colonise (Andrew and Underwood 1993; Hill et al. 2003), with recent evidence indicating that this may be due to the remaining urchins compensating for the reduced abundance with faster growth rates and higher fecundity, which would be associated with increased herbivory. Although ineffective at transforming barrens, removal of some urchins can improve the quality of commercial product (roe) in the remaining animals (Blount et al. 2017). A global review found that removing sea urchins is unlikely to be a long-term solution to support kelp forests because it does not address the underlying cause of high urchin abundance (Miller et al. 2022). There has been research in Victoria and Tasmania showing varying degrees of success with C. rodgersii removal and kelp recovery (Gorfine et al. 2012; Sanderson et al. 2015; Tracey et al. 2015; Kriegisch et al. 2016), but it is challenging to compare these results to those in NSW because of different measurements (i.e. NSW surveys presented as percentage of original population reduction, whereas Tasmania surveys presented as urchin density) and ecosystems (i.e. C. rodgersii is native to NSW). Moreover, the range extension of C. rodgersii in Tasmania is driven by larval dispersal through the strengthening of the EAC (Johnson et al. 2005), an ecological construct different from the situation in NSW.
With the commencement of marine parks in NSW, it was initially predicted that the protection of urchin predators in no-take sanctuary zones may lead to a reduction in urchin numbers and associated barrens areas (Babcock et al. 1999; Ling and Johnson 2012). This prediction was based on findings from overseas, in which the restoration of predators within marine protected areas led to a shift from barrens to seaweed forests (Sangil et al. 2012; Edgar et al. 2017; Kawamata and Taino 2021), with similar patterns also found in New Zealand (Kerr et al. 2024). This is a field of research that is quickly developing, with improved understanding of predator–prey dynamics in NSW (Day et al. 2021, 2024b, 2024c) and Tasmania (Smith et al. 2022b, 2023), as well as the multi-decadal analysis of urchins, barrens and kelp habitat-mosaic in NSW (Glasby and Gibson 2020; Davis et al. 2022b, 2023).
In NSW, there is no evidence of reductions in barrens areas or urchin numbers in Marine Park Sanctuary Zones (Glasby and Gibson 2020), despite significant and widespread increases in the abundance in these no-take zones of the predators, C. auratus, Achoerodus viridis and potentially Sagmariasus verreauxi (Lee et al. 2015; Day 2020; Knott et al. 2021). Barrens are even more abundant inside of the sanctuary zones for Jervis Bay Marine Park than they are outside (Barrett et al. 2008). Research suggests that the control of C. rodgersii by lobsters has been overestimated, at least with contemporary lobster size distributions. Although S. verreauxi can eat up to three urchins per day when starved in aquaria (Provost et al. 2017), gut content analysis of this species suggests that urchins may not be a key food item, with C. rodgersii detected in only 1–2% of lobsters collected over a wide latitudinal range (Day et al. 2021, 2024b). This was corroborated by feeding trials, in which H. erythrogramma was eaten more regularly than C. rodgersii by S. verreauxi in NSW (Day et al. 2021), and small urchins of either species were more frequently consumed than were their larger counterparts (Day et al. 2024c). Moreover, because S. verreauxi undertakes extensive migrations (Booth 1997), it is not a permanent resident of C. rodgersii barrens.
Surprisingly, most predation of urchins occurred by small lobsters (<125-mm carapace length, CL) rather than larger individuals (Day et al. 2021, 2024c). Results of urchin tethering experiments in open barrens and fringe habitats (Day et al. 2023) and outside of an inhabited lobster den (Day et al. 2024b) support this result of low urchin predation by lobsters, as fish predators ate most tethered urchins irrespective of urchin size or species. Overall, these studies suggest lobsters as capable but hesitant C. rodgersii predators (Day et al. 2021). However, S. verreauxi is reportedly the largest growing lobster in the world (Montgomery and Liggins 2012) and research is ongoing into whether urchins including C. rodgersii are a significant food source for very large lobsters of >180 mm CL, whose populations have become reduced since European colonisation. Fish predators (including horned sharks) have more potential impact on urchin populations than do lobsters, because they ate the majority of urchins in tethering experiments (Day et al. 2023, 2024b, 2024c).
Overall, management of NSW barrens through the removal of C. rodgersii from subtidal reefs requires justification. Barrens formation and changes result from various mechanisms and vary over decades (Glasby and Gibson 2020). The expansion of barrens at a local scale, resulting in an undesirable outcome (e.g. a persistent loss of macroalgae), may support a management response (e.g. removal of urchins). To effect a change from barrens to macroalgal beds requires the removal of >70% urchins at a given site (Fletcher 1987; Andrew 1993; Hill et al. 2003). Removal efforts need to be sustained over time and are costly so the feasibility of long-term urchin removal and maintenance must be considered. Importantly, robust monitoring of the manipulated and control areas must occur to assess the effectiveness of urchin removals.
Future research needs: what are the knowledge gaps?
Owing to the different roles of C. rodgersii and urchin barrens between NSW and other parts of Australia, some research needs are unique to NSW whereas others are common to jurisdictions throughout this species’ range.
