Trends in eastern blue groper (Achoerodus viridis) abundance along south-eastern Australia (New South Wales): the influence of latitude, climate change and potential depth refuges
Nathan A. Knott A * , Matthew J. Rees
A * , Matthew J. Rees  A , Tom R. Davis
A , Tom R. Davis  B , David Harasti
B , David Harasti  C , Hamish A. Malcolm B , Matthew D. Taylor
C , Hamish A. Malcolm B , Matthew D. Taylor  C , Belinda G. Curley D , Stephen Morris E , Neville S. Barrett F , Rick D. Stuart-Smith F , Graham J. Edgar F and Rachel Przeslawski
C , Belinda G. Curley D , Stephen Morris E , Neville S. Barrett F , Rick D. Stuart-Smith F , Graham J. Edgar F and Rachel Przeslawski  A G
A G
A
B
C
D
E
F
G
Abstract
Eastern blue groper (Achoerodus viridis) is an iconic Australian fish and a trial prohibition of fishing for it has been implemented in New South Wales (NSW). A review of available data on this species is needed to inform future management.
To assess the temporal and spatial patterns in the abundance of A. viridis.
Data collected across four NSW bioregions from two systematic sampling programs, namely, baited remote underwater video (2010–23) and underwater visual census (2008–23), were analysed with the inclusion of two other common wrasse species as references.
Achoerodus viridis showed strong latitudinal variation: being least abundant in the warmer northern bioregion and peaking in abundance in the central Manning and southern Batemans Bioregions. Temporal trajectories for A. viridis were mixed with significant declines on shallow reefs in the Manning and Hawkesbury Bioregions, whereas abundances on deeper reefs were stable. Similar patterns of decline were observed for the two reference species, although both species were far more abundant than was A. viridis.
Achoerodus viridis, like other temperate wrasse, appears to be declining because of warming oceans, although depth may provide a thermal refuge
These analyses should assist decision-making for future management regulations for A. viridis.
Keywords: climate-change shifts, crimson banded wrasse, fishing regulations, Great Souther Reef, labrids, monitoring, Notolabrus gymnogenis, Ophthalmolepis lineolata, refugia, southern Maori wrasse.
Introduction
The eastern blue groper (Achoerodus viridis) is a rocky-reef species endemic to south-eastern Australia, from southern Queensland to Victoria. Males are iridescent blue, large and long-lived, growing up to 1.2 m in length (Gillanders 1999), and show very high levels of site-attachment on rocky reefs (Lee et al. 2015). This leads to a unique situation, whereby snorkelers and divers can recognise individual fish, and through ongoing interactions at times develop ‘friendships’ with these fish (Gillanders 1999; Dengate 2014). Achoerodus viridis is a protogynous hermaphrodite with individuals transforming from functional females (<500 mm) to functional males as they grow in size (Gillanders 1995a), and this process is also influenced by low numbers of males in an area (as seen in other labrids; Warner and Swearer 1991; Gillanders 1995a). This large wrasse is a benthic carnivore that eats a wide range of invertebrates on rocky reefs, including small amphipods through to large barnacles, mussels, snails and urchins (Gillanders 1995b), and may play an important functional role in temperate rocky-reef ecosystems (Gillanders 1999; Last et al. 2011; Young et al. 2014). Early settlers and juveniles can be found in estuaries on seagrass or rocky reefs and on open coastal rocky reefs, where they can move to rocky reefs within estuaries or on the open coast – from shallow waters to depths of 60 m (Gillanders 1997).
The life-history traits of A. viridis include late reproductive maturity (>10 years for males) and long lifespan. These traits make the species susceptible to overfishing (Gillanders 1995a; Coulson et al. 2009; Curley et al. 2013a; Young et al. 2014) and the IUCN Red List classifies the species as ‘Near Threatened’ (Choat and Pollard 2010). In the 1960s, signs of depletion were initially identified by spearfishers, who lobbied for the protection of the species (Young et al. 2014). Consequently, it was protected from harvest by all methods in New South Wales (NSW) in 1969, although later these protections were eased to allow recreational line fishing (Gillanders 1999; Young et al. 2014). There is some anecdotal evidence of recovery of A. viridis since these protections were implemented (Newbery 2023), although no formal analysis has been completed (Young et al. 2014).
Until recently (prior to 23 February 2024), recreational harvest of A. viridis was permitted within NSW, with a limit of two fish per person and a minimum legal size of 30 cm (NSW Government 2019). Over the past decade, only a small number of recreational fishers have reported catching A. viridis in fishing surveys (Murphy et al. 2023) and, as a consequence, the estimates of recreational harvest for this species carry high uncertainty. However, the trend over the past 10 years has been a consistent decline in estimated recreational catch in NSW, from 3529 ± 1985 fish (mean ± s.e.) in 2013–14 (West et al. 2015) to 164 ± 164 fish in 2021/22 (Murphy et al. 2023) by licensed recreational fishers. The most recent recreational fishing survey indicates that only ~0.2% of surveyed households reported any interactions with the species, which suggests very low targeted fishing effort, consistent with previous recreational-fishing surveys (Kingsford et al. 1991; West et al. 2015; Murphy et al. 2023).
Over the past two decades, A. viridis has been illegally speared and killed (Jones 2009; Trembath 2023) and two recent, well-publicised events (within weeks of each other) have heightened public concern about this issue (Newbery 2023; Chung 2024). As a result of the high social value and the concern around this iconic species, the NSW Government has implemented a 12-month trial of new fishing rules prohibiting harvest of the species by any method in NSW, which is intended to continue alongside stakeholder consultation on future rules. A review of available data on population trends has not yet been undertaken for A. viridis, but such information is important to support decision-making on future regulations and conservation of this iconic species. The current study draws on data from two long-term monitoring programs to assess evidence of temporal trends in the abundance of A. viridis along the NSW coastline. To aid the contextual interpretation of A. viridis abundance, data for two other common wrasse species, i.e. Ophthalmolepis lineolata (southern Maori wrasse) and Notolabrus gymnogenis (crimson banded wrasse), are also analysed. The observed patterns are considered in light of the various stressors that are likely to be exerting pressure on the species, and the implications for future management arrangements.
