A compendium of ecological knowledge for restoration of freshwater fishes in Australia’s Murray–Darling Basin
John D. Koehn A B T , Scott M. Raymond A , Ivor Stuart A , Charles R. Todd
A B T , Scott M. Raymond A , Ivor Stuart A , Charles R. Todd  A , Stephen R. Balcombe C , Brenton P. Zampatti D R , Heleena Bamford E S , Brett A. Ingram
A , Stephen R. Balcombe C , Brenton P. Zampatti D R , Heleena Bamford E S , Brett A. Ingram  F , Christopher M. Bice D G , Kate Burndred H , Gavin Butler I , Lee Baumgartner B , Pam Clunie A , Iain Ellis J , Jamin P. Forbes B , Michael Hutchison K , Wayne M. Koster
F , Christopher M. Bice D G , Kate Burndred H , Gavin Butler I , Lee Baumgartner B , Pam Clunie A , Iain Ellis J , Jamin P. Forbes B , Michael Hutchison K , Wayne M. Koster  A , Mark Lintermans L , Jarod P. Lyon A , Martin Mallen-Cooper M , Matthew McLellan N , Luke Pearce O , Jordi Ryall A , Clayton Sharpe P , Daniel J. Stoessel
A , Mark Lintermans L , Jarod P. Lyon A , Martin Mallen-Cooper M , Matthew McLellan N , Luke Pearce O , Jordi Ryall A , Clayton Sharpe P , Daniel J. Stoessel  A , Jason D. Thiem N , Zeb Tonkin A , Anthony Townsend Q and Qifeng Ye D
A , Jason D. Thiem N , Zeb Tonkin A , Anthony Townsend Q and Qifeng Ye D
A Applied Aquatic Ecology, Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, 123 Brown Street, Heidelberg, Vic. 3084, Australia.
B Institute for Land, Water and Society, Charles Sturt University, PO Box 789, Albury, NSW 2640, Australia.
C Australian Rivers Institute, Griffith University, 170 Kessels Road, Nathan, Qld 4111, Australia.
D Inland Waters and Catchment Ecology Program, South Australian Research and Development Institute, Aquatic Sciences, PO Box 120, Henley Beach, SA 5022, Australia.
E Environmental Watering Plan Implementation, Murray–Darling Basin Authority, GPO Box 1801, Canberra, ACT 2601, Australia.
F Victorian Fisheries Authority, Private Bag 20, Alexandra, Vic. 3714, Australia.
G School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
H Land and Water Science, Department of Natural Resources, Mines and Energy, Level 1, 44 Nelson Street, Mackay, Qld 4740, Australia.
I NSW Department of Primary Industries, Fisheries, Grafton Fisheries Centre, Private Mail Bag 2, Grafton, NSW 2460, Australia.
J Murray–Darling Unit, NSW Department of Primary Industries, Fisheries, 32 Enterprise Way, Buronga, NSW 2739, Australia.
K Bribie Island Research Centre, Department of Agriculture and Fisheries, PO Box 2066, Woorim, Qld 4507, Australia.
L Centre for Applied Water Science, Institute for Applied Ecology, University of Canberra, Canberra, ACT 2601, Australia.
M Fishway Consulting Services, 8 Tudor Place, Saint Ives Chase, NSW 2075, Australia.
N NSW Department of Primary Industries, Fisheries, Narrandera Fisheries Centre, PO Box 182, Narrandera, NSW 2700 Australia.
O Aquatic Ecosystems, NSW Department of Primary Industries, Unit 5, 620 Macauley Street, Albury, NSW 2640, Australia.
P NSW Water & Wetlands Conservation Branch, National Parks and Wildlife Service, PO Box 363, Buronga, NSW 2730, Australia.
Q Murray–Darling Unit, NSW Department of Primary Industries, Fisheries, 4 Marsden Park Road, Calala, NSW 2340, Australia.
R Present address: CSIRO – Land and Water, Locked Bag 2, Glen Osmond, SA 5064, Australia.
S Present address: Murray–Darling Unit, NSW Department of Primary Industries, Fisheries, TAFE Building, K Block, New England Institute, 116 Allingham Street, Armidale, NSW 2350, Australia.
T Corresponding author. Email: john.koehn@delwp.vic.gov.au
Marine and Freshwater Research 71(11) 1391-1463 https://doi.org/10.1071/MF20127
Submitted: 28 April 2020 Accepted: 3 August 2020 Published: 9 October 2020
Journal Compilation © CSIRO 2020 Open Access CC BY
Abstract
Many freshwater fishes are imperilled globally, and there is a need for easily accessible, contemporary ecological knowledge to guide management. This compendium contains knowledge collated from over 600 publications and 27 expert workshops to support the restoration of 9 priority native freshwater fish species, representative of the range of life-history strategies and values in south-eastern Australia’s Murray–Darling Basin. To help prioritise future research investment and restoration actions, ecological knowledge and threats were assessed for each species and life stage. There is considerable new knowledge (80% of publications used were from the past 20 years), but this varied among species and life stages, with most known about adults, then egg, juvenile and larval stages (in that order). The biggest knowledge gaps concerned early life stage requirements, survival, recruitment, growth rates, condition and movements. Key threats include reduced longitudinal and lateral connectivity, altered flows, loss of refugia, reductions in both flowing (lotic) and slackwater riverine habitats, degradation of wetland habitats, alien species interactions and loss of aquatic vegetation. Examples and case studies illustrating the application of this knowledge to underpin effective restoration management are provided. This extensive ecological evidence base for multiple species is presented in a tabular format to assist a range of readers.
Keywords: Australia, environmental flows, functional traits, knowledge transfer, native freshwater fish, rehabilitation.
Introduction
Globally, freshwater biota and their ecosystems are under severe threat and in need of conservation and restoration (Dudgeon et al. 2006; Flitcroft et al. 2019). Although the threats to freshwater fishes and their habitats have been extensively documented (e.g. Cadwallader 1978; Malmqvist and Rundle 2002; Koehn and Lintermans 2012), there is often limited understanding or an unconsolidated knowledge base for the biology and ecology of many species (Cooke et al. 2012). Furthermore, natural resource management should be guided by cohesive, contemporary science (Ryder et al. 2010), but incorporating ecological knowledge into practical management strategies, and thus investment and action plans, remains a challenge. This can be due to several factors, including scientific knowledge quickly being superseded (Stoffels et al. 2018) and managers considering scientific literature time consuming to read and complex to interpret (Pullin et al. 2004). In addition, the time between undertaking research and the publication of findings can be considerable, meaning that results are often unavailable to managers within appropriate time frames or are in difficult-to-access reports. Furthermore, assessment of management interventions can require studies that span many years, which can delay the uptake of promising approaches. Much research is also confined to a single species or site, resulting in disparate knowledge sources with findings that may not be transferable system wide, or applicable to multispecies or multisite management efforts. Therefore, there is a need to consolidate the outcomes of research (Cooke et al. 2017) and to improve knowledge transfer between researchers and managers (Cvitanovic et al. 2015).
Conceptual models can be useful for synthesising and explicitly defining ecological relationships and responses, and are particularly needed for guiding water management (Likens et al. 2009; Poff and Zimmerman 2010). Internationally, effective management of riverine ecosystems and the species they support is often compromised by poor coordination of science and management efforts. There are rarely efforts to collate and compile research findings across multiple researchers for the benefit of multiple users (Counihan et al. 2018). Summarising such research outcomes into a single publication can provide a useful resource for decision makers.
Large river ecosystems are heavily affected by the cumulative and potentially synergistic effects of multiple stressors (Tockner et al. 2010), but research and monitoring programs are often designed to investigate only single stressors or impacts. Consequently, although such datasets may contribute to the overall body of knowledge, they may not be fit for purpose in terms of being comprehensive, scalable or transferable to other restoration programs. Efforts to combine such datasets and knowledge have been successful in the US (Ward et al. 2017; Counihan et al. 2018) and Europe (Catalán et al. 2019), but there have been no recent similar peer-reviewed publications that consolidate existing data or contemporary knowledge for inland fishes in Australia (but see Pusey et al. 2004) to directly assist current management.
Australia’s Murray–Darling has been listed among the world’s top 10 river systems at environmental risk (Wong et al. 2007), with the Murray–Darling Basin (MDB) being one of the most regulated river basins (Nilsson et al. 2005). Only 40–50% of the MDB’s main stem rivers remain free flowing (Liermann et al. 2012; Grill et al. 2015, 2019), and many of those have their hydrology altered to some degree by regulation or extraction. The MDB covers >1×106 km2, involves six jurisdictions and is known as ‘Australia’s food bowl’ (Koehn 2015). Its rivers and catchments are now mostly in poor ecological condition (Davies et al. 2008, 2010, 2012). Long-held concerns continue regarding the overallocation of water, flow regulation and environmental damage (Walker et al. 1995; Kingsford 2000; Lester et al. 2011). End-of-system flows are now zero for 40% of the time, compared with 1% of the time under natural flow conditions (CSIRO 2008), and extensive river reaches have been converted from lotic (flowing water) to lentic (still water) environments by weirs and reduced flows (Maheshwari et al. 1995; Mallen-Cooper and Zampatti 2018). The effects of these anthropogenic flow alterations were worsened during the Millennium Drought (1997–2010; Murphy and Timbal 2008; van Dijk et al. 2013), further affecting environmental assets (Kingsford et al. 2011). Of considerable concern are predictions that indicate climate change will exacerbate such climatic extremes, affecting not only river flows (CSIRO 2008; Fiddes and Timbal 2017), but also fishes and their habitats (Balcombe et al. 2011; Morrongiello et al. 2011). Indeed, severe drought conditions, large-scale fish kills and bushfires during late 2019 and early 2020 have further heightened these concerns (Vertessy et al. 2019; Legge et al. 2020).
However, flow alteration is only one of the many threats to MDB fishes. Of additional concern are barriers to movements (4000 major barriers in the MDB; Baumgartner et al. 2014b), interactions with alien species, habitat loss and alteration, cold-water pollution, fish kills and commercial (past) and recreational fishing, all of which have heavily affected populations (Koehn and Lintermans 2012). MDB fish populations have suffered substantial declines, with almost half the species now being listed as threatened under state or national legislation (Lintermans 2007; Table 1). Consequently, remediation of these threats has been identified as being necessary for the recovery of MDB fishes (Koehn et al. 2014b; Baumgartner et al. 2020), and this resulted in the development of a comprehensive restoration program (the Native Fish Strategy; Barrett 2004; Murray–Darling Basin Commission 2004; Koehn and Lintermans 2012; Koehn et al. 2019b) which has recently been revised to The Native Fish Recovery Strategy (Murray–Darling Basin Authority 2020). In addition, the Murray–Darling Basin Plan (Murray–Darling Basin Authority 2011) has the objective of improving flows through increased delivery of water for the environment (Hart 2016a, 2016b; Stewardson and Guarino 2018). Both restoration programs recognise the requirement for policy setting and decision making to have a strong scientific foundation and to be guided by contemporary knowledge (Murray–Darling Basin Commission 2004; Swirepik et al. 2016).

|
A key purpose of such restoration programs is to consider the ecological requirements of the fishes affected by human-induced ecosystem alterations (Cooke et al. 2012; Baumgartner et al. 2020). Water for the environment can be used to re-establish critical components of flow regimes to benefit biota (Bunn and Arthington 2002), but ideally this would be based on detailed knowledge of flow–ecology relationships (Davies et al. 2014). Such ecological knowledge is not always readily available or reported in a consistent manner that is useful to management, particularly for all species and their various life stages. This rapidly developing sphere of water management (Arthington 2012) requires a range of data sources and knowledge to guide decision making (King and Louw 1998). Considerable knowledge gaps remain regarding both fundamental and applied ecology that could hinder restoration for many MDB species (Stoffels et al. 2018; Koehn et al. 2019a), and the need for access to the most up-to-date knowledge to support restorative management for native fishes has driven the creation of this knowledge compendium.
Through this compendium, we aim to at least partially address the overall need for knowledge for nine priority MDB freshwater fish species. The species were chosen in conjunction with national (Murray–Darling Basin Authority) and state fish and water management agencies to meet their priorities and to represent both large- and small-bodied species across a range of habitats, life-history types and public values (biodiversity, conservation, cultural, recreational; Koehn et al. 2019a). We collated information from a wide range of scientific journals, reports and studies, incorporated knowledge provided by experts through workshops and organised the currently available key conceptual and empirical ecological knowledge to provide easy access. Knowledge categories were chosen to support the rebuilding of fish populations through environmental flow and complementary restoration programs. Assessments of the impacts of threats and the status of our current knowledge of the various ecological components were made to guide management priorities and future research directions and investment. This compendium will be of value to a diverse audience, including researchers, students, policy makers and water and other natural resources managers, and it contains case studies to illustrate how the compiled scientific information can be used to better inform management (Fig. 1).

|
Materials and methods
The MDB
The MDB covers 14% of Australia’s land area and is a predominantly semi-arid environment, with the Darling River (2740 km long) system in the north, largely fed by semi-monsoonal summer rainfall, and the Murray River (2530 km long) system in the south, fed by winter and spring rainfall (Fig. 2; see Mackay and Eastburn 1990; Breckwoldt et al. 2004). Hence, the MDB is often classified into southern (SMDB) and northern (NMDB) components. There are considerable spatial and temporal differences in the key ecological drivers (seasonal, climatic, geomorphological and hydrological drivers) across the MDB. The most notable differences relate to the temperature gradient from north to south (higher in the north) and the rainfall gradient from east to west (high to low, coinciding with high to low elevation; Balcombe et al. 2011). The significant environmental and ecological differences between the SMDB and NMDB are briefly summarised in Table S1, available as Supplementary material to this paper. These strong climatic gradients influence the hydrological variability throughout the systems and determine how water is managed within the MDB.
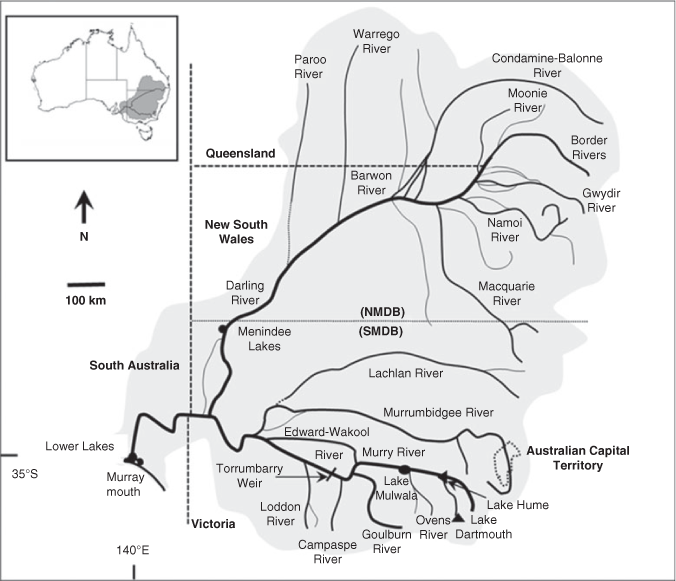
|
The SMDB includes the Murray River and tributaries and, for practical management rather than ecological reasons, the lower Darling River up to Menindee Lakes (Fig. 2). The natural hydrology of the Murray River and major tributaries (e.g. Goulburn and Murrumbidgee rivers) was characterised by interannual variability, but had consistent seasonal patterns with permanent lotic habitats (Mallen-Cooper and Zampatti 2018). These rivers are now highly regulated by headwater dams that store and re-regulate flows to support irrigated agriculture, which has two major effects on hydrology: (1) seasonal reversal of flows (high summer flows, low winter flows) immediately downstream of dams; and (2) the loss of flow in all seasons downstream of irrigation areas (Mackay and Eastburn 1990; Thoms et al. 2000; Nilsson et al. 2005).
The NMDB is considered as the entire catchment of the Barwon–Darling rivers and tributaries downstream to Menindee Lakes (Fig. 2). The hydrology and river ecology of the NMDB has two natural groupings: (1) the westward-flowing tributaries from the elevated tablelands, which are near perennial, have high gradient upland reaches and have some upland-specific species; and (2) arid rivers of the north and north-west that are intermittent rivers that may not flow half the time, have low gradients and have some specific arid species. Under natural conditions, the Barwon–Darling river system itself was near perennial, receiving flow from all westward-flowing tributaries (Mallen-Cooper and Zampatti, in press). The hydrology of the arid rivers exhibits greater intra- and interannual variability than the westward-flowing tributaries, and the NMDB in general exhibits greater variability than the SMDB. Flow is now highly affected by storage dams in the westward-flowing tributaries and by direct water abstraction and off-stream storage in the arid rivers (Breckwoldt et al. 2004).
MDB fishes and species selection
Because of the generally semi-arid nature of the MDB, its variable climate and moderate flow volumes, the native fish fauna is reasonably depauperate. Defining an exact number of native fish species for the MDB is surprisingly difficult, given ongoing taxonomic revisions and the inclusion (or not) of diadromous, estuarine and translocated native species. Until recently, only 46 obligate native freshwater species were described (Unmack 2001, 2013; Lintermans 2007). The further description of three new Galaxias species (Raadik 2014) increased this number to 49, which is likely to increase further with a number of cryptic species of Galaxiidae, Hypseleotris and Gadopsis currently being described (T. Raadik, Arthur Rylah Institute for Environmental Research, pers. comm., M. Lintermans and P. Unmack, University of Canberra, unpubl. data). Distributions vary from species that span almost the entire MDB (e.g. golden perch, Macquaria ambigua) to those with more restricted ranges that are limited to either the NMDB (e.g. Hyrtl’s tandan (catfish), Neosilurus hyrtlii) or the SMDB (e.g. southern pygmy perch, Nannoperca australis). There have been marked reductions in abundance and contractions in distribution for many species, with numerous localised extinctions. Almost half (47%) the recognised species are now considered threatened taxa (Lintermans 2007; Table 1) by the International Union for Conservation of Nature (IUCN), many having been of concern for considerable time (Ingram et al. 2000). Overall, native fish populations have been estimated to be at <10% of pre-European settlement levels (mid-1800s; Murray–Darling Basin Commission 2004, Koehn et al. 2014b; Murray–Darling Basin Authority 2020). In addition, there are 12 alien fish species present in the MDB (Lintermans 2007; M. Lintermans, University of Canberra, unpubl. data).
Although ecosystem and multispecies management is considered the ideal, most focus in the MDB pertains to individual species in specific locations, and popular, large-bodied, recreationally fished species in particular (Saddlier et al. 2013; Ebner et al. 2016; Koehn et al. 2019a). The ecological knowledge detailed in this compendium relates to nine native freshwater fishes recognised as high-priority species within the MDB (Koehn et al. 2019a), namely Murray cod (Maccullochella peelii), trout cod (Maccullochella macquariensis), golden perch, silver perch (Bidyanus bidyanus), Macquarie perch (Macquaria australasica), freshwater catfish (Tandanus tandanus), southern pygmy perch, Murray hardyhead (Craterocephalus fluviatilis) and olive perchlet (Ambassis agassizii) (Fig. 3). These species represent a range of life-history strategies (e.g. Winemiller and Rose 1992; Humphries et al. 1999; Growns 2004; Baumgartner et al. 2014a; Mallen-Cooper and Zampatti 2015), sizes (adult lengths ranging from 60 mm to >1 m), habitat preferences (rivers and wetlands), values (cultural, recreational, conservation) and management status (e.g. threatened species, those sought by recreational anglers). However, all nine species have some form of conservation listing at either the national or state level, with many listings being accompanied by local or national recovery plans (Table 1). All have been identified as likely to benefit from water for the environment (Koehn et al. 2014a) and other restoration efforts (Koehn and Lintermans 2012).
We recognise that this selection of species does not fully represent the MDB fish community in its entirety. Four of the species considered occur only in the SMDB, whereas only one occurs solely in the NMDB; the remaining four species occur in both the SMDB and NMDB. However, this selection does approximate the regional distribution of non-diadromous MDB species, with 34% occurring only in the SMDB, 20% occurring only in the NMDB and 46% occurring basin wide (M. Lintermans, University of Canberra, unpubl. data). Some key omissions that should be addressed in future reviews include the specious families of Eleotridae and Galaxiidae, including many of the most threatened small-bodied species (Lintermans et al. 2020), important highly abundant species, such as bony herring (Nematalosa erebi), and diadromous and estuarine species (Zampatti et al. 2010).
Conservation status listings
Among conservation listings there is considerable variation in composition, listing status and current relevance. The national Environment Protection and Biodiversity Conservation Act 1999 and state jurisdictional listings (Table 1) are driven by public nominations and are therefore not necessarily comprehensive or contemporary: there are species not listed that may be at higher risk of extinction than some of those listed. The list of the Australian Society for Fish Biology (https://www.asfb.org.au/committees/#ThreatnedFishesCommittee, accessed 19 August 2020), the national professional society for fish and fisheries, has been collated using a more comprehensive and intentional assessment process, and has informed the most recent IUCN assessment (2019; M. Lintermans, University of Canberra, unpubl. data), where almost all of Australia’s freshwater fish were assessed. However, this IUCN assessment only assesses the status of species within the national or international setting, without considering the status in particular regions, or of populations or subspecies. There are several species for which MDB populations are under significant threat (e.g. freshwater catfish, southern pygmy perch), despite these species being relatively secure in coastal catchments outside the MDB (Gilligan and Clunie 2019; Pearce et al. 2019). It is important to note that the effects of fish kills from blackwater, drought and the recent fires have not yet been considered in any assessments (e.g. Vertessy et al. 2019; Legge et al. 2020) and that most recovery plans have not been funded or implemented and are generally in need of revision. This highlights the need for dedicated expert processes to ensure that the conservation status of all species (not just popular ones) is regularly assessed to ensure listings remain contemporary.
Knowledge collation
Ecological and biological knowledge was initially collated with the aim of supporting the development of population models to inform environmental flow and conservation planning in the MDB for the nine selected species (e.g. see Todd and Lintermans 2015; Todd et al. 2017). However, the potential for this knowledge to support restoration efforts more widely was soon recognised by the managers and researchers involved in that process, resulting in this publication. Managers clearly identified their need for easy access to such information, preferably from one authoritative source, to help them make informed decisions. In this study, knowledge was summarised from more than 600 publications (both peer-reviewed publications and reports, >80% of which had been published in the past 20 years, i.e. from the year 2000 onwards) and from 27 species-specific expert workshops involving more than 63 individual fish ecologists and fisheries and water managers from across the MDB. Publications were identified from known existing publications (e.g. Humphries and Walker 2013), systematic database searches (Google Scholar, Web of Science) and the knowledge and publication collections held by the authors and their associates. This approach was deemed to be the most appropriate to produce a comprehensive compendium of the available literature (especially reports). On rare occasions, where studies were not available within the MDB, information was inferred from closely related taxa or areas outside the MDB. This information was clearly identified as from such sources. Although efforts were made to include all available studies during the systematic collations process, it is recognised that occasional publications or details may have been missed, or are not referred to for brevity or to avoid duplication.
Understanding ecological concepts and principles is essential to managing freshwater fishes (Lapointe et al. 2014) and our inclusive, structured, facilitated workshops were organised with the aim of developing an up-to-date conceptual understanding of the ecology of each species. Each workshop provided a forum where conclusions could be arrived at by consensus around components needed to support the requirements of both population models and management (see King and Louw 1998). The particular aim of workshop discussions and knowledge inputs on the ecological requirements was the construction of knowledge of the life stages (eggs, larvae, juveniles and adults) and population drivers (determining survival, recruitment and movements), conceptually outlined in Fig. 4, especially the factors affecting successful recruitment (King et al. 2009b).

|
Unfortunately, in studies relating to the breeding of fishes, a range of definitions is often used to describe recruitment (e.g. ‘to the postlarval life stages’, ‘to age 0+’, ‘into a fishery’). To avoid confusion, the stage to which recruitment is being referred should be clearly stated in studies because, although the presence of single life-history stages (eggs, larvae) may be useful interim outcomes measures, it is the combined survival of all life stages that determines population recovery. This paper generally considers recruitment as progression into the adult population, with the combination of the species fecundity and the number of adults contributing to the outputs from spawning (number of eggs; Fig. 4).
The use of experts within the collaborative workshop framework encouraged wide-ranging discussions regarding the ecology of species, research results and concepts, and the inclusion of information from across the large spatial scales of the MDB. This allowed the refinement of an updated conceptual ecological understanding for each species. Recent or unpublished knowledge and data, revised data relationships (e.g. age–fecundity relationships) and expert opinion (used minimally and denoted as such in Tables 4–12) has been included if it could be verified in the workshops. Although some caution should always be used regarding expert opinion or reports not supported by peer-reviewed literature (Morgan 2014), the inclusion of such information following workshop vetting met the urgent need to access recent (and in some cases unpublished) science by managers (Koehn et al. 2019a). Other information relating to non-ecological aspects of the species in this paper (e.g. phylogeny (see Humphries and Walker 2013 and chapters therein) and aquaculture production) was only included if it related to the population processes outlined above (Fig. 4).
Knowledge components of this paper
General species information
For each fish species, we a provide brief description and details of distribution and abundance. Fig. 3 contains a photograph, general distribution map and summary of biological information (life span, length, weight; for an additional summary and location details, see also Lintermans 2007; https://fishesofaustralia.net.au, accessed 19 August 2020). Conservation status and other values (recreation, hatchery production etc.) are provided in Table 1. However, distribution and conservation status are dependent on the availability of data from contemporary surveys. In some cases, these data are limited by a lack of attention to some regions or habitats (especially wetlands), inadequate effort (Lintermans and Robinson 2018; Scheele et al. 2019) or consideration of sampling method detection rates (e.g. Lyon et al. 2014a). Further illustrations, identification keys, biogeography and taxonomic relationships are available elsewhere (e.g. Cadwallader and Backhouse 1983; Merrick and Schmida 1984; McDowall 1996; Allen et al. 2002; Pusey et al. 2004; Lintermans 2007; Unmack 2013).
Species detailed ecological knowledge
For each species, detailed ecological knowledge is included with regard to prespawning (maturity, fecundity and eggs), spawning (description, season, conditions, location), post-spawning (hatching, larvae), recruitment (survival), growth, habitats, movements (eggs and larvae, juveniles, adults) and behaviour.
Assessments of knowledge status and threats
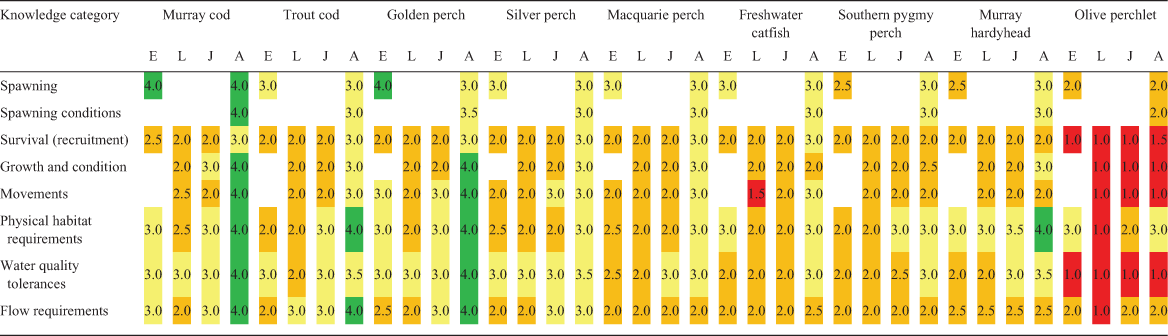
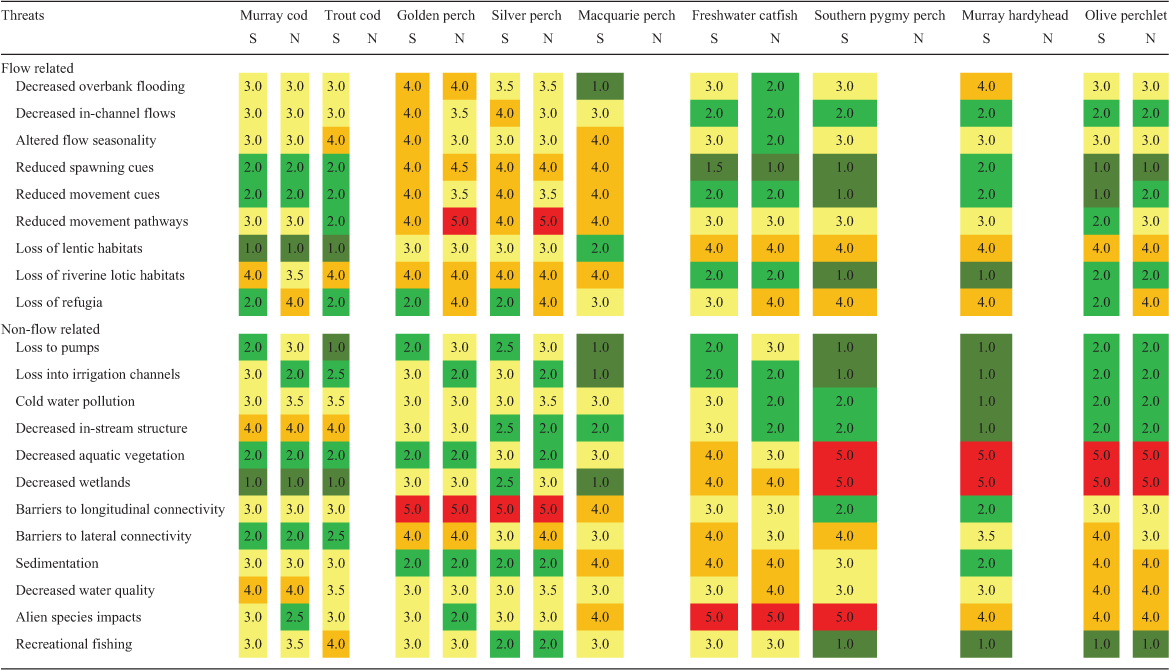
For each species and life stage (eggs, larvae, juveniles and adults), knowledge status for each category was independently assessed by the 29 authors. Assessments were only undertaken by those authors confident of their ability to do so in each case. This resulted in a difference in the mean number of assessments of SMDB (n = 18) and NMDB (n = 9) species. The available knowledge was scored as a proportion of the knowledge that the authors considered is required to adequately manage the species’ recovery from 1 to 5 as follows: 1, 0–19% of knowledge required; 2, 20–39% of knowledge required; 3, 40–59% of knowledge required; 4, 60–79% of knowledge required; 5, 80–100% of knowledge required. For each knowledge category for each species and life stage, median values were calculated from all assessment scores. Based on the findings of the workshops, assessment of the potential effects of a range of threats (flow related and non-flow related) on each species was also independently undertaken by the authors for both the SMDB and NMDB. Some threats only occur in some areas, or at particular times, and brief descriptors have been provided to indicate the scope of each threat, in addition to its likely impact (Table S2). Each threat was scored on a scale of 1–5 for each species (1, low level of impact; 5, high level of impact) with median values calculated from all assessment scores.