Driver(s) of ecosystem shifts in NSW
We have yet to understand the drivers of barrens distribution and kelp–barrens dynamics in NSW, particularly over long timescales (hundreds of years) and depth gradients. Subtidal scientific surveys only began in the 1970s, so historic data on barrens extent in NSW do not exist, making it challenging to identify causes of any changes. Manipulative experiments and observational research can help establish the drivers behind shifts between barrens and kelp ecosystems, thereby facilitating better predictions of future barren presence and extent and informing management interventions if needed. We also need to better understand the role that depth and reef morphology (particularly slope, topographic complexity, wave exposure and sand movement) might play in influencing urchin behaviour, kelp recruitment and barrens persistence and coverage (Glasby and Gibson 2020). Most studies on C. rodgersii and barrens have been confined to shallow waters (<15 m) (Table S1), with comparatively little being known about spatiotemporal variation of habitat on deeper rocky reefs, despite their prevalence on the NSW continental shelf (Jordan et al. 2010; Davies et al. 2016).
Best-practice monitoring for populations
The most suitable methods required to monitor urchin barrens and C. rodgersii populations depend on the hypothesis or monitoring purpose, spatial scale of interest, and region (e.g. C. rodgersii flourishes in deeper waters in Tasmania than NSW). Urchin populations are usually quantified using underwater visual census or imagery in transects that are at least 1 × 5 m, and it is unknown whether these techniques may compromise estimates considering the cryptic and nocturnal habits of C. rodgersii. Barrens are quantified using aerial imagery in shallow and clear waters, usually up to 10-m depth. Given that the dimensions of barrens can be large in NSW, estimates of extent should ideally use large grain sizes (e.g. minimum 1-m2 sample points replicated along transects; e.g. Connell and Irving 2008). Ideally, this would be undertaken using divers or towed video, but of course any historical assessment of change, or estimates over particularly large spatial scales, will require a remote sampling methods such as aerial imagery, ideally combined with Lidar to record depth and estimate reef slope and rugosity. Studies aiming to investigate temporal change in barrens should also consider the value of sampling fixed v. random areas; fixed areas are likely to be better for providing the most accurate estimates of rate of change. Finally, the extent of what is defined as a barren compared with a mosaic habitat has yet to be consistently agreed on and may contribute to discrepancies among research findings.
Role of predators on NSW urchin densities
Although evidence from elsewhere in the world suggests that predators control urchins and the associated barrens, it is unclear whether the current large extent of barrens in NSW is a consequence of reduced predation pressure, particularly reductions in maximum size of predators since European colonisation. A broad suite of predators eat urchins in NSW (Day et al. 2021, 2023, 2024b), unlike in other regions where urchin predators are dominated by particular species. Food-web dynamics of urchin barrens habitats is a developing field of research, and stable isotope analysis may help identify the relative roles of predation in structuring C. rodgersii populations. Manipulative experiments would then be required to test the abilities of these predators to influence the extent of barrens under different scenarios. If possible, more detailed analysis of historical records of fish catches and the potential reduction in mean or maximum size of key urchin predators would be useful.
Integration of Indigenous knowledge in research projects
Urchins and kelp are important for many Aboriginal communities along south-eastern Australia. The local knowledge and oral histories held by these communities will prove invaluable in establishing long-term patterns and implementing suitable management actions. Preliminary yarning circles have been conducted with several Traditional Owners and cultural fishers from the NSW southern coast (Chewying et al., in press), and an expanded study that includes more people from different communities would help identify similarities and differences in knowledge across NSW and other areas of south-eastern Australia.
Cumulative or multiple threats to kelp
Decreases in kelp cover are likely to be influenced by concurrent or cumulative stressors (Young et al. 2023), including those related to climate change (e.g. ocean warming, marine heatwaves, flooding, storms). In combination, the more severe disturbance regimes associated with climate change are likely to drive shifts from kelp to barrens ecosystems (Carnell and Keough 2020) and will affect the success of kelp restoration efforts (Layton et al. 2020). In addition, multiple stressors may increase the grazing pressure on kelp; a combination of warming waters and artificial light at night increased herbivory rates of C. rodgersii on kelp (Caley et al. 2024).
Efficacy of marine parks in maintaining barrens and kelp forests
In other parts of the world, marine parks have been shown to decrease barrens by providing refuge for predators that feed on urchins, but there has been no evidence of this in studies of the NSW sanctuary zones. The reasons for this are unknown, and future research can help determine whether NSW barrens are naturally persistent, size of sanctuary zones precludes effects, habitat complexity is high so small urchins can hide, or time scale too short to detect change either in abundance or maximum size of predators.
Feasibility of culling and harvesting to eco-engineer barrens to kelp
In NSW, we have yet to fully understand the logistics, efficacy, and potential impacts of removing urchins and how this may vary among stakeholder groups (Traditional Owners, commercial fishers, community groups). It remains uncertain whether urchin population reduction should be maintained over large scales by culling, in what part of the barrens the culling would have to occur, and whether other concurrent measures would need to be undertaken (e.g. seeding reefs with kelp via green gravel, Fredriksen et al. 2020; crustose coralline algae recovery, Twist et al. 2024).