Methods
Two major survey programs systematically report on fish assemblages on NSW rocky reefs, including (a) NSW Statewide Baited Remote Underwater Video (BRUV) program (Knott et al. 2021), and (b) the Australian Temperate Reef Collaboration (ATRC) and Reef Life Survey (RLS) programs (effectively a joint program, see https://atrc.au/ and https://reeflifesurvey.com/; IMOS – National Reef Monitoring Network – Global reef fish abundance and biomass (dataset 19/05/2024); Edgar et al. 2023). The NSW Statewide BRUV program samples the relative abundance of rocky-reef fishes between 20- and 40-m depth across four of the NSW bioregional divisions (Interim Marine and Coastal Regionalisation for Australia Technical Group 1998; Fig. 1), whereas the ATRC and RLS programs use underwater visual census (UVC, i.e. dive surveys) to sample the relative abundances of fishes (and also invertebrates and algae) over depths of 3–20 m (although mostly between 5 and 10 m) within the same bioregions. These programs cover a diverse coastline with a significant temperature gradient from north to south (Fig. 1; Zann 2000; Davis et al. 2023).
Sites and bioregions sampled using baited remote underwater video (BRUV; red dots) and underwater visual census (UVC diver surveys: ATRC and RLS; blue dots) along the NSW coastline. Average sea-surface temperatures are shown to indicate the thermal gradient across the state’s coastline and bioregions. Sea-surface water temperatures (2007–24) were extracted from the EU Copernicus Marine Service (see http://marine.copernicus.eu, accessed 9 May 2024).
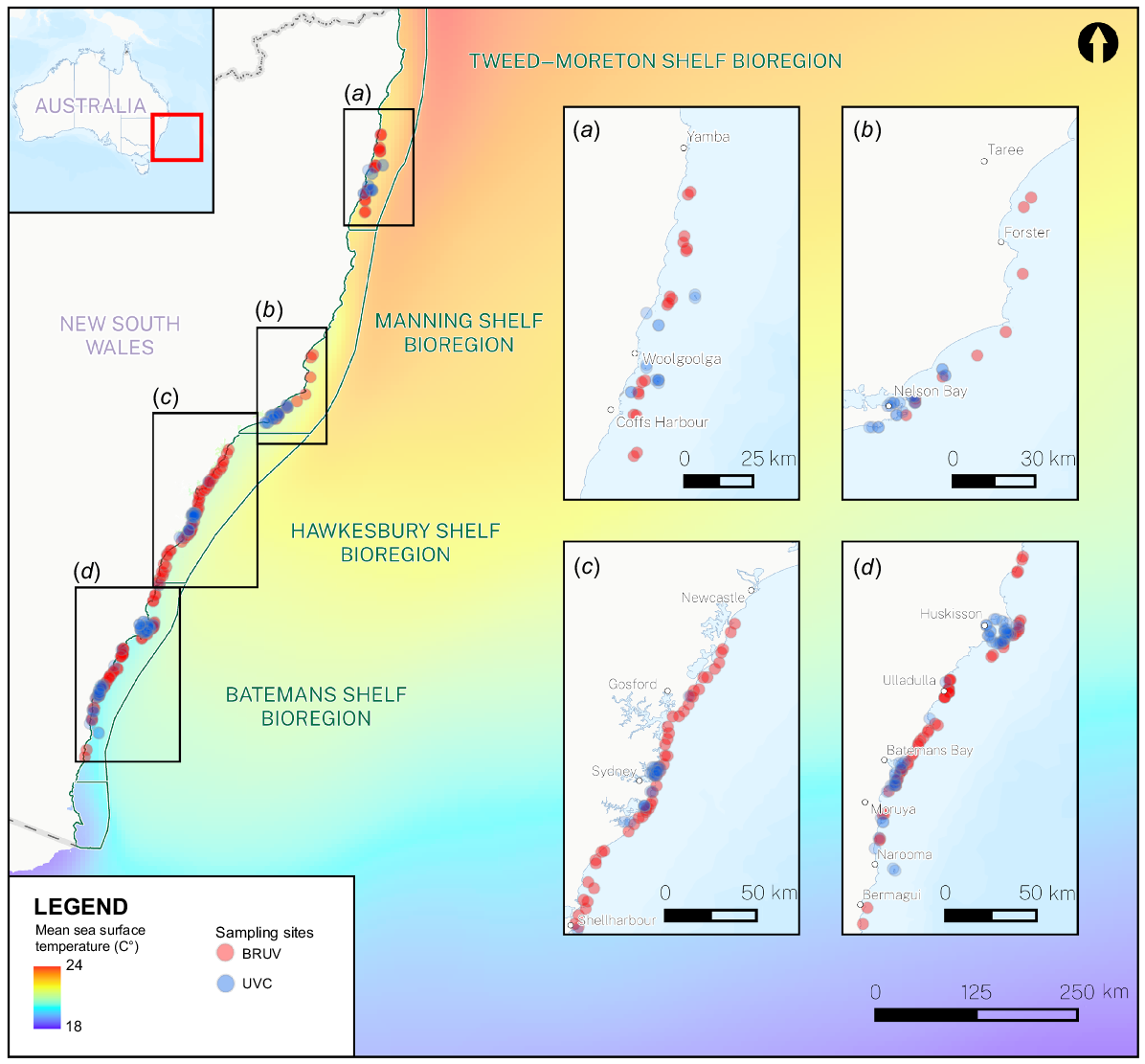
Baited remote underwater video (BRUV) sampling has been consistently conducted in the Batemans, Manning and Tweed–Moreton (hereafter referred to as Tweed) Bioregions since 2010 (Kelaher et al. 2014; Malcolm et al. 2015; Harasti et al. 2018; Knott et al. 2021) and in the Hawkesbury Bioregion since 2019 (Rees et al. 2021). Numerous sites have been sampled in each bioregion to provide representative coverage of the fish assemblages across the NSW coastline and the same sites were sampled in each year and bioregion (Fig. 1; Knott et al. 2021; Rees et al. 2021). BRUV units consisted of a single or stereo-cameras within a metal frame, with a bait placed at 1.5 m from the cameras. Each unit was deployed on the seafloor for a minimum of 30 min (Harasti et al. 2015), with a general separation of 200 m between units and baited with ~500 g of crushed pilchards (Sardinops sagax). A field of view of 5-m distance from the camera was either estimated visually (i.e. with single-camera BRUV units) or through stereo-camera measurement (i.e. with stereo-camera BRUV units) and the maximum number of individuals of a particular species (including A. viridis) encountered in this field of view at any point during the deployment was recorded as the MaxN metric for that species (Ellis and DeMartini 1995; Cappo et al. 2001, 2003, 2004). Although BRUV sampling uses bait to attract piscivores and scavenging fishes to the camera and A. viridis is a benthic invertivore, relative abundance estimates for the species are likely to still be robust and informative because (i) numerous fish swim in towards the BRUV to investigate the structure and the feeding commotion (regardless of their feeding guilds, including A. viridis), (ii) numerous fish haphazardly pass by the cameras and, hence, are recorded (Harvey et al. 2007), and (iii) there is a high level of replication (i.e. ~200 BRUV samples per year; see Supplementary Table S1 for the number of sites per bioregion per year).