Results
Assessment of knowledge
Assessment scores for each knowledge category for each species and life stage, indicating the available level of knowledge and highlighting key gaps, are presented as a heat grid in Table 2. Only 7% of life stage knowledge cells had a score indicating a ‘high’ level of knowledge available (≥80% of required knowledge available; score ≥4). A high level of life stage knowledge was mostly associated with the adults of larger key recreational or threatened species (i.e. Murray cod, trout cod and golden perch). At the other end of the scale, 7% of life stage knowledge cells had median scores of ≤1.5, indicating generally poor knowledge (<20% of required knowledge available). Approximately 55% of cells had median scores of ≤2.5, indicating limited knowledge was available, whereas the remaining cells (~31%) scored 2.5–4, indicating moderate knowledge was available (Table 2). The knowledge available varied between species, with most known about the large-bodied Murray cod and the least known about the small-bodied olive perchlet. Across all species, most was known about adults, followed by egg, juvenile and larval stages (in that order; Fig. S1a). The gaps in knowledge about survival and recruitment, growth and fish condition, movements and flow requirements, especially for larvae and juveniles, require the most attention (Fig. S1b).
There was surprisingly little difference in the knowledge assessment scores for species between the NMDB and the SMDB (Fig. S2). However, we recognise the potential bias here relating to the greater number of SMBD-focused species and knowledge sources. Assessment of the literature used to collate technical knowledge of species for this paper indicated that 70% of research studies had been conducted in or addressed only SMDB species. Only 14% of studies had been solely conducted in or addressed NMDB species, with a further 16% of studies including both SMDB and NMDB species.
Assessment of threats
Assessment scores for the threats to each species are presented in a heat grid in Table 3 to indicate the threats of greatest potential impact, and there is further ranking of scores in Fig. 5. Golden perch and silver perch had the greatest number of individual potential threats, mostly related to water management, such as altered flows, the loss of lotic habitats, movement cues and pathways and barriers to longitudinal and lateral connectivity. Across all species, flow-related threats resulted in reduced movement pathways, altered flow seasonality and loss of refugia (Fig. 5). Additional flow-related impacts included loss of riverine backwater and slackwater habitats (see Humphries et al. 2006; Vietz et al. 2013) resulting from high irrigation flows and, conversely, loss of riverine lotic habitats by the creation of weir pools. Other lentic habitats, such as wetlands, are also reduced by river regulation and reductions in high flows. Key non-flow-related threats included the effects of alien species, barriers to longitudinal and lateral connectivity, decreased water quality and loss of wetlands aquatic vegetation (Fig. 5).
The loss of wetlands and aquatic vegetation most affected freshwater catfish, southern pygmy perch, Murray hardyhead and olive perchlet. These species were also considered to be at most risk from the effects of alien carp (Cyprinus carpio) (Table 3). Altered flow seasonality and loss of aquatic vegetation were the greatest threats in the SMDB (Fig. 5). The loss of refugia, the loss of early life stages to irrigation diversions and pumps and the loss of movement pathways and longitudinal connectivity were more evident in the NMDB (Fig. 5). The species subject to recreational fishing (Murray cod and golden perch) were considered vulnerable to overfishing, especially in isolated waterholes. Overall, the greatest variation in assessment scores related to the effects of loss of spawning and movement cues and the loss of lotic habitats, with generally greater variation observed in the scores for the NMDB (Fig. S3). This likely reflects the lesser certainty of knowledge and the lower number of assessment scores applicable to the NMDB. It must be noted that these assessments only apply to the species in this study and although the ranking of scores may alter slightly if all MDB species were included, post-assessment expert discussions considered them to be generally representative of threats to fishes in MDB river systems.
General species information
General information for each species (a general description and information on its distribution and abundance) is provided in the text below, and this is complemented by the detailed species ecological knowledge (referenced) presented in Tables 4–12. This general information supplements Fig. 3 and provides a summary for those readers less familiar with these species. Distributional data were summarised from existing texts (e.g. Gehrke and Harris 2000, 2001; Lintermans 2007) and recent surveys.

|
Murray cod Maccullochella peelii (Mitchell, 1838) (Fig. 3a)
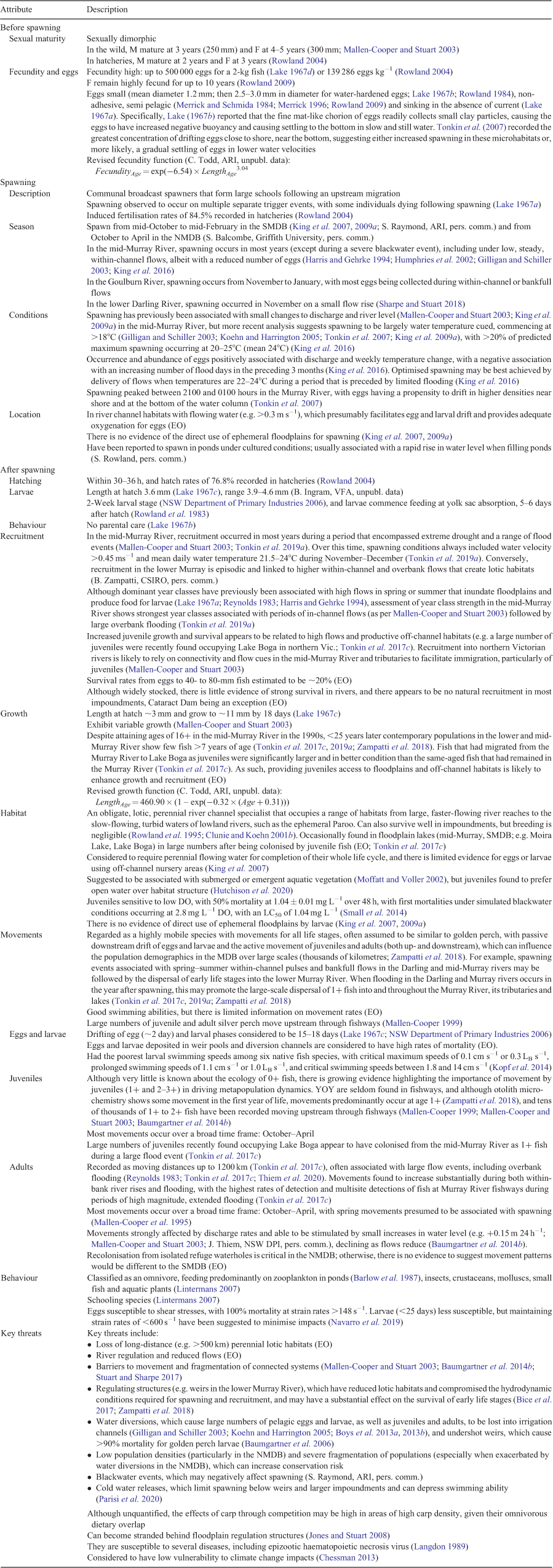
General description. The Murray cod is long-lived, large-bodied, demersal (Koehn 2009a), apex predator (Ebner 2006; Baumgartner 2007) that is considered a river channel specialist with a high affinity for in-stream woody habitat (Koehn 2009b). It lays demersal adhesive eggs on hard substrates, with the male providing parental care to eggs and larvae (Rowland 1983a, 1998b). Larvae can undergo drift after hatch (Koehn and Harrington 2006). This iconic species has high conservation, biodiversity, cultural and recreational values (Ebner et al. 2016). It is currently conservation listed nationally, but is also extremely popular with recreational fishers (Koehn and Todd 2012), which has prompted substantial investment in stock enhancement. All jurisdictions have fishery regulations that attempt to manage harvesting and to protect breeding stocks (Rowland 1985, 2005). Detailed knowledge on the ecological attributes of the Murray cod is given in Table 4.
Distribution and abundance. The Murray cod remains widely distributed throughout most of its natural range (most of the MDB), but there have been some localised extinctions, such as has been suggested for the Paroo River (Sarac et al. 2011), and considerable declines in abundance (Cadwallader and Gooley 1984; Ye et al. 2000, 2008; Rowland 2005; Mallen-Cooper and Brand 2007; Zampatti et al. 2014). Murray cod once supported a considerable commercial fishery across its southern range (Reid et al. 1997; Rowland 2005; Humphries and Winemiller 2009), but concerns about overfishing and declining catches have been reported since the early 1900s (Dannevig 1903; Dakin and Kesteven 1938; Rowland 1989, 2005). Declines in catches eventually saw all commercial fisheries closed by the early 2000s (Rowland 2005). Widely produced in hatcheries and stocked for recreational fishing (Rowland 2005; Ingram et al. 2011; Forbes et al. 2015a, 2015b; Hunt and Jones 2018), Murray cod have been translocated and stocked outside the MDB (Cadwallader and Gooley 1984), as well as farmed for human consumption (Haldane 2014). There has been a partial recovery of populations in some areas, and the species was not considered threatened in the 2019 IUCN Red List assessment (Gilligan et al. 2019), but there have been numerous large-scale fish kills in the past two decades (Koehn 2005b; King et al. 2012; Thiem et al. 2017; Vertessy et al. 2019). Although there has been no MDB-wide dedicated long-term Murray cod-specific monitoring, it is a key species considered under broad-scale monitoring, such as in the Sustainable Rivers Audit from 2004 to 2013 (Davies et al. 2008, 2010, 2012; Murray–Darling Basin Authority, unpubl. data), the unpublished Murray–Darling Basin Fish Survey from 2015 to 2020 (see Gwinn et al. 2019, 2020) and state monitoring programs. However, the stock status across the MDB has been described as ‘poorly understood and undefined’ (Ye et al. 2018). It is worth noting that all four members of the genus Maccullochella are considered threatened, with Murray cod and trout cod being present in the MDB (Lintermans et al. 2004).
Trout cod Maccullochella macquariensis (Cuvier, 1829) (Fig. 3b)
General description. The trout cod is a moderate- to large-bodied, long-lived apex predator, mainly found in the SMDB (but also the Macquarie River) that is a river channel specialist with a high affinity for in-stream woody habitat (Nicol et al. 2007; Baumgartner et al. 2014a; Koehn and Nicol 2014). Like other cod species, trout cod lay demersal adhesive eggs on hard substrates, with the male presumably providing parental care (see Table 3). Larvae can undergo drift after hatch (Koehn and Harrington 2006). Closely related to Murray cod, and only being formally described in 1972 (Berra and Weatherley 1972), trout cod has long been considered threatened (Berra 1974) and is listed as a threatened species (Table 1). Renowned for its aggression and fighting qualities as a sport fish (Berra 1974; Cadwallader 1977), the trout cod is readily captured by recreational fishers, although now largely protected from harvest. Detailed knowledge on the ecological attributes of the trout cod is given in Table 5.
Distribution and abundance. Historically, trout cod were widespread in the mid-upper reaches of the Murray, Murrumbidgee and Macquarie river systems (Douglas et al. 1994), but they are now reduced to a single truly natural population in the Murray River downstream of Lake Mulwala (Douglas et al. 1994). Over the past few decades this population has expanded downstream for several hundred kilometres (Douglas et al. 2012; Koehn et al. 2013). There was early taxonomic confusion and misidentification of cod, so some details about their past distribution remain uncertain, but an extensive decline in the range and abundance of trout cod occurred from the 1950s to the 1970s (Cadwallader and Gooley 1984; Faragher et al. 1993; Douglas et al. 1994; Trueman 2012a). Sites of significant population losses in the past 60 years include the Murray River (Cadwallader 1977), Lachlan River (late 1960s; Trueman 2012a), Lake Sambell (apparently 1970; Cadwallader and Gooley 1984), the upper Murrumbidgee River (1970s; Lintermans et al. 1988) and the lower Mitta Mitta River downstream of Lake Dartmouth (following its construction, completed in 1979; Koehn et al. 1995). Conservation stocking and translocation programs (mainly 0+ fish) have recently resulted in populations being re-established in the mid- and upper Murrumbidgee (including tributaries), upper Murray, lower Goulburn and lower Ovens rivers (Koehn et al. 2013), Seven Creeks (Goulburn catchment, Vic.), Cataract Dam (Nepean catchment, NSW; Douglas et al. 1994; Lintermans et al. 2015) and Lake Sambell (Ovens catchment, Vic.). The successful re-establishment of the Ovens River population (Bearlin et al. 2002; Lyon et al. 2012) has led to expansion into the Murray River upstream of Lake Mulwala. Stocking of on-grown hatchery-reared fish (age 2+) has had limited success (Ebner et al. 2007; Ebner and Thiem 2009).
Golden perch Macquaria ambigua (Richardson, 1845) (Fig. 3c)
General description. The golden perch is a medium- to large-bodied, long-lived top-level predator and river channel and floodplain specialist that can move large distances (Reynolds 1983; Ebner 2006; Koehn and Nicol 2016) and typically prefers in-stream structure (mostly wood) as daytime habitat (Crook et al. 2001; Koehn and Nicol 2014; Koster et al. 2020). The golden perch has pelagic eggs and larvae that undergo passive drift in flowing waters. It is keenly sought by anglers and is widely produced by hatcheries and stocked into rivers and impoundments (Rowland et al. 1983; Rowland 2013; Forbes et al. 2015b; Crook et al. 2016; Hunt and Jones 2018). Spawning, recruitment and movement responses are typically linked to elevated flows in the SMDB (Reynolds 1983; O’Connor et al. 2005; Zampatti and Leigh 2013b; Baumgartner et al. 2014b; Koster et al. 2014, 2017; Llewellyn 2014; Zampatti et al. 2018; Thiem et al. 2020), but in the NMDB reproduction can occur in the absence of connecting flows in isolated waterholes (Balcombe et al. 2006); this has also been observed in some impoundments in the SMDB (Battaglene 1991; M. Lintermans, University of Canberra, unpubl. data). Detailed knowledge on the ecological attributes of golden perch is given in Table 6.
Distribution and abundance. The golden perch remains widespread throughout most of the MDB (Lintermans 2007; Trueman 2012a), although it is likely absent above some barriers (Brumley 1987). It once supported an extensive commercial fishery in the SMDB (Cadwallader 1977) in the lower Murray River channel in South Australia (SA), but this was closed in 2002 (Ferguson and Ye 2012), and the commercial fishery is now restricted to lakes Alexandrina and Albert (the lower lakes), with annual catches of ~50–150 tonnes (Mg) in 2002–13, peaking at 150 Mg in 2005–06 (Earl 2019).
Silver perch Bidyanus bidyanus (Mitchell, 1838) (Fig. 3d)
General description. The silver perch is a long-lived, medium- to large-bodied, omnivorous, schooling river channel specialist with spawning and movements linked to rising flows (Tonkin et al. 2017a). It is a highly mobile species, with pelagic eggs and larvae that undergo passive drift in flowing waters (Rowland 2009). Nationally listed as threatened, the silver perch is cultured in hatcheries for the restaurant trade (Rowland et al. 1995; Rowland 2004, 2009) and is widely stocked (mainly in impoundments) throughout the MDB and outside its natural range for conservation and recreational purposes (Clunie and Koehn 2001c, 2001d). Detailed knowledge on the ecological attributes of the silver perch is given in Table 7.
Distribution and abundance. Once widespread in most MDB lowland river reaches, the silver perch has suffered substantial declines in abundance and range (Lintermans 2007; Trueman 2012a), especially in the mid-Darling River and NMDB, where it is now rare (Clunie and Koehn 2001d), and there is concern for its future. The mid-reaches of the Murray River support the highest relative abundances (Tonkin et al. 2017a), although even this population has declined substantially from historical levels (94% reduction at the Euston fishway over the past 50 years; Mallen-Cooper and Brand 2007). Variable numbers of fish occupy the NMDB (Warrego–Condamine, Macquarie, Namoi and Border rivers), Edward–Wakool, lower Darling, Murrumbidgee, Loddon, Campaspe and Goulburn rivers and the lower Murray River reaches (all SMDB; Tonkin et al. 2019a).
Macquarie perch Macquaria australasica Cuvier, 1830 (Fig. 3e)
General description. The Macquarie perch is a long-lived, moderate-bodied, schooling (when juvenile) or solitary (when adult) riverine species (Lintermans 2007) that has also flourished in several reservoirs where there is access to riverine habitats for spawning (e.g. Wharton 1973; Cadwallader 1981; Appleford et al. 1998; Tonkin et al. 2010; Ebner et al. 2011; Lintermans 2012). A macro- and microinvertebrate carnivore, the Macquarie perch has large demersal eggs and exhibits no parental care (Cadwallader and Rogan 1977; Lintermans 2007). It has long been recognised as a threatened species (Burbidge and Jenkins 1984), but is still subject to limited recreational fishing in Victoria and is stocked for conservation purposes (Lintermans et al. 2015). Detailed knowledge on the ecological attributes of the Macquarie perch is given in Table 8.
Distribution and abundance. The Macquarie perch is mostly endemic to the SMDB (also the Lachlan and Macquarie rivers), where it was historically widespread and abundant and supported a popular recreational fishery (Cadwallader and Rogan 1977). Since the 1950s, the Macquarie perch has undergone major declines in range (including a reduction of hundreds of kilometres in the Murray River) and abundance, and its distribution has been fragmented into small, discrete, reproductively isolated populations (Cadwallader 1981; Ingram et al. 1990, 2000; Pavlova et al. 2017). The Macquarie perch survives well in some impoundments (e.g. Lake Dartmouth, Vic.; Cotter Reservoir, ACT), but these populations fluctuate (Tonkin et al. 2014). Smaller populations around the ACT have been subject to extirpations (e.g. Ebner et al. 2011; Lintermans 2012, 2013b). The Macquarie perch has been translocated within and outside its natural range (e.g. to the Yarra River, Vic.; Cadwallader 1981; Lintermans 2007, 2008, 2013b; Lintermans et al. 2015). Reintroduction through translocation and stocking of hatchery-produced juveniles in an attempt to re-establish populations occurs in the Ovens (Vic.), Cotter, Molonglo (ACT) and Retreat (NSW) rivers (Lintermans 2013a; Todd and Lintermans 2015; Pearce 2013).
Freshwater catfish Tandanus tandanus Mitchell 1838 (Fig. 3f)
General description. The freshwater catfish is a medium-sized, largely benthic species that occurs in rivers, wetlands and impoundments (Lintermans 2007). A macrocarnivore, the freshwater catfish deposits large demersal, non-adhesive eggs in a nest depression constructed from pebbles and gravel, and exhibits extended parental care (Davis 1977a, 1977b; Clunie and Koehn 2001b; Lintermans 2007). The freshwater catfish can be quite territorial and aggressive, especially when guarding a nest. The MDB population of the freshwater catfish is considered as threatened in NSW, Vic. and SA, but it is a popular recreational fishing species, although fishing is now mostly limited to impoundments. Juveniles may form loose schools, but adults tend to be solitary when they are not breeding (Cadwallader and Backhouse 1983). Detailed knowledge on the ecological attributes of the freshwater catfish is given in Table 9.
Distribution and abundance. The freshwater catfish is native to the MDB and eastern coastal drainages from central NSW to northern Queensland (Clunie and Koehn 2001a; Gilligan and Clunie 2019). Previously abundant (Roberts and Sainty 1996; Copeland et al. 2003; Trueman 2012a), the freshwater catfish once supported occasional commercial fisheries (e.g. on Lake Brewster in NSW and the lower Murray River; Roberts and Sainty 1996; Ye et al. 2015) but has experienced significant declines throughout most of its MDB range over the past 60 years (Lake 1971; Reynolds 1976; Pollard et al. 1996), especially in regulated rivers. It has been suggested as being extinct in the Paroo River (Sarac et al. 2011). The remaining riverine populations that have relatively high abundances largely now occur above impoundments in the NMDB (Clunie and Koehn 2001b). The freshwater catfish is rarely abundant in unimpounded main river channels in the SMDB; most populations are found in weir pools or wetland habitats (Clunie and Koehn 2001b). Conservation concerns have resulted in a national recovery plan being produced, despite the freshwater catfish having no national conservation listing (Clunie and Koehn 2001b).
Southern pygmy perch Nannoperca australis Gunther 1861 (Fig. 3g)
General description. The southern pygmy perch is a short-lived, small-bodied, microcarnivorous wetland specialist (Kuiter and Allen 1986; Baumgartner et al. 2014a; Whiterod 2019) that also occurs in slow-flowing creeks and is commonly associated with aquatic vegetation (Humphries 1995; Koster 1997). Flooding is not required for spawning, but it may support recruitment and dispersal (Tonkin et al. 2008). Demersal non-adhesive eggs are scattered on the substrate with no parental care (Llewellyn 1974; Lintermans 2007). Detailed knowledge on the ecological attributes of the southern pygmy perch is given in Table 10.
Distribution and abundance. The southern pygmy perch is endemic to south-eastern Australia (Llewellyn 1974; Humphries 1995; Hammer 2002) and was once widely distributed in south eastern Australia (not the NMDB), but its range in the SMDB has contracted greatly (Bray and Thompson 2019), where it has been reduced to a series of fragmented and regionally threatened populations (Pearce 2014; Hammer et al. 2009; Pearce et al. 2019). The southern pygmy perch remains reasonably common in Victorian coastal areas (Lintermans 2007). Translocations to establish new populations are being considered (Whiterod 2019; S. Raymond, Arthur Rylah Institute for Environmental Research, pers. comm).
Murray hardyhead Craterocephalus fluviatilis McCulloch, 1913 (Fig. 3h)
General description. The Murray hardyhead is a small-bodied, short-lived, highly mobile, schooling ‘wetland specialist’ (Ebner et al. 2003; Hammer and Wedderburn 2008) found most frequently in freshwater to brackish, still or slow-flowing waters of off-channel habitats, including floodplain billabongs, wetlands and lakes (Ebner et al. 2003; Ellis 2005; Hammer and Wedderburn 2008). The Murray hardyhead is listed as threatened in NSW, Vic. and SA. It is a batch spawner, with eggs deposited on aquatic vegetation during a prolonged breeding season from September to March (Ellis 2005). Its diet consists predominantly of microcrustaceans, with larger individuals also consuming items such as dipteran larvae (Ellis 2006; Wedderburn et al. 2013). Detailed knowledge on the ecological attributes of the Murray hardyhead is given in Table 11.
Distribution and abundance. The Murray hardyhead is endemic to the lowland floodplains of the Murray and Murrumbidgee river systems (SMDB), and it has been recorded from Yarrawonga downstream to Lake Alexandrina. Once widespread and abundant, the Murray hardyhead has suffered serious reductions in both distribution and abundance in the past two decades, particularly during the Millennium Drought (Ivantsoff and Crowley 1996; Ebner et al. 2003; Wedderburn and Hammer 2003; Lyon and Ryan 2005). Its current distribution is limited to a small number of saline wetlands on the Murray River floodplain downstream of Kerang, where its populations are highly fragmented (Lloyd and Walker 1986; Ebner et al. 2003; Wedderburn 2009; Ellis et al. 2013). The Murray hardyhead is now locally extinct from at least 17 historically documented sites (Ellis et al. 2013) and is presumed extinct in NSW (Gilligan 2005), although reintroductions are now occurring (Stoessel et al. 2019; Ellis et al. 2020). The remaining populations of Murray hardyhead are fragmented and now largely confined to discrete off-channel habitats upstream of the SA border to the mid-Murray River. These populations are predominantly disconnected from the main river channel, except during flood (Ellis et al. 2013). In the lower lakes of the Murray River, the species is patchily distributed across a broad area of connected off-channel and lake edge sites in Lake Alexandrina (Wedderburn and Hammer 2003; Wedderburn et al. 2007; Wedderburn 2009), and it is being considered for translocations (Whiterod 2019).
Olive perchlet Ambassis agassizii Steindachner 1866 (Fig. 3i)
General description. The olive perchlet is a small-bodied schooling species that inhabits freshwater pools and slow-flowing reaches in rivers, streams and wetlands (Leggett 1984; Allen and Burgess 1990; Allen 1996). The MDB of olive perchlet population is listed as threatened in NSW, SA and Vic. (considered extinct). The olive perchlet occupies littoral vegetation (Hutchison et al. 2020), often mid-water (Milton and Arthington 1985), and is most active at night (Allen et al. 2002; D. Moffatt (Department of Environment and Science, Qld), pers. comm.). The olive perchlet is a microcarnivore that lays small, adhesive eggs on aquatic plants and rocks on the streambed (Lintermans 2007; Llewellyn 2008). Detailed knowledge on the ecological attributes of the olive perchlet is given in Table 12.
Distribution and abundance. Historically widespread throughout the MDB north of the Murray River, the range of the olive perchlet has contracted to small, patchy populations (Lintermans 2007; Llewellyn 2008). The Border Rivers catchments (NMDB; especially the mid-upper Condamine catchment) remain strongholds, where the olive perchlet are more abundant in tributary streams and floodplain wetlands than in the main river channel (Hutchison et al. 2008; Norris et al. 2015). The olive perchlet also occurs in coastal drainages from northern NSW to north Queensland (Lintermans 2007; McNeil et al. 2008).
Detailed species ecological knowledge
Significant new ecological knowledge has become available over the past two decades, with >80% of references cited in this paper being published since 2000. This compendium provides a ready information source for individual species, but reference to the original publications is recommended when more detailed knowledge is required. Tables 4–12 include new summary equations on growth and fecundity relationships for seven of the species, derived using methodology similar to Todd and Lintermans (2015). In some cases, we found data and information from studies on the same species to vary, particularly across different regions. Such differing information was included, but it is strongly recommended that the original publications are accessed for more complete interpretation for a particular region or species. Tables 4–12 also include key knowledge gaps and potential threats. Because drought and climate change impacts may exacerbate many other threats (especially those that are water related), susceptibility assessments for most species have also been included (from Crook et al. 2010 and Chessman 2013). Key messages for restoration for each species are provided in the ‘Key knowledge gaps and messages for restoration for each species’ section in the Supplementary material and Koehn et al. (2020).
Case studies illustrating the relevance of new knowledge to management
To illustrate the significance and relevance of using this contemporary information, case study examples of applications of this new knowledge to future management are provided below.
Cast study 1: age of silver perch
Despite being long-lived in some impoundments (up to 27 years), in the 1990s silver perch within the Murray River, although estimated to live up to 17 years, rarely exceeded 11 years (Mallen-Cooper and Stuart 2003, fig. 3). Contemporary sampling indicates that silver perch now rarely exceed 7 years (Tonkin et al. 2019a). If this is a true reflection of the age structure of the population, this finding highlights a need for flow sequencing to promote more regular spawning and recruitment than previously thought for silver perch. These spatially and temporally differing age estimates highlight the need for appropriate and contemporary data to be used in modelling and population estimates, and for management.
Cast study 2: spawning and salinity tolerances of Murray hardyhead
Increased knowledge of the spawning biology and salinity tolerances of the early life stages (i.e. eggs, larvae and juveniles) of Murray hardyhead (Fig. 6) provides opportunities to improve management at saline sites where the species is present or reintroduced (Ellis et al. 2020). Water delivery can (and is) now be targeted towards attaining suitable salinities at critical times for the benefit of spawning, egg development and larval and juvenile survival, and to the detriment of alien competitors and predators.
|
Case study 3: growth differences
Regional differences in growth rates of golden perch, although originally indicated for three MDB rivers (Mallen-Cooper and Stuart 2003), have now been demonstrated across the species’ geographic range (Fig. 7; Wright et al. 2020) and do not follow a north–south (latitudinal) divide, but differences are likely a result of variable habitats and associated productivity within regions. Variability in growth rates also occurs across temporal scales and for other widespread species, such as Murray cod (Rowland 1989; G. Butler (NSW Department of Primary Industries, Fisheries), unpubl. data). Because these biological parameters (and perhaps other variables, such as fecundity) control important population processes (Fig. 4), we advocate using river-specific data where possible (e.g. to develop population models; Todd et al. 2017).

|
Case study 4: recruitment variability
Recruitment rates of golden perch are highly variable spatially and temporally. Recruitment in the less unregulated productive rivers and floodplain lakes of the NMDB appears more frequent than in many rivers of the highly regulated SMDB (Sharpe 2011; Zampatti et al. 2019; Stuart and Sharpe 2020). In addition, within both regions, the natal origin of recruits in given localities may be temporally variable. Individuals spawned in certain rivers can substantially augment populations in other rivers, and the contribution of different recruitment sources varies as a function of hydrology, nursery habitats and connectivity (Zampatti et al. 2018; Stuart and Sharpe 2020). For golden perch populations, this knowledge reinforces the need for management over broad spatial scales and consideration of inter-regional movements.