Conclusions
Barrens are a conspicuous part of the mosaic of habitats on NSW rocky reefs. Generally, the extent of barrens and densities of C. rodgersii in NSW increase from north to south. There is a lack of evidence that C. rodgersii abundance or barrens area have increased along the NSW coast since the 1960s. However, historical patterns are unable to be assessed, and shifting baselines may affect our understanding of barrens as a natural characteristic of NSW rocky reefs.
In conjunction with recent perspective pieces (Andrew 2022; Kingsford and Byrne 2023), this review has shown that barrens and the associated urchin C. rodgersii are ecologically important to NSW rocky ecosystems. In most areas across the state, they do not seem to be a problem to be managed as they are in other regions (Gorfine et al. 2012; Balemi and Shears 2023; Ling and Keane 2024). These findings can inform broader discussions at a national and international level to ensure that appropriate research, management, and policies are being implemented for each jurisdiction. Importantly, the temporal dynamics of any potential ecosystem shift from kelp forest to barrens will be influenced by climate-driven habitat warming because of the thermal sensitivity of kelp (Provost et al. 2017) and will be influenced by the frequency and severity of storms, with storms having the potential to clear large areas of kelp forest (Davis et al. 2022a). Without reducing these stressors or otherwise mitigating their effects, the removal of urchins and the restoration of kelp beds may yield few tangible benefits in the long-term.
Supplementary material
All published studies focussing on barrens or C. rodgersii in New South Wales as of June 2024 are available in Table S1 of the Supplementary material, which is available online.
Data availability
No new data were generated from this study. Key information from previous studies is provided in Supplementary material.
Declaration of funding
Maria Byrne, Rowan Chick and Jeremy Day received in-kind support from FRDC project 2021/060.
Acknowledgements
Neil Andrew (University of Wollongong), Jacquomo Monk (University of Tasmania), Adriana Vergès (University of New South Wales), and Mel Coleman (NSW DPIRD) reviewed and provided valuable input to the research summary on which this review was based (see https://www.dpi.nsw.gov.au/fishing/fisheries-research/fisheries-research-summaries). Craig Blount (NSW DPIRD) and Neil Andrew provided data for Supplementary Table S1. Greg West created Fig. 1 from available imagery from T. Glasby and T. Davis. Two anonymous reviewers provided helpful comments on an earlier draft of this paper.
References
Agnetta D, Bonaviri C, Badalamenti F, Di Trapani F, Gianguzza P (2024) Coralline barrens and benthic mega-invertebrates: an intimate connection. Marine Environmental Research 199, 106579.
| Crossref | Google Scholar |
Andrew NL (1991) Changes in subtidal habitat following mass mortality of sea urchins in Botany Bay, New South Wales. Australian Journal of Ecology 16, 353-362.
| Crossref | Google Scholar |
Andrew NL (1993) Spatial heterogeneity, sea urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74, 292-302.
| Crossref | Google Scholar |
Andrew NL (1994) Survival of kelp adjacent to areas grazed by sea urchins in New South Wales, Australia. Australian Journal of Ecology 19, 466-472.
| Crossref | Google Scholar |
Andrew N (2022) Sea urchins have invaded Tasmania and Victoria, but we can’t work out what to do with them. In The Conversation, 6 December 2022. Available at https://theconversation.com/sea-urchins-have-invaded-tasmania-and-victoria-but-we-cant-work-out-what-to-do-with-them-194534
Andrew NL, O’Neill AL (2000) Large-scale patterns in habitat structure on subtidal rocky reefs in New South Wales. Marine and Freshwater Research 51, 255-263.
| Crossref | Google Scholar |
Andrew NL, Underwood AJ (1989) Patterns of abundance of the sea urchin Centrostephanus rodgersii (Agassiz) on the central coast of New South Wales, Australia. Journal of Experimental Marine Biology and Ecology 131, 61-80.
| Crossref | Google Scholar |
Andrew NL, Underwood AJ (1992) Associations and abundance of sea urchins and abalone on shallow subtidal reefs in southern New South Wales. Marine and Freshwater Research 43, 1547-1559.
| Crossref | Google Scholar |
Andrew NL, Underwood AJ (1993) Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Marine Ecology Progress Series 99, 89-98.
| Crossref | Google Scholar |
Andrew NL, Worthington DG, Brett PA, Bentley N, Chick RC, Blount C (1998) Interactions between the abalone fishery and sea urchins in New South Wales. FRDC Project Number 93/102, NSW Fisheries Final Report Series Number 12. (Fisheries Research and Development Corporation) Available at https://www.frdc.com.au/sites/default/files/products/1993-102-DLD.pdf
Atmore LM, Aiken M, Furni F (2021) Shifting baselines to thresholds: reframing exploitation in the marine environment. Frontiers in Marine Science 8, 742188.
| Crossref | Google Scholar |
Babcock RC, Kelly S, Shears NT, Walker JW, Willis TJ (1999) Changes in community structure in temperate marine reserves. Marine Ecology Progress Series 189, 125-134.
| Crossref | Google Scholar |
Balemi CA, Shears NT (2023) Emergence of the subtropical sea urchin Centrostephanus rodgersii as a threat to kelp forest ecosystems in northern New Zealand. Frontiers in Marine Science 10, 1224067.