Underwater visual census sampling has been conducted as part of the ATRC in Jervis Bay since 1996 and across the southern Batemans Bioregion since 2005, and RLS in Jervis Bay (Batemans Bioregion; in March annually), Sydney (Hawkesbury Bioregion; generally January–May), Port Stephens (Manning Bioregion; generally April–May) and Coffs Harbour (Tweed Bioregion; generally April–June) since 2008. Both programs sample fish along a single or multiple 50- × 5-m transect(s) at a site (i.e. a density estimate per 250-m2 block; see Stuart-Smith et al. 2017 and https://reeflifesurvey.com/methods/; generally between depths of 5–10 m). ATRC sampling has been conducted over differing numbers of years within the Batemans Bioregion (annually in the early periods and then less frequently) but at many sites within the bioregion (between 30 and 40 sites; Jervis Bay in May, Batemans Bay generally in November, and Narooma generally in November), whereas RLS has been conducted annually and generally at fewer sites (between 12 and 20) in each of the four bioregions. To produce a suitable temporal series, only data from sites with >6 years of sampling were included in the analyses (see Table S2 for the number of sites per bioregion per year). The ATRC program involves research staff from the University of Tasmania (UTAS) and Fisheries NSW (see https://atrc.au/), whereas RLS is a combined citizen and scientist program (University of Tasmania), with RLS members being trained and certified (Stuart-Smith et al. 2017; see https://reeflifesurvey.com/).
Both BRUV and UVC data are presented for the four sampled NSW bioregions to assess the generality of patterns in A. viridis relative abundances among bioregions and through time. The presence of similar patterns from the two sampling techniques improves confidence that the underlying patterns are general, although any differences in the trends could also be due to the different depth strata that are targeted by the two programs (UVC targets shallower reef than does BRUV).
To evaluate whether the temporal and spatial patterns of A. viridis reflect those of the temperate wrasses more generally, abundance indices for two other common wrasse species, namely, southern Maori wrasse (Ophthalmolepis lineolata, Labridae) and crimson banded wrasse (Notolabrus gymnogenis, Labridae), were also considered. To assess temporal and spatial patterns in the abundance indices, we used generalised linear mixed modelling (GLMM) for the BRUV data (because there were fewer sampling times in this dataset) and generalised additive mixed modelling (GAMM) for the UVC data. For each species, the main factors were bioregion (fixed categorical) and year (fixed categorical for BRUV: 2010–22, 5 years of annual data; fixed continuous for UVC: 2008–23, 15 years of annual data). Because both BRUV and UVC samples were spatially clustered within sites, a random factor of ‘site’ was included in each analysis. The UVC model also included ‘year’ as a random effect so as to partition the trends into smooth and non-smooth components. The models were fitted with a Poisson (BRUV) or negative binomial distribution (UVC) and log-link functions. The residuals of each model were checked for overdispersion, outliers and heterogeneity. All GLMMs were run using the lme4 package (ver. 1.1-69, see https://cran.r-project.org/web/packages/lme4/index.html; Bates et al. 2015) and GAMMs with the gamm4 package (ver. 0.2-6, see https://CRAN.R-project.org/package=gamm4). The emmeans package (ver. 1.10.0, see https://CRAN.R-project.org/package=emmeans) was used to obtain back-transformed model estimates with 95% confidence intervals for plotting.