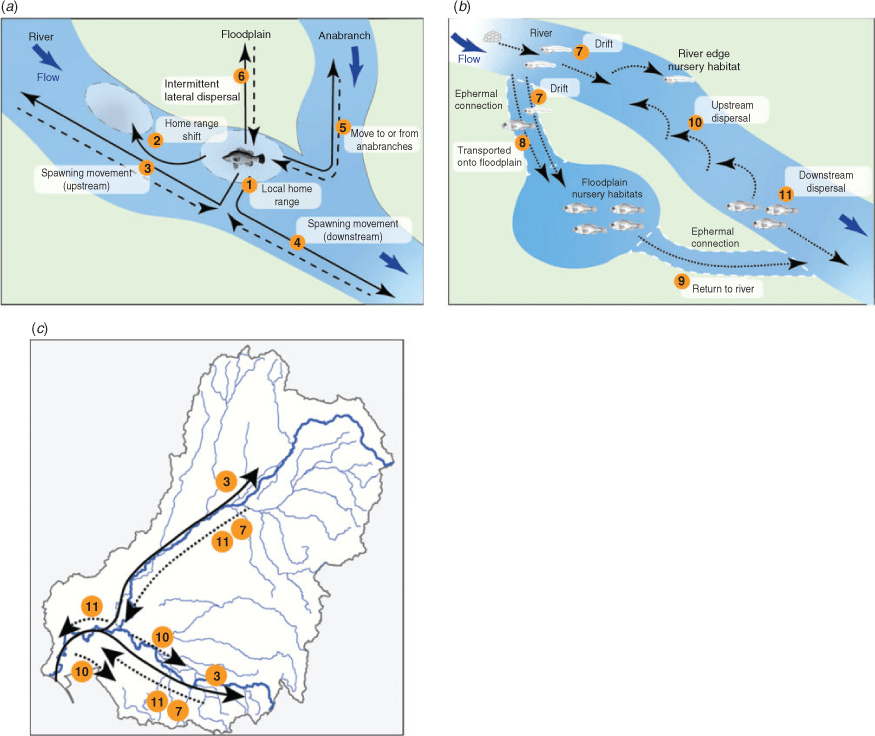
Case study 5: movements of golden perch
Imperfect knowledge on the spatial ecology of freshwater fishes often hinders their management (Cooke et al. 2016). The recent increase in knowledge about the complex movements of golden perch has been brought together in this paper (Table 6) to provide an updated conceptual movement model (from Koehn and Crook 2013; Koster and Crook 2017) for adults and juveniles (Fig. 8a), as well as eggs and larvae (Fig 8b), with application of the larger-scale movements to golden perch metapopulations across the MDB (Fig. 8c). The flow-related ecology and population dynamics of golden perch operate over greater spatial scales than previously thought (Zampatti et al. 2018, 2019; Stuart and Sharpe 2020), and understanding these dynamics, including the variety of movement types exhibited by different life stages, is essential to improving fish passage science (White et al. 2011; O’Connor et al. 2015), managing river connectivity issues and maintaining population structure for this wide-ranging species (Silva et al. 2018). This knowledge is increasingly important as water management planning progresses from site to connected catchment, and to basin scales (Stewardson and Guarino 2018). Importantly, these movements indicate that there are significant population interactions between the Darling River and the heavily regulated Murray River, including inputs of recruitment pulses (Zampatti et al. 2018, 2019; Stuart and Sharpe 2020).
Case study 6: different movement strategies
Although the golden perch example above highlights the progress made in our understanding of the movements for this species over time, similar details are not available for all species (Tables 4–12). There are also other aspects of movement that need to be considered: seasonal and diel patterns of different life stages; intraspecific differences for lakes v. river populations; intra- v. intergenerational movements; lateral movements to and from river floodplains and side channel habitats; and the variation in movement among individuals within populations (e.g. scale, extremes and averages, proportions of the population moving). We provide some examples of these types of movements below.
Macquarie perch in reservoirs have unique requirements compared with riverine populations. Fish in impoundments must leave the reservoir to spawn in inflowing streams (Cadwallader and Rogan 1977; Douglas 2002; Tonkin et al. 2010; Lintermans 2012), and such movements are restricted to the spawning season (October–December). Movements within impoundments can be wide ranging and occur throughout the year (Thiem et al. 2013). Riverine populations may or may not demonstrate spawning migrations (Koster et al. 2014, 2017; Kearns et al. 2015), perhaps depending on whether their requirements can be met near their home location. Movements to spawning habitats can be limited by instream barriers unless fish passage or inundation of such barriers occurs (Tonkin et al. 2010; Broadhurst et al. 2013; Lintermans 2012, 2013b).
Freshwater catfish exhibit variable movement patterns across their range. Although recorded moving widely from rivers onto floodplain wetlands, and between refugial waterholes, most individuals are predominantly sedentary and nocturnal (Koster et al. 2015; Burndred et al. 2018; Carpenter-Bundhoo et al. 2020a, 2020b). Most larger-scale movements are associated with flow events, particularly the first post-winter flows (e.g. Marshall et al. 2016; Burndred et al. 2018), which do not appear to be consistently related to spawning.
Murray cod move both upstream and downstream, with greatest movements prior to spawning and at night (Koehn et al. 2009). Downstream larval drift is an efficient form of dispersal and is affected by rates of flow (Koehn and Harrington 2006; Koehn 2011), and larvae can be damaged passing instream (especially undershot) weirs or entrained by pumps or diversions (Baumgartner et al. 2006, 2009).
There is a paucity of movement data for most smaller species (<150-mm total length; e.g. olive perchlet, southern pygmy perch and Murray hardyhead), not just due to size-related limitations of tagging methods (but see Allan et al. 2018), but also because they generally receive less attention than larger-bodied species (Saddlier et al. 2013; Lintermans et al. 2020). However, when studies have occurred, large numbers of individuals of many small species have been found to move (Stuart and Berghuis 2002; Lyon et al. 2008). It is often assumed that movements for these species are limited to a local scale, but intergenerational movements caused by high-flow events may be critical for dispersal to, and recolonisation of, isolated habitats. Although these movements may be small compared with that of larger fishes, they should be considered relative to body size, and movements between habitats may be critical (e.g. main river channel to vegetated wetlands).
The diversity of these movement types and the prevailing threats to them (e.g. barriers and loss of connectivity, loss of flow components and alien fish species limiting the ranges of small-bodied fish) indicate that they cannot be remediated by the provision of fish passage alone. Flow cues are required and, in some cases, this may necessitate overbank flows to connect floodplain wetlands, with the appropriate timing, duration and frequency for these connections. Further, the exclusivity of some movements and their cues to individual species or life stages means that management must ensure that all critical movements can be achieved. These case studies highlight the importance of refining key movement and ecological concepts as new knowledge becomes available and using this to maximise restoration outcomes.
Discussion
This compendium of contemporary ecological knowledge integrates scientific outputs, 80% of which were published since 2000, into one peer-reviewed paper. It synthesises our conceptual ecological understanding of nine Australian freshwater fish species to inform improved natural resource management capability and decision making. This specific ecological knowledge can be readily used in conjunction with broader ecological concepts, such as habitat use, movements, reproduction and population dynamics (see Humphries and Walker 2013; Humphries et al. 2020).
Knowledge assessment and priority gaps
Overall, our assessment indicates there is wide-ranging, although incomplete, ecological knowledge across all life stages of the nine native fish species. This highlights the need for further targeted research. Not surprisingly, the knowledge gaps identified in this study correspond generally with gaps previously identified by MDB fisheries and water managers (Koehn et al. 2019a). Specifically, we found that the survival rates of life stages and recruitment to adults (population dynamics), movement (especially movement of larvae and juveniles) and factors affecting fish growth and condition require the most research attention. Further knowledge of the relationships linking these components to flow is vital to improving the outcomes of management of water for the environment, including fish population recovery (Poff and Zimmerman 2010; Davies et al. 2014; Stewart-Koster et al. 2014). We have explicitly identified these key knowledge limitations, which will help set the agenda for future research investment. Filling these gaps could be achieved by an adequately funded, coordinated research plan with clear reference to management needs (Likens et al. 2009).
Among the species studied, the greatest knowledge is available for the larger fishes that are popular for recreational fishing, despite the generally recognised need for greater consideration and research attention to smaller species (i.e. adult length <150 mm; Saddlier et al. 2013; Lintermans et al. 2020). The three small species we studied (southern pygmy perch, Murray hardyhead and olive perchlet) consistently scored poorly in our assessment of current ecological knowledge relative to the larger species. Small fishes comprise over half the MDB species and are among the most threatened, yet their perceived insignificance and the difficulty in applying many research approaches (e.g. electronic tagging; but see Allan et al. 2018) mean that they have received limited research attention. This must be redressed.
For the larger species, considerable knowledge of spawning, egg and larval survival and water quality requirements comes largely from hatcheries, with less known from the wild. This knowledge is especially needed to understand important issues such as egg and larval dispersal, including drift distances (passive or active drift), and survival in weir pools. The knowledge obtained from hatchery studies, although valuable, must be cautiously applied when managing wild fish populations. Even for well-studied and widespread species, there is a need to revisit fundamental research and generate important data for understudied aspects of their biology or regions within their range. For example, most Murray cod growth and fecundity data were sourced from hatcheries rather than from a broad geographic sample of wild fish, and the data are now fairly dated (Rowland 1998b). Such information may not reflect changes in population structures (e.g. fewer and larger individuals) or river conditions (e.g. lower river productivity from reduced flows and flooding) over time. Our understanding of Murray cod ecology in upland habitats is also limited, which raises concern regarding the transferability of our knowledge to these areas. Although there is now considerable information available about longitudinal movements for some larger species, movements of smaller species, especially between river and off-channel habitats, remain a neglected area of research (Lyon et al. 2010).
There was surprisingly little difference in our assessment of the current knowledge between the SMDB and NMDB, despite the disparity in the number of studies in each region. However, it was generally agreed that additional NMDB studies are required, with a consensus that knowledge cannot always simply be transferred across such broad regions. There are important regional ecological differences between the SMDB and NMDB (e.g. the greater importance of refuge pools in the NMDB, Table S1; and differences in growth rates, Fig. 8). These differences are more likely to occur along a gradient than according to a simplistic north–south delineation (see Wright et al. 2020). Conversely, the workshop discussions concluded that although there were occasional, regional species-specific differences in ecology (e.g. fecundity, growth), there were also many general similarities in the population drivers across the MDB. Although considerable knowledge gaps were recognised, it was agreed that there was substantial knowledge already available to inform restoration decisions, but that more refined information would reduce the uncertainty of those decisions and maximise beneficial outcomes.
New technologies
Opportunities to address some knowledge gaps will come from new and emerging technologies. Although genetics and genomics were not extensively covered in this paper, this rapidly expanding field has many potential applications (Moore et al. 2010; Grummer et al. 2019). These approaches continue to reveal new and often cryptic species, increasing the known fish diversity (including the description of new species in the MDB), setting new conservation priorities (Adams et al. 2011, 2014; Raadik 2014) and informing stocking programs (Bearlin and Tikel 2003; Welsh et al. 2020). A revised understanding of the phylogenetics (Nock and Baverstock 2008; Nock et al. 2010) and genetic connectivity can provide insights into species’ resilience to disturbance and their capacity for adaptation to climate change effects (Beheregaray et al. 2017; Harrisson et al. 2017; Attard et al. 2018). Genetic rescue (Pavlova et al. 2017) and mapping of whole genomes (Austin et al. 2016, 2017) are additional techniques, and such tools may be used to determine connectivity rates, effective population sizes (the proportion of fish reproductively contributing to the population; Faulks et al. 2010a, 2010b, 2011; Farrington et al. 2014), population structure (Hill et al. 2015), survival rates and the effects of stocking and translocations (Rourke et al. 2010, 2011; Weeks et al. 2011). This can be used together with otolith microchemistry to determine natal origin and life-history movement patterns (Zampatti et al. 2018, 2019). Further use of passive integrated transponders (Allan et al. 2018), acoustic and radio tags (Adams et al. 2012; McKenzie et al. 2012), with accompanying remote data collection, can enhance our understanding of connectivity as a key population process over multiple spatial and temporal scales. Otoliths can also be used to determine a range of life-history traits (see Starrs et al. 2016) and responses to environmental change (e.g. flows, temperature; Izzo et al. 2016a, 2016b). The development of environmental DNA (eDNA) techniques may enable cost-effective biodiversity assessments (Bylemans et al. 2018), the detection of threatened or alien species (MacDonald et al. 2014; Janosik and Johnston 2015 Shaw et al. 2017; Hinlo et al. 2018) and measurement of breeding status (Bylemans et al. 2017) or biodiversity (Civade et al. 2016).
Remote monitoring using drones (Tyler et al. 2018) and underwater video can help our understanding of aspects such as spawning behaviour and habitat use (Butler and Rowland 2009; Ebner et al. 2014). Other evolving options are worth exploring to further understand ecological processes, such as floodplain productivity (Rees et al. 2020), including the use of bulk and compound-specific tracers, stable isotopes and fatty and amino acids to investigate productivity, food web resources, trophic requirements, fish condition and trophic ecology (e.g. Jardine et al. 2020; Twining et al. 2020). The continued development and adoption of these and other technologies will provide more holistic knowledge to inform management actions across a range of spatial and temporal scales.
Improvements to monitoring
The quantification of catch effectiveness metrics for species by different sampling methods (expressed as detection or capture probability; Bearlin et al. 2008; Ebner et al. 2008; Lyon et al. 2014a) strengthens the ability of monitoring data to estimate population abundances (Gwinn et al. 2019) and to assess the presence or recruitment status of rare species (Lintermans 2016). Currently many large-scale environmental or threatened species monitoring programs (e.g. the Sustainable Rivers Audit; Davies et al. 2008, 2010) do not adequately consider this (but see Harris and Gehrke 1997, chapter 1; Lintermans and Robinson 2018). Rare fish species or those with low detectability may be undersampled in general or non-targeted surveys (Ebner et al. 2008; Lintermans and Robinson 2018; Wedderburn 2018; Scheele et al. 2019). In addition, some habitats (e.g. wetlands) are often entirely overlooked or sampled with inappropriate techniques or effort. The need for targeted, robust monitoring (i.e. not just generic river monitoring) is now recognised and recommended to assess the status of threatened species’ populations (Scheele et al. 2019), as well as to provide data to judge the success of recovery actions (Lintermans 2013c).
The need for Indigenous knowledge
The objective of this paper was to collate published data as a basis for population models; however, a collation of Indigenous knowledge would be an important addition to this compendium and provide an additional perspective on native fish population restoration. If undertaken by traditional owners, this would increase ecological knowledge (e.g. Dargin 1976), respect cultural values (e.g. Ginns 2012; Jackson et al. 2014; Jackson and Moggridge 2019; Moggridge et al. 2019) and provide a traditional ecological management viewpoint for restoration (Trueman 2012b; Pascoe 2017). This knowledge, along with other historical information (e.g. Trueman 2012a), would also inform natural native fish population abundances and correct post-European settlement perspectives that have occurred due to based shifting baselines (lack of recognition of gradual changes from natural conditions; Humphries and Winemiller 2009).
Threat assessment and priority restoration actions
A holistic approach is required to mitigate key threats to fishes and rehabilitate populations in the MDB (Lintermans 2013a). Many common and well-understood threats were confirmed as high priorities for attention. Reduced movement pathways through barriers to longitudinal and lateral connectivity, altered flow seasonality, loss of refugia, loss of both lotic and lentic habitats, alien species, decreased water quality and losses of aquatic vegetation are examples of such key threats. Most of these threats have long been recognised (Cadwallader 1978). Some have been partially addressed (e.g. fish passage in the Murray River; Barrett and Mallen-Cooper 2006; Baumgartner et al. 2014b), but others, such as cold water pollution (Lugg and Copeland 2014; but see Michie et al. 2020), alien species control (e.g. carp, redfin, eastern gambusia; Lintermans 2013a) and undershot weirs (Baumgartner et al. 2006), remain as future challenges. The more recently recognised threats, such as loss of early fish life stages to irrigation pumps (Baumgartner et al. 2009; Boys et al. 2012, 2013a, 2013b) and infrastructure diversions (King and O’Connor 2007), appear significant but require quantification of their effect on populations.
Some threats were found to exhibit regional differences. For example, recreational fishing regulations and harvest rates are not uniform across the MDB. Combined with regional growth rate variations, these are likely to have different effects on population structures (Nicol et al. 2005). In the NMDB, loss of refugia, loss to pumps, movement pathways and barriers to longitudinal connectivity are considered principal threats, whereas altered flow seasonality and loss of aquatic vegetation are considered more prominent in the more regulated SMDB. Some historical, widespread threats, such as river desnagging and cold water releases (which can have severe effects in particular river reaches), may now have less of an impact due to the poor status of remaining populations (Lugg and Copeland 2014). Remediation of these issues provides proven opportunities to increase fish populations (Todd et al. 2005; Sherman et al. 2007; Gray et al. 2019; Lyon et al. 2019; Michie et al. 2020). Water extraction is a widespread threat that can be especially damaging to vital refuge habitats during low flows (Bond et al. 2015). Floodplain harvesting (retaining overbank flood flows using water diversion and storage structures) was considered an acute issue in the NMDB, resulting in the loss of floodplain wetland habitats, loss of connectivity to those habitats (floodplain channels), reduced flooding and downstream flows and restricted useable floodplain area (Thoms et al. 2005). Climate change poses risks to most species (Balcombe et al. 2011; Morrongiello et al. 2011; Pratchett et al. 2011) and was considered likely to exacerbate (but not supersede) the existing flow-related threats associated with river regulation and water extraction (McMahon and Finlayson 2003; Koehn et al. 2011).
Application of this knowledge to management
Although access to a comprehensive, contemporary knowledge base increases confidence in ecological decision making (Stoffels et al. 2018; Koehn et al. 2019a), it is also important to facilitate effective use of that knowledge. The detailed technical aspects of the biology, ecology and life-history processes (Tables 4–12) readily inform restoration actions for each individual species, support management decisions or can parameterise tools such as population models. Indeed, the knowledge collated for this paper has already supported published population models (Todd and Lintermans 2015; Todd et al. 2017) and been used to inform ecological outcomes from environmental flow delivery in the MDB (Koehn et al. 2014c). However, more ‘collective’ use of this knowledge can increase our conceptual understanding of how to manage species and ecosystems. In the following section, we describe case studies illustrating the use of the collated knowledge and outputs from workshop discussions to inform management. The case studies relate to: (1) species-specific management; (2) the timing of key ecological events; (3) population processes; and (4) managing flows for native fish (designed hydrograph).
Case study 1: species-specific management
The detailed knowledge highlights some striking ecological differences between closely related species that are often managed concurrently. Some management responses prefer to deal with groups of ‘similar’ species (a grouping approach) rather than addressing the different needs of a larger number of individual species. Grouping can be useful in a management context because it allows more ‘similar’ species to be assigned to a ‘guild’, where membership depends on the ecological traits selected or management actions being considered (e.g. Winemiller and Rose 1992; Humphries et al. 1999; Growns 2004; Baumgartner et al. 2014a; Mallen-Cooper and Zampatti 2015). Managing a group of ‘similar species’ often appeals under resource-constrained management conditions, where development and implementation of fewer actions can occur, rather than more actions for numerous individual species. Although this may achieve efficiencies for water delivery, we must ensure that ecological outcomes are maximised. However, individual native fish species have a range of different environmental requirements, life histories, habitat preferences, trophic positions, predator defences and metabolic rates, all of which influence the specific niche of any given species (Winemiller et al. 2015). Thus, there are inherent assumptions and risks associated with the oversimplification of species’ needs. Although there is an argument to group species into guilds for ease of management, a more plausible argument could be made to manage species individually so as to maximise benefits to them, this being especially so if they are threatened. There is no ‘one-size-fits-all’ approach to fish and flows (Poff et al. 1997; Yen et al. 2013).
Two very closely related species often considered ecologically ‘similar’, namely the Murray cod and trout cod (both being described as habitat and river channel specialists), exhibit significant ecological differences (Table 13), despite them being within the same genera and being so closely related that they can hybridise. For example, trout cod have a much smaller distribution than Murray cod, they may spawn earlier and they have lower fecundity; the two species also have different microhabitat preferences, movements, temperature tolerances and maximum lengths. These differences contributed to trout cod being at a significantly greater conservation risk than Murray cod. Transferring knowledge from the ‘better-known’ Murray cod to manage trout cod may have critical implications for recovery management decisions because there are clearly significant species-specific ecological differences that have allowed the Murray cod to persist while trout cod declined. For example, based on a nuanced understanding of habitat preferences, instream woody habitat reconstruction (i.e. resnagging; Lyon et al. 2014b, 2019) should occur further from the bank and in faster water if remediation outcomes are targeted at trout cod rather than Murray cod (Koehn and Nicol 2014). In addition, due to lesser dispersal rates for trout cod, such habitat patches should be constructed closer together and in closer proximity to existing habitat patches (Koehn et al. 2008, 2009; Koehn and Nicol 2016). Evidence from resnagging programs suggests that the dispersal and subsequent population recovery times are slower for trout cod than Murray cod (Lyon et al. 2019; Raymond et al. 2019).

|
Case study 2: timing of key ecological events
Consideration of the temporal occurrence and extent of key biological events is critical to successful ecosystem management, both for removing threats (e.g. loss to pumps and water diversions during peak periods of egg or larval drift) and supporting ecological processes (e.g. reinstating spawning or movement cues). In addition, other operational works that may affect fishes (e.g. water level lowering) should be undertaken at the appropriate times of the year. To raise awareness of these issues and to inform management scheduling, Fig. 9 provides a calendar of key biological events for the nine species considered here.
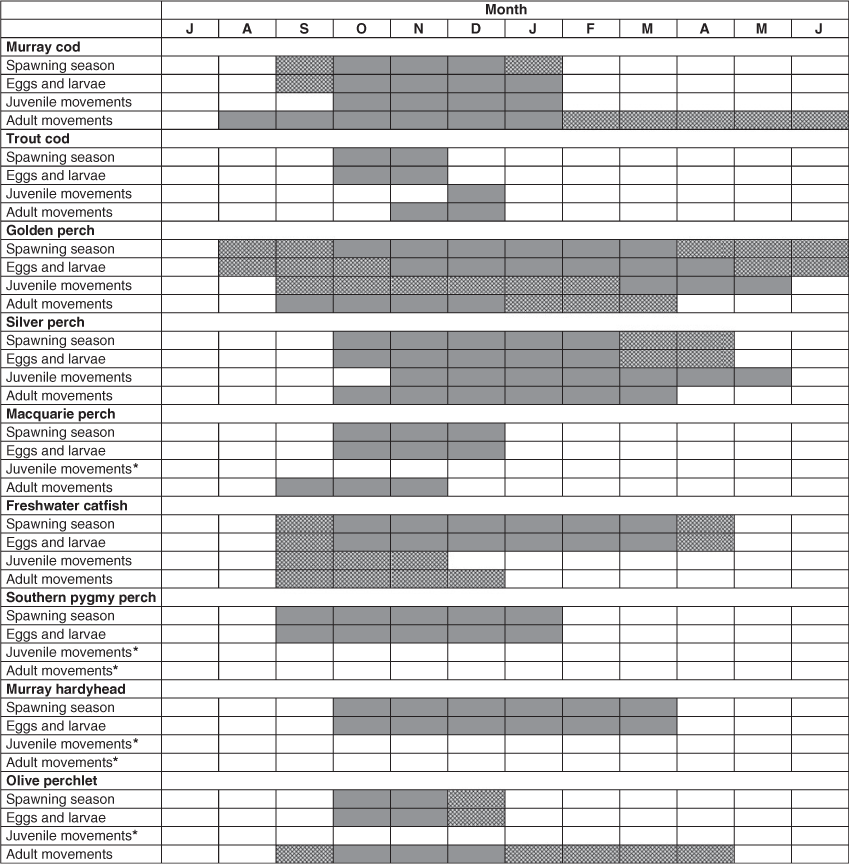
|
Case study 3: population processes
Understanding the temporal and spatial scales of ecological processes that drive population dynamics for species enables researchers and managers to better predict the likelihood and progression of success following implementation of restoration actions. To illustrate this, we provide examples of how these processes operate differently across the life-history strategies of three species from this study (southern pygmy perch, Murray cod and golden perch; Fig. 10). The different ages of maturity and fecundities of these three species affect their reproductive output and recruitment into adult populations; thus, it is necessary to account for the size of the original spawning stock, the survival of all life stages and the contribution of each life stage to population outcomes. It is important to consider the wider impact of reproductive output and recruitment across the whole range, especially for widespread species. For example, the suggested minimum spatial scale to manage highly mobile species such as golden perch is >500 km (see Fig. 8; Table 6); for Murray cod it may be <50 km (Table 4) and for southern pygmy perch (Table 10) it may be at the local site scale (e.g. within a wetland or creek reach; <1 km). However, it must be recognised that local populations often occur within a hierarchy of habitats and larger distributions, which can be enhanced by broader-scale ecological functions (such as productivity and connectivity). Therefore, monitoring needs to be undertaken at the appropriate scale for each species, because what happens in one region may provide benefits elsewhere (e.g. golden perch spawning may result in the recruitment of juveniles into populations downstream).
The production and use of conceptual models for all MDB species would enable managers to synthesise issues related to individual species’ life cycle, threats and population dynamics for recovery. We chose not to produce figures for all nine species, and indeed believe it is a useful process for managers to complete prior to considering restoration options. Regardless, the similarities and differences among the other species in this paper should be considered, especially in light of the ecological differences exemplified in Table 13 for Murray cod and trout cod. It should be noted that models in this compendium were constructed from current knowledge and species’ distributions. Hence, they may have been constrained by historical reductions in range and habitat occupancy, and should be updated as more knowledge becomes available.
Case study 4: managing flows for native fish
One approach for the effective delivery of environmental flows to improve fish outcomes is to design hydrographs that reflect aspects of the natural flow regime associated with key biological events (Poff et al. 1997; Stuart et al. 2019). For riverine species, flow recommendations aimed at achieving native fish outcomes must consider the suite of drivers that flows create within an appropriate spatial scale (i.e. a river reach; interconnected valleys for golden perch). This is in acknowledgment that aquatic biota do not respond to discharge per se, but to the conditions that it creates, such as connectivity, water velocity, turbulence, depth and the availability of key habitats (Mallen-Cooper and Zampatti 2018). Because flow discharge is the currency of water management, unfortunately this concept of hydraulic complexity and change is often missed. As flow is delivered as discharge, most recommendations are expressed in this language (ML day–1), although increasingly more detailed flow plans do use mechanistic links between river flows and some of the ecological drivers or processes they influence (e.g. cues to movement; e.g. Victorian Environmental Water Holder 2020). Some such links to the flow are well established (e.g. height to fill for wetlands), whereas others, such as areas of particular water velocity or turbulence, are not (Mallen-Cooper and Zampatti 2018). Despite the loss of lotic habitats being widely acknowledged as altering biodiversity in rivers (Walker 2006), quantitative studies that link the interaction of discharge, hydraulics and biotic processes are lacking in the MDB (Davies et al. 2014). The updated knowledge collated here is used to provide a specific case study of a conceptual hydrograph for the SMDB that includes some key components of hydraulic diversity important for three species, namely the Murray cod, a riverine nesting species, and golden perch and silver perch, which are pelagic spawners, indicating the likely benefits to these and other species (Fig. 11; Table 14). These species all require lotic habitats (identified as a key habitat loss in regulated rivers; Table 3), which could be achieved with increased flows or a combination of flow and weir lowering or removal (Bice et al. 2017; Mallen-Cooper and Zampatti 2018). Wetland specialist species would require a different emphasis for their hydrograph, which may describe hydraulic characteristics such as wetland depth, area and persistence, partial wetting and the spatial connective links to the river.

|

|
Delivering a designed hydrograph with flow components that meet each species’ life stage requirements, including spawning, survival of the various life stages (and hence recruitment) and movements, can contribute to beneficial population outcomes. The present example is focused over a reasonably short spatial scale (river reach) in the SMDB, where the common environmental flow mechanism is the delivery of water from an upland storage (which should be at a natural temperature). Other management options can protect important flow components from water extraction, such as pumping, diversion and floodplain harvesting (capture of overbank flows). This is particularly applicable in the NMDB, where there is less capacity for stored water to be delivered for the environment and greater extraction of flows from the river. ‘Designed’ hydrographs (sensu Acreman et al. 2014) can be used to inform flow management, including the use of modified irrigation flows to provide benefits to fishes. Note that this conceptual hydrograph (Fig. 11) is designed for the SMDB, and careful implementation is needed for any individual river reach, with the spatial scale, magnitude and timing of flow and hydraulic components adjusted to suit that reach and fish community.
On the basis of our review, we suggest that efforts should be made to further incorporate a range of key ecological concepts into management and recovery actions, including survival of all life stages and their recruitment through to adults (see Fig. 4), movements of all life stages and spatial management cognisant of the riverscape scales of life-history processes (e.g. Fig. 8), quantification of flow–ecology relationships that link volumetric recommendations (e.g. discharge) to the specific drivers they are trying to influence (e.g. water velocity, habitat area, connectivity; Mallen-Cooper and Zampatti 2018), the use of stochastic population models to explore outcomes from a number of possible management actions (e.g. Todd et al. 2017), the coordination of flow management over appropriate spatial and temporal scales to meet population requirements (e.g. year to year; decadal flow sequences) and the need to undertake accurate population assessments (Lyon et al. 2019) that include abundance, diversity, distribution and structure (age and size) and account for factors affecting populations, such as recreational harvest, larval mortality and stocking (see Todd and Koehn 2009).