| Crossref | Google Scholar |
Banks SC, Piggott MP, Williamson JE, Bové U, Holbrook NJ, Beheregaray LB (2007) Oceanic variability and coastal topography shape genetic structure in a long-dispersing sea urchin. Ecology 88, 3055-3064.
| Crossref | Google Scholar | PubMed |
Barrett N, Edgar G, Polacheck A, Lynch T, Clements F (2008) Ecosystem Monitoring of Subtidal Reefs in the Jervis Bay Marine Park 1996–2007. TAFI Internal Report. (Tasmanian Aquaculture and Fisheries Institute, University of Tasmania: Taroona, Tas., Australia) Available at https://hdl.handle.net/102.100.100/490052
Blount C, Worthington D (2002) Identifying individuals of the sea urchin Centrostephanus rodgersii with high-quality roe in New South Wales, Australia. Fisheries Research 58, 341-348.
| Crossref | Google Scholar |
Blount C, Chick RC, Worthington DG (2017) Enhancement of an underexploited fishery – improving the yield and colour of roe in the sea urchin Centrostephanus rodgersii by reducing density or transplanting individuals. Fisheries Research 186, 586-597.
| Crossref | Google Scholar |
Blount C, Worthington DG, Byrne M, Chick RC, Andrew NL (2024a) Aging of the sea urchin Centrostephanus rodgersii using demi-pyramid microstructure. Fisheries Research 270, 106899.
| Crossref | Google Scholar |
Blount C, Worthington DG, Byrne M, Chick RC, Organ K, Knott N, Andrew NL (2024b) Abundance, size and biomass of long-spined sea urchins (Centrostephanus rodgersii) and red sea urchins (Heliocidaris tuberculata) in New South Wales, Australia. Marine and Freshwater Research 75, MF23212.
| Crossref | Google Scholar |
Booth JD (1997) Long-distance movements in Jasus spp. and their role in larval recruitment. Bulletin of Marine Science 61, 111-128.
| Google Scholar |
Bosch NE, Mclean M, Zarco-Perello S, Bennett S, Stuart-Smith RD, Vergés A, Pessarrodona A, Tuya F, Langlois T, Spencer C, Bell S, Saunders BJ, Harvey ES, Wernberg T (2022) Persistent thermally driven shift in the functional trait structure of herbivorous fishes: evidence of top-down control on the rebound potential of temperate seaweed forests? Global Change Biology 28, 2296-2311.
| Crossref | Google Scholar | PubMed |
Byrne M, Andrew NL, Worthington DG, Brett PA (1998) Reproduction in the diadematoid sea urchin Centrostephanus rodgersii in contrasting habitats along the coast of New South Wales, Australia. Marine Biology 132, 305-318.
| Crossref | Google Scholar |
Byrne M, Gall ML, Campbell H, Lamare MD, Holmes SP (2022) Staying in place and moving in space: contrasting larval thermal sensitivity explains distributional changes of sympatric sea urchin species to habitat warming. Global Change Biology 28, 3040-3053.
| Crossref | Google Scholar | PubMed |
Caley A, Marzinelli EM, Byrne M, Mayer-Pinto M (2024) Artificial light at night and warming impact grazing rates and gonad index of the sea urchin Centrostephanus rodgersii. Proceedings of the Royal Society of London – B. Biological Sciences 291, 20240415.
| Crossref | Google Scholar |
Carnell PE, Keough MJ (2019) Reconstructing historical marine populations reveals major decline of a kelp forest ecosystem in Australia. Estuaries and Coasts 42, 765-778.
| Crossref | Google Scholar |
Carnell PE, Keough MJ (2020) More severe disturbance regimes drive the shift of a kelp forest to a sea urchin barren in south-eastern Australia. Scientific Reports 10, 11272.
| Crossref | Google Scholar |
Carnell PE, Ierodiaconou D, Atwood TB, Macreadie PI (2020) Overgrazing of seagrass by sea urchins diminishes blue carbon stocks. Ecosystems 23, 1437-1448.
| Crossref | Google Scholar |
Chewying K, Walbunja Rangers, Gibbs M, Przeslawski R, Rogers K (in press) Braiding Indigenous oral histories and habitat mapping to understand urchin barrens in southern New South Wales. Marine and Freshwater Research. doi:10.1071/MF24117
Coleman MA, Kennelly SJ (2019) Microscopic assemblages in kelp forests and urchin barrens. Aquatic Botany 154, 66-71.
| Crossref | Google Scholar |
Coleman MA, Kelaher BP, Steinberg PD, Millar AJK (2008) Absence of a large brown macroalga on urbanized rocky reefs around Sydney, Australia, and evidence for historical decline. Journal of Phycology 44, 897-901.
| Crossref | Google Scholar | PubMed |
Coleman MA, Palmer-Brodie A, Kelaher BP (2013) Conservation benefits of a network of marine reserves and partially protected areas. Biological Conservation 167, 257-264.
| Crossref | Google Scholar |
Connell SD, Irving AD (2008) Integrating ecology with biogeography using landscape characteristics: a sase study of subtidal habitat across continental Australia. Journal of Biogeography 35, 1608-1621.