Results and discussion
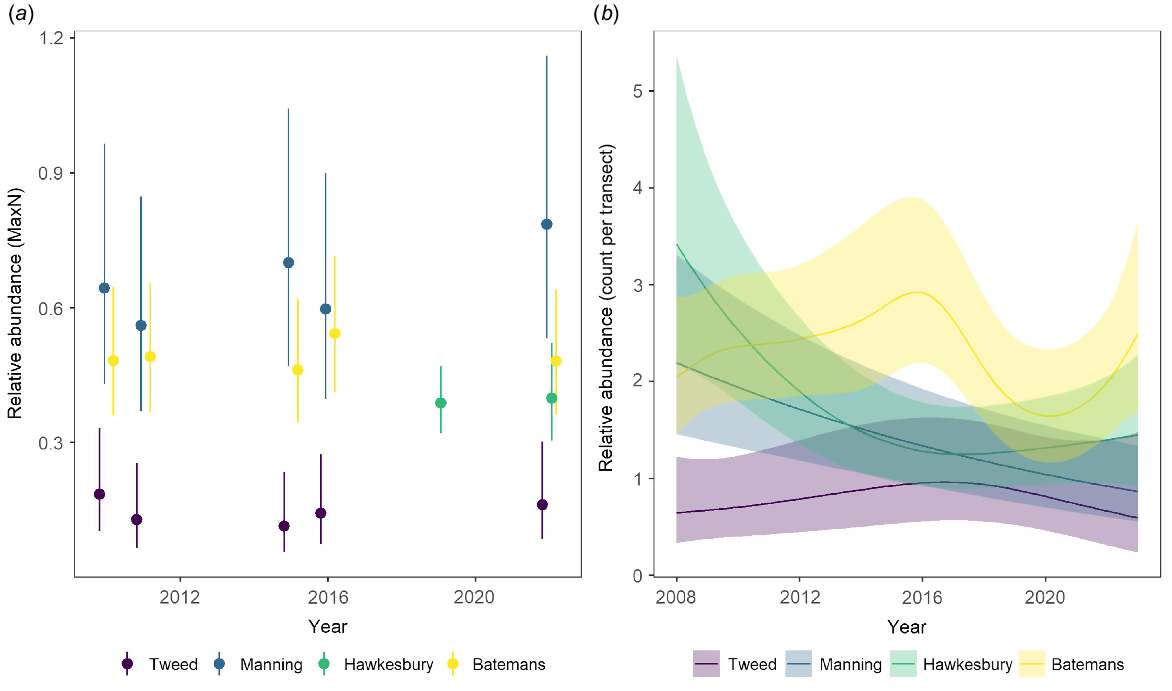
Relative abundance estimates for Achoerodus viridis indicated that populations along the NSW coastline showed a mix of stability and variability through time, depending on the bioregion and depth of reef (or technique) (Fig. 2a, b, Supplementary Fig. S1 and S2). The Tweed Bioregion consistently had the lowest abundances (Fig. 2a, b), which was unsurprising for a temperate wrasse species and the warmer waters occurring in this bioregion (Fig. 1). On deeper reefs (BRUV: 20–40 m), abundances were highest in the Manning Bioregion, followed closely by the more southern Batemans Bioregion (Fig. 2a). There was substantial variation among sites in each bioregion (indicated by the large confidence intervals), but the mean abundance estimates and the bioregional order remained consistent through time and were very stable (Fig. 2a). On shallow rocky reefs (UVC: 5–10 m), A. viridis was generally most abundant in the southern bioregion (Batemans; Fig. 2b). However, its abundances did fluctuate within this bioregion through time, increasing from 2008 and peaking in 2016, declining through to 2020 and then increasing again (Fig. 2b). In the Hawkesbury and Manning Bioregions, A. viridis declined through time by ~50%, with abundances at the end of the time series being similar to those observed in the northern Tweed Bioregion (Fig. 2b).
Abundance patterns for Achoerodus viridis (eastern blue groper) along the New South Wales coastline over the past decade. (a) Modelled relative abundance (predicted mean MaxN ± 95% confidence intervals) from baited remote underwater video (2010–22). (b) Modelled density estimates per 250-m2 transects from underwater visual census (2008–23; predicted mean ± 95% confidence intervals).
There was no evidence to suggest that A. viridis was overly abundant on any of the sampled rocky reefs along the NSW coastline compared with the other wrasse species Ophthalmolepis lineolata and Notolabrus gymnogenis (Fig. 3a, b, S1 and S2). The abundances of A. viridis were almost always lower than those of these two other wrasse species (Fig. 3a, b). Generally, abundance patterns observed for A. viridis and the other wrasse species (Fig. 3) were consistent with previous spatial and temporal estimates collected over smaller time scales along the NSW coastline (Curley et al. 2002, 2013b; Coleman et al. 2013), including the substantial variation among sites (Fig. 3a, b).
Comparison of the relative abundance patterns of Achoerodus viridis (eastern blue groper), Notolabrus gymnogenis (crimson banded wrasse) and Ophthalmolepis lineolata (southern Maori wrasse) on New South Wales coastline through time. (a) Modelled relative abundances (predicted mean MaxN ± 95% confidence intervals) from baited remote underwater video (2010–2022). (b) Modelled density estimates per 250-m2 transects from underwater visual census (2008–2023; predicted mean ± 95% confidence intervals).