Integrated restoration management
The multijurisdictional nature of the MDB, together with the broad distribution and movements of many fishes, necessitates integrated management through multiagency coordination of environmental flows and other actions to restore populations (Murray–Darling Basin Authority 2011; Koehn and Lintermans 2012; Stuart and Sharpe 2017; Reid et al. 2019; Baumgartner et al. 2020). Flow management occurs at spatial scales ranging from individual sites to the entire MDB, over time frames of 1–10 years (Stewardson and Guarino 2018; Koehn et al. 2019a). Collaborations between managers and researchers are most fruitful when they integrate knowledge such as species’ requirements and appropriate timing and spatial and temporal scales into restoration management actions (Gibbons et al. 2008). This is particularly so in the complex space of delivering environmental flows, and further efforts in this regard can only improve environmental outcomes.
Examples of previous successful collaborations for Australian restoration programs include the Sea to Lake Hume Lake Hume Murray Fishway Program (Baumgartner et al. 2014b), the MDB Native Fish Strategy (Koehn and Lintermans 2012, Koehn et al. 2014b), coordinated and well-designed conservation stocking regimes (Bearlin et al. 2002; Todd et al. 2004), recovery plans (Trout Cod Recovery Team 2008a, 2008b; National Murray Cod Recovery Team 2010a, 2010b; Koehn et al. 2013) and improved, multi-State stocking and size regulations for Murray cod recreational fisheries management (Rogers et al. 2010; Koehn and Todd 2012; Lintermans 2013c). Ecological modelling can be used to evaluate the likely outcomes of various combinations of management options (Koehn and Todd 2012; Todd et al. 2017), and these will be most successful if the models integrate contemporary ecological knowledge with up-to-date rainfall, flow, climate patterns and climate change predictions (Neave et al. 2015).
To help managers integrate knowledge and priorities into decisions, a stepwise framework for restoration is presented in Table 15. This is supplemented by a case study example for Murray cod management in the mid-Murray River system. In this example, the key ecological elements necessary for maintaining Murray cod populations were identified (Table 15) and an assessment was undertaken for each river reach to highlight the key limiting elements (Table 16). Although some key elements or components pose ‘threshold’ effects (e.g. low base flows, rise and fall rates, cold water pollution) that can be population limiting (e.g. by reducing recruitment; Fig. 11), once these are restored, other actions (e.g. habitat improvement) can be successfully implemented (Table 16). Some threats occur widely across river reaches (e.g. recreational fishing), whereas others, such as cold water pollution, only apply downstream of major storages. Some effects will be more easily remedied than others. For example, extensive river reaches (≥1000 km) of the lower Murray and Barwon–Darling rivers were converted from lotic to lentic environments by the imposition of weirs and reduced flows (Maheshwari et al. 1995; Walker 2006; Mallen-Cooper and Zampatti, in press) Remediation of hydraulic effects occurring within these reaches is unlikely from a generically designed hydrograph or by changes to river flows alone. A combination of integrated actions, such as weir removal or lowering in combination with flow restorations, may be required (Bice and Zampatti 2015; Bice et al. 2017; Mallen-Cooper and Zampatti 2018). A further summary perspective on broader restoration actions for MDB fishes is provided in Koehn et al. (2020)
The need for access to research results, scientific concepts and assessments is essential to support major environmental restoration programs underway within the MDB (Murray–Darling Basin Authority 2011). The knowledge presented in this paper partially meets this need, yet additional methods of communication are required to facilitate knowledge transfer to decision makers and the public (e.g. communication plans, knowledge brokers, video clips etc.). In addition to further knowledge to inform policy and management, managers require timely advice during the planning and implementation of management actions, based on robust research and monitoring. The uptake of research is greatest when projects incorporate a specific knowledge transfer component (Koehn et al. 2019a), where dialogue and collaborative relationships between researchers and managers can improve ecological outcomes (Gibbons et al. 2008; Cvitanovic et al. 2015).
Conclusion
Given the poor and declining status of native fish populations in the MDB, there is an urgent need for restoration policy, management and community actions; we cannot just manage for the status quo. We need to build resilient fish populations able to withstand and recover from the multitude of human-induced impacts and disturbances. There is a need for an integrated approach to address flow- and non-flow-related stressors, which requires ready access to contemporary knowledge. This paper offers direction and scientific support to maximise restoration outcomes by providing an accessible compendium of up-to-date ecological knowledge. A conceptual ecological understanding of the nine key fish species provides a basis from which recovery management can be planned. Assessing the potential effects of threats to these species guides the prioritisation of restoration actions. Identification of key knowledge gaps highlighted the need for continued investment in knowledge generation and dissemination. The compendium format publishes the current ecological knowledge synthesis, together with a bibliography of the associated primary literature, in a format accessible to a range of readers (e.g. students, researchers, natural resource managers and funders) that may be involved in native fish population recovery in the MDB. The species-specific approach supports nuanced management, with information provided on multiple species and life stages at a variety of spatial scales to inform the processes needed to ensure population recovery. This approach is applicable to many other fishes, regions and integrated restoration programs, both in Australia and worldwide.
Conflicts of interest
Lee J. Baumgartner is an Associate Editor of Marine and Freshwater Research. Despite this relationship, he did not at any stage have Associate Editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this Journal. Marine and Freshwater Research encourages its editors to publish in the Journal and they are kept totally separate from the decision-making process for their manuscripts. The authors declare that they have no further conflicts of interest.
Declaration of funding
This paper was funded by the Murray–Darling Basin Authority through the Native Fish Population Modelling Project and the Arthur Rylah Institute for Environmental Research. Most authors have also received support from their individual workplaces.
Acknowledgements
The authors recognise and thank all those scientists and managers who have contributed to the research, knowledge and management of freshwater fishes in the Murray–Darling Basin and across Australia. The authors particularly thank the following additional Murray–Darling Basin fish ecologists and managers for their generous contributions to this paper through the expert workshops and provision of knowledge, information or data: Craig Boys, Steven Brooks, Peter Brownhalls, Luke Carpenter-Bundhoo, Katherine Cheshire, Bernie Cockayne, Anthony Conallin, Meaghan Duncan, James Fawcett, Neal Foster, Kate Hodges, Tim Hosking, Paul Humphries, Scott Huntly, Peter Jackson, Peter Kind, Jason Lieschke, Stuart Little, Jaye Lobegeiger, Jonathan Marshall, Prue McGuffie, Dale McNeil, Norbert Menke, Christine Mercer, David Moffatt, John Morrongiello, Andrew Norris, Justin O’Connor, Andrea Prior, Lara Suitor, Paul Webb, Nick Whiterod, Sueanne Williams and Ryan Woods. Thanks also to Brian Lawrence and Stuart Little (Murray–Darling Basin Authority) and the project steering committee for support, Josh Barrow for literature collation and assistance with the manuscript, Aart Mostead, Michael Hammer and Gunther Schmida for use of their fish photographs, Ben Fanson for assistance with analysis, Andrew Geschke for help with the figures, Jeanette Birtles for editorial assistance, Max Finlayson (Editor-in-Chief, Marine and Freshwater Research) for suggesting and supporting this concept. Thanks also to Andrew Boulton, Gabriel Cornell (Arthur Rylah Institute for Environmental Research), Justin O’Connor (Arthur Rylah Institute for Environmental Research), Brendan Ebner (Commonwealth Scientific and Industrial Research Organisation), Mark Kennard (Griffith University) and the three other anonymous reviewers for their constructive comments on drafts of this paper, support staff at ARI and anyone else who has been missed but assisted with this large effort.
References
Acreman, M., Arthington, A. H., Colloff, M. J., Couch, C., Crossman, N. D., Dyer, F., Overton, I., Pollino, C. A., Stewardson, M. J., and Young, W. (2014). Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world. Frontiers in Ecology and the Environment 12, 466–473.| Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world.Crossref | GoogleScholarGoogle Scholar |
ACT Government (2017). Native species conservation plan: Murray cod (Maccullochella peelii). Available at https://www.environment.act.gov.au/__data/assets/pdf_file/0013/1124005/2017-Murray-Cod-Conservation-Plan_Access.pdf [Verified 13 August 2020].
ACT Government (2018). ACT aquatic and riparian conservation strategy and action plans. Available at https://www.environment.act.gov.au/__data/assets/pdf_file/0011/1244729/ACT-Aquatic-and-Riparian-Conservation-Strategy.pdf [Verified 13 August 2020].
Adams, M., Wedderburn, S. D., Unmack, P. J., Hammer, M. P., and Johnson, J. B. (2011). Use of congeneric assessment to reveal the linked genetic histories of two threatened fishes in the Murray–Darling Basin, Australia. Conservation Biology 25, 767–776.
| Use of congeneric assessment to reveal the linked genetic histories of two threatened fishes in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 21676026PubMed |
Adams, N. S., Beeman, J. W., and Eiler, J. H. (Eds) (2012). ‘Telemetry Techniques: A User’s Guide for Fisheries Research.’ (American Fisheries Society: Bethesda, MD, USA.)
Adams, M., Raadik, T. A., Burridge, C., and Georges, A. (2014). Global biodiversity assessment and hyper-cryptic species complexes: more than one species of elephant in the room? Systematic Biology 63, 518–533.
| Global biodiversity assessment and hyper-cryptic species complexes: more than one species of elephant in the room?Crossref | GoogleScholarGoogle Scholar | 24627185PubMed |
Allan, H., Unmack, P., Duncan, R. P., and Lintermans, M. (2018). Potential impacts of PIT tagging on a critically endangered small-bodied fish: a trial on the surrogate mountain Galaxias. Transactions of the American Fisheries Society 147, 1078–1084.
| Potential impacts of PIT tagging on a critically endangered small-bodied fish: a trial on the surrogate mountain Galaxias.Crossref | GoogleScholarGoogle Scholar |
Allen, G. R. (1996). Family Chandidae: glassfishes, chanda perches. In ‘Freshwater Fishes of South-eastern Australia’. (Ed. R. McDowall.) pp. 146–149. (Reed Books: Sydney, NSW, Australia.)
Allen, G. R., and Burgess, W. E. (1990). A review of the glassfishes (Chandidae) of Australia and New Guinea. Records of the Western Australian Museum 34, 139–206.
Allen, G. R., Midgley, S. H., and Allen, M. (2002). ‘Field Guide to the Freshwater Fishes of Australia.’ (Western Australian Museum: Perth, WA, Australia.)
Allen-Ankins, S., Stoffels, R. J., Pridmore, P. A., and Vogel, M. T. (2012). The effects of turbidity, prey density and environmental complexity on the feeding of juvenile Murray cod Maccullochella peelii. Journal of Fish Biology 80, 195–206.
| The effects of turbidity, prey density and environmental complexity on the feeding of juvenile Murray cod Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar | 22220898PubMed |
Anderson, H. K. (1915). Rescue operations on the Murrumbidgee River. Royal Zoological Society of New South Wales 1, 157–160.
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Validation of the use of thin-sectioned otoliths for determining the age and growth of golden perch, Macquaria ambigua, in the lower Murray–Darling Basin, Australia. Marine and Freshwater Research 43, 1103–1128.
| Validation of the use of thin-sectioned otoliths for determining the age and growth of golden perch, Macquaria ambigua, in the lower Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Anon, (1971). Murray cod takes the long distance title. The Fisherman 4, 24.
Appleford, P., Anderson, T. A., and Gooley, G. J. (1998). Reproductive cycle and gonadal development of Macquarie perch Macquaria australasica Cuvier (Pisces: Percichthydae), in Lake Dartmouth and tributaries of the Murray–Darling Basin, Victoria, Australia. Marine and Freshwater Research 49, 163–169.
| Reproductive cycle and gonadal development of Macquarie perch Macquaria australasica Cuvier (Pisces: Percichthydae), in Lake Dartmouth and tributaries of the Murray–Darling Basin, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Arthington, A. H. (2012). ‘Environmental Flows: Saving Rivers in the Third Millennium.’ (University of California Press: Berkeley, CA, USA.)
Arthington, A. H., McKay, R. J., Russell, D. J., and Milton, D. A. (1984). Occurrence of the introduced cichlid Oreochromis mossambicus in Queensland. Australian Journal of Marine and Freshwater Research 35, 267–272.
| Occurrence of the introduced cichlid Oreochromis mossambicus in Queensland.Crossref | GoogleScholarGoogle Scholar |
Arumugam, P. T., and Geddes, M. C. (1987). Feeding and growth of golden perch larvae and fry (Macquaria ambigua Richardson). Transactions of the Royal Society of South Australia 111, 59–66.
Attard, C. R. M., Brauer, C. J., Sandoval-Castillo, J., Faulks, L. K., Unmack, P. J., Gilligan, D. M., and Beheregaray, L. B. (2018). Ecological disturbance influences adaptive divergence despite high gene flow in golden perch (Macquaria ambigua): implications for management and resilience to climate change. Molecular Ecology 27, 196–215.
| Ecological disturbance influences adaptive divergence despite high gene flow in golden perch (Macquaria ambigua): implications for management and resilience to climate change.Crossref | GoogleScholarGoogle Scholar |
Austin, C. M., Tan, M. H., Lee, Y. P., Croft, L. J., and Gan, H. M. (2016). The complete mitogenome of the Murray cod, Maccullochella peelii (Mitchell, 1838) (Teleostei: Percichthyidae). Mitochondrial DNA – A. DNA Mapping, Sequencing, and Analysis 27, 729–730.
| The complete mitogenome of the Murray cod, Maccullochella peelii (Mitchell, 1838) (Teleostei: Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Austin, C. M., Tan, M. H., Harrisson, K. A., Lee, Y. P., Croft, L. J., Sunnucks, P., Pavlova, A., and Gan, H. M. (2017). De novo genome assembly and annotation of Australia’s largest freshwater fish, the Murray cod (Maccullochella peelii), from Illumina and Nanopore sequencing read. GigaScience 6, gix063.
| De novo genome assembly and annotation of Australia’s largest freshwater fish, the Murray cod (Maccullochella peelii), from Illumina and Nanopore sequencing read.Crossref | GoogleScholarGoogle Scholar | 28873963PubMed |
Backhouse, G., Lyon, J., and Cant, B. (2008). National recovery plan for the Murray hardyhead Craterocephalus fluviatilis. (Department of Sustainability and Environment: Melbourne, Vic., Australia.) Available at http://www.environment.gov.au/biodiversity/threatened/publications/national-recovery-plan-murray-hardyhead-craterocephalus-fluviatilis-2008 [Verified 27 July 2020].
Balcombe, S. R., Arthington, A. H., Foster, N. D., Thoms, M. C., Wilson, G. G., and Bunn, S. E. (2006). Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin. Marine and Freshwater Research 57, 619–633.
| Fish assemblages of an Australian dryland river: abundance, assemblage structure and recruitment patterns in the Warrego River, Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Bunn, S. E., Arthington, A. H., Fawcett, J. H., McKenzie-Smith, F. J., and Wright, A. (2007). Fish larvae, growth and biomass relationships in an Australian arid zone river: links between floodplains and waterholes. Freshwater Biology 52, 2385–2398.
| Fish larvae, growth and biomass relationships in an Australian arid zone river: links between floodplains and waterholes.Crossref | GoogleScholarGoogle Scholar |
Balcombe, S. R., Sheldon, F., Capon, S. J., Bond, N. R., Hadwen, W. L., Marsh, N., and Bernays, S. J. (2011). Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia. Marine and Freshwater Research 62, 1099–1114.
| Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Barlow, C. C., McLoughlin, R. J., and Bock, K. (1987). Complementary feeding habits of golden perch Macquaria ambigua (Richardson) (Percichthyidae) and silver perch Bidyanus bidyanus (Mitchell) (Teraponidae) in farm dams. Proceedings of the Linnean Society of New South Wales 109, 143–152.
Barrett, J. (2004). Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches. Ecological Management & Restoration 5, 15–23.
| Introducing the Murray–Darling Basin Native Fish Strategy and initial steps towards demonstration reaches.Crossref | GoogleScholarGoogle Scholar |
Barrett, J., and Mallen-Cooper, M. (2006). The Murray River’s ‘Sea to Hume Dam’ fish passage program: progress to date and lessons learned. Ecological Management & Restoration 7, 173–183.
| The Murray River’s ‘Sea to Hume Dam’ fish passage program: progress to date and lessons learned.Crossref | GoogleScholarGoogle Scholar |
Battaglene, S. C. (1991). The golden perch, Macquaria ambigua (Pisces: Percichthyidae) of Lake Keepit, NSW. M.Sc. Thesis, University of New South Wales, Sydney, NSW, Australia.
Baumgartner, L. J. (2007). Diet and feeding habits of predatory fishes upstream and downstream of a low-level weir. Journal of Fish Biology 70, 879–894.
| Diet and feeding habits of predatory fishes upstream and downstream of a low-level weir.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Reynoldson, N., and Gilligan, D. M. (2006). Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs. Marine and Freshwater Research 57, 187–191.
| Mortality of larval Murray cod (Maccullochella peelii peelii) and golden perch (Macquaria ambigua) associated with passage through two types of low-head weirs.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Reynoldson, N. K., Cameron, L., and Stanger, J. (2009). Effects of irrigation pumps on riverine fish. Fisheries Management and Ecology 16, 429–437.
| Effects of irrigation pumps on riverine fish.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Conallin, J., Wooden, I., Campbell, B., Gee, R., Robinson, W. A., and Mallen-Cooper, M. (2014a). Using flow guilds of freshwater fish in adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems. Fish and Fisheries 15, 410–427.
| Using flow guilds of freshwater fish in adaptive management framework to simplify environmental flow delivery for semi-arid riverine systems.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L., Zampatti, B., Jones, M., Stuart, I., and Mallen-Cooper, M. (2014b). Fish passage in the Murray–Darling Basin, Australia: not just an upstream battle. Ecological Management & Restoration 15, 28–39.
| Fish passage in the Murray–Darling Basin, Australia: not just an upstream battle.Crossref | GoogleScholarGoogle Scholar |
Baumgartner, L. J., Gell, P., Thiem, J. D., Finlayson, C. M., and Ning, N. (2020). Ten complementary measures to assist with environmental watering programs in the Murray–Darling river system, Australia. River Research and Applications 36, 645–655.
| Ten complementary measures to assist with environmental watering programs in the Murray–Darling river system, Australia.Crossref | GoogleScholarGoogle Scholar |
Bearlin, A. R., and Tikel, D. (2003). Conservation genetics of Murray–Darling Basin fish: silver perch, Murray cod and trout cod. In ‘Managing Fish Translocation and Stocking in the Murray–Darling Basin, Workshop: Statement, Recommendations and Supporting Papers’, 25–26 September 2002, Canberra, ACT, Australia. (Ed. B. Phillips.) pp. 59–82. (World Wildlife Fund: Sydney, NSW, Australia.)
Bearlin, A. R., Schreiber, E. S., Nicol, S. J., Starfield, A. M., and Todd, C. R. (2002). Identifying the weakest link: simulating adaptive management of the reintroduction of a threatened fish. Canadian Journal of Fisheries and Aquatic Sciences 59, 1709–1716.
| Identifying the weakest link: simulating adaptive management of the reintroduction of a threatened fish.Crossref | GoogleScholarGoogle Scholar |
Bearlin, A. R., Nicol, S. J., and Glenane, T. (2008). Behavioral responses of Murray cod Maccullochella peelii peelii to pulse frequency and pulse width from electric fishing machines. Transactions of the American Fisheries Society 137, 107–113.
| Behavioral responses of Murray cod Maccullochella peelii peelii to pulse frequency and pulse width from electric fishing machines.Crossref | GoogleScholarGoogle Scholar |
Beheregaray, L. B., Pfeiffer, L. V., Attard, C. R. M., Sandoval-Castillo, J., Domingos, F. M. C. B., Faulks, L. K., Gilligan, D. M., and Unmack, P. J. (2017). Genome-wide data delimits multiple climate-determined species ranges in a widespread Australian fish, the golden perch (Macquaria ambigua). Molecular Phylogenetics and Evolution 111, 65–75.
| Genome-wide data delimits multiple climate-determined species ranges in a widespread Australian fish, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 28347889PubMed |
Belton, E. J. (2015). Diet and prey selectivity of larval Murray cod Maccullochella peelii (Mitchell 1938) in an upland and lowland river. B.Sc.(Hons) Thesis, University of Canberra, Canberra, ACT, Australia.
Berra, T. M. (1974). The trout cod, Maccullochella macquariensis, a rare freshwater fish of eastern Australia. Biological Conservation 6, 53–56.
| The trout cod, Maccullochella macquariensis, a rare freshwater fish of eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Berra, T. M., and Weatherley, A. H. (1972). A systematic study of the Australian freshwater serranid fish genus Maccullochella. Copeia 1972, 53–64.
| A systematic study of the Australian freshwater serranid fish genus Maccullochella.Crossref | GoogleScholarGoogle Scholar |
Bice, C. (2010). Biological information and age structure analysis of large-bodied fish species captured during the Lake Albert trial fish-down, October 2009. Report to Primary Industries and Resources South Australia – Biosecurity, SARDI Research Report Series number 430, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Bice, C., and Ye, Q. (2007). Monitoring threatened fish communities on Hindmarsh Island, in the Lower Lakes of the River Murray, South Australia, in the summers of 2006 and 2007 with reference to baseline data from 2005. SARDI Publication number F2007/000551-2, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Bice, C. M., and Zampatti, B. P. (2015). The influence of weir pool raising on main channel hydraulics in the lower River Murray. SARDI Publication number F2015/000381-1, SARDI Research Report Series number 840, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Bice, C., Hammer, M., Wilson, P., and Zampatti, B. (2011). Fish monitoring for the ‘Drought Action Plan for South Australian Murray–Darling Basin threatened freshwater fish populations’: summary for 2010/11. SARDI Publication number F2010/000647-2, SARDI Research Report Series number 564, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Bice, C., Whiterod, N., and Zampatti, B. (2014). The Critical Fish Habitat Project: Assessment of the success of reintroduction of threatened fish species in the Coorong, Lower Lakes and Murray Mouth region 2011–2014. SARDI Publication number F2012/000348-3, SARDI Research Report Series number 792, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Bice, C. M., Gibbs, M. S., Kilsby, N. N., Mallen-Cooper, M., and Zampatti, B. P. (2017). Putting the ‘river’ back into the lower River Murray: quantifying the hydraulic impact of river regulation to guide ecological restoration. Transactions of the Royal Society of South Australia 141, 108–131.
| Putting the ‘river’ back into the lower River Murray: quantifying the hydraulic impact of river regulation to guide ecological restoration.Crossref | GoogleScholarGoogle Scholar |
Bishop, K. A., and Bell, J. D. (1978). Observations on the fish fauna below Tallowa Dam (Shoalhaven River, New South Wales) during river flow stoppages. Marine and Freshwater Research 29, 543–549.
| Observations on the fish fauna below Tallowa Dam (Shoalhaven River, New South Wales) during river flow stoppages.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Thomson, J., Reich, P., and Stein, J. (2011). Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research 62, 1043–1061.
| Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bond, N. R., Balcombe, S. R., Crook, D. A., Marshall, J. C., Menke, N., and Lobegeiger, J. S. (2015). Fish population persistence in hydrologically variable landscapes. Ecological Applications 25, 901–913.
| Fish population persistence in hydrologically variable landscapes.Crossref | GoogleScholarGoogle Scholar | 26465032PubMed |
Boys, C. A., and Thoms, M. C. (2006). A large-scale, hierarchical approach for assessing habitat associations of fish assemblages in large dryland rivers. Hydrobiologia 572, 11–31.
| A large-scale, hierarchical approach for assessing habitat associations of fish assemblages in large dryland rivers.Crossref | GoogleScholarGoogle Scholar |
Boys, C. A., Rowland, S. J., Gabor, M., Gabor, L., Marsh, I. B., Hum, S., and Callinan, R. B. (2012). Emergence of epizootic ulcerative syndrome in native fish of the Murray–Darling river system, Australia: hosts, distribution and possible vectors. PLoS One 7, e35568.
| Emergence of epizootic ulcerative syndrome in native fish of the Murray–Darling river system, Australia: hosts, distribution and possible vectors.Crossref | GoogleScholarGoogle Scholar | 22558170PubMed |
Boys, C. A., Robinson, W., Baumgartner, L. J., Rampano, B., and Lowry, M. (2013a). Influence of approach velocity and mesh size on the entrainment and contact of a lowland river fish assemblage at a screened irrigation pump. PLoS One 8, e67026.
| Influence of approach velocity and mesh size on the entrainment and contact of a lowland river fish assemblage at a screened irrigation pump.Crossref | GoogleScholarGoogle Scholar | 23818975PubMed |
Boys, C. A., Baumgartner, L., Miller, B., Deng, Z., Brown, R., and Pflugrath, B. (2013b). Protecting downstream migrating fish at mini hydropower and other river infrastructure. Fisheries Final Report Series number 137, NSW Department of Primary Industries, Port Stephens Fisheries Institute, Nelson Bay, NSW, Australia.
Bray, D. J., and Thompson, V. J. (2019). Southern pygmy perch Nannoperca australis Günther 1861. In ‘Fishes of Australia.’ Available at https://fishesofaustralia.net.au/home/species/1828 [Verified 31 March 2020].
Breckwoldt, R., Boden, R., and Andrew, J. (Eds) (2004). ‘The Darling.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Broadhurst, B. T., Ebner, B. C., and Clear, R. C. (2012). A rock-ramp fishway expands nursery grounds of the endangered Macquarie perch (Macquaria australasica). Australian Journal of Zoology 60, 91–100.
| A rock-ramp fishway expands nursery grounds of the endangered Macquarie perch (Macquaria australasica).Crossref | GoogleScholarGoogle Scholar |
Broadhurst, B. T., Ebner, B. C., Lintermans, M., Thiem, J. D., and Clear, R. C. (2013). Jailbreak: a fishway releases the endangered Macquarie perch from confinement below an anthropogenic barrier. Marine and Freshwater Research 64, 900–908.
| Jailbreak: a fishway releases the endangered Macquarie perch from confinement below an anthropogenic barrier.Crossref | GoogleScholarGoogle Scholar |
Broadhurst, B. T., Clear, R. C., Fulton, C., and Lintermans, M. (2017). Enlarged Cotter Reservoir ecological monitoring program. Technical report 2017, Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia.
Broadhurst, B. T., Clear, R. C., Fulton, C., and Lintermans, M. (2018). Enlarged Cotter Reservoir ecological monitoring program: Technical report 2018, Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia.
Broadhurst, B. T., Lintermans, M., Clear, R. C., and Jekabsons, M. (2019). Spawning habitat of Macquarie perch in the Cotter River 2018. Report to Icon Water. Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia.
Brown, P., and Hall, K. C. (2003). Lake Eppalock golden perch stock assessment. Fisheries Victoria Research Report Series number 6, Department of Primary Industries, Melbourne, Vic., Australia.
Brown, P., and Wooden, I. (2007). Age at first increment formation and validation of daily growth increments in golden perch (Macquaria ambigua: Percichthyidae) otoliths. New Zealand Journal of Marine and Freshwater Research 41, 157–161.
| Age at first increment formation and validation of daily growth increments in golden perch (Macquaria ambigua: Percichthyidae) otoliths.Crossref | GoogleScholarGoogle Scholar |
Brumley, A. R. (1987). Past and present distributions of golden perch Macquaria ambigua (Pisces: Percichthyidae) in Victoria, with reference to releases of hatchery-produced fry. Proceedings of the Royal Society of Victoria 99, 111–116.
Bunn, S. E., and Arthington, A. H. (2002). Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30, 492–507.
| Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity.Crossref | GoogleScholarGoogle Scholar | 12481916PubMed |
Burbidge, A. A., and Jenkins, R. W. G. (1984). ‘Endangered Vertebrates of Australia and Its Island Territories.’ (Australian National Parks and Wildlife Service: Canberra, ACT, Australia.)
Burndred, K. R., Cockayne, B. J., and Lou, D. C. (2017). Early development of eel-tailed catfish, Tandanus tandanus (Mitchell) (Teleostei:Plotosidae), with validation of daily otolith increment formation. Australian Journal of Zoology 65, 12–20.
| Early development of eel-tailed catfish, Tandanus tandanus (Mitchell) (Teleostei:Plotosidae), with validation of daily otolith increment formation.Crossref | GoogleScholarGoogle Scholar |
Burndred, K. R., Cockayne, B. J., Donaldson, J. A., and Ebner, B. C. (2018). Natural flow events influence the behaviour and movement patterns of eel-tailed catfish (Tandanus tandanus) in a subtropical Queensland river. Australian Journal of Zoology 66, 185–194.
| Natural flow events influence the behaviour and movement patterns of eel-tailed catfish (Tandanus tandanus) in a subtropical Queensland river.Crossref | GoogleScholarGoogle Scholar |
Butler, G. L., and Rowland, S. J. (2009). Using underwater cameras to describe the reproductive behaviour of the endangered eastern freshwater cod Maccullochella ikei. Ecology Freshwater Fish 18, 337–349.
| Using underwater cameras to describe the reproductive behaviour of the endangered eastern freshwater cod Maccullochella ikei.Crossref | GoogleScholarGoogle Scholar |
Bylemans, J., Furlan, E., Hardy, C., McGuffie, P., Lintermans, M., and Gleeson, D. (2017). An environmental DNA (eDNA) based method for monitoring spawning activity: a case study using the endangered Macquarie perch (Macquaria australasica). Methods in Ecology and Evolution 8, 646–655.
| An environmental DNA (eDNA) based method for monitoring spawning activity: a case study using the endangered Macquarie perch (Macquaria australasica).Crossref | GoogleScholarGoogle Scholar |
Bylemans, J., Gleeson, D. M., Hardy, C. M., and Furlan, E. (2018). Toward an ecoregion scale evaluation of eDNA metabarcoding primers: a case study for the freshwater fish biodiversity of the Murray–Darling Basin (Australia). Ecology and Evolution 8, 8697–8712.
| Toward an ecoregion scale evaluation of eDNA metabarcoding primers: a case study for the freshwater fish biodiversity of the Murray–Darling Basin (Australia).Crossref | GoogleScholarGoogle Scholar | 30271538PubMed |
Cadwallader, P. L. (1977). J. O. Langtry’s 1949–50 Murray River investigations. Fisheries and Wildlife Paper Victoria number 13, Fisheries and Wildlife Division, Melbourne, Vic., Australia.)