| Crossref | Google Scholar |
Curley BG, Kingsford MJ, Gillanders BM (2002) Spatial and habitat-related patterns of temperate reef fish assemblages: implications for the design of Marine Protected Areas. Marine and Freshwater Research 53, 1197-1210.
| Crossref | Google Scholar |
Davis TR, Cadiou G, Champion C, Coleman MA (2020) Environmental drivers and indicators of change in habitat and fish assemblages within a climate change hotspot. Regional Studies in Marine Science 36, 101295.
| Crossref | Google Scholar |
Davis TR, Larkin MF, Forbes A, Veenhof RJ, Scott A, Coleman MA (2022a) Extreme flooding and reduced salinity causes mass mortality of nearshore kelp forests. Estuarine, Coastal and Shelf Science 275, 107960.
| Crossref | Google Scholar |
Davis TR, Champion C, Coleman MA (2022b) Ecological interactions mediate projected loss of kelp biomass under climate change. Diversity and Distributions 28, 306-317.
| Crossref | Google Scholar |
Davis TR, Knott NA, Champion C, Przeslawski R (2023) Impacts of climate change on densities of the urchin Centrostephanus rodgersii vary among marine regions in Eastern Australia. Diversity 15, 419.
| Crossref | Google Scholar |
Day JK, Knott NA, Swadling DS, Ayre DJ (2021) Dietary analysis and mesocosm feeding trials confirm the eastern rock lobster Sagmariasus verreauxi as a generalist predator that can avoid ingesting urchin spines during feeding. Marine and Freshwater Research 72, 1220-1232.
| Crossref | Google Scholar |
Day J, Knott NA, Ayre D, Byrne M (2023) Effects of habitat on predation of ecologically important sea urchin species on east coast Australian temperate reefs in tethering experiments. Marine Ecology Progress Series 714, 71-86.
| Crossref | Google Scholar |
Day JK, Knott NA, Swadling DS, Ayre DJ, Huggett MJ, Gaston TF (2024a) Investigating the diets and condition of Centrostephanus rodgersii (long-spined urchin) in barrens and macroalgae habitats in south-eastern Australia. Marine Ecology Progress Series 729, 167-183.
| Crossref | Google Scholar |
Day JK, Knott NA, Swadling DS, Huggett MJ, Gaston TF (2024b) Assessing lobster and co-predator feeding rates on barrens-forming sea urchins in South East Australia. Frontiers in Marine Science 11, 1418506.
| Crossref | Google Scholar |
Day JK, Knott NA, Swadling DS, Ayre D, Huggett MJ, Gaston TF (2024c) Spiny lobster predation of barrens-forming sea urchins is not limited by body size, but may be overstated. Ecosphere 15, e4960.
| Crossref | Google Scholar |
Dowdy AJ, Pepler A, Di Luca A, Cavicchia L, Mills G, Evans JP, Louis S, Mcinnes KL, Walsh K (2019) Review of Australian east coast low pressure systems and associated extremes. Climate Dynamics 53, 4887-4910.
| Crossref | Google Scholar |
Ebeling AW, Laur DR, Rowley RJ (1985) Severe storm disturbances and reversal of community structure in a southern California kelp forest. Marine Biology 84, 287-294.
| Crossref | Google Scholar |
Edgar GJ, Stuart-Smith RD, Thomson RJ, Freeman DJ (2017) Consistent multi-level trophic effects of marine reserve protection across northern New Zealand. PLoS ONE 12, e0177216.
| Crossref | Google Scholar |
Eger AM, Marzinelli EM, Christie H, Fagerli CW, Fujita D, Gonzalez AP, Hong SW, Kim JH, Lee LC, Mchugh TA, Nishihara GN, Tatsumi M, Steinberg PD, Vergés A (2022) Global kelp forest restoration: past lessons, present status, and future directions. Biological Reviews 97, 1449-1475.
| Crossref | Google Scholar | PubMed |
Eger AM, Blain CO, Brown AL, Chan SSW, Miller KI, Vergés A (2024) Kelp forests versus urchin barrens: a comparison of ecosystem functions and services provided by two alternative stable marine habitats. Proceedings of the Royal Society of London – B. Biological Sciences 291, 20241539.
| Crossref | Google Scholar |
Environment and Communications References Committee (2023) Win–win under our oceans: climate-related marine invasive species. Senate enquiry report (Commonwealth of Australia) Available at https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Environment_and_Communications/Invasivemarinespecies/Report
Ettinger-Epstein P, Kingsford MJ (2008) Effects of the El Niño–Southern Oscillation on Turbo torquatus (Gastropoda) and their kelp habitat. Austral Ecology 33, 594-606.
| Crossref | Google Scholar |
Eurich JG, Selden RL, Warner RR (2014) California spiny lobster preference for urchins from kelp forests: implications for urchin barren persistence. Marine Ecology Progress Series 498, 217-225.
| Crossref | Google Scholar |
Filbee-Dexter K, Scheibling RE (2014) Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series 495, 1-25.
| Crossref | Google Scholar |
Fletcher WJ (1987) Interactions among subtidal Australian sea urchins, gastropods, and algae: effects of experimental removals. Ecological Monographs 57, 89-109.