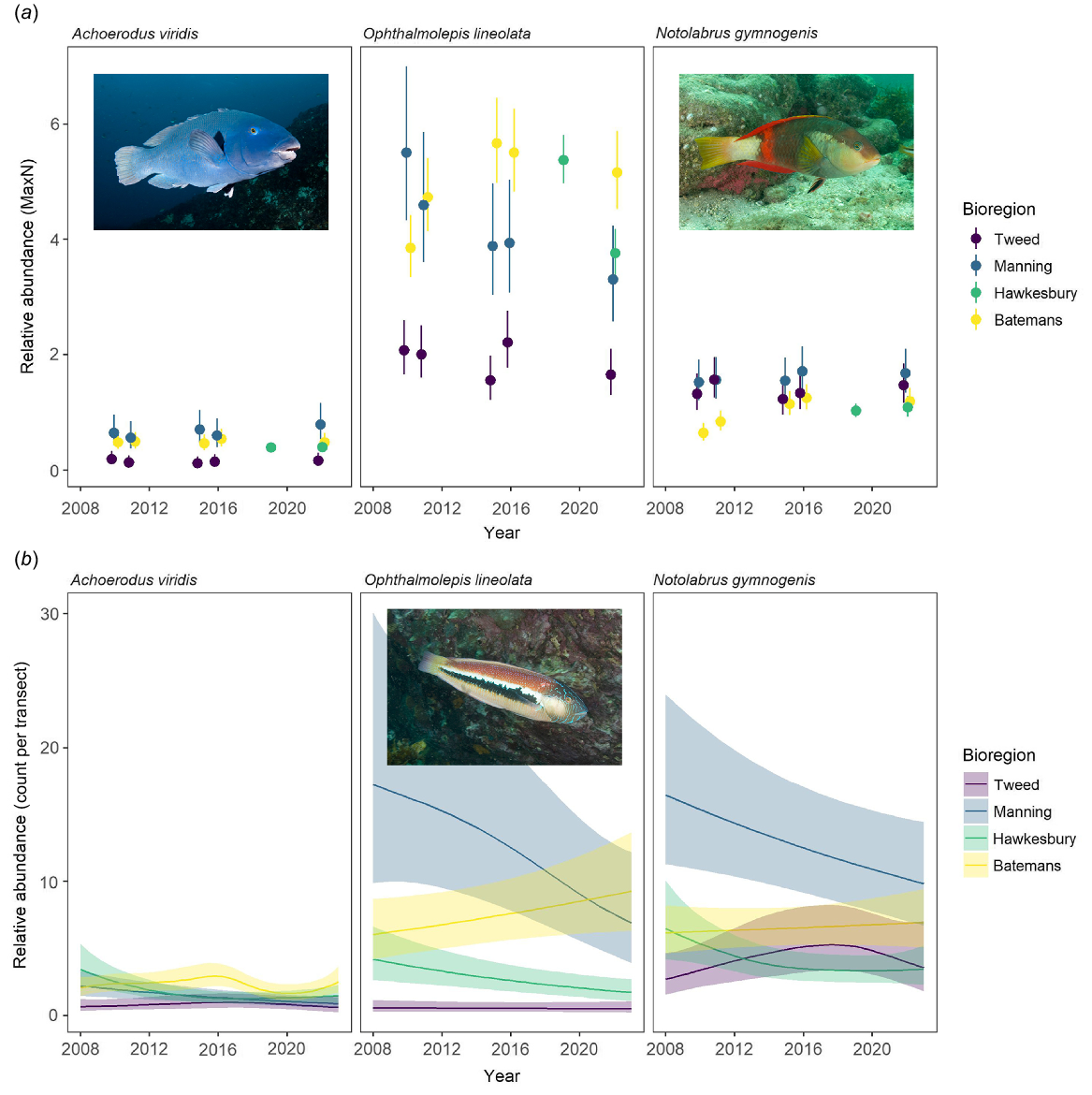
Ophthalmolepis lineolata (southern Maori wrasse), on deep reefs, had the lowest abundances in the Tweed Bioregion and the highest in the Batemans Bioregion (Fig. 3a and S1). The Hawkesbury Bioregion had abundances similar to or slightly lower than those in the Batemans Bioregion (Fig. 3a and S1). The Manning Bioregion initially had high abundances in the time series, but then they reduced by ~50%, on average, over the 12-year period (Fig. 3a and S1). Similarly, on shallow reefs, the Tweed had very few O. lineolata and the Manning initially had high abundances, but these dwindled significantly through time (>50% reduction; Fig. 3b and S2). In the Batemans Bioregion, the relative abundances showed a general increase (Fig. 3b and S2), whereas the Hawkesbury Bioregion showed much lower abundances that were not intermediate to the Batemans and Manning Bioregions, as would be expected with a latitudinal pattern (Fig. 3b and S2). Notolabrus gymnogenis (crimson banded wrasse), on deep reefs, had a slightly higher abundance in the warmer waters of the Manning and the Tweed, than in the Hawkesbury and Batemans Bioregions (Fig. 3a, S1). The abundance of N. gymnogenis in Batemans showed a trend of a slight increase through time, whereas its abundances in the Hawkesbury Bioregion appeared to be at least similar to, or slightly lower than, those in Batemans (Fig. 3a and S1). On shallow reefs, it was clearly most abundant in the Manning Bioregion (Fig. 3b, S2), followed by Batemans, whereas the Tweed and Hawkesbury Bioregions had the lowest abundances (Fig. 3b and S2). Intriguingly, the abundance in the Manning showed a significant decline through time similar to that observed with A. viridis and O. lineolata on shallow reefs (Fig. 3b and S2).
The abundances of A. viridis, O. lineolata and N. gymnogenis varied with latitude or sea temperatures, generally being least abundant in the warmest waters and most abundant in the cooler bioregions (Fig. 2 and 3; N. gymnogenis on deep reefs in the Tweed being the exception). Furthermore, there were substantial and consistent decreases with each of the species on the shallower reefs in the Manning Bioregion, and, to a certain degree, Hawkesbury Bioregion, through time (Fig. 2 and 3). These decreases correspond to generally warming waters experienced along the south-eastern coast of Australia over this period (Hobday and Pecl 2014; Davis et al. 2023). This pattern is consistent with similar latitudinal patterns and temporal changes related to water temperature and warming waters with the congeneric temperate western blue grouper (Achoerodus gouldii) (Parker et al. 2021), and temperate fish assemblages more generally along the eastern and western coasts of Australia (Edgar et al. 2023). However, on deep reefs, their abundances were generally stable, with O. lineolata being the only species to show declines (only in the Manning; Fig. 3a). The cooler water on these deeper reefs may provide more stable cooler water conditions for A. viridis populations, whereas the shallower reefs may be more affected by warmer waters. These patterns are consistent with the theory that deeper coastal reefs may offer a refuge for temperate species as oceans warm as a result of climate change, as has been identified for the temperate kelp Ecklonia radiata along this coastline (Davis et al. 2021).