Cadwallader, P. L. (1978). Some causes of the decline in range and abundance of native fish in the Murray–Darling river system. Proceedings of the Royal Society of Victoria 90, 211–224.
Cadwallader, P. L. (1979). Distribution of native and introduced fish in the Seven Creeks river system, Victoria. Australian Journal of Ecology 4, 361–385.
| Distribution of native and introduced fish in the Seven Creeks river system, Victoria.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, P. L. (1981). Past and present distributions and translocations of Macquarie perch Macquaria australasica (Pisces: Percicthyidae), with particular reference to Victoria. Proceedings of the Royal Society of Victoria 93, 23–30.
Cadwallader, P. L. (1996). ‘Overview of the Impacts of Introduced Salmonids on Australian Native Fauna.’ (Australian Nature Conservation Agency: Canberra, ACT, Australia.)
Cadwallader, P. L., and Backhouse, G. N. (1983). ‘A Guide to the Freshwater Fish of Victoria.’ (Victorian Government Printing Office: Melbourne, Vic., Australia.)
Cadwallader, P. L., and Douglas, J. (1986). Changing food habits of Macquarie perch, Macquaria australasica Cuvier (Pisces: Percichthyidae), during the initial filling phase of Lake Dartmouth, Victoria. Marine and Freshwater Research 37, 647–657.
| Changing food habits of Macquarie perch, Macquaria australasica Cuvier (Pisces: Percichthyidae), during the initial filling phase of Lake Dartmouth, Victoria.Crossref | GoogleScholarGoogle Scholar |
Cadwallader, P. L., and Gooley, G. J. (1984). Past and present distributions and translocations of Murray cod Maccullochella peeli and trout cod M. macquariensis (Pisces: Percichthyidae) in Victoria. Proceedings of the Royal Society of Victoria 96, 33–43.
Cadwallader, P. L., and Gooley, G. J. (1985). Propagation and rearing of Murray Cod Maccullochella peeli at the Warmwater Fisheries Station Pilot Project, Lake Charlegrark. Fisheries and Wildlife Service, Department of Conservation, Forests and Lands, Melbourne, Vic., Australia.
Cadwallader, P. L., and Rogan, P. L. (1977). The Macquarie perch, Macquaria australasica (Pisces: Percichthyidae), of Lake Eildon, Victoria. Australian Journal of Ecology 2, 409–418.
| The Macquarie perch, Macquaria australasica (Pisces: Percichthyidae), of Lake Eildon, Victoria.Crossref | GoogleScholarGoogle Scholar |
Carpenter-Bundhoo, L., Butler, G. L., Espinoza, T., Bond, N. R., Bunn, S. E., and Kennard, M. J. (2020a). Reservoir to river: quantifying fine scale fish movements after translocation. Ecology Freshwater Fish 29, 89–102.
| Reservoir to river: quantifying fine scale fish movements after translocation.Crossref | GoogleScholarGoogle Scholar |
Carpenter-Bundhoo, L., Butler, G. L., Bond, N. R., Bunn, S. E., Reinfelds, I. V., and Kennard, M. J. (2020b). Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species. Scientific Reports 10, 6817.
| Effects of a low-head weir on multi-scaled movement and behavior of three riverine fish species.Crossref | GoogleScholarGoogle Scholar | 32321932PubMed |
Catalán, I. A., Auch, D., Kamermans, P., Morales-Nin, B., Angelopoulos, N. V., Reglero, P., Sandersfeld, T., and Peck, M. A. (2019). Critically examining the knowledge base required to mechanistically project climate impacts: a case study of Europe’s fish and shellfish. Fish and Fisheries 20, 501–517.
| Critically examining the knowledge base required to mechanistically project climate impacts: a case study of Europe’s fish and shellfish.Crossref | GoogleScholarGoogle Scholar |
Cheshire, K. J. M., Ye, Q., Wilson, P., and Bucater, L. (2012). From drought to flood: annual variation in larval fish assemblages in a heavily regulated lowland temperate river. Goyder Institute for Water Research Technical Report Series number 12/6, Goyder Institute for Water Research, Adelaide, SA, Australia.
Chessman, B. C. (2013). Identifying species at risk from climate change: traits predict the drought vulnerability of freshwater fishes. Biological Conservation 160, 40–49.
| Identifying species at risk from climate change: traits predict the drought vulnerability of freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C., and Williams, W. D. (1974). Distribution of fish in inland saline waters in Victoria, Australia. Australian Journal of Marine and Freshwater Research 25, 167–172.
| Distribution of fish in inland saline waters in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Chotipuntu, P. (2003). Salinity sensitivity in early life stages of an Australian freshwater fish, Murray cod (Maccullochella peelii peelii Mitchell 1838). Ph.D. Thesis, University of Canberra, ACT, Australia.
Civade, R., Dejean, T., Valentini, A., Roset, N., Raymond, J. C., Bonin, A., Taberlet, P., and Pont, D. (2016). Spatial representativeness of environmental DNA metabarcoding signal for fish biodiversity assessment in a natural freshwater system. PLoS One 11, e0157366.
| Spatial representativeness of environmental DNA metabarcoding signal for fish biodiversity assessment in a natural freshwater system.Crossref | GoogleScholarGoogle Scholar | 27359116PubMed |
Clark, T. D., Ryan, T., Ingram, B. A., Woakes, A. J., Butler, P. J., and Frappell, P. B. (2005). Factorial aerobic scope is independent of temperature and primarily modulated by heart rate in exercising Murray cod (Maccullochella peelii peelii). Physiological and Biochemical Zoology 78, 347–355.
| Factorial aerobic scope is independent of temperature and primarily modulated by heart rate in exercising Murray cod (Maccullochella peelii peelii).Crossref | GoogleScholarGoogle Scholar | 15887081PubMed |
Closs, G. P., Balcombe, S. R., Driver, P., McNeil, D. G., and Shirley, M. J. (2006). The importance of floodplain wetlands to Murray–Darling fish: What’s there? What do we know? What do we need to know? In ‘Native Fish and Wetlands in the Murray–Darling Basin: Action Plan, Knowledge Gaps and Supporting Papers. Proceedings of a Workshop Held in Canberra ACT, 7–8 June 2005’. (Ed. B. Phillips.) pp. 14–28. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Clunie, P., and Koehn, J. (2001a). Freshwater catfish: a recovery plan. Final report to the Murray–Darling Basin Commission, Canberra, ACT, Australia. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Clunie, P., and Koehn, J. (2001b). Freshwater catfish: a resource document. Final report to the Murray–Darling Basin Commission, Canberra, ACT, Australia. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Clunie, P., and Koehn, J. (2001c). Silver perch: a recovery plan. Final report to the Murray–Darling Basin Commission, Canberra, ACT, Australia. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Clunie, P., and Koehn, J. (2001d). Silver perch: a resource document. Final report to the Murray–Darling Basin Commission, Canberra, ACT, Australia. Arthur Rylah Institute for Environmental Melbourne, Vic., Australia, Victoria.
Cockayne, B. J., McDougall, A. J., Espinoza, T., Burndred, K. R., Thrupp, C. L., Broadfoot, C. D., and Finn, M. (2013). Riverine flow and spawning requirements of Macquaria ambigua oriens: implications for conservation and management. Marine and Freshwater Research 64, 42–53.
| Riverine flow and spawning requirements of Macquaria ambigua oriens: implications for conservation and management.Crossref | GoogleScholarGoogle Scholar |
Cockayne, B. J., Sternberg, D., Schmarr, D. W., Duguid, A. W., and Mathwin, R. (2015). Lake Eyre golden perch (Macquaria sp.) spawning and recruitment is enhanced by flow events in the hydrologically variable rivers of Lake Eyre Basin, Australia. Marine and Freshwater Research 66, 822.
| Lake Eyre golden perch (Macquaria sp.) spawning and recruitment is enhanced by flow events in the hydrologically variable rivers of Lake Eyre Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Cole, T. L., Hammer, M. P., Unmack, P. J., Teske, P. R., Brauer, C. J., Adams, M., and Beheregaray, L. B. (2016). Range-wide fragmentation in a threatened fish associated with post-European settlement modification in the Murray–Darling Basin, Australia. Conservation Genetics 17, 1377–1391.
| Range-wide fragmentation in a threatened fish associated with post-European settlement modification in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Collins, A. L., and Anderson, T. A. (1995). The regulation of endogenous energy stores during starvation and refeeding in the somatic tissues of the golden perch. Journal of Fish Biology 47, 1004–1015.
| The regulation of endogenous energy stores during starvation and refeeding in the somatic tissues of the golden perch.Crossref | GoogleScholarGoogle Scholar |
Collins, A. L., and Anderson, T. A. (1999). The role of food availability in regulating reproductive development in female golden perch. Journal of Fish Biology 55, 94–104.
| The role of food availability in regulating reproductive development in female golden perch.Crossref | GoogleScholarGoogle Scholar |
Cook, B. D., Bunn, S. E., and Hughes, J. M. (2007). Molecular genetic and stable isotope signatures reveal complementary patterns of population connectivity in the regionally vulnerable southern pygmy perch (Nannoperca australis). Biological Conservation 138, 60–72.
| Molecular genetic and stable isotope signatures reveal complementary patterns of population connectivity in the regionally vulnerable southern pygmy perch (Nannoperca australis).Crossref | GoogleScholarGoogle Scholar |
Cooke, S. J., Paukert, C., and Hogan, Z. (2012). Endangered river fish: factors hindering conservation and restoration. Endangered Species Research 17, 179–191.
| Endangered river fish: factors hindering conservation and restoration.Crossref | GoogleScholarGoogle Scholar |
Cooke, S. J., Martins, E. G., Struthers, D. P., Gutowsky, L. F. G., Power, M., Doka, S. E., Dettmers, J. M., Crook, D. A., Lucas, M. C., Holbrook, C. M., and Krueger, C. C. (2016). A moving target – incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environmental Monitoring and Assessment 188, 239.
| A moving target – incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations.Crossref | GoogleScholarGoogle Scholar | 27004432PubMed |
Cooke, S. J., Wesch, S., Donaldson, L. A., Wilson, A. D. M., and Haddaway, N. R. (2017). A call for evidence-based conservation and management of fisheries and aquatic resources. Fisheries 42, 143–149.
| A call for evidence-based conservation and management of fisheries and aquatic resources.Crossref | GoogleScholarGoogle Scholar |
Copeland, C., Schoonevelt-Reid, E., and Neller, S. (2003). ‘Fish Everywhere – An Oral History of Fish and Their Habitats in the Gwydir River.’ (NSW Fisheries: Ballina, NSW, Australia.)
Couch, A. J. (2018). Murray cod: distribution, dispersal and hybridisation in the upper Murrumbidgee River. Ph.D. Thesis, University of Canberra, ACT, Australia.
Couch, A. J., Unmack, P. J., Dyer, F. J., and Lintermans, M. (2016). Who’s your mama? Riverine hybridisation of threatened freshwater trout cod and Murray cod. PeerJ 4, e2593.
| Who’s your mama? Riverine hybridisation of threatened freshwater trout cod and Murray cod.Crossref | GoogleScholarGoogle Scholar | 27812407PubMed |
Counihan, T. D., Waite, I. R., Casper, A. F., Ward, D. L., Sauer, J. S., Irwin, E. R., Chapman, C. G., Ickes, B. S., Paukert, C. P., Kosovich, J. J., and Bayer, J. M. (2018). Can data from disparate long-term fish monitoring programs be used to increase our understanding of regional and continental trends in large river assemblages? PLoS One 13, e0191472.
| Can data from disparate long-term fish monitoring programs be used to increase our understanding of regional and continental trends in large river assemblages?Crossref | GoogleScholarGoogle Scholar | 29364953PubMed |
Crook, D. A. (2004). Is the home range concept compatible with the movements of two species of lowland river fish? Journal of Animal Ecology 73, 353–366.
| Is the home range concept compatible with the movements of two species of lowland river fish?Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., Robertson, A. I., King, A. J., and Humphries, P. (2001). The influence of spatial scale and habitat arrangement on diel patterns of habitat use by two lowland river fishes. Oecologia 129, 525–533.
| The influence of spatial scale and habitat arrangement on diel patterns of habitat use by two lowland river fishes.Crossref | GoogleScholarGoogle Scholar | 24577692PubMed |
Crook, D. A., Reich, P., Bond, N. R., McMaster, D., Koehn, J. D., and Lake, P. S. (2010). Using biological information to support proactive strategies for managing freshwater fish during drought. Marine and Freshwater Research 61, 379–387.
| Using biological information to support proactive strategies for managing freshwater fish during drought.Crossref | GoogleScholarGoogle Scholar |
Crook, D. A., O’Mahony, D. J., Gillanders, B. M., Munro, A. R., Sanger, A. C., Thurstan, S., and Baumgartner, L. J. (2016). Contribution of stocked fish to riverine populations of golden perch (Macquaria ambigua) in the Murray–Darling Basin, Australia. Marine and Freshwater Research 67, 1401–1409.
| Contribution of stocked fish to riverine populations of golden perch (Macquaria ambigua) in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
CSIRO (2008). Water availability in the Murray–Darling Basin. A report to the Australian Government from the CSIRO Sustainable Yields Project. (CSIRO: Canberra, ACT, Australia.) Available at http://www.clw.csiro.au/publications/waterforahealthycountry/mdbsy/pdf/WaterAvailabilityInTheMDB-FinalReport.pdf [Verified 13 August 2020].
Cvitanovic, C., Hobday, A. J., van Kerkoff, L., and Marshall, N. A. (2015). Overcoming barriers to knowledge exchange for adaptive resource management; the perspectives of Australian marine scientists. Marine Policy 52, 38–44.
| Overcoming barriers to knowledge exchange for adaptive resource management; the perspectives of Australian marine scientists.Crossref | GoogleScholarGoogle Scholar |
Dakin, W. J., and Kesteven, G. L. (1938). The Murray cod [Maccullochella macquariensis (Cuv. et Val.)] State Fisheries Research Bulletin 1, 1–18.
Dannevig, H. C. (1903). Summary of evidence regarding the Murray cod fisheries, with notes. In ‘Murray Cod Fisheries’. pp. 3–32. (Government Printer: Sydney, NSW, Australia.)
Dargin, P. (1976). ‘Aboriginal Fisheries of the Darling–Barwon Rivers.’ (Brewarrina Historical Society: Brewarrina, NSW, Australia.)
Davies, P., Harris, J., Hillman, T., and Walker, K. (2008). SRA Report 1: a report on the ecological health of rivers in the Murray–Darling Basin, 2004–2007. Prepared by the Independent Sustainable Rivers Audit Group for the Murray–Darling Ministerial Council. Murray–Darling Basin Commission, Canberra, ACT, Australia.
Davies, P. E., Harris, J. H., Hillman, T. J., and Walker, K. F. (2010). The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia. Marine and Freshwater Research 61, 764–777.
| The Sustainable Rivers Audit: assessing river ecosystem health in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Davies, P., Stewardson, M., Hillman, T., Roberts, J., and Thoms, M. (2012). Sustainable Rivers Audit 2: The ecological health of rivers in the Murray–Darling Basin at the end of the Millennium Drought (2008–2010). Prepared by the Independent Sustainable Rivers Audit Group for the Murray–Darling Basin Ministerial Council.
Davies, P. M., Naiman, R. J., Warfe, D. M., Pettit, N. E., Arthington, A. H., and Bunn, S. E. (2014). Flow–ecology relationships: closing the loop on effective environmental flows. Marine and Freshwater Research 65, 133–141.
| Flow–ecology relationships: closing the loop on effective environmental flows.Crossref | GoogleScholarGoogle Scholar |
Davis, T. L. O. (1977a). Reproductive biology of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia. I. Structure of the gonads. Australian Journal of Marine and Freshwater Research 28, 139–158.
| Reproductive biology of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia. I. Structure of the gonads.Crossref | GoogleScholarGoogle Scholar |
Davis, T. L. O. (1977b). Reproductive biology of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia. II. Gonadal cycle and fecundity. Australian Journal of Marine and Freshwater Research 28, 159–169.
| Reproductive biology of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia. II. Gonadal cycle and fecundity.Crossref | GoogleScholarGoogle Scholar |
Davis, T. L. O. (1977c). Age determination and growth of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia. Marine and Freshwater Research 28, 119–137.
| Age determination and growth of the freshwater catfish, Tandanus tandanus Mitchell, in the Gwydir River, Australia.Crossref | GoogleScholarGoogle Scholar |
Department of Agriculture, Water and the Environment (2018). National recovery plan for Macquarie perch (Macquaria australasica). (Commonwealth of Australia: Canberra, ACT, Australia.) Available at https://www.environment.gov.au/biodiversity/threatened/publications/recovery/macquaria-australasica-2018 [Verified 27 July 2020].
Department of Environment and Resource Management (2010). Environmental conditions and spawning of golden perch (Macquaria ambigua Richardson, 1845) in the Border Rivers. DERM, Brisbane, Qld, Australia.
Department of Environment, Land, Water and Planning (2017). Draft national recovery plan for the Murray hardyhead Craterocephalus fluviatilis. DELWP for the Australian Government Department of the Environment and Energy, Canberra, ACT, Australia.
Department of Natural Resources and Mines and Energy (2016). Technical summary for eel-tailed catfish (T. tandanus). DNRME, Mackay, Qld, Australia.
Department of Sustainability and Environment (2013). Advisory list of threatened vertebrate fauna in Victoria. Available at https://www.environment.vic.gov.au/__data/assets/pdf_file/0014/50450/Advisory-List-of-Threatened-Vertebrate-Fauna_FINAL-2013.pdf [Verified 31 March 2020].
Dexter, T., Bond, N., Hale, R., and Reich, P. (2014). Dispersal and recruitment of fish in an intermittent stream network. Austral Ecology 39, 225–235.
| Dispersal and recruitment of fish in an intermittent stream network.Crossref | GoogleScholarGoogle Scholar |
Douglas, J. W. (2002). Observations on aspects of Macquarie Perch Macquaria australasica (Cuvier) spawning, natural recruitment and selected population attributes in Lake Dartmouth and the Mitta Mitta River between 1994 and 1998. Marine and Freshwater Resources Institute Freshwater Fisheries Report number 02/7, Marine and Freshwater Resources Institute, Department of Natural Resources and Environment, Melbourne, Vic., Australia.
Douglas, J. (2009). Movement and habitat preferences of golden perch in Lake Eildon. Fisheries Victoria Research Report Series number 38, Department of Primary Industries, Snobs Creek, Vic., Australia.
Douglas, J. W., Gooley, G. J., and Ingram, B. A. (1994). ‘Trout cod, Maccullochella macquariensis (Cuvier) (Pisces: Percichthyidae). Resource Handbook and Research and Recovery Plan.’ (Victorian Fisheries Research Institute, Department of Conservation and Natural Resources: Melbourne, Vic., Australia.)
Douglas, J. W., Gooley, G. J., Ingram, B. A., Murray, N. D., and Brown, L. D. (1995). Natural hybridization between Murray cod, Maccullochella peelii peelii (Mitchell), and trout cod, Maccullochella macquariensis (Cuvier) (Percichthyidae), in the Murray River, Australia. Marine and Freshwater Research 46, 729–734.
| Natural hybridization between Murray cod, Maccullochella peelii peelii (Mitchell), and trout cod, Maccullochella macquariensis (Cuvier) (Percichthyidae), in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Douglas, J., Gooley, G., Kemp, D., and Ingram, B. (1998). An ecological survey of three major Murray River anabranches in North-Western Victoria with reference to Ecosystem Condition Indices. Marine and Freshwater Resources Institute, Sydney, NSW, Australia.
Douglas, J., Giles, A., and Strongman, R. (2002). Lake Dartmouth multi-species fishery assessment. Marine and Freshwater Resources Institute Freshwater Fisheries Report number 02/2, Marine and Freshwater Resources Institute, Melbourne Vic., Australia.
Douglas, J., Brown, P., Hunt, T., Rogers, M., and Allen, M. (2010). Evaluating relative impacts of recreational fishing harvest and discard mortality on Murray cod (Maccullochella peelii peelii). Fisheries Research 106, 18–21.
| Evaluating relative impacts of recreational fishing harvest and discard mortality on Murray cod (Maccullochella peelii peelii).Crossref | GoogleScholarGoogle Scholar |
Douglas, J., Hunt, T., and Trueman, W. (2012). Confirmed records of the endangered trout cod Maccullochella macquariensis from the Murray River at Gunbower Island, Victoria. Victorian Naturalist 129, 152–155.
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z. I., Knowler, D. J., Lévêque, C., Naiman, R. J., Prieur-Richard, A.-H., Soto, D., Stiassny, M. L. J., and Sullivan, C. A. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar | 16336747PubMed |
Earl, J. (2019). Fisheries statistics, stock status and performance indicators for South Australian Lakes and Coorong Fishery. Report to PIRSA Fisheries and Aquaculture. SARDI Publication number F2009/000669-10, SARDI Research Report Series number 1020, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ebner, B. C. (2006). Murray cod an apex predator in the Murray River, Australia. Ecology Freshwater Fish 15, 510–520.
| Murray cod an apex predator in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., and Lintermans, M. (2007). Fish passage, movement requirements and habitat use for Macquarie perch. Final Report to the Department of Agriculture, Fisheries and Forestry Australia. Parks, Conservation and Lands, Canberra, ACT, Australia.
Ebner, B. C., and Thiem, J. D. (2009). Monitoring by telemetry reveals differences in movement and survival following hatchery or wild rearing of an endangered fish. Marine and Freshwater Research 60, 45–57.
| Monitoring by telemetry reveals differences in movement and survival following hatchery or wild rearing of an endangered fish.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Raadik, T., and Ivantsoff, W. (2003). Threatened fishes of the world: Craterocephalus fluviatilis McCulloch, 1913 (Atherinidae). Environmental Biology of Fishes 68, 390.
| Threatened fishes of the world: Craterocephalus fluviatilis McCulloch, 1913 (Atherinidae).Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Thiem, J. D., and Lintermans, M. (2007). Fate of 2 year-old, hatchery-reared trout cod Maccullochella macquariensis (Percichthyidae) stocked into two upland rivers. Journal of Fish Biology 71, 182–199.
| Fate of 2 year-old, hatchery-reared trout cod Maccullochella macquariensis (Percichthyidae) stocked into two upland rivers.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Thiem, J. D., Gilligan, D. M., Lintermans, M., Wooden, I. J., and Linke, S. (2008). Estimating species richness and catch per unit effort from boat electro-fishing in a lowland river in temperate Australia. Austral Ecology 33, 891–901.
| Estimating species richness and catch per unit effort from boat electro-fishing in a lowland river in temperate Australia.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Johnston, L., and Lintermans, M. (2009a). Radio-tagging and tracking of translocated trout cod (Maccullochella macquariensis: Percichthyidae) in an upland river. Marine and Freshwater Research 60, 346–355.
| Radio-tagging and tracking of translocated trout cod (Maccullochella macquariensis: Percichthyidae) in an upland river.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Scholz, O., and Gawne, B. (2009b). Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia. New Zealand Journal of Marine and Freshwater Research 43, 571–578.
| Golden perch Macquaria ambigua are flexible spawners in the Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Lintermans, M., Jekabsons, M., and Dunford, M. (2010). Convoluted shorelines confound diel-range estimates of radio-tracked fish. Marine and Freshwater Research 61, 1360–1365.
| Convoluted shorelines confound diel-range estimates of radio-tracked fish.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Lintermans, M., and Dunford, M. (2011). A reservoir serves as refuge for adults of the endangered Macquarie perch. Lakes and Reservoirs: Research and Management 16, 23–33.
| A reservoir serves as refuge for adults of the endangered Macquarie perch.Crossref | GoogleScholarGoogle Scholar |
Ebner, B. C., Starrs, D., Morgan, D. L., Fulton, C., Donaldson, J., Doody, J., Cousins, S., Kennard, M., Butler, G., Tonkin, Z., Beatty, S., Broardhurst, B., Lintermans, M., and Fletcher, C. (2014). Emergence of field-based underwater video for understanding the ecology of freshwater fishes and crustaceans in Australia. Journal of the Royal Society of Western Australia 97, 287–296.
Ebner, B. C., Morgan, D. L., Kerezsy, A., Hardie, S., Beatty, S. J., Seymour, J. E., Donaldson, J. A., Linke, S., Peverell, S., Roberts, D., Espinoza, T., Marshall, N., Kroon, F. J., Burrows, D. W., and McAllister, R. R. J. (2016). Enhancing conservation of Australian freshwater ecosystems: identification of freshwater flagship fishes and relevant target audiences. Fish and Fisheries 17, 1134–1151.
| Enhancing conservation of Australian freshwater ecosystems: identification of freshwater flagship fishes and relevant target audiences.Crossref | GoogleScholarGoogle Scholar |
Ellis, I. (2005). Ecology and breeding seasonality of the Murray hardyhead Craterocephalus fluvialtilis (McCulloch), Family Atherinidae, in two lakes near Mildura, Victoria. Publication number 5/2005, Murray–Darling Freshwater Research Centre, Mildura, Vic., Australia.
Ellis, I. (2006). Age structure and dietary analysis of the Murray hardyhead Craterocephalus fluvialtilis (McCulloch), Family Atherinidae, in two lakes near Mildura, Victoria. Publication number 10/2006, Murray Darling Freshwater Research Centre, Mildura, Vic., Australia.
Ellis, I., and Kavanagh, M. (2014). A review of the biology and status of the endangered Murray hardyhead: streamlining recovery processes. Final report prepared for the Murray–Darling Basin Authority. MDFRC Publication 19/2014, Murray–Darling Freshwater Research Centre, Mildura, Vic., Australia.
Ellis, I., Carr, L., and Pyke, L. (2011). Conservation of Murray hardyhead Craterocephalus fluviatilis in status of population monitoring, translocation and captive breeding programs 2010/11. Publication number 26/2011, Murray–Darling Freshwater Research Centre, Mildura, Vic., Australia.
Ellis, I., Carr, L., and Pyke, L. (2012). Murray hardyhead Craterocephalus fluviatilis rejuvenation in Victoria: population monitoring, translocation and captive breeding programs 2011–12. Publication number 20/2012, Murray–Darling Freshwater Research Centre, Mildura, Vic., Australia.
Ellis, I. M., Stoessel, D., Hammer, M. P., Wedderburn, S. D., Suitor, L., and Hall, A. (2013). Conservation of an inauspicious endangered freshwater fish, Murray hardyhead (Craterocephalus fluviatilis), during drought and competing water demands in the Murray–Darling Basin, Australia. Marine and Freshwater Research 64, 792–806.
| Conservation of an inauspicious endangered freshwater fish, Murray hardyhead (Craterocephalus fluviatilis), during drought and competing water demands in the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Ellis, I., Whiterod, N., and Nias, D. (2020). Short-term intervention monitoring associated with the translocation of the Murray Hardyhead into Little Frenchmans Creek, Wingillie Station NSW. Report to the Commonwealth Environmental Water Office. Murray Wetlands Working Group, NSW, Australia.
Faragher, R. A., Brown, P., and Harris, J. H. (1993). Population surveys of the endangered fish species trout cod (Maccullochella macquariensis) and eastern cod (M. ikei). NSW Fisheries Research Institute, Sydney, NSW, Australia.
Farrington, L., Lintermans, M., and Ebner, B. C. (2014). Characterising genetic diversity and effective population size in one reservoir and two riverine populations of the threatened Macquarie perch. Conservation Genetics 15, 707–716.
| Characterising genetic diversity and effective population size in one reservoir and two riverine populations of the threatened Macquarie perch.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010a). Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica. Conservation Genetics 11, 921–934.
| Evolution and maintenance of divergent lineages in an endangered freshwater fish, Macquaria australasica.Crossref | GoogleScholarGoogle Scholar |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2010b). Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua). Molecular Ecology 19, 4723–4737.
| Islands of water in a sea of dry land: hydrological regime predicts genetic diversity and dispersal in a widespread fish from Australia’s arid zone, the golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 20887362PubMed |
Faulks, L. K., Gilligan, D. M., and Beheregaray, L. B. (2011). The role of anthropogenic vs. natural in-stream structures in determining connectivity and genetic diversity in an endangered freshwater fish, Macquarie perch (Macquaria australasica). Evolutionary Applications 4, 589–601.
| The role of anthropogenic vs. natural in-stream structures in determining connectivity and genetic diversity in an endangered freshwater fish, Macquarie perch (Macquaria australasica).Crossref | GoogleScholarGoogle Scholar | 25568007PubMed |
Ferguson, G. J., and Ye, Q. (2012). Stock assessment of golden perch (Macquaria ambigua). Stock Assessment Report for PIRSA Fisheries and Aquaculture. F2007/01051-1, SARDI Research Report Series number 656, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ferguson, G. J., and Ye, Q. (2016). Influences of drought and high flow on the large-bodied fish assemblage in the Lower Lakes. Report to the Department of Environment, Water and Natural Resources. SARDI Publication number F2016/000021-1, SARDI Research Report Series number 888, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ferguson, M., Power, T., Jennings, D., O’Brien, A., and Marsden, T. (2008). St Lawrence Wetland fish passage improvement. Department of Primary Industries and Fisheries, Brisbane, Qld, Australia.