| Crossref | Google Scholar |
Flukes EB, Johnson CR, Ling SD (2012) Forming sea urchin barrens from the inside out: an alternative pattern of overgrazing. Marine Ecology Progress Series 464, 179-194.
| Crossref | Google Scholar |
Foo SA, Dworjanyn SA, Poore AGB, Byrne M (2012) Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: performance of early embryos. PLoS ONE 7, e42497.
| Crossref | Google Scholar |
Fredriksen S, Filbee-Dexter K, Norderhaug KM, Steen H, Bodvin T, Coleman MA, Moy F, Wernberg T (2020) Green gravel: a novel restoration tool to combat kelp forest decline. Scientific Reports 10, 3983.
| Crossref | Google Scholar |
Galloway AWE, Gravem SA, Kobelt JN, Heady WN, Okamoto DK, Sivitilli DM, Saccomanno VR, Hodin J, Whippo R (2023) Sunflower sea star predation on urchins can facilitate kelp forest recovery. Proceedings of the Royal Society of London – B. Biological Sciences 290, 20221897.
| Crossref | Google Scholar |
Gillanders BM (1995) Feeding ecology of the temperate marine fish Achoerodus viridis (Labridae): size, seasonal and site-specific differences. Marine and Freshwater Research 46, 1009-1020.
| Crossref | Google Scholar |
Gillies CL, Mcleod IM, Alleway HK, Cook P, Crawford C, Creighton C, Diggles B, Ford J, Hamer P, Heller-Wagner G, Lebrault E, Le Port A, Russell K, Sheaves M, Warnock B (2018) Australian shellfish ecosystems: past distribution, current status and future direction. PLoS ONE 13, e0190914.
| Crossref | Google Scholar |
Glasby TM, Gibson PT (2020) Decadal dynamics of subtidal barrens habitat. Marine Environmental Research 154, 104869.
| Crossref | Google Scholar |
Glasby TM, Gibson PT, Cruz-Motta JJ (2017) Differences in rocky reef habitats related to human disturbances across a latitudinal gradient. Marine Environmental Research 129, 291-303.
| Crossref | Google Scholar | PubMed |
Gorman D, Russell BD, Connell SD (2009) Land-to-sea connectivity: linking human-derived terrestrial subsidies to subtidal habitat change on open rocky coasts. Ecological Applications 19, 1114-1126.
| Crossref | Google Scholar | PubMed |
Guidetti P, Dulčić J (2007) Relationships among predatory fish, sea urchins and barrens in Mediterranean rocky reefs across a latitudinal gradient. Marine Environmental Research 63, 168-184.
| Crossref | Google Scholar | PubMed |
Hill NA, Blount C, Poore AGB, Worthington D, Steinberg PD (2003) Grazing effects of the sea urchin Centrostephanus rodgersii in two contrasting rocky reef habitats: effects of urchin density and its implications for the fishery. Marine and Freshwater Research 54, 691-700.
| Crossref | Google Scholar |
Huggett MJ, King CK, Williamson JE, Steinberg PD (2005) Larval development and metamorphosis of the Australian diadematid sea urchin Centrostephanus rodgersii. Invertebrate Reproduction & Development 47, 197-204.
| Crossref | Google Scholar |
Jones GP, Andrew NL (1990) Herbivory and patch dynamics on rocky reefs in temperate Australasia: the roles of fish and sea urchins. Australian Journal of Ecology 15, 505-520.
| Crossref | Google Scholar |
Kawamata S, Taino S (2021) Trophic cascade in a marine protected area with artificial reefs: spiny lobster predation mitigates urchin barrens. Ecological Applications 31, e02364.
| Crossref | Google Scholar |
Kerr VC, Grace RV, Shears NT (2024) Estimating the extent of urchin barrens and kelp forest loss in northeastern Aotearoa, New Zealand. New Zealand Journal of Marine and Freshwater Research [Published online early 23 April 2024].
| Crossref | Google Scholar |
King CK, Hoegh-Guldberg O, Byrne M (1994) Reproductive cycle of Centrostephanus rodgersii (Echinoidea), with recommendations for the establishment of a sea urchin fishery in New South Wales. Marine Biology 120, 95-106.
| Crossref | Google Scholar |
Kingsford MJ, Byrne M (2023) New South Wales rocky reefs are under threat. Marine and Freshwater Research 74, 95-98.
| Crossref | Google Scholar |
Knott NA, Williams J, Harasti D, Malcolm HA, Coleman MA, Kelaher BP, Rees MJ, Schultz A, Jordan A (2021) A coherent, representative, and bioregional marine reserve network shows consistent change in rocky reef fish assemblages. Ecosphere 12, e03447.
| Crossref | Google Scholar |
Kriegisch N, Reeves S, Johnson CR, Ling SD (2016) Phase-shift dynamics of sea urchin overgrazing on nutrified reefs. PLoS ONE 11, e0168333.
| Crossref | Google Scholar |
Layton C, Coleman MA, Marzinelli EM, Steinberg PD, Swearer SE, Vergés A, Wernberg T, Johnson CR (2020) Kelp forest restoration in Australia. Frontiers in Marine Science 7, 74.