Interestingly, O. lineolata and N. gymnogenis on shallow reefs in Hawkesbury Bioregion did not have abundances that were intermediate of those in the Manning and Batemans Bioregions (Fig. 3b), as would have been expected with a latitudinal gradient. The lower-than-expected abundances of these species in the Hawkesbury Bioregion suggests that high human populations of the metropolis of Greater Sydney may be having a negative effect on their abundance. Similar reduced abundance patterns related to human population densities have been found for the recreationally and commercially important Chrysophrys auratus (Australasian snapper) in the Hawkesbury Bioregion (Rees et al. 2021). Human impacts were also implicated in declines in A. gouldii abundance (western blue groper; Coulson et al. 2009; Parker et al. 2021) along the Western Australian coastline; however, in NSW, the relative abundance data for A. viridis did not suggest any such effect.
The information provided here presents new insights into spatial and temporal population variability in A. viridis, and temperate wrasses more broadly. Whereas there is evidence for significant declines in shallow waters of the Manning and Hawkesbury, population abundances on deeper inshore reefs appear to have been comparatively stable over the past 12 years. The most likely cause of the observed declines in shallow waters of the central NSW bioregions is ocean warming on this section of the coast, although additional research is required to further confirm these climate-related relationships.
The analyses presented here highlight the value of long-term monitoring such as NSW DPI’s Statewide Baited Remote Underwater Video (BRUV) program, the Australian Temperate Reef Collaboration (DPI and UTAS in NSW) and Reef Life Survey (UTAS and citizens). Systematic scientific and specialist citizen-science monitoring programs provide invaluable quantitative information on the state or condition of the marine environment and its biological diversity that would otherwise go unreported (Last et al. 2011; Young et al. 2014; Stuart-Smith et al. 2017; Edgar et al. 2023). The quantitative and systematic collection of data on a wide range of biodiversity by these programs allows an enormous array of topical questions to be answered, and species-specific information to be provided to support decision-making (Thornborough et al. 2023).
Data availability
The BRUV data that support this study are available from the corresponding author on reasonable request. The UVC data can be accessed from the Australian Ocean Data Network (AODN) site – https://portal.aodn.org.au/: IMOS – National Reef Monitoring Network Sub-Facility – Global reef fish abundance and biomass.
Declaration of funding
Funding for the NSW DPI Statewide BRUV program is funded by the NSW Government including Fisheries NSW and the NSW Marine Estate Management Strategy. Australian Temperate Reef Collaboration (ATRC) and Reef Life Survey (RLS) programs were funded by the NSW Government, including Fisheries NSW and the University of Tasmania and grant funding from the Australian Research Council (Linkage Project LP150100761) and an Ian Potter Foundation Grant.
Acknowledgements
We recognise, and thank, a large number of NSW DPIRD (Fisheries) staff for assisting with the BRUV fieldwork and video processing, including those acknowledged in Knott et al. (2021) and additionally Jason Delamont, Art Shultz, Martin Hing, Matt Hammond, Adrian Ferguson, Georgia Hall and Paul Brown. We acknowledge, and thank, Reef Life Survey for the usage of data collected by numerous citizens and scientists, in particular Antonia Cooper, Elizabeth Oh (UTas: RLS and ATRC) and John Turnbull, Andrew Green and Bill Barker (RLS). Data from RLS and ATRC are managed through, and were sourced from, Australia’s Integrated Marine Observing System (IMOS); IMOS is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). We also thank Andrew Green for the use of his images of Ophthalmolepis lineolata and Notolabrus gymnogenis and David Harasti for images of Achoerodus viridis; Gerard Stewart and Joe Neilson for producing the site maps with average sea-surface temperatures; Dr Thor Saunders from Fisheries NSW for his internal review of this manuscript; and for Hugh Kearns for important insights into scientific writing. The final version of the paper was improved by two anonymous reviewers.
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1-48.
| Crossref | Google Scholar |
Cappo M, Speare P, Wassenberg T, Harvey E, Rees M, Heyward A, Pitcher R (2001) The use of baited remote underwater video stations (BRUVS) to survey demersal fish stocks – How deep and meaningful? In ‘Video sensing of the size and abundance of target and non-target Fauna in Australian Fisheries: A National Workshop’, 1 January 2001, Rottnest Island, WA, Australia. (Eds ES Harvey, M Cappo) pp. 63–71. (Fisheries Research Development Corporation)
Cappo M, Harvey E, Malcolm H, Speare P (2003) Potential of video techniques to monitor diversity, abundance and size of fish in studies of marine protected areas. In ‘Aquatic protected areas: what works best and how do we know’, 1 January 2003, Cairns, Qld, Australia. (Eds JP Beumer, A Grant, DC Smith) pp. 455–464. (Australian Society for Fish Biology)
Cappo M, Speare P, De’ath G (2004) Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. Journal of Experimental Marine Biology and Ecology 302, 123-152.