Fiddes, S., and Timbal, B. (2017). Future impacts of climate change on streamflows across Victoria, Australia: making use of statistical downscaling. Climate Research 71, 219–236.
| Future impacts of climate change on streamflows across Victoria, Australia: making use of statistical downscaling.Crossref | GoogleScholarGoogle Scholar |
Flitcroft, R., Cooperman, M. S., Harrison, I. J., Juffe-Bignoli, D., and Boon, P. J. (2019). Theory and practice to conserve freshwater biodiversity in the Anthropocene. Aquatic Conservation: Marine and Freshwater Ecosystem 29, 1013–1021.
| Theory and practice to conserve freshwater biodiversity in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Forbes, J. P., Watts, R. J., Robinson, W. A., Baumgartner, L. J., Allen, M. S., McGuffie, P., Cameron, L. M., and Crook, D. A. (2015a). System-specific variability in Murray cod and golden perch maturation and growth influences fisheries management options. North American Journal of Fisheries Management 35, 1226–1238.
| System-specific variability in Murray cod and golden perch maturation and growth influences fisheries management options.Crossref | GoogleScholarGoogle Scholar |
Forbes, J. P., Watts, R. J., Robinson, W. A., Baumgartner, L. J., and Steffe, A. S. (2015b). Recreational fishing effort, catch, and harvest for Murray cod and Golden perch in the Murrumbidgee River, Australia. North American Journal of Fisheries Management 35, 649–658.
| Recreational fishing effort, catch, and harvest for Murray cod and Golden perch in the Murrumbidgee River, Australia.Crossref | GoogleScholarGoogle Scholar |
Forbes, J., Watts, R. J., Robinson, W. A., Baumgartner, L. J., McGuffie, P., Cameron, L. M., and Crook, D. A. (2016). Assessment of stocking effectiveness for Murray cod (Maccullochella peelii) and golden perch (Macquaria ambigua) in rivers and impoundments of south-eastern Australia. Marine and Freshwater Research 67, 1410–1419.
| Assessment of stocking effectiveness for Murray cod (Maccullochella peelii) and golden perch (Macquaria ambigua) in rivers and impoundments of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Forbes, J. P., Todd, C. R., Baumgartner, L. J., Watts, R. J., Robinson, W. A., Steffe, A. S., Murphy, J. J., Asmus, M. W., and Thiem, J. D. (2020). Simulation of different fishery regulations to prevent population decline in a large freshwater invertebrate, the Murray crayfish (Euastacus armatus). Marine and Freshwater Research 71, 962–971.
| Simulation of different fishery regulations to prevent population decline in a large freshwater invertebrate, the Murray crayfish (Euastacus armatus).Crossref | GoogleScholarGoogle Scholar |
Francis, C., and Sheldon, F. (2002). River red gum (Eucalyptus camaldulensis Dehnh.) organic matter as a carbon source in the lower Darling River, Australia. Hydrobiologia 481, 113–124.
| River red gum (Eucalyptus camaldulensis Dehnh.) organic matter as a carbon source in the lower Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C. (1991). Avoidance of inundated floodplain habitat by larvae of golden perch (Macquaria ambigua Richardson): influence of water quality or food distribution? Marine and Freshwater Research 42, 707–719.
| Avoidance of inundated floodplain habitat by larvae of golden perch (Macquaria ambigua Richardson): influence of water quality or food distribution?Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2000). Large-scale patterns in species richness and composition of temperate riverine fish communities, south-eastern Australia. Marine and Freshwater Research 51, 165–182.
| Large-scale patterns in species richness and composition of temperate riverine fish communities, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Gehrke, P. C., and Harris, J. H. (2001). Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia. Regulated Rivers: Research and Management 17, 369–391.
| Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Gibbons, P., Zammit, C., Youngentob, K., Possingham, H. P., Lindenmayer, D. B., Bekessy, S., Burgman, M., Colyvan, M., Considine, M., Felton, A., Hobbs, R., Hurley, K., Mcalpine, C., Mccarthy, M. A., Moore, J., Robinson, D., Salt, D., and Wintle, B. (2008). Some practical suggestions for improving engagement between researchers and policy-makers in natural resource management. Ecological Management & Restoration 9, 182–186.
| Some practical suggestions for improving engagement between researchers and policy-makers in natural resource management.Crossref | GoogleScholarGoogle Scholar |
Gibbs, M. S., Bice, C., Brookes, J., Furst, D., Gao, L., Joehnk, K., Marklund, M., Nicol, J., Pethybridge, H., Szarvas, S., Wallace, T., Welti, N., and Zampatti, B. (2020). Ecological connectivity of the River Murray: managing ecological outcomes and water quality risks through integrated river management. Goyder Institute for Water Research Technical Report Series number 20/03, Goyder Institute for Water Research, Adelaide, SA, Australia.
Gilligan, D. (2005). Fish communities of the Murrumbidgee catchment: status and trends. Fisheries Final Report Series number 75, NSW Department of Primary Industries, Narrandera, NSW, Australia.
Gilligan, D., and Clunie, P. (2019). Tandanus tandanus. In ‘The IUCN Red List of Threatened Species 2019’. e.T122902003A123382071. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/122902003/123382071 [Verified 13 April 2020].
Gilligan, D., and Schiller, C. (2003). Downstream transport of larval and juvenile fish in the Murray River. NSW Fisheries Final Report Series number 50, NSW Department of Primary Industries, Sydney, NSW, Australia.
Gilligan, D., Zampatti, B., Lintermans, M., Koehn, J., Butler, G., and Brooks, S. (2019). Murray River cod Maccullochella peelii. In ‘The IUCN Red List of Threatened Species 2019’. e.T12576A103325360. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/12576/103325360 [Verified 13 April 2020].
Ginns, A. (2012). Murray cod – creator of the river. RipRap 34, 42–43.
Gooley, G. J. (1992). Validation of the use of otoliths to determine the age and growth of Murray cod, Maccullochella peelii (Mitchell) (Percichthyidae), in Lake Charlegrark, Western Victoria. Marine and Freshwater Research 43, 1091–1102.
| Validation of the use of otoliths to determine the age and growth of Murray cod, Maccullochella peelii (Mitchell) (Percichthyidae), in Lake Charlegrark, Western Victoria.Crossref | GoogleScholarGoogle Scholar |
Gooley, G. J., and McDonald, G. L. (1988). Preliminary study on the hormone-induced spawning of Macquarie perch, Macquaria australasica (Cuvier) (Percichthyidae), from Lake Dartmouth, Victoria. Arthur Rylah Institute for Environmental Research Technical Report Series number 80, Department of Conservation, Forests and Lands, Alexandra, Vic., Australia.
Gooley, G. J., Anderson, T. A., and Appleford, P. (1995). Aspects of the reproductive cycle and gonadal development of Murray cod, Maccullochella peelii peelii (Mitchell) (Percichthyidae), in Lake Charlegrark and adjacent farm ponds, Victoria, Australia. Marine and Freshwater Research 46, 723–728.
| Aspects of the reproductive cycle and gonadal development of Murray cod, Maccullochella peelii peelii (Mitchell) (Percichthyidae), in Lake Charlegrark and adjacent farm ponds, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Grant, E. M. (1987). ‘Fishes of Australia.’ (EM Grant: Scarborough, Qld, Australia.)
Gray, S. C., De Silva, S. S., Ingram, B. A., and Gooley, G. J. (2000). Effects of river impoundment on body condition and reproductive performance of the Australian native fish, Macquarie perch (Macquaria australasica). Lakes and Reservoirs: Research and Management 5, 281–291.
| Effects of river impoundment on body condition and reproductive performance of the Australian native fish, Macquarie perch (Macquaria australasica).Crossref | GoogleScholarGoogle Scholar |
Gray, R., Jones, H. A., Hitchcock, J. N., Hardwick, L., Pepper, D., Lugg, A., Seymour, J. R., and Mitrovic, S. M. (2019). Mitigation of cold-water thermal pollution downstream of a large dam with the use of a novel thermal curtain. River Research and Applications 35, 855–866.
| Mitigation of cold-water thermal pollution downstream of a large dam with the use of a novel thermal curtain.Crossref | GoogleScholarGoogle Scholar |
Grill, G., Lehner, B., Lumsdon, A. E., MacDonald, G. K., Zarfl, C., and Liermann, C. R. (2015). An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales. Environmental Research Letters 10, 015001.
| An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales.Crossref | GoogleScholarGoogle Scholar |
Grill, G., Lehner, B., Thieme, M., Geenen, B., Tickner, D., Antonelli, F., Babu, S., Borrelli, P., Cheng, L., Crochetiere, H., Ehalt Macedo, H., Filgueiras, R., Goichot, M., Higgins, J., Hogan, Z., Lip, B., McClain, M. E., Meng, J., Mulligan, M., Nilsson, C., Olden, J. D., Opperman, J. J., Petry, P., Liermann, C. R., Sáenz, L., Salinas-Rodríguez, S., Schelle, P., Schmitt, R. J. P., Snider, J., Tan, F., Tockner, K., Valdujo, P. H., van Soesbergen, A., and Zarfl, S. C. (2019). Mapping the world’s free-flowing rivers. Nature 569, 215–221.
| Mapping the world’s free-flowing rivers.Crossref | GoogleScholarGoogle Scholar | 31068722PubMed |
Growns, I. (2004). A numerical classification of reproductive guilds of the freshwater fishes of south-eastern Australia and their application to river management. Fisheries Management and Ecology 11, 369–377.
| A numerical classification of reproductive guilds of the freshwater fishes of south-eastern Australia and their application to river management.Crossref | GoogleScholarGoogle Scholar |
Growns, I., Wooden, I., and Schiller, C. (2004). Use of instream wood habitat by trout cod Maccullochella macquariensis (Cuvier) in the Murrumbidgee River. Pacific Conservation Biology 10, 261–265.
| Use of instream wood habitat by trout cod Maccullochella macquariensis (Cuvier) in the Murrumbidgee River.Crossref | GoogleScholarGoogle Scholar |
Grummer, J. A., Beheregaray, L. B., Bernatchez, L., Hand, B. K., Luikart, G., Narum, S. R., and Taylor, E. B. (2019). Aquatic landscape genomics and environmental effects on genetic variation. Trends in Ecology & Evolution 34, 641–654.
| Aquatic landscape genomics and environmental effects on genetic variation.Crossref | GoogleScholarGoogle Scholar |
Gwinn, D. C., Todd, C. R., Brown, P., Hunt, T. L., Butler, G., Kitchingman, A., Koehn, J. D., and Ingram, B. (2019). Assessing a threatened fish species under budgetary constraints: evaluating the use of existing monitoring data. North American Journal of Fisheries Management 39, 315–327.
| Assessing a threatened fish species under budgetary constraints: evaluating the use of existing monitoring data.Crossref | GoogleScholarGoogle Scholar |
Gwinn, D. C., Butler, G. L., Ingram, B. A., Raymond, S., Lintermans, M., and Ye, Q. (2020). Borrowing external information to estimate angler size selectivity: model development and application to Murray cod. Canadian Journal of Fisheries and Aquatic Sciences 77, 425–437.
| Borrowing external information to estimate angler size selectivity: model development and application to Murray cod.Crossref | GoogleScholarGoogle Scholar |
Haldane, J. (2014). Cod capacity. Fish 22, 32–33.
Hall, K. C., Broadhurst, M. K., and Butcher, P. A. (2012). Post-release mortality of angled golden perch Macquaria ambigua and Murray cod Maccullochella peelii. Fisheries Management and Ecology 19, 10–21.
| Post-release mortality of angled golden perch Macquaria ambigua and Murray cod Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar |
Hall, K. C., Broadhurst, M. K., and Cullis, B. R. (2015). Deep hooking and post-release mortality of two Australian native freshwater fishes angled from rivers by using natural baits. Transactions of the American Fisheries Society 144, 860–871.
| Deep hooking and post-release mortality of two Australian native freshwater fishes angled from rivers by using natural baits.Crossref | GoogleScholarGoogle Scholar |
Hammer, M. (2002). Recovery outline for the southern pygmy perch in the Mount Lofty Ranges, South Australia. Department of Environmental Biology, University of Adelaide and Native Fish Australia (SA) Inc., Adelaide, SA, Australia.
Hammer, M., and Wedderburn, S. D. (2008). The threatened Murray hardyhead: natural history and captive rearing. Fishes of Sahul 22, 390–399.
Hammer, M., Wedderburn, S., and van Weenen, J. (2009). ‘Action Plan for South Australian Freshwater Fishes.’ (Native Fish Australia (SA) Inc.: Adelaide, SA, Australia.)
Hammer, M. P., Bice, C. M., Hall, A., Frears, A., Watt, A., Whiterod, N. S., Beheregaray, L. B., Harris, J. O., and Zampatti, B. P. (2013). Freshwater fish conservation in the face of critical water shortages in the southern Murray–Darling Basin, Australia. Marine and Freshwater Research 64, 807–821.
| Freshwater fish conservation in the face of critical water shortages in the southern Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Harris, J. H., and Dixon, P. I. (1988). Hybridization between trout cod and Murray cod. Isozyme Bulletin 19, 39.
Harris, J. H., and Gehrke, P. C. (1994). Modelling the relationship between streamflow and population recruitment to manage freshwater fisheries. Australian Fisheries 6, 28–30.
Harris, J. H., and Gehrke, P. C. (Eds) (1997). ‘Fish and Rivers in Stress. The NSW Rivers Survey.’ (NSW Fisheries Office of Conservation and the CRC for Freshwater Ecology: Sydney, NSW, Australia.)
Harrisson, K. A., Amish, S. J., Pavlova, A., Narum, S. R., Telonis-Scott, M., Rourke, M. L., Lyon, J., Tonkin, Z., Gilligan, D. M., Ingram, B. A., Lintermans, M., Gan, H. M., Austin, C. M., Luikart, G., and Sunnucks, P. (2017). Signatures of polygenic adaptation associated with climate across the range of a threatened fish species with high genetic connectivity. Molecular Ecology 26, 6253–6269.
| Signatures of polygenic adaptation associated with climate across the range of a threatened fish species with high genetic connectivity.Crossref | GoogleScholarGoogle Scholar | 28977721PubMed |
Hart, B. T. (2016a). The Australian Murray–Darling Basin plan: challenges in its implementation (part 1). International Journal of Water Resources Development 32, 819–834.
| The Australian Murray–Darling Basin plan: challenges in its implementation (part 1).Crossref | GoogleScholarGoogle Scholar |
Hart, B. T. (2016b). The Australian Murray–Darling Basin plan: challenges in its implementation (part 2). International Journal of Water Resources Development 32, 835–852.
| The Australian Murray–Darling Basin plan: challenges in its implementation (part 2).Crossref | GoogleScholarGoogle Scholar |
Henry, G. W., and Lyle, J. M. (Eds) (2003). ‘The National Recreational and Indigenous Fishing Survey.’ (Australian Government Department of Agriculture, Fisheries and Forestry: Canberra, ACT, Australia.)
Hill, E., Ingram, B. A., Rourke, M., Mitchell, J., and Strugnell, J. M. (2015). Genetic diversity and population structure of the threatened freshwater catfish, Tandanus tandanus, in Victoria, Australia. Conservation Genetics 16, 317–329.
| Genetic diversity and population structure of the threatened freshwater catfish, Tandanus tandanus, in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Hinlo, R., Lintermans, M., Gleeson, D., Broadhurst, B., and Furlan, E. (2018). Performance of eDNA assays to detect and quantify an elusive benthic fish in upland streams. Biological Invasions 20, 3079–3093.
| Performance of eDNA assays to detect and quantify an elusive benthic fish in upland streams.Crossref | GoogleScholarGoogle Scholar |
Humphries, P. (1995). Life history, food and habitat of southern pygmy perch, Nannoperca australis, in the Macquarie River, Tasmania. Marine and Freshwater Research 46, 1159–1169.
| Life history, food and habitat of southern pygmy perch, Nannoperca australis, in the Macquarie River, Tasmania.Crossref | GoogleScholarGoogle Scholar |
Humphries, P. (2005). Spawning time and early life history of Murray cod, Maccullochella peelii peelii (Mitchell) in an Australian river. Environmental Biology of Fishes 72, 393–407.
| Spawning time and early life history of Murray cod, Maccullochella peelii peelii (Mitchell) in an Australian river.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., and Lake, P. S. (2000). Fish larvae and the management of regulated rivers. Regulated Rivers: Research and Management 16, 421–432.
| Fish larvae and the management of regulated rivers.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., and Walker, K. (Eds) (2013). ‘Ecology of Australian Freshwater Fishes.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Humphries, P., and Winemiller, K. O. (2009). Historical impacts on river fauna, shifting baselines and challenges for restoration. Bioscience 59, 673–684.
| Historical impacts on river fauna, shifting baselines and challenges for restoration.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., King, A. J., and Koehn, J. D. (1999). Fish, flows and flood plains: links between freshwater fishes and their environment in the Murray–Darling river system, Australia. Environmental Biology of Fishes 56, 129–151.
| Fish, flows and flood plains: links between freshwater fishes and their environment in the Murray–Darling river system, Australia.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., Serafini, L. G., and King, A. J. (2002). River regulation and fish larvae: variation through space and time. Freshwater Biology 47, 1307–1331.
| River regulation and fish larvae: variation through space and time.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., Cook, R. A., Richardson, A. J., and Serafini, L. G. (2006). Creating a disturbance: manipulating slackwaters in a lowland river. River Research and Applications 22, 525–542.
| Creating a disturbance: manipulating slackwaters in a lowland river.Crossref | GoogleScholarGoogle Scholar |
Humphries, P., King, A., McCasker, N., Kopf, R. K., Stoffels, R., Zampatti, B., and Price, A. (2020). Riverscape recruitment: a conceptual synthesis of drivers of fish recruitment in rivers. Canadian Journal of Fisheries and Aquatic Sciences 77, 213–225.
| Riverscape recruitment: a conceptual synthesis of drivers of fish recruitment in rivers.Crossref | GoogleScholarGoogle Scholar |
Hunt, T. L., and Jones, P. (2018). Informing the great fish stocking debate: an Australian case study. Reviews in Fisheries Science & Aquaculture 26, 275–308.
| Informing the great fish stocking debate: an Australian case study.Crossref | GoogleScholarGoogle Scholar |
Hunt, T. L., Douglas, J. W., Allen, M. S., Gwinn, D. C., Tonkin, Z., Lyon, J., and Pickworth, A. (2011). Evaluation of population decline and fishing sustainability of the endangered Australian freshwater fish Macquaria australasica. Fisheries Management and Ecology 18, 513–520.
| Evaluation of population decline and fishing sustainability of the endangered Australian freshwater fish Macquaria australasica.Crossref | GoogleScholarGoogle Scholar |
Hutchison, M. (2014). Fish assemblages as indicators of ecosystem health in the Condamine–Balonne River system. A guide prepared for the Department of Science Information Technology Innovation and the Arts. Department of Agriculture and Fisheries, Brisbane, Qld, Australia.
Hutchison, M., Butcher, A., Kirkwood, J., Mayer, D., Chilcott, K., and Backhouse, S. (2008). Mesoscale movements of small and medium-sized fish in the Murray–Darling Basin. MDBC Publication 41/08, Murray–Darling Basin Commission, Canberra, ACT, Australia.
Hutchison, M., Norris, A., and Nixon, D. (2020). Habitat preferences and habitat restoration options for small-bodied and juvenile fish species in the northern Murray–Darling Basin. Ecological Management & Restoration 21, 51–57.
| Habitat preferences and habitat restoration options for small-bodied and juvenile fish species in the northern Murray–Darling Basin.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A. (2004). Murray cod fingerling production and growout. In ‘Development of Intensive Commercial Aquaculture Production Technology for Murray Cod. Final Report to the Fisheries Research and Development Corporation (Project number 1999/328)’. (Eds B. A. Ingram and S. S. De Silva.) pp. 25–65. (Primary Industries Research: Alexandra, Vic., Australia.)
Ingram, B. A. (2009). Culture of juvenile Murray cod, trout cod and Macquarie perch (Percichthyidae) in fertilised earthen ponds. Aquaculture 287, 98–106.
| Culture of juvenile Murray cod, trout cod and Macquarie perch (Percichthyidae) in fertilised earthen ponds.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., and De Silva, S. S. (2007). Diet composition and preference of juvenile Murray cod, trout cod and Macquarie perch (Percichthyidae) reared in fertilised earthen ponds. Aquaculture 271, 260–270.
| Diet composition and preference of juvenile Murray cod, trout cod and Macquarie perch (Percichthyidae) reared in fertilised earthen ponds.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., and Douglas, J. W. (1995). Threatened fishes of the world: Maccullochella macquariensis (Cuvier, 1829) (Percichthyidae). Environmental Biology of Fishes 43, 38.
| Threatened fishes of the world: Maccullochella macquariensis (Cuvier, 1829) (Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Ingram, B., and Gooley, G. (1996). Hormone-induced spawning of the threatened Macquarie perch (Macquaria australasica): an Australian native freshwater fish. In ‘Developing and Sustaining World Fisheries Resources: The State of Science and Management. Proceedings Vol. 1’. (Eds D. A. Hancock and J. P. Beumer.) p. 97. (CSIRO Publishing: Melbourne, Vic., Australia.)
Ingram, B. A., and Rimmer, M. A. (1992). Induced breeding and larval rearing of the endangered Australian freshwater fish trout cod, Maccullochella macquariensis (Cuvier) (Percichthyidae). Aquaculture and Fisheries Management 24, 7–17.
Ingram, B. A., Barlow, C. G., Burchmore, J. J., Gooley, G. J., Rowland, S. J., and Sanger, A. C. (1990). Threatened native freshwater fishes in Australia – some case histories. Journal of Fish Biology 37, 175–182.
| Threatened native freshwater fishes in Australia – some case histories.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., Rimmer, M. A., and Rowland, S. J. (1994). Induced spawning trials with captive Macquarie perch, Macquaria australasica (Percichthyidae). Proceedings of the Linnean Society of New South Wales 114, 109–116.
Ingram, B. A., Douglas, J. W., and Lintermans, M. (2000). Threatened fishes of the world: Macquaria australasica Cuvier, 1830 (Percichthyidae). Environmental Biology of Fishes 59, 68.
| Threatened fishes of the world: Macquaria australasica Cuvier, 1830 (Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., McPartlan, H., Ho, H. K., Rourke, M., and Bravington, W. (2007). High value aquaculture in sustainable rural landscapes. Our rural landscape: sustainable development through innovation. Final report for Project 05194. Department of Primary Industries, Melbourne, Vic., Australia.
Ingram, B. A., Hayes, B., and Rourke, M. L. (2011). Impacts of stock enhancement strategies on the effective population size of Murray cod, Maccullochella peelii, a threatened Australian fish. Fisheries Management and Ecology 18, 467–481.
| Impacts of stock enhancement strategies on the effective population size of Murray cod, Maccullochella peelii, a threatened Australian fish.Crossref | GoogleScholarGoogle Scholar |
Ingram, B. A., Ho, H. K., Turchini, G. M., and Holland, M. (2012a). Gamete quality and spawning in captive Murray cod broodstock. Fisheries Victoria Research Report number 58, Department of Primary Industries, Melbourne, Vic., Australia.
Ingram, B. A., Ho, H. K., Nguyen, T. T. T., Turchini, G., Bradley, T., and Blume, A. (2012b). Current research in Murray cod aquaculture. In ‘Proceedings of the Australasian Aquaculture Conference 2012’, 1–4 May 2012, Melbourne, Vic., Australia. (National Aquaculture Council and World Aquaculture Society & World Aquaculture Society – Asian Pacific Chapter: Melbourne, Vic., Australia.)
Ingram, B. A., Charles, I., and McInnes, B. (2014). New insights into hatchery production of freshwater catfish (Tandanus tandanus). Recreational Fishing Grants Program research report, Department of Environment and Primary Industries, Melbourne, Vic., Australia.
Ingram, B. A., Hunt, T. L., Lieschke, J., and Douglas, J. (2015). Monitoring fish stockings in Victoria: 2014 native fish surveys. Recreation Fishing Grants Program research report, Department of Economic Development, Jobs, Transport and Resources, Queenscliff, Vic., Australia.
Ivantsoff, W., and Crowley, L. (1996). Family Atherinidae. Silversides or hardyheads. In ‘Freshwater Fishes of South-eastern Australia’. (Ed. R. M. McDowall.) pp. 123–133. (Reed Books: Sydney, NSW, Australia.)
Izzo, C., Doubleday, Z. A., Grammer, G. L., Barnes, T. C., Delean, S., Ferguson, G. J., Ye, Q., and Gillanders, B. M. (2016a). Multi-species response to rapid environmental change in a large estuary system: a biochronological approach. Ecological Indicators 69, 739–748.
| Multi-species response to rapid environmental change in a large estuary system: a biochronological approach.Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Doubleday, Z. A., Grammer, G. L., Gilmore, K. L., Alleway, H. K., Barnes, T. C., Disspain, M. C. F., Giraldo, A. J., Mazioumi, N., and Gillanders, B. M. (2016b). Fish as proxies of ecological and environmental change. Reviews in Fish Biology and Fisheries 26, 265–286.
| Fish as proxies of ecological and environmental change.Crossref | GoogleScholarGoogle Scholar |
Jackson, S., and Moggridge, B. (2019). Indigenous water management. Australasian Journal of Environmental Management 26, 193–196.
| Indigenous water management.Crossref | GoogleScholarGoogle Scholar |
Jackson, S. E., Douglas, M. M., Kennard, M. J., Pusey, B. J., Huddleston, J., Harney, B., Liddy, L., Liddy, M., Liddy, R., Sullivan, L., Huddleston, B., Banderson, M., McMah, A., and Allsop, Q. (2014). ‘We like to listen to stories about fish’: integrating Indigenous ecological and scientific knowledge to inform environmental flow assessments. Ecology and Society 19, art43.
| ‘We like to listen to stories about fish’: integrating Indigenous ecological and scientific knowledge to inform environmental flow assessments.Crossref | GoogleScholarGoogle Scholar |
Janosik, A. M., and Johnston, C. E. (2015). Environmental DNA as an effective tool for detection of imperiled fishes. Environmental Biology of Fishes 98, 1889–1893.
| Environmental DNA as an effective tool for detection of imperiled fishes.Crossref | GoogleScholarGoogle Scholar |
Jardine, T. D., Galloway, A. W. E., and Kainz, M. J. (2020). Unlocking the power of fatty acids as dietary tracers and metabolic signals in fishes and aquatic invertebrates. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 375, 20190639.
| Unlocking the power of fatty acids as dietary tracers and metabolic signals in fishes and aquatic invertebrates.Crossref | GoogleScholarGoogle Scholar | 32536302PubMed |
Jenkins, K. M., and Boulton, A. J. (2003). Connectivity in a dryland river: short-term aquatic microinvertebrate recruitment following floodplain inundation. Ecology 84, 2708–2723.
| Connectivity in a dryland river: short-term aquatic microinvertebrate recruitment following floodplain inundation.Crossref | GoogleScholarGoogle Scholar |
Jones, M. J., and Stuart, I. G. (2007). Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river. Ecology Freshwater Fish 16, 210–220.
| Movements and habitat use of common carp (Cyprinus carpio) and Murray cod (Maccullochella peelii peelii) juveniles in a large lowland Australian river.Crossref | GoogleScholarGoogle Scholar |
Jones, M. J., and Stuart, I. G. (2008). Regulated floodplains – a trap for unwary fish. Fisheries Management and Ecology 15, 71–79.
| Regulated floodplains – a trap for unwary fish.Crossref | GoogleScholarGoogle Scholar |
Jones, M., Tinkler, P., Lindeman, M., Hackett, G., and Pickworth, A. (2008). Threats, distribution and abundance of Yarra pygmy perch in Victoria during a drought period. Arthur Rylah Institute for Environmental Research Technical Report Series number 184, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Kaminskas, S., and Humphries, P. (2009). Diet of Murray cod (Maccullochella peelii peelii) (Mitchell) larvae in an Australian lowland river in low flow and high flow years. Hydrobiologia 636, 449–461.
| Diet of Murray cod (Maccullochella peelii peelii) (Mitchell) larvae in an Australian lowland river in low flow and high flow years.Crossref | GoogleScholarGoogle Scholar |
Kearns, J., Tonkin, Z., O’Mahony, J., and Lyon, J. (2012). Identification and protection of key spawning habitats for Macquarie Perch in King Parrot Creek: Black Saturday Victoria 2009. Department of Natural Resources and Environment, Melbourne, Vic., Australia.
Kearns, J., Ayres, R., O’Mahony, J., Hackett, G., Liu, C., and Tonkin, Z. (2015). Investigating movement and connectivity of Macquarie perch in King Parrot Creek, northern Victoria. Client Report for the Goulburn Broken Catchment Management Authority. (Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning: Melbourne, Vic., Australia.) Available at https://www.ari.vic.gov.au/__data/assets/pdf_file/0026/34964/VBRRA-P16-web-rev2.pdf [Verified 15 September 2020].
Keenan, C., Watts, R., and Serafini, L. (1996). Population genetics of golden perch, silver perch and eel-tailed catfish within the Murray Darling Basin. In ‘Proceedings of the 1995 Riverine Environment Research Forum’, 4–6 October 1995, Melbourne, Vic., Australia. (Eds J. R. Banes and R. Lehane.) pp. 17–26. (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
King, J., and Louw, D. (1998). Instream flow assessments for regulated rivers in South Africa using the building block methodology. Aquatic Ecosystem Health & Management 1, 109–124.
| Instream flow assessments for regulated rivers in South Africa using the building block methodology.Crossref | GoogleScholarGoogle Scholar |
King, A. J., and O’Connor, J. P. (2007). Native fish entrapment in irrigation systems: a step towards understanding the significance of the problem. Ecological Management & Restoration 8, 32–37.
| Native fish entrapment in irrigation systems: a step towards understanding the significance of the problem.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Tonkin, Z., and Mahoney, J. (2007). Assessing the effectiveness of environmental flows on fish recruitment in Barmah–Millewa Forest. A report to the Murray–Darling Basin Commission. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
King, A., Tonkin, Z., and Mahoney, J. (2009a). Environmental flow enhances native fish spawning and recruitment in the Murray River, Australia. River Research and Applications 25, 1205–1218.
| Environmental flow enhances native fish spawning and recruitment in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
King, A. J., Ramsey, D., Baumgartner, L., Humphries, P., Jones, M., Koehn, J., Lyon, J., Mallen-Cooper, M., Meredith, S., Vilizzi, L., Ye, Q., and Zampatti, B. (2009b). Environmental requirements for managing successful fish recruitment in the Murray River Valley – review of existing knowledge. Arthur Rylah Institute for Environmental Research Technical Report Series number 197, Department of Sustainability and Environment, Melbourne, Vic., Australia.