| Crossref | Google Scholar |
Lee KA, Huveneers C, Macdonald T, Harcourt RG (2015) Size isn’t everything: movements, home range, and habitat preferences of eastern blue gropers (Achoerodus viridis) demonstrate the efficacy of a small marine reserve. Aquatic Conservation: Marine and Freshwater Ecosystems 25, 174-186.
| Crossref | Google Scholar |
Ling SD (2008) Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156, 883-894.
| Crossref | Google Scholar | PubMed |
Ling SD, Johnson CR (2009) Population dynamics of an ecologically important range-extender: kelp beds versus sea urchin barrens. Marine Ecology Progress Series 374, 113-125.
| Crossref | Google Scholar |
Ling SD, Johnson CR (2012) Marine reserves reduce risk of climate-driven phase shift by reinstating size- and habitat-specific trophic interactions. Ecological Applications 22, 1232-1245.
| Crossref | Google Scholar | PubMed |
Ling SD, Keane JP (2024) Climate-driven invasion and incipient warnings of kelp ecosystem collapse. Nature Communications 15, 400.
| Crossref | Google Scholar |
Ling SD, Johnson CR, Frusher SD, Ridgway KR (2009) Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proceedings of the National Academy of Sciences 106, 22341-22345.
| Crossref | Google Scholar |
Ling SD, Scheibling RE, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon AK, Norderhaug KM, Pérez-Matus A, Hernández JC, Clemente S, Blamey LK, Hereu B, Ballesteros E, Sala E, Garrabou J, Cebrian E, Zabala M, Fujita D, Johnson LE (2015) Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 370, 20130269.
| Crossref | Google Scholar |
Ling SD, Barrett NS, Edgar GJ (2018) Facilitation of Australia’s southernmost reef-building coral by sea urchin herbivory. Coral Reefs 37, 1053-1073.
| Crossref | Google Scholar |
Mabin CJT, Johnson CR, Wright JT (2019) Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera from Tasmania, Australia. Marine Ecology Progress Series 614, 1-19.
| Crossref | Google Scholar |
McLaren E, Bronstein O, Kroh A, Winkler V, Miskelly A, Sommer B, Byrne M (2023) Hidden in plain sight: Tripneustes kermadecensis (Echinodermata: Echinoidea) is a junior synonym of the eastern Australian sea urchin Evechinus australiae described in 1878. Invertebrate Systematics 37, 741-754.
| Crossref | Google Scholar |
McLaren E, Sommer B, Pandolfi JM, Beger M, Byrne M (2024) Taxa-dependent temporal trends in the abundance and size of sea urchins in subtropical eastern Australia. Ecology and Evolution 14, e11412.
| Crossref | Google Scholar |
McLaughlin RH, O’Gower AK (1971) Life history and underwater studies of a heterodont shark. Ecological Monographs 41, 271-289.
| Crossref | Google Scholar |
McPherson ML, Finger DJI, Houskeeper HF, Bell TW, Carr MH, Rogers-Bennett L, Kudela RM (2021) Large-scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Communications Biology 4, 298.
| Crossref | Google Scholar |
Medrano A, Hereu B, Cleminson M, Pagès-Escolà M, Rovira GL, Solà J, Linares C (2020) From marine deserts to algal beds: Treptacantha elegans revegetation to reverse stable degraded ecosystems inside and outside a no-take marine reserve. Restoration Ecology 28, 632-644.
| Crossref | Google Scholar |
Miller KI, Blain CO, Shears NT (2022) Sea urchin removal as a tool for macroalgal restoration: a review on removing “the spiny enemies”. Frontiers in Marine Science 9, 831001.
| Crossref | Google Scholar |
Montgomery SS, Liggins GW (2012) Recovery of the eastern rock lobster Sagmariasus verreauxi off New South Wales, Australia. Marine Biology Research 9(1), 104-115.
| Crossref | Google Scholar |
Mos B, Byrne M, Dworjanyn SA (2020) Effects of low and high pH on sea urchin settlement, implications for the use of alkali to counter the impacts of acidification. Aquaculture 528, 735618.
| Crossref | Google Scholar |
Perkins NR, Hill NA, Foster SD, Barrett NS (2015) Altered niche of an ecologically significant urchin species, Centrostephanus rodgersii, in its extended range revealed using an Autonomous Underwater Vehicle. Estuarine, Coastal and Shelf Science 155, 56-65.
| Crossref | Google Scholar |
Perkins NR, Hosack GR, Foster SD, Monk J, Barrett NS (2020) Monitoring the resilience of a no-take marine reserve to a range extending species using benthic imagery. PLoS ONE 15, e0237257.
| Crossref | Google Scholar |
Provost EJ, Kelaher BP, Dworjanyn SA, Russell BD, Connell SD, Ghedini G, Gillanders BM, Figueira W, Coleman MA (2017) Climate-driven disparities among ecological interactions threaten kelp forest persistence. Global Change Biology 23, 353-361.
| Crossref | Google Scholar | PubMed |
Rogers-Bennett L, Catton CA (2019) Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Scientific Reports 9, 15050.
| Crossref | Google Scholar |
Sanderson JC, Ling SD, Dominguez JG, Johnson CR (2015) Limited effectiveness of divers to mitigate ‘barrens’ formation by culling sea urchins while fishing for abalone. Marine and Freshwater Research 67, 84-95.