| Crossref | Google Scholar |
Choat JH, Pollard D (2010) Eastern blue groper Achoerodus viridis. In ‘The IUCN Red List of Threatened Species 2010’. e.T187572A8572139. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/187572/8572139
Chung L (2024) Blue groper spearfishing sparks investigation in Sydney’s south. In The Sydney Morning Herald, 1 January 2024. Available at https://www.smh.com.au/national/nsw/blue-groper-spearfishing-sparks-investigation-in-sydney-s-south-20240101-p5euha.html
Coleman MA, Palmer-Brodie A, Kelaher BP (2013) Conservation benefits of a network of marine reserves and partially protected areas. Biological Conservation 167, 257-264.
| Crossref | Google Scholar |
Coulson PG, Hesp SA, Hall NG, Potter IC (2009) The western blue groper (Achoerodus gouldii), a protogynous hermaphroditic labrid with exceptional longevity, late maturity, slow growth, and both late maturation and sex change. Fishery Bulletin 107(1), 57-75.
| Google Scholar |
Curley BG, Kingsford MJ, Gillanders BM (2002) Spatial and habitat-related patterns of temperate reef fish assemblages: implications for the design of Marine Protected Areas. Marine and Freshwater Research 53(8), 1197-1210.
| Crossref | Google Scholar |
Curley BG, Jordan AR, Figueira WF, Valenzuela VC (2013a) A review of the biology and ecology of key fishes targeted by coastal fisheries in south-east Australia: identifying critical knowledge gaps required to improve spatial management. Reviews in Fish Biology and Fisheries 23, 435-458.
| Crossref | Google Scholar |
Curley BG, Glasby TM, Curley AJ, Creese RG, Kingsford MJ (2013b) Enhanced numbers of two temperate reef fishes in a small, partial-take marine protected area related to spearfisher exclusion. Biological Conservation 167, 435-445.
| Crossref | Google Scholar |
Davis TR, Champion C, Coleman MA (2021) Climate refugia for kelp within an ocean warming hotspot revealed by stacked species distribution modelling. Marine Environmental Research 166, 105267.
| Crossref | Google Scholar | PubMed |
Davis TR, Knott NA, Champion C, Przeslawski R (2023) Impacts of climate change on densities of the urchin Centrostephanus rodgersii vary among marine regions in Eastern Australia. Diversity 15(3), 419.
| Crossref | Google Scholar |
Dengate C (2014) The distinctive blue groper loves the camera and is renowed for its Elvis impression. In Manly Daily, 8 August 2014. Available at https://www.dailytelegraph.com.au/newslocal/northern-beaches/the-distinctive-blue-groper-loves-the-camera-and-is-renowed-for-its-elvis-impression/news-story/cc0f9496c7f16a3fd90e88e6cd07e1e0
Edgar GJ, Stuart-Smith RD, Heather FJ, Barrett NS, Turak E, Sweatman H, Emslie MJ, Brock DJ, Hicks J, French B, Baker SC, Howe SA, Jordan A, Knott NA, Mooney P, Cooper AT, Oh ES, Soler GA, Mellin C, Ling SD, Dunic JC, Turnbull JW, Day PB, Larkin MF, Seroussi Y, Stuart-Smith J, Clausius E, Davis TR, Shields J, Shields D, Johnson OJ, Fuchs YH, Denis-Roy L, Jones T, Bates AE (2023) Continent-wide declines in shallow reef life over a decade of ocean warming. Nature 615(7954), 858-865.
| Crossref | Google Scholar | PubMed |
Ellis DM, DeMartini EE (1995) Evaluation of a video camera technique for indexing abundances of juvenile pink snapper, Pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Fishery Bulletin 93(1), 67-77.
| Google Scholar |
Gillanders BM (1995a) Reproductive biology of the protogynous hermaphrodite Achoerodus viridis (Labridae) from south-eastern Australia. Marine and Freshwater Research 46(7), 999-1008.
| Crossref | Google Scholar |
Gillanders BM (1995b) Feeding ecology of the temperate marine fish Achoerodus viridis (Labridae): size, seasonal and site-specific differences. Marine and Freshwater Research 46(7), 1009-1020.
| Crossref | Google Scholar |
Gillanders BM (1997) Patterns of abundance and size structure in the blue groper, Achoerodus viridis (Pisces, Labridae): evidence of links between estuaries and coastal reefs. Environmental Biology of Fishes 49(2), 153-173.
| Crossref | Google Scholar |
Harasti D, Malcolm H, Gallen C, Coleman MA, Jordan A, Knott NA (2015) Appropriate set times to represent patterns of rocky reef fishes using baited video. Journal of Experimental Marine Biology and Ecology 463, 173-180.
| Crossref | Google Scholar |
Harasti D, Williams J, Mitchell E, Lindfield S, Jordan A (2018) Increase in relative abundance and size of snapper Chrysophrys auratus within partially protected and no-take areas in a temperate marine protected area. Frontiers in Marine Science 5, 208.
| Crossref | Google Scholar |
Harvey ES, Cappo M, Butler JJ, Hall N, Kendrick GA (2007) Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Marine Ecology Progress Series 350, 245-254.
| Crossref | Google Scholar |
Hobday AJ, Pecl GT (2014) Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Reviews in Fish Biology and Fisheries 24(2), 415-425.
| Crossref | Google Scholar |
Interim Marine and Coastal Regionalisation for Australia Technical Group (1998) Interim Marine and Coastal Regionalisation for Australia: an Ecosystem-Based Classification for Marine and Coastal Environments. Version 3.3. (Environment Australia, Commonwealth Department of the Environment: Canberra, ACT, Australia)
Jones G (2009) Fifteen eastern blue gropers found with horrific spear wounds at Wattamolla in Royal National Park. In Daily Telegraph, 27 October 2009. Available at https://www.dailytelegraph.com.au/fifteen-eastern-blue-gropers-found-with-horrific-spear-wounds-at-wattamolla-in-royal-national-park-/news-story/7742b39c9443c0b33a6026020c61b77e
Kelaher BP, Coleman MA, Broad A, Rees MJ, Jordan A, Davis AR (2014) Changes in fish assemblages following the establishment of a network of no-take marine reserves and partially protected areas. PLoS ONE 9(1), e85825.