King, A. J., Tonkin, Z., and Lieshcke, J. (2012). Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events. Marine and Freshwater Research 63, 576–586.
| Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events.Crossref | GoogleScholarGoogle Scholar |
King, A., Humphries, P., and McCasker, N. (2013). Reproduction and early life history. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 159–193. (CSIRO Publishing: Melbourne, Vic., Australia.)
King, A. J., Gwinn, D. C., Tonkin, Z., Mahoney, J., Raymond, S., and Beesley, L. (2016). Using abiotic drivers of fish spawning to inform environmental flow management. Journal of Applied Ecology 53, 34–43.
| Using abiotic drivers of fish spawning to inform environmental flow management.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T. (2000). Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecology 25, 109–127.
| Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia.Crossref | GoogleScholarGoogle Scholar |
Kingsford, R. T., Walker, K. F., Lester, R. E., Young, W. J., Fairweather, P. G., Sammut, J., and Geddes, M. C. (2011). A Ramsar wetland in crisis – the Coorong, Lower Lakes and Murray Mouth, Australia. Marine and Freshwater Research 62, 255–265.
| A Ramsar wetland in crisis – the Coorong, Lower Lakes and Murray Mouth, Australia.Crossref | GoogleScholarGoogle Scholar |
Knight, J. T., Butler, G. L., Smith, P. S., and Wager, R. N. E. (2007). Reproductive biology of the endangered Oxleyan pygmy perch Nannoperca oxleyana Whitley. Journal of Fish Biology 71, 1494–1511.
| Reproductive biology of the endangered Oxleyan pygmy perch Nannoperca oxleyana Whitley.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2005a). Threats to Murray cod. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop’, 4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) pp. 30–37. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra, ACT, Australia.)
Koehn, J. D. (2005b). The loss of valuable Murray cod in fish kills: a science and management perspective. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) pp. 73–82. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra, ACT, Australia.)
Koehn, J. D. (2006). The ecology and conservation management of Murray Cod Maccullochella peelii peelii. Ph.D. Thesis, The University of Melbourne, Melbourne, Vic., Australia.
Koehn, J. D. (2009a). Using radio telemetry to evaluate the depths inhabited by Murray cod (Maccullochella peelii peelii). Marine and Freshwater Research 60, 317–320.
| Using radio telemetry to evaluate the depths inhabited by Murray cod (Maccullochella peelii peelii).Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D. (2009b). Multi-scale habitat selection by Murray cod Maccullochella peelii peelii in two lowland rivers. Journal of Fish Biology 75, 113–129.
| Multi-scale habitat selection by Murray cod Maccullochella peelii peelii in two lowland rivers.Crossref | GoogleScholarGoogle Scholar | 20738486PubMed |
Koehn, J. D. (2011). Spatial management of freshwater fish: a case study for Murray cod. In ‘Spatial Management in Fisheries. Australian Society for Fish Biology 2007 Workshop Proceedings’, 11–12 September, Canberra, ACT, Australia. (Eds M. A. Treloar and R. Tilzey.) pp. 14–30. (Australian Society for Fish Biology: Canberra, ACT, Australia.)
Koehn, J. D. (2015). Managing people, water, food and fish in the Murray–Darling Basin, southeastern Australia. Fisheries Management and Ecology 22, 25–32.
| Managing people, water, food and fish in the Murray–Darling Basin, southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J., and Crook, D. (2013). Movements and migration. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 105–128. (CSIRO Publishing: Melbourne, Vic., Australia.)
Koehn, J. D., and Harrington, D. J. (2005). Collection and distribution of the early life stages of the Murray cod (Maccullochella peelii peelii) in a regulated river. Australian Journal of Zoology 53, 137–144.
| Collection and distribution of the early life stages of the Murray cod (Maccullochella peelii peelii) in a regulated river.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Harrington, D. J. (2006). Environmental conditions and timing for the spawning of Murray cod (Maccullochella peelii peelii) and the endangered trout cod (M. macquariensis) in southeastern Australian rivers. River Research and Applications 22, 327–342.
| Environmental conditions and timing for the spawning of Murray cod (Maccullochella peelii peelii) and the endangered trout cod (M. macquariensis) in southeastern Australian rivers.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Lintermans, M. (2012). A strategy to rehabilitate fishes of the Murray–Darling Basin, south-eastern Australia. Endangered Species Research 16, 165–181.
| A strategy to rehabilitate fishes of the Murray–Darling Basin, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Nicol, S. J. (2014). Comparative habitat use by large riverine fishes. Marine and Freshwater Research 65, 164–174.
| Comparative habitat use by large riverine fishes.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., and Nicol, S. J. (2016). Comparative movements of four large fish species in a lowland river. Journal of Fish Biology 88, 1350–1368.
| Comparative movements of four large fish species in a lowland river.Crossref | GoogleScholarGoogle Scholar | 26919062PubMed |
Koehn, J. D., and O’Connor, W. G. (1990). ‘Biological Information for Management of Native Freshwater Fish in Victoria.’ (Government Printer: Melbourne, Vic., Australia.)
Koehn, J. D., and Todd, C. R. (2012). Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii. Fisheries Management and Ecology 19, 410–425.
| Balancing conservation and recreational fishery objectives for a threatened fish species, the Murray cod, Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Doeg, T. J., Harrington, D. J., and Milledge, G. A. (1995). The effects of Dartmouth Dam on the aquatic fauna of the Mitta Mitta River. Report to the Murray–Darling Basin Commission. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Koehn, J. D., Nicol, S. J., McKenzie, J. A., Lieschke, J. A., Lyon, J. P., and Pomorin, K. (2008). Spatial ecology of an endangered native Australian Percichthyid fish, the trout cod Maccullochella macquariensis. Endangered Species Research 4, 219–225.
| Spatial ecology of an endangered native Australian Percichthyid fish, the trout cod Maccullochella macquariensis.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., McKenzie, J. A., O’Mahony, D. J., Nicol, S. J., O’Connor, J. P., and O’Connor, W. G. (2009). Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river. Ecology Freshwater Fish 18, 594–602.
| Movements of Murray cod (Maccullochella peelii peelii) in a large Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Hobday, A. J., Pratchett, M. S., and Gillanders, B. M. (2011). Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation. Marine and Freshwater Research 62, 1148–1164.
| Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Lintermans, M., Lyon, J. P., Ingram, B. A., Gilligan, D. M., Todd, C. R., and Douglas, J. W. (2013). Recovery of the endangered trout cod, Maccullochella macquariensis: what have we achieved in more than 25 years? Marine and Freshwater Research 64, 822–837.
| Recovery of the endangered trout cod, Maccullochella macquariensis: what have we achieved in more than 25 years?Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., King, A. J., Beesley, L., Copeland, C., Zampatti, B. P., and Mallen-Cooper, M. (2014a). Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management. Ecological Management & Restoration 15, 40–50.
| Flows for native fish in the Murray–Darling Basin: lessons and considerations for future management.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Lintermans, M., and Copeland, C. (2014b). Laying the foundations for fish recovery: the first 10 years of the Native Fish Strategy for the Murray–Darling Basin, Australia. Ecological Management & Restoration 15, 3–12.
| Laying the foundations for fish recovery: the first 10 years of the Native Fish Strategy for the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Koehn, J. D., Stuart, I. G., Bamford, H., Bice, C. M., Hodges, K., Jackson, P., Lieschke, J., Lovett, S., Mallen-Cooper, M., Raadik, T., Thiem, J., Todd, C., Tonkin, Z., and Zampatti, B. (2014c). Quantifiable environmental outcomes for fish. Client Report for the Murray–Darling Basin Authority, Arthur Rylah Institute for Environmental Research, Department of Environment and Primary Industries, Melbourne, Vic., Australia.) Available at https://www.researchgate.net/publication/274998593_Quantifiable_environmental_outcomes_for_fish [Verified 15 September 2020].
Koehn, J. D., Balcombe, S. R., and Zampatti, B. P. (2019a). Fish and flow management in the Murray–Darling Basin: directions for research. Ecological Management & Restoration 20, 142–150.
| Fish and flow management in the Murray–Darling Basin: directions for research.Crossref | GoogleScholarGoogle Scholar |
Koehn, J., Lintermans, M., and Copeland, C. (2019b). Recovering Murray–Darling Basin fishes by revitalizing a Native Fish Strategy. EMR Project Summaries. Available at https://site.emrprojectsummaries.org/2019/09/13/recovering-murray-darling-basin-fishes-by-revitalizing-a-native-fish-strategy-update-of-emr-feature/ [Verified 19 August 2020].
Koehn, J. D., Balcombe, S. R., Baumgartner, L. J., Bice, C. M., Burndred, K., Ellis, I., Koster, W. M., Lintermans, M., Pearce, L., Sharpe, C., Stuart, I., and Todd, C. (2020). What is needed to restore native fishes in the Murray–Darling Basin? Marine and Freshwater Research 71, 1464–1468.
| What is needed to restore native fishes in the Murray–Darling Basin?Crossref | GoogleScholarGoogle Scholar |
Kopf, S. M., Humphries, P., and Watts, R. J. (2014). Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species. Journal of Fish Biology 84, 1820–1841.
| Ontogeny of critical and prolonged swimming performance for the larvae of six Australian freshwater fish species.Crossref | GoogleScholarGoogle Scholar | 24814314PubMed |
Koster, W. M. (1997). A study of the interactions between the dwarf galaxias (Galaxiella pusilla), southern pygmy perch (Nannoperca australis) and eastern gambusia (Gambusia holbrooki). B.Sc.(Hons) Thesis, Deakin University, Geelong, Vic., Australia.
Koster, W. M., and Crook, D. A. (2017). Using telemetry data to develop conceptual models of movement to support the management of riverine fishes. Marine and Freshwater Research 68, 1567–1575.
| Using telemetry data to develop conceptual models of movement to support the management of riverine fishes.Crossref | GoogleScholarGoogle Scholar |
Koster, W., Crook, D., and Dawson, D. (2009). Lower Goulburn fish communities project 2009. Annual report, Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Koster, W. M., Dawson, D. R., Morrongiello, J. R., and Crook, D. A. (2013). Spawning season movements of Macquarie perch (Macquaria australasica) in the Yarra River, Victoria. Australian Journal of Zoology 61, 386–394.
| Spawning season movements of Macquarie perch (Macquaria australasica) in the Yarra River, Victoria.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Dawson, D. R., O’Mahony, D. J., Moloney, P. D., and Crook, D. A. (2014). Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua). PLoS One 9, e96044.
| Timing, frequency and environmental conditions associated with mainstem–tributary movement by a lowland river fish, golden perch (Macquaria ambigua).Crossref | GoogleScholarGoogle Scholar | 24788137PubMed |
Koster, W. M., Dawson, D. R., Clunie, P., Hames, F., McKenzie, J., Moloney, P. D., and Crook, D. (2015). Movement and habitat use of the freshwater catfish (Tandanus tandanus) in a remnant floodplain wetland. Ecology Freshwater Fish 24, 443–455.
| Movement and habitat use of the freshwater catfish (Tandanus tandanus) in a remnant floodplain wetland.Crossref | GoogleScholarGoogle Scholar |
Koster, W. M., Dawson, D. R., Liu, C., Moloney, P. D., Crook, D. A., and Thomson, J. R. (2017). Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia. Journal of Fish Biology 90, 93–108.
| Influence of streamflow on spawning-related movements of golden perch Macquaria ambigua in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 27734494PubMed |
Koster, W. M., Dawson, D. R., Kitchingman, A., Moloney, P. D., and Hale, R. (2020). Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool. Journal of Fish Biology 96, 782–794.
| Habitat use, movement and activity of two large-bodied native riverine fishes in a regulated lowland weir pool.Crossref | GoogleScholarGoogle Scholar | 32017088PubMed |
Kuiter, R. H., and Allen, G. R. (1986). A synopsis of the Australian pygmy perches (Percichthyidae), with the description of a new species. Revue Française d’Aquariologie Herpétologie 12, 109–116.
Kuiter, R. H., Humphries, P., and Arthington, A. H. (1996). Pygmy perch. In ‘Freshwater Fishes of South-Eastern Australia’. (Ed. R. M. McDowall.) pp. 168–175. (Reed Books: Sydney, NSW, Australia.)
Lake, J. S. (1959). The freshwater fishes of New South Wales. New South Wales State Fisheries Bulletin 5, 1–20.
Lake, J. S. (1967a). Principal fishes of the Murray–Darling River system. In ‘Australian Inland Waters and Their Fauna’. (Ed. A. H. Weatherly.) pp. 192–203. (Australian National University Press: Canberra, ACT, Australia.)
Lake, J. S. (1967b). Rearing experiments with five species of Australian freshwater fishes: II. Morphogenesis and ontogeny. Australian Journal of Marine and Freshwater Research 18, 155–173.
| Rearing experiments with five species of Australian freshwater fishes: II. Morphogenesis and ontogeny.Crossref | GoogleScholarGoogle Scholar |
Lake, J. S. (1967c). Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning. Australian Journal of Marine and Freshwater Research 18, 137–153.
| Rearing experiments with five species of Australian freshwater fishes. I. Inducement to spawning.Crossref | GoogleScholarGoogle Scholar |
Lake, J. S. (1967d). Freshwater fish of the Murray–Darling River system: the native and introduced fish species. NSW Chief Secretary’s Department: Sydney, NSW, Australia.
Lake, J. S. (1967e). Freshwater catfish. Freshwater fish of the Murray Darling River system. State Fisheries Research Bulletin number 7, New South Wales State Fisheries, Sydney, NSW, Australia.
Lake, J. S. (1971). ‘Freshwater Fishes of Rivers of Australia.’ (Thomas Nelson: Sydney, NSW, Australia.)
Langdon, J. S. (1989). Experimental transmission and pathogenicity of epizootic haematopoietic necrosis virus (EHNV) in redfin perch, Perca fluviatilis L., and 11 other teleosts. Journal of Fish Diseases 12, 295–310.
| Experimental transmission and pathogenicity of epizootic haematopoietic necrosis virus (EHNV) in redfin perch, Perca fluviatilis L., and 11 other teleosts.Crossref | GoogleScholarGoogle Scholar |
Lapointe, N. W. R., Cooke, S. J., Imhof, J. G., Boisclair, D., Casselman, J. M., Curry, R. A., Langer, O. E., McLaughlin, R. I., Minns, C. K., Post, J. R., Power, M., Rasmussen, J. B., Reynolds, J. D., Richardson, J. S., and Tonn, W. M. (2014). Principles for ensuring healthy and productive freshwater ecosystems that support sustainable fisheries. Environmental Reviews 22, 110–134.
| Principles for ensuring healthy and productive freshwater ecosystems that support sustainable fisheries.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Woinarski, J., Garnett, S., Nimmo, D., Scheele, B., Lintermans, M., Mitchell, N., Whiterod, N., and Ferris, J. (2020). Rapid analysis of impacts of the 2019–20 fires on animal species, and prioritisation of species for management response. Report prepared for the Wildlife and Threatened Species Bushfire Recovery Expert Panel 14 March 2020. (Department of Agriculture, Water and the Environment.) Available at https://www.environment.gov.au/system/files/pages/ef3f5ebd-faec-4c0c-9ea9-b7dfd9446cb1/files/assessments-species-vulnerability-fire-impacts-14032020.pdf [Verified 21 August 2020].
Leggett, R. (1984). The olive perch Ambassis nigripinnis. Fishes of Sahul 1, 29–30.
Leigh, S. J., and Zampatti, B. P. (2013). Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia. Australian Journal of Zoology 61, 160–169.
| Movement and mortality of Murray cod, Maccullochella peelii, during overbank flows in the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Leigh, S. J., Zampatti, B. P., and Nicol, J. M. (2008). Spatial and temporal variation in larval fish assemblage structure in the Chowilla Anabranch system: with reference to water physico-chemistry and stream hydrology. SARDI Publication number F2008/000051-1, SARDI Report Series number 286, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Lester, R. E., Webster, I. T., Fairweather, P. G., and Young, W. J. (2011). Linking water-resource models to ecosystem-response models to guide water-resource planning – an example from the Murray–Darling Basin, Australia. Marine and Freshwater Research 62, 279–289.
| Linking water-resource models to ecosystem-response models to guide water-resource planning – an example from the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Liermann, C. R., Nilsson, C., Robertson, J., and Ng, R. Y. (2012). Implications of dam obstruction for global freshwater fish diversity. Bioscience 62, 539–548.
| Implications of dam obstruction for global freshwater fish diversity.Crossref | GoogleScholarGoogle Scholar |
Lieschke, J. A., Lyon, J. P., Moloney, P. D., and Nicol, S. J. (2016). Spatial partitioning in the use of structural woody habitat supports the cohabitation of two cod species in a large lowland river. Marine and Freshwater Research 67, 1835–1843.
| Spatial partitioning in the use of structural woody habitat supports the cohabitation of two cod species in a large lowland river.Crossref | GoogleScholarGoogle Scholar |
Likens, G. E., Walker, K. F., Davies, P. E., Brookes, J., Olley, J., Young, W. J., Thoms, M. C., Lake, P. S., Gawne, B., Davis, J., Arthington, A. H., Thompson, R., and Oliver, R. L. (2009). Ecosystem science: toward a new paradigm for managing Australia’s inland aquatic ecosystems. Marine and Freshwater Research 60, 271–279.
| Ecosystem science: toward a new paradigm for managing Australia’s inland aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2004). Rehabilitation of fish habitats in the Murrumbidgee River, ACT. Final report to the MD 2001 FishRehab Program. Environment ACT, Canberra, ACT, Australia.
Lintermans, M. (2007). ‘Fishes of the Murray–Darling Basin: An Introductory Guide.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Lintermans, M. (2008). The status of Macquarie Perch Macquaria australasica in the Mongarlowe River in 2007 and 2008. Report to the Friends of the Mongarlowe River Inc. (Landcare.) Available at https://landcare.nsw.gov.au/groups/friends-of-the-mongarlowe-river-inc/reports/The%20Status%20of%20Macquarie%20Perch%20Macquaria%20australasica%20in%20the%20Mongarlowe%20River%20Final%20report/view [Verified 21 August 2020].
Lintermans, M. (2012). Managing potential impacts of reservoir enlargement on threatened Macquaria australasica and Gadopsis bispinosus in southeastern Australia. Endangered Species Research 16, 1–16.
| Managing potential impacts of reservoir enlargement on threatened Macquaria australasica and Gadopsis bispinosus in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2013a). Conservation and management. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 283–316. (CSIRO Publishing: Melbourne, Vic., Australia.)
Lintermans, M. (2013b). The rise and fall of a translocated population of the endangered Macquarie perch, Macquaria australasica, in south-eastern Australia. Marine and Freshwater Research 64, 838–850.
| The rise and fall of a translocated population of the endangered Macquarie perch, Macquaria australasica, in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2013c). A review of on-ground recovery actions for threatened freshwater fish in Australia. Marine and Freshwater Research 64, 775–791.
| A review of on-ground recovery actions for threatened freshwater fish in Australia.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M. (2016). Finding the needle in the haystack: comparing sampling methods for detecting an endangered freshwater fish. Marine and Freshwater Research 67, 1740–1749.
| Finding the needle in the haystack: comparing sampling methods for detecting an endangered freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Lintermans, M., and Pearce, L. (2017). Southern Pygmy Perch Nannoperca australis – a local action plan for Gunning District Landcare. Consultancy report prepared for Gunning District Landcare. University of Canberra, Canberra, ACT, Australia.
Lintermans, M., and Robinson, W. (2018). The extent and adequacy of monitoring of Australian threatened freshwater fish. In ‘Monitoring Threatened Species and Ecological Communities’. (Eds S. Legge, D. Lindenmayer, N. Robinson, B. Scheele, D. Southwell, and B. Wintle.) pp. 85–99. (CSIRO Publishing: Melbourne, Vic., Australia.)
Lintermans, M., Kukolic, K., and Rutzou, T. (1988). The status of the trout cod Maccullochella macquariensis in the Australian Capital Territory. Victorian Naturalist 105, 205–207.
Lintermans, M., Rowland, S., Koehn, J., Butler, G. L., Simpson, B., and Wooden, I. (2004). The status, threats and management of freshwater cod species Maccullochella spp. in Australia. In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) pp. 15–29. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra, ACT, Australia.)
Lintermans, M., Broadhurst, B., Thiem, J. D., Ebner, B. C., Wright, D., Clear, R., and Norris, R. (2010). Constructed homes for threatened fishes in the Cotter River catchment: phase 2 final report. Report to ACTEW Corporation. Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia.
Lintermans, M., Broadhurst, B., and Clear, R. (2011). Characterising potential predation of Macquarie perch Macquaria australasica by cormorants in Cotter Reservoir. Report to ACTEW Corporation. Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia.
Lintermans, M., Lyon, J. P., Hammer, M. P., Ellis, I., and Ebner, B. C. (2015). Chapter 17. Underwater, out of sight: lessons from threatened freshwater fish translocations in Australia. In ‘Advances in Reintroduction Biology of Australian and New Zealand Fauna’. (Eds D. P. Armstrong, M. W. Hayward, D. Moro, and P. J. Seddon.) pp. 237–253. (CSIRO Publishing: Melbourne, Vic., Australia.)
Lintermans, M., Geyle, H. M., Beatty, S., Brown, C., Ebner, B. C., Freeman, R., Hammer, M. P., Humphreys, W. F., Kennard, M. J., Kern, P., Martin, K., Morgan, D. L., Raadik, T. A., Unmack, P. J., Wager, R., Woinarski, J. C. Z., and Garnett, S. T. (2020). Big trouble for little fish: identifying Australian freshwater fishes in imminent risk of extinction. Pacific Conservation Biology. , .
| Big trouble for little fish: identifying Australian freshwater fishes in imminent risk of extinction.Crossref | GoogleScholarGoogle Scholar |
Llewellyn, L. C. (1968). Tagging gives answers to fish growth queries. Fisherman – Journal of State Fisheries, New South Wales 3, 1–4.
Llewellyn, L. C. (1974). Spawning, development and distribution of the southern pigmy perch Nannoperca australis australis Gunther from inland waters in eastern Australia. Marine and Freshwater Research 25, 121–149.
| Spawning, development and distribution of the southern pigmy perch Nannoperca australis australis Gunther from inland waters in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Llewellyn, L. C. (1983). The distribution of fish in New South Wales. Australian Society for Limnology, Special Publication 7, 1–68.
Llewellyn, L. C. (2008). Observations on the breeding biology of Ambassis agassizii Steindachner, 1867 (Teleostei: Ambassidae) from the Murray Darling Basin in New South Wales. Australian Zoologist 34, 476–498.
| Observations on the breeding biology of Ambassis agassizii Steindachner, 1867 (Teleostei: Ambassidae) from the Murray Darling Basin in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Llewellyn, L. C. (2014). Movements of golden perch Macquaria ambigua (Richardson) in the mid Murray and lower Murrumbidgee rivers (New South Wales) with notes on other species. Australian Zoologist 37, 139–156.
| Movements of golden perch Macquaria ambigua (Richardson) in the mid Murray and lower Murrumbidgee rivers (New South Wales) with notes on other species.Crossref | GoogleScholarGoogle Scholar |
Llewellyn, L. C., and MacDonald, M. C. (1980). Family Percichthyidae. Australian freshwater basses and cods. In ‘Freshwater Fishes of South-eastern Australia’. (Ed. R. M. McDowall.) pp. 142–149. (Reed: Sydney, NSW, Australia.)
Lloyd, L. N., and Walker, K. F. (1986). Distribution and conservation status of small freshwater fish in the River Murray, South Australia. Transactions of the Royal Society of South Australia 110, 49–57.
Lugg, A., and Copeland, C. (2014). Review of cold water pollution in the Murray–Darling Basin and the impacts on fish communities. Ecological Management & Restoration 15, 71–79.
| Review of cold water pollution in the Murray–Darling Basin and the impacts on fish communities.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., and O’Connor, J. P. (2008). Smoke on the water: can riverine fish populations recover following a catastrophic fire-related sediment slug? Austral Ecology 33, 794–806.
| Smoke on the water: can riverine fish populations recover following a catastrophic fire-related sediment slug?Crossref | GoogleScholarGoogle Scholar |
Lyon, J., and Ryan, T. (2005). Observations of the nationally threatened freshwater fish, Murray hardyhead Craterocephalus fluviatilis McCulloch 1913, in three Victorian salt lakes. Victorian Naturalist 122, 78–84.
Lyon, J. P., Ryan, T. J., and Scroggie, M. P. (2008). Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecology Freshwater Fish 17, 184–188.
| Effects of temperature on the fast-start swimming performance of an Australian freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Lyon, J., Stuart, I., Ramsey, D., and O’Mahony, J. (2010). The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management. Marine and Freshwater Research 61, 271.
| The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management.Crossref | GoogleScholarGoogle Scholar |
Lyon, J., Todd, C., Nicol, S. J., Macdonald, A., Stoessel, D., Ingram, B. A., Barker, R. J., and Bradshaw, C. J. A. (2012). Reintroduction success of threatened Australian trout cod (Maccullochella macquariensis) based on growth and reproduction. Marine and Freshwater Research 63, 598–605.
| Reintroduction success of threatened Australian trout cod (Maccullochella macquariensis) based on growth and reproduction.Crossref | GoogleScholarGoogle Scholar |
Lyon, J., Kearns, J., Bird, T., Tonkin, Z., Mahony, J. O., Nicol, S., Hackett, G., Raymond, S., Todd, C., Kitchingman, A., Lyon, J., Kearns, J., Bird, T., Tonkin, Z., Mahony, J. O., Nicol, S., Hackett, G., Raymond, S., Todd, C., and Kitchingman, A. (2014a). Monitoring of resnagging between Lake Hume and Yarrawonga: final report 2014. Arthur Rylah Institute for Environmental Research Report to the Murray–Darling Basin Authority, The Living Murray Program. Department of Environment and Primary Industries, Melbourne, Vic., Australia.
Lyon, J. P., Bird, T., Nicol, S., Kearns, J., Mahony, J. O., Todd, C. R., Cowx, I. G., and Bradshaw, C. J. A. (2014b). Efficiency of electrofishing in turbid lowland rivers: implications for measuring temporal change in fish populations. Canadian Journal of Fisheries and Aquatic Sciences 71, 878–886.
| Efficiency of electrofishing in turbid lowland rivers: implications for measuring temporal change in fish populations.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., Tonkin, Z., Moloney, P. D., Todd, C., and Nicol, S. (2018). Conservation implications of angler misidentification of an endangered fish. Aquatic Conservation 28, 1396–1402.
| Conservation implications of angler misidentification of an endangered fish.Crossref | GoogleScholarGoogle Scholar |
Lyon, J. P., Bird, T. J., Kearns, J., Nicol, S., Tonkin, Z., Todd, C. R., O’Mahony, J., Hackett, G., Raymond, S., Lieschke, J., Kitchingman, A., and Bradshaw, C. J. A. (2019). Increased population size of fish in a lowland river following restoration of structural habitat. Ecological Applications 29, e01882.
| Increased population size of fish in a lowland river following restoration of structural habitat.Crossref | GoogleScholarGoogle Scholar | 30946514PubMed |
MacDonald, A. J., Young, M. J., Lintermans, M., and Sarre, S. D. (2014). Primers for detection of Macquarie perch from environmental and trace DNA samples. Conservation Genetics Resources 6, 551–553.
| Primers for detection of Macquarie perch from environmental and trace DNA samples.Crossref | GoogleScholarGoogle Scholar |
Mackay, N. J. (1973). Histological changes in the ovaries of the golden perch, (Plectroplites ambiguus), associated with the reproductive cycle. Marine and Freshwater Research 24, 95–102.
| Histological changes in the ovaries of the golden perch, (Plectroplites ambiguus), associated with the reproductive cycle.Crossref | GoogleScholarGoogle Scholar |
Mackay, N., and Eastburn, D. R. (Eds) (1990). ‘The Murray.’ (Murray–Darling Basin Commission: Canberra, ACT, Australia.)
Maheshwari, B. L., Walker, K. F., and McMahon, T. A. (1995). Effects of regulation on the flow regime of the River Murray, Australia. Regulated Rivers: Research and Management 10, 15–38.
| Effects of regulation on the flow regime of the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M. (1999). Developing fishways for non-salmonid fishes: a case study from the Murray River in Australia. In ‘Innovations in Fish Passage Technology’. (Ed. M. Odeh.) pp. 173–195. (American Fisheries Society: Bethesda, MD, USA.)
Mallen-Cooper, M., and Brand, D. A. (2007). Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage? Fisheries Management and Ecology 14, 319–332.
| Non-salmonids in a salmonid fishway: what do 50 years of data tell us about past and future fish passage?Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Stuart, I. G. (2003). Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system. River Research and Applications 19, 697–719.
| Age, growth and non-flood recruitment of two potamodromous fishes in a large semi-arid/temperate river system.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. (2015). Background paper: use of life history conceptual models of fish in flow management in the Murray–Darling Basin. Murray–Darling Basin Authority, Canberra, ACT, Australia.