| Crossref | Google Scholar |
Sangil C, Clemente S, Martín-García L, Hernández JC (2012) No-take areas as an effective tool to restore urchin barrens on subtropical rocky reefs. Estuarine, Coastal and Shelf Science 112, 207-215.
| Crossref | Google Scholar |
Scheibling RE, Lauzon-Guay J-S (2010) Killer storms: North Atlantic hurricanes and disease outbreaks in sea urchins. Limnology and Oceanography 55, 2331-2338.
| Crossref | Google Scholar |
Smith JE, Keane J, Mundy C, Gardner C, Oellermann M (2022b) Spiny lobsters prefer native prey over range-extending invasive urchins. ICES Journal of Marine Science 79, 1353-1362.
| Crossref | Google Scholar |
Smith JE, Keane J, Oellermann M, Mundy C, Gardner C (2023) Lobster predation on barren-forming sea urchins is more prevalent in habitats where small urchins are common: a multi-method diet analysis. Marine and Freshwater Research 74, 1493-1505.
| Crossref | Google Scholar |
Smith JE, Flukes E, Keane JP (2024) The risky nightlife of undersized sea urchins. Marine and Freshwater Research 75, MF23189.
| Crossref | Google Scholar |
Strain EMA, Johnson CR, Thomson RJ (2013) Effects of a range-expanding sea urchin on behaviour of commercially fished abalone. PLoS ONE 8, e73477.
| Crossref | Google Scholar |
Sward D, Monk J, Barrett NS (2022) Regional estimates of a range-extending ecosystem engineer using stereo-imagery from ROV transects collected with an efficient, spatially balanced design. Remote Sensing in Ecology and Conservation 8, 105-118.
| Crossref | Google Scholar |
Thomas LJ, Liggins L, Banks SC, Beheregaray LB, Liddy M, McCulloch GA, Waters JM, Carter L, Byrne M, Cumming RA, Lamare MD (2021) The population genetic structure of the urchin Centrostephanus rodgersii in New Zealand with links to Australia. Marine Biology 168, 138.
| Crossref | Google Scholar |
Tracey SR, Baulch T, Hartmann K, Ling SD, Lucieer V, Marzloff MP, Mundy C (2015) Systematic culling controls a climate driven, habitat modifying invader. Biological Invasions 17, 1885-1896.
| Crossref | Google Scholar |
Twist BA, Mazel F, Zaklan Duff S, Lemay MA, Pearce CM, Martone PT (2024) Kelp and sea urchin settlement mediated by biotic interactions with benthic coralline algal species. Journal of Phycology 60, 363-379.
| Crossref | Google Scholar | PubMed |
Underwood AJ, Kingsford MJ, Andrew NL (1991) Patterns in shallow subtidal marine assemblages along the coast of New South Wales. Australian Journal of Ecology 16, 231-249.
| Crossref | Google Scholar |
Valentine JP, Edgar GJ (2010) Impacts of a population outbreak of the urchin Tripneustes gratilla amongst Lord Howe Island coral communities. Coral Reefs 29, 399-410.
| Crossref | Google Scholar |
Valentine JP, Johnson CR (2004) Establishment of the introduced kelp Undaria pinnatifida following dieback of the native macroalga Phyllospora comosa in Tasmania, Australia. Marine and Freshwater Research 55, 223-230.
| Crossref | Google Scholar |
Veenhof RJ, Coleman MA, Champion C, Dworjanyn SA (2023) Urchin grazing of kelp gametophytes in warming oceans. Journal of Phycology 59, 838-855.
| Crossref | Google Scholar | PubMed |
Vergés A, Doropoulos C, Malcolm HA, Skye M, Garcia-Pizá M, Marzinelli EM, Campbell AH, Ballesteros E, Hoey AS, Vila-Concejo A, Bozec Y-M, Steinberg PD (2016) Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences 113, 13791-13796.
| Crossref | Google Scholar |
Webb M, Byrne M (2025) Impact of hyposalinity on the barrens-forming sea urchin Centrostephanus rodgersii in context with flooding events. Marine Environmental Research 207, 107012.
| Crossref | Google Scholar |
Williams J, Coleman MA, Jordan A (2020) Depth, nutrients and urchins explain variability in Ecklonia radiata (laminariales) distribution and cover across ten degrees of latitude. Aquatic Botany 166, 103274.
| Crossref | Google Scholar |
Wright JT, Benkendorff K, Davis AR (1997) Habitat associated differences in temperate sponge assemblages: the importance of chemical defence. Journal of Experimental Marine Biology and Ecology 213, 199-213.
| Crossref | Google Scholar |
Wright JT, Dworjanyn SA, Rogers CN, Steinberg PD, Williamson JE, Poore AGB (2005) Density-dependent sea urchin grazing: differential removal of species, changes in community composition and alternative community states. Marine Ecology Progress Series 298, 143-156.
| Crossref | Google Scholar |
Young MA, Critchell K, Miller AD, Treml EA, Sams M, Carvalho R, Ierodiaconou D (2023) Mapping the impacts of multiple stressors on the decline in kelps along the coast of Victoria, Australia. Diversity and Distributions 29, 199-220.
| Crossref | Google Scholar |