| Crossref | Google Scholar | PubMed |
Kingsford MJ, Underwood AJ, Kennelly SJ (1991) Humans as predators on rocky reefs in New South Wales, Australia. Marine Ecology Progress Series 72(1–2), 1-14.
| Crossref | Google Scholar |
Knott NA, Williams J, Harasti D, Malcolm HA, Coleman MA, Kelaher BP, Rees MJ, Schultz A, Jordan A (2021) A coherent, representative, and bioregional marine reserve network shows consistent change in rocky reef fish assemblages. Ecosphere 12(4), e03447.
| Crossref | Google Scholar |
Last PR, White WT, Gledhill DC, Hobday AJ, Brown R, Edgar GJ, Pecl G (2011) Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecology and Biogeography 20(1), 58-72.
| Crossref | Google Scholar |
Lee KA, Huveneers C, Macdonald T, Harcourt RG (2015) Size isn’t everything: movements, home range, and habitat preferences of eastern blue gropers (Achoerodus viridis) demonstrate the efficacy of a small marine reserve. Aquatic Conservation: Marine and Freshwater Ecosystems 25(2), 174-186.
| Crossref | Google Scholar |
Malcolm HA, Schultz AL, Sachs P, Johnstone N, Jordan A (2015) Decadal changes in the abundance and length of snapper (Chrysophrys auratus) in subtropical marine sanctuaries. PLoS ONE 10(6), e0127616.
| Crossref | Google Scholar | PubMed |
Murphy JJ, Ochwada-Doyle FA, Hughes JM, West LD, Miles NG, Fowler AM, Stark KE, Taylor MD (2023) Survey of recreational fishing in NSW, 2021/22 – key results. (Department of Regional NSW, NSW Government) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/1550631/NSW-Recreational-Fisheries-Monitoring-Program-Survey-of-recreational-fishing-in-NSW-202122.pdf
Newbery J (2023) The groper dilemma. In Fishing World, 16 August 2023. Available at https://fishingworld.com.au/news/environment/environment-the-groper-dilemma/
NSW Government (2019) Fisheries Management (General) Regulation 2019. In ‘NSW Legislation’. (NSW Government) Available at https://legislation.nsw.gov.au/view/html/inforce/current/sl-2019-0407#sec.234
Parker JRC, Saunders BJ, Bennett S, Harvey ES (2021) Successful establishment of range-shifting, warm-water Labridae in temperate South Western Australia. Marine Ecology Progress Series 667, 161-175.
| Crossref | Google Scholar |
Rees MJ, Knott NA, Hing ML, Hammond M, Williams J, Neilson J, Swadling DS, Jordan A (2021) Habitat and humans predict the distribution of juvenile and adult snapper (Sparidae: Chrysophrys auratus) along Australia’s most populated coastline. Estuarine, Coastal and Shelf Science 257, 107397.
| Crossref | Google Scholar |
Stuart-Smith RD, Edgar GJ, Barrett NS, Bates AE, Baker SC, Bax NJ, Becerro MA, Berkhout J, Blanchard JL, Brock DJ, Clark GF, Cooper AT, Davis TR, Day PB, Duffy JE, Holmes TH, Howe SA, Jordan A, Kininmonth S, Knott NA, Lefcheck JS, Ling SD, Parr A, Strain E, Sweatman H, Thomson R (2017) Assessing national biodiversity trends for rocky and coral reefs through the integration of citizen science and scientific monitoring programs. BioScience 67(2), 134-146.
| Crossref | Google Scholar | PubMed |
Thornborough K, Bourner J, Cadiou G, Coleman M, Crocetti S, Davis TR, Folpp HR, Glasby TM, Johnson C, Johnson DD, Jordan A, Knott NA, Pini-Fitzsimmons J, Przeslawski R, Scanes P, Wright A (2023) Environmental condition framework: marine integrated monitoring program. (NSW Marine Estate Management Strategy) Available at https://www.marine.nsw.gov.au/__data/assets/pdf_file/0007/1550761/ECF_full_report.pdf
Trembath M (2023) Spearfisherman could have been fined up to $22,000 for killing Blue Groper at Hungry Point. In St George & Sutherland Shire Leader, 1 May 2023 (updated 5 May 2023). Available at https://www.theleader.com.au/story/8165175/gentle-giant-killed-blue-groper-illegally-speared-at-hungry-point/
Warner RR, Swearer SE (1991) Social control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae). The Biological Bulletin 181(2), 199-204.
| Crossref | Google Scholar | PubMed |
West LD, Stark KE, Murphy JJ, Lyle JM, Ochwada-Doyle FA (2015) Survey of recreational fishing in New South Wales and the ACT, 2013/14. (NSW Department of Primary Industries: Sydney, NSW, Australia) Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0010/600130/West-et-al-Survey-of-rec-fishing-in-NSW-ACT-2013-14-2016_04_05.pdf
Young MAL, Foale S, Bellwood DR (2014) Impacts of recreational fishing in Australia: historical declines, self-regulation and evidence of an early warning system. Environmental Conservation 41(4), 350-356.
| Crossref | Google Scholar |
Zann LP (2000) The eastern Australian region: a dynamic tropical/temperate biotone. Marine Pollution Bulletin 41(1), 188-203.
| Crossref | Google Scholar |