Mallen-Cooper, M., and Zampatti, B. P. (2018). History, hydrology and hydraulics: rethinking the ecological management of large rivers. Ecohydrology 11, e1965.
| History, hydrology and hydraulics: rethinking the ecological management of large rivers.Crossref | GoogleScholarGoogle Scholar |
Mallen-Cooper, M., and Zampatti, B. P. (in press). Restoring the ecological integrity of a dryland river: why low flows in the Barwon–Darling River must flow. Ecological Management & Restoration , .
Mallen-Cooper, M., Stuart, I. G., Hides-Pearson, F., and Harris, J. H. (1995). Fish migration in the Murray River and assessment of the Torrumbarry fishway. Final report for Natural Resources Management Strategy Project. NSW Fisheries, Sydney, NSW, Australia.
Mallen-Cooper, M., Koehn, J., King, A., Stuart, I., and Zampatti, B. (2008). Risk assessment of the proposed Chowilla regulator and managed floodplain inundations for fish. Report to Department of Water, Land and Biodiversity, South Australia. Fishway Consulting, Sydney, NSW, Australia.
Mallen-Cooper, M., Zampatti, B., Hillman, T., King, A., Koehn, J., Saddlier, S., Sharpe, S., and Stuart, I. (2011). Managing the Chowilla Creek environmental regulator for fish species at risk. Technical report prepared for the South Australian Murray–Darling Basin Natural Resources Management Board. Fishway Consulting, Sydney, NSW, Australia.
Malmqvist, B., and Rundle, S. (2002). Threats to the running water ecosystems of the world. Environmental Conservation 29, 134–153.
| Threats to the running water ecosystems of the world.Crossref | GoogleScholarGoogle Scholar |
Marsden, T., Berghuis, A., and Stuart, I. (2015). Fish Friendly Fitzroy Project, Fitzroy barrage fishway sampling and recommended upgrades. Report to the Fitzroy Basin Association for the Fish Friendly Fitzroy Project. The Fisheries Collective, Melbourne, Vic., Australia.
Marshall, J. C., Menke, N., Crook, D. A., Lobegeiger, J. S., Balcombe, S. R., Huey, J. A., Fawcett, J. H., Bond, N. R., Starkey, A. H., Sternberg, D., Linke, S., and Arthington, A. H. (2016). Go with the flow: the movement behaviour of fish from isolated waterhole refugia during connecting flow events in an intermittent dryland river. Freshwater Biology 61, 1242–1258.
| Go with the flow: the movement behaviour of fish from isolated waterhole refugia during connecting flow events in an intermittent dryland river.Crossref | GoogleScholarGoogle Scholar |
Mayrhofer, M. (2007). Comparison of the life history and population biology of golden perch, Macquaria ambigua (Richardson 1845) (Perciformes: Perchthyidae). B.Sc.(Hons) Thesis, University of Adelaide, Adelaide, SA, Australia.
McCasker, N., Humphries, P., Meredith, S., and Klomp, N. (2014). Contrasting patterns of larval mortality in two sympatric riverine fish species: a test of the critical period hypothesis. PLoS One 9, e109317.
| Contrasting patterns of larval mortality in two sympatric riverine fish species: a test of the critical period hypothesis.Crossref | GoogleScholarGoogle Scholar | 25299441PubMed |
McDowall, R. M. (Ed.) (1996). ‘Freshwater Fishes of South-Eastern Australia.’ (Reed Books: Sydney, NSW, Australia.)
McDowall, R. M. (2006). Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes? Reviews in Fish Biology and Fisheries 16, 233–422.
| Crying wolf, crying foul, or crying shame: alien salmonids and a biodiversity crisis in the southern cool-temperate galaxioid fishes?Crossref | GoogleScholarGoogle Scholar |
McKenzie, J. R., Parsons, B., Seitz, A. C., Kopf, R. K., Mesa, M., and Phelps, Q. (Eds) (2012). ‘Proceedings of the 2nd International Symposium on Advances in Fish Tagging and Marking Technology, Auckland, New Zealand. American Fisheries Society Symposium 76.’ (American Fisheries Society: Bethesda, MD, USA.)
McKinnon, L. J. (1997). Monitoring of fish aspects of the flooding of Barmah Forest. Final report to the Murray–Darling Basin Commission for Natural Resources, Management Strategy Project V014. Marine and Freshwater Resources Institute, Queenscliff, Vic., Australia.
McMahon, T. A., and Finlayson, B. L. (2003). Droughts and anti-droughts: the low-flow hydrology of Australian rivers. Freshwater Biology 48, 1147–1160.
| Droughts and anti-droughts: the low-flow hydrology of Australian rivers.Crossref | GoogleScholarGoogle Scholar |
McNeil, D. G., and Closs, G. P. (2007). Behavioural responses of a south-east Australian floodplain fish community to gradual hypoxia. Freshwater Biology 52, 412–420.
| Behavioural responses of a south-east Australian floodplain fish community to gradual hypoxia.Crossref | GoogleScholarGoogle Scholar |
McNeil, D., Wilson, P., Hartwell, D., and Pellizzare, M. (2008). Olive perchlet (Ambassis agassizii) in the Lachlan River: population status and sustainability in the Lake Brewster region. A report submitted to the Lachlan Catchment Management Authority, SARDI Aquatic Sciences Publication number F2008/000846-1, SARDI Research Report Series number 309, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Merrick, J. R. (1996). Family Terapontidae – freshwater grunters or perches, Chapter 26. In ‘Freshwater Fishes of South-eastern Australia’. (Ed. R. M. McDowall.) pp. 164–166. (Reed Books: Sydney, NSW, Australia.)
Merrick, J. R., and Midgley, S. H. (1981). Spawning behaviour of the freshwater catfish Tandanus tandanus (Plotosidae). Marine and Freshwater Research 32, 1003–1006.
| Spawning behaviour of the freshwater catfish Tandanus tandanus (Plotosidae).Crossref | GoogleScholarGoogle Scholar |
Merrick, J. R., and Schmida, G. E. (1984). ‘Australian Freshwater Fishes: Biology and Management.’ (Griffin Press: Adelaide, SA, Australia.)
Michie, L. E., Hitchcock, J. N., Thiem, J. D., Boys, C. A., and Mitrovic, S. M. (2020). The effect of varied dam release mechanisms and storage volume on downstream river thermal regimes. Limnologica 81, 125760.
| The effect of varied dam release mechanisms and storage volume on downstream river thermal regimes.Crossref | GoogleScholarGoogle Scholar |
Milton, D. A., and Arthington, A. H. (1985). Reproductive strategy and growth of the Australian smelt, Retropinna semoni (Weber) (Pisces: Retropinnidae), and the olive perchlet, Ambassis nigripinnis (De Vis) (Pisces: Ambassidae) in Brisbane, southeastern Queensland. Australian Journal of Marine and Freshwater Research 36, 329–341.
| Reproductive strategy and growth of the Australian smelt, Retropinna semoni (Weber) (Pisces: Retropinnidae), and the olive perchlet, Ambassis nigripinnis (De Vis) (Pisces: Ambassidae) in Brisbane, southeastern Queensland.Crossref | GoogleScholarGoogle Scholar |
Mitchell, P. A. (1976). A study of aspects of the behaviour and breeding biology of the southern pigmy perch Nannoperca australis australis (Gunther) (Teleostei, Nannopercidae). B.Sc.(Hons) Thesis, The University of Melbourne, Melbourne, Vic., Australia.
Moffatt, D., and Voller, J. (2002). ‘Fish and Fish Habitat of the Queensland Murray–Darling Basin.’ Information series. (Department of Primary Industries: Brisbane, Qld, Australia.)
Moggridge, B. J., Betterridge, L., and Thompson, R. M. (2019). Integrating Aboriginal cultural values into water planning; a case study from New South Wales, Australia. Australasian Journal of Environmental Management 26, 273–286.
| Integrating Aboriginal cultural values into water planning; a case study from New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Moore, M., and Marsden, T. (2013). Moores Creek Fishway sampling report. Queensland Department of Agriculture Fisheries and Forestry, Brisbane, Qld, Australia.
Moore, A., Ingram, B. A., Friend, S., Ho, H. K., Robinson, N., McCormack, R., Coughran, J., and Hayes, B. (2010). ‘Management of Genetic Resources for Fish and Crustaceans in the Murray–Darling Basin.’ (Bureau of Rural Sciences: Canberra, ACT, Australia).
Morgan, M. G. (2014). Use (and abuse) of expert elicitation in support of decision making for public policy. Proceedings of the National Academy of Sciences of the United States of America 111, 7176–7184.
| Use (and abuse) of expert elicitation in support of decision making for public policy.Crossref | GoogleScholarGoogle Scholar | 24821779PubMed |
Morrongiello, J. R., Bond, N. R., Crook, D. A., and Wong, B. B. M. (2010). Nuptial coloration varies with ambient light environment in a freshwater fish. Journal of Evolutionary Biology 23, 2718–2725.
| Nuptial coloration varies with ambient light environment in a freshwater fish.Crossref | GoogleScholarGoogle Scholar | 20964785PubMed |
Morrongiello, J. R., Beatty, S. J., Bennett, J. C., Crook, D. A., Ikedife, D. N. E. N., Kennard, M. J., Kerezsy, A., Lintermans, M., McNeil, D. G., Pusey, B. J., and Rayner, T. S. (2011). Climate change and its implications for Australia’s freshwater fish. Marine and Freshwater Research 62, 1082–1098.
| Climate change and its implications for Australia’s freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Crook, D. A., King, A. J., Ramsey, D. S. L., and Brown, P. (2011b). Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes. Global Change Biology 17, 745–755.
| Impacts of drought and predicted effects of climate change on fish growth in temperate Australian lakes.Crossref | GoogleScholarGoogle Scholar |
Morrongiello, J. R., Bond, N. R., Crook, D. A., and Wong, B. B. M. (2012). Spatial variation in egg size and egg number reflects trade-offs and bet-hedging in a freshwater fish. Journal of Animal Ecology 81, 806–817.
| Spatial variation in egg size and egg number reflects trade-offs and bet-hedging in a freshwater fish.Crossref | GoogleScholarGoogle Scholar | 22309288PubMed |
Murphy, B. F., and Timbal, B. (2008). A review of recent climate variability and climate change in southeastern Australia. International Journal of Climatology 28, 859–879.
| A review of recent climate variability and climate change in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Murray–Darling Basin Authority (2011). Delivering a healthy working basin. About the draft basin plan. (MDBA: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/sites/default/files/pubs/delivering-a-healthy-working-basin.pdf [Verified 16 April 2020].
Murray–Darling Basin Authority (2020). Native fish recovery strategy. Working together for the future of native fish. (MDBA: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/node/5971/ [Verified 23 June 2020].
Murray–Darling Basin Commission (2004). Native fish strategy for the Murray–Darling Basin 2003–2013. (MDBC: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/sites/default/files/pubs/NFS-for-MDB-2003-2013.pdf [Verified 23 May 2017].
National Murray Cod Recovery Team (2010a). National recovery plan for the Murray cod Maccullochella peelii peelii. (Department of Sustainability and Environment: Melbourne, Vic., Australia.) Available at https://www.environment.gov.au/system/files/resources/bcc0fbf6-279b-4c52-88c5-42ce4d44b864/files/murray-cod.pdf [Verified 1 April 2020].
National Murray Cod Recovery Team (2010b). Background and implementation information for the national recovery plan for the Murray cod Maccullochella peelii peelii. (Department of Sustainability and Environment: Melbourne, Vic., Australia.) Available at https://www.environment.gov.au/system/files/resources/bcc0fbf6-279b-4c52-88c5-42ce4d44b864/files/murray-cod-background.pdf [Verified 1 April 2020].
Navarro, A., Boys, C. A., Robinson, W., Baumgartner, L. J., Miller, B., Deng, Z. D., and Finlayson, C. M. (2019). Tolerable ranges of fluid shear for early life-stage fishes: implications for safe fish passage at hydropower and irrigation infrastructure. Marine and Freshwater Research 70, 1503–1512.
| Tolerable ranges of fluid shear for early life-stage fishes: implications for safe fish passage at hydropower and irrigation infrastructure.Crossref | GoogleScholarGoogle Scholar |
Neave, I., McLeod, A., Raisin, G., and Swirepik, J. (2015). Managing water in the Murray–Darling Basin under a variable climate. Dealing with climate change in the 2012 Basin Plan and into the future. AWA Water Journal 42, 102–107.
Neira, F. J., Miskiewicz, A. G., and Trnski, T. (Eds) (1998). ‘Larvae of Temperate Australian Fishes – Laboratory Guide for Larval Fish Identification.’ (University of Western Australia Press: Perth, WA, Australia.)
Nicol, S. J., Lieschke, J. A., Lyon, J. P., and Koehn, J. D. (2004). Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia. New Zealand Journal of Marine and Freshwater Research 38, 541–551.
| Observations on the distribution and abundance of carp and native fish, and their responses to a habitat restoration trial in the Murray River, Australia.Crossref | GoogleScholarGoogle Scholar |
Nicol, S., Todd, C., Koehn, J., and Lieschke, J. (2005). How can recreational angling regulations help meet the multiple objectives of Murray cod populations? In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) pp. 98–106. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology: Canberra, ACT, Australia.)
Nicol, S. J., Barker, R. J., Koehn, J. D., and Burgman, M. A. (2007). Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis, Cuvier. Biological Conservation 138, 30–37.
| Structural habitat selection by the critically endangered trout cod, Maccullochella macquariensis, Cuvier.Crossref | GoogleScholarGoogle Scholar |
Nilsson, C. C., Reidy, A., Dynesius, M., and Revenga, C. (2005). Fragmentation and flow regulation of the world’s large river systems. Science 308, 405–408.
| Fragmentation and flow regulation of the world’s large river systems.Crossref | GoogleScholarGoogle Scholar |
Nock, C. J., and Baverstock, P. R. (2008). Polymorphic microsatellite loci for species of Australian freshwater cod, Maccullochella. Conservation Genetics 9, 1353–1356.
| Polymorphic microsatellite loci for species of Australian freshwater cod, Maccullochella.Crossref | GoogleScholarGoogle Scholar |
Nock, C. J., Elphinstone, M. S., Rowland, S. J., and Baverstock, P. R. (2010). Phylogenetics and revised taxonomy of the Australian freshwater cod genus, Maccullochella (Percichthyidae). Marine and Freshwater Research 61, 980–991.
| Phylogenetics and revised taxonomy of the Australian freshwater cod genus, Maccullochella (Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Norris, A., Hutchison, M., and Chilcott, K. (2015). Dewfish demonstration reach monitoring and evaluation report, Autumn 2015. Department of Agriculture and Fisheries, Brisbane, Qld, Australia.
NSW Department of Primary Industries (2006). Trout cod Maccullochella macquariensis recovery plan. NSW DPI, Port Stephens, NSW, Australia.
NSW Department of Primary Industries (2014). Priorities action statement – southern pygmy perch. (NSW DPI: Port Stephens, NSW, Australia.) Available at https://www.dpi.nsw.gov.au/fishing/threatened-species/what-current/endangered-species2/southern-pygmy-perch/priorities-action-statement-actions-for-southern-pygmy-perch [Verified 13 August 2020].
O’Brien, T. A., and Ryan, T. J. (1997). Impact of saline drainage on key Murray–Darling fish species. Final Report NMRS Project R5004, Freshwater Ecology Division, Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
O’Connor, J. P., O’Mahony, D. J., and O’Mahony, J. M. (2005). Movements of Macquaria ambigua, in the Murray River, south-eastern Australia. Journal of Fish Biology 66, 392–403.
| Movements of Macquaria ambigua, in the Murray River, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
O’Connor, J. P., O’Mahony, D. J., O’Mahony, J. M., and Glenane, T. J. (2006). Some impacts of low and medium head weirs on downstream fish movement in the Murray–Darling Basin in southeastern Australia. Ecology Freshwater Fish 15, 419–427.
| Some impacts of low and medium head weirs on downstream fish movement in the Murray–Darling Basin in southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
O’Connor, J., Amtstaetter, F., Jones, M., and Mahoney, J. (2015). Prioritising the rehabilitation of fish passage in a regulated river system based on fish movement. Ecological Management & Restoration 16, 67–72.
| Prioritising the rehabilitation of fish passage in a regulated river system based on fish movement.Crossref | GoogleScholarGoogle Scholar |
Parisi, M. A., Cramp, R. L., Gordos, M. A., and Franklin, C. E. (2020). Can the impacts of cold-water pollution on fish be mitigated by thermal plasticity? Conservation Physiology 8, coaa005.
| Can the impacts of cold-water pollution on fish be mitigated by thermal plasticity?Crossref | GoogleScholarGoogle Scholar | 32099655PubMed |
Pascoe, B. (2017). ‘Dark Emu Black Seeds: Agriculture or Accident?’ (Magabala Books: Broome, WA, Australia.)
Pavlova, A., Beheregaray, L. B., Coleman, R., Gilligan, D., Harrisson, K. A., Ingram, B. A., Kearns, J., Lamb, A. M., Lintermans, M., Lyon, J., Nguyen, T. T. T., Sasaki, M., Tonkin, Z., Yen, J. D. L., and Sunnucks, P. (2017). Severe consequences of habitat fragmentation on genetic diversity of an endangered Australian freshwater fish: a call for assisted gene flow. Evolutionary Applications 10, 531–550.
| Severe consequences of habitat fragmentation on genetic diversity of an endangered Australian freshwater fish: a call for assisted gene flow.Crossref | GoogleScholarGoogle Scholar | 28616062PubMed |
Pearce, L. K. (2013). Macquarie perch refuge project final report for the Lachlan Catchment Management Authority. NSW Department of Primary Industries, Albury, NSW, Australia.
Pearce, L. K. (2014). Conservation management of southern pygmy perch (Nannoperca australis) in NSW, in the context of climactic extremes and alien species. M.Sc. Thesis, Charles Sturt University, Albury, NSW, Australia.
Pearce, L. K. (2015a). Coppabella Creek fish sampling 2014. Summary of findings report. NSW Department of Primary Industries, Albury, NSW, Australia.
Pearce, L. (2015b). Surveys, monitoring and conservation status of southern pygmy perch (Nannoperca australis) within Blakney and Pudman creeks. NSW Department of Primary Industries, Albury, NSW, Australia.
Pearce, L., Bice, C., Whiterod, N., and Raadik, T. (2019). Southern pygmy perch Nannoperca australis. In ‘The IUCN Red List of Threatened Species 2019’. e.T123358579A123382811. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/123358579/123382811 [Verified 13 April 2020].
Pierce, B. E. (1990). Chowilla fisheries investigations. South Australian Department of Fisheries, Adelaide, SA, Australia.
Poff, N. L., and Zimmerman, J. K. H. (2010). Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55, 194–205.
| Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Poff, N. L., Allan, J. D., Bain, M. B., Karr, J. R., Prestegaard, K. L., Richter, B. D., Sparks, R. E., and Stromberg, J. C. (1997). The natural flow regime. Bioscience 47, 769–784.
| The natural flow regime.Crossref | GoogleScholarGoogle Scholar |
Pollard, D. A., Davis, T. L. O., and Llewellyn, L. C. (1996). Eel-tailed catfishes. In ‘Freshwater Fishes of South East Australia’. (Ed. R. M. McDowall.) pp. 109–113. (Reed: Sydney, NSW, Australia.)
Pratchett, M. S., Bay, L. K., Gehrke, P. C., Koehn, J. D., Osborne, K., Pressey, R. L., Sweatman, H. P. A., and Wachenfeld, D. (2011). Contribution of climate change to habitat degradation and loss in Australia’s aquatic ecosystems. Marine and Freshwater Research 62, 1062–1081.
| Contribution of climate change to habitat degradation and loss in Australia’s aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Pritchard, J. C. (2004). Linking fish growth and climate across modern space and through evolutionary time: otolith chronologies of the Australian freshwater fish, golden perch (Macquaria, ambigua, Percichthyidae). Ph.D. Thesis, Australian National University, Canberra, ACT, Australia.
Pullin, A. S., Knight, T. M., Stone, D. A., and Charman, K. (2004). Do conservation managers use scientific evidence to support their decision-making? Biological Conservation 119, 245–252.
| Do conservation managers use scientific evidence to support their decision-making?Crossref | GoogleScholarGoogle Scholar |
Pusey, B., Kennard, M., and Arthington, A. (2004). ‘Freshwater Fishes of North-Eastern Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Raadik, T. A. (2014). Fifteen from one: a revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 3898, 1–198.
| Fifteen from one: a revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species.Crossref | GoogleScholarGoogle Scholar | 25543673PubMed |
Raymond, S., Koehn, J., Tonkin, Z., Todd, C., Stoessel, D., Hackett, G., O’Mahony, J., Berry, K., Lyon, J., Sharley, J., and Moloney, P. (2019). Differential responses by two closely related native fishes to restoration actions. Restoration Ecology 27, 1463–1472.
| Differential responses by two closely related native fishes to restoration actions.Crossref | GoogleScholarGoogle Scholar |
Rees, G. N., Cooke, R. A., Ning, N. S. P., McInerney, P. J., Pertie, R. T., and Nielson, D. L. (2020). Manage floodplain inundation maintains ecological function in lowland rivers. The Science of the Total Environment 727, 138469.
| Manage floodplain inundation maintains ecological function in lowland rivers.Crossref | GoogleScholarGoogle Scholar | 32330710PubMed |
Reid, D. D., Harris, J. H., and Chapman, D. J. (1997). NSW inland commercial fishery data analysis. Fisheries Research and Development Corporation, Canberra, ACT, Australia.
Reid, A. J., Carlson, A. K., Creed, I. F., Eliason, E. J., Gell, P. A., Johnson, P. T. J., Kidd, K. A., MacCormack, T. J., Olden, J. D., Ormerod, S. J., Smol, J. P., Taylor, W. W., Tockner, K., Vermaire, J. C., Dudgeon, D., and Cooke, S. J. (2019). Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews of the Cambridge Philosophical Society 94, 849–873.
| Emerging threats and persistent conservation challenges for freshwater biodiversity.Crossref | GoogleScholarGoogle Scholar | 30467930PubMed |
Renfree, M., and Marsden, T. (2006). Clare Weir fishlock monitoring. Department of Primary Industries and Fisheries, Mackay, Qld, Australia.
Reynolds, F. R. (1976). Tagging important in River Murray fish study. Australian Fisheries 35, 22.
Reynolds, L. F. (1983). Migration patterns of five fish species in the Murray–Darling River system. Australian Journal of Marine and Freshwater Research 34, 857–871.
| Migration patterns of five fish species in the Murray–Darling River system.Crossref | GoogleScholarGoogle Scholar |
Rimmer, M. A. (1987). Trout cod bred for the first time at Narrandera. Australian Fisheries 46, 33–34.
Roberts, J., and Sainty, G. (1996). ‘Listening to the Lachlan.’ (Sainty and Associates: Sydney, NSW, Australia.)
Roberts, D. T., Duivenvoorden, L. J., and Stuart, I. G. (2008). Factors influencing recruitment patterns of golden perch (Macquaria ambigua oriens) within a hydrologically variable and regulated Australian tropical river system. Ecology Freshwater Fish 17, 577–589.
| Factors influencing recruitment patterns of golden perch (Macquaria ambigua oriens) within a hydrologically variable and regulated Australian tropical river system.Crossref | GoogleScholarGoogle Scholar |
Rogers, M. W., Allen, M. S., Brown, P., Hunt, T., Fulton, W., and Ingram, B. A. (2010). A simulation model to explore the relative value of stock enhancement versus harvest regulations for fishery sustainability. Ecological Modelling 221, 919–926.
| A simulation model to explore the relative value of stock enhancement versus harvest regulations for fishery sustainability.Crossref | GoogleScholarGoogle Scholar |
Rolls, R., and Wilson, G. (2010). Spatial and temporal patterns in fish assemblages following an artificially extended floodplain inundation event, northern Murray–Darling Basin, Australia. Environmental Management 45, 822–833.
| Spatial and temporal patterns in fish assemblages following an artificially extended floodplain inundation event, northern Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar | 20127088PubMed |
Rourke, M. L., and Gilligan, D. (2010). Population genetic structure of freshwater catfish (Tandanus tandanus) in the Murray–Darling Basin and coastal catchments of New South Wales: implications for future re-stocking programs. Industry and Investment NSW – Fisheries Final Report Series, NSW Fisheries, Narrandera, NSW, Australia.
Rourke, M. L., and Gilligan, D. M. (2015). Complex biogeography and historic translocations lead to complicated phylogeographic structure of freshwater eel-tailed catfish (Tandanus spp.) in south-eastern Australia. Conservation Genetics 16, 777–790.
| Complex biogeography and historic translocations lead to complicated phylogeographic structure of freshwater eel-tailed catfish (Tandanus spp.) in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Rourke, M. L., McPartlan, H. C., Ingram, B. A., and Taylor, A. C. (2009). Polygamy and low effective population size in a captive Murray cod (Maccullochella peelii peelii) population: genetic implications for wild restocking programs. Marine and Freshwater Research 60, 873–883.
| Polygamy and low effective population size in a captive Murray cod (Maccullochella peelii peelii) population: genetic implications for wild restocking programs.Crossref | GoogleScholarGoogle Scholar |
Rourke, M. L., McPartlan, H. C., Ingram, B. A., and Taylor, A. C. (2010). Biogeography and life history ameliorate the potentially negative genetic effects of stocking on Murray cod (Maccullochella peelii peelii). Marine and Freshwater Research 61, 918–927.
| Biogeography and life history ameliorate the potentially negative genetic effects of stocking on Murray cod (Maccullochella peelii peelii).Crossref | GoogleScholarGoogle Scholar |
Rourke, M. L., McPartlan, H. C., Ingram, B. A., and Taylor, A. C. (2011). Variable stocking effect and endemic population genetic structure in Murray cod Maccullochella peelii. Journal of Fish Biology 79, 155–177.
| Variable stocking effect and endemic population genetic structure in Murray cod Maccullochella peelii.Crossref | GoogleScholarGoogle Scholar | 21722117PubMed |
Rowland, S. J. (1983a). Spawning of the Australian freshwater fish Murray cod Maccullochella peeli (Mitchell), in earthen ponds. Journal of Fish Biology 23, 525–534.
| Spawning of the Australian freshwater fish Murray cod Maccullochella peeli (Mitchell), in earthen ponds.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (1983b). The hormone-induced ovulation and spawning of the Australian freshwater fish golden perch, Macquaria ambigua (Richardson) (Percichthyidae). Aquaculture 35, 221–238.
| The hormone-induced ovulation and spawning of the Australian freshwater fish golden perch, Macquaria ambigua (Richardson) (Percichthyidae).Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (1984). The hormone induced spawning of silver perch Bidyanus bidyanus (Mitchell) (Teraponidae). Aquaculture 42, 83–86.
Rowland, S. J. (1985). Aspects of the biology and artificial breeding of the Murray cod, Maccullochella peeli, and the eastern freshwater cod, M. ikei sp. nov. (Pisces: Percichthyidae). Ph.D. Thesis, Macquarie University, Sydney, NSW, Australia.
Rowland, S. J. (1989). Aspects of the history and fishery of the Murray cod, Maccullochella peelii (Michell) (Percichthyidae). Proceedings of the Linnean Society of New South Wales 111, 202–213.
Rowland, S. J. (1998a). Age and growth of the Australian freshwater fish Murray cod, Maccullochella peelii peelii. Proceedings of the Linnean Society of New South Wales 120, 163–180.
Rowland, S. J. (1998b). Aspects of the reproductive biology of Murray cod Maccullochella peelii peelii. Proceedings of the Linnean Society of New South Wales 120, 147–162.
| Aspects of the reproductive biology of Murray cod Maccullochella peelii peelii.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (2004). Domestication of silver perch, Bidyanus bidyanus, broodfish. Journal of Applied Aquaculture 16, 75–83.
| Domestication of silver perch, Bidyanus bidyanus, broodfish.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (2005). Overview of the history, fishery, biology and aquaculture of Murray cod (Maccullochella peelii peelii). In ‘Management of Murray Cod in the Murray–Darling Basin: Statement, Recommendations and Supporting Papers. Proceedings of a Workshop’, 3–4 June 2004, Canberra, ACT, Australia. (Eds M. Lintermans and B. Phillips.) pp. 38–61. (Murray–Darling Basin Commission and Cooperative Research Centre for Freshwater Ecology, University of Canberra: Canberra, ACT, Australia.)
Rowland, S. J. (2009). Review of aquaculture research and development of the Australian freshwater fish silver perch, Bidyanus bidyanus. Journal of the World Aquaculture Society 40, 291–324.
| Review of aquaculture research and development of the Australian freshwater fish silver perch, Bidyanus bidyanus.Crossref | GoogleScholarGoogle Scholar |
Rowland, S. J. (2013). Hatchery production for conservation and stock enhancement: the case of Australian freshwater fish. In ‘Advances in Aquaculture Hatchery Technology’. (Eds G. Allan and G. Burnell.) pp. 557–595. (Woodhead Publishing: Cambridge, UK.)
Rowland, S., and Ingram, B. (1991). Diseases of Australian native freshwater fishes, with particular emphasis on the ectoparasitic and fungal diseases of Murray cod (Maccullochella peeli), golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus). NSW Fisheries Fisheries Bulletin 4, 1–33.
Rowland, S., Dirou, J., and Selosse, P. (1983). Production and stocking of golden and silver perch in NSW. Australian Fisheries 42, 24–28.
Rowland, S. J., Allan, G. L., Hollis, M., and Pontifex, T. (1995). Production of the Australian freshwater silver perch, Bidyanus bidyanus (Mitchell), at two densities in earthen ponds. Aquaculture 130, 317–328.
| Production of the Australian freshwater silver perch, Bidyanus bidyanus (Mitchell), at two densities in earthen ponds.Crossref | GoogleScholarGoogle Scholar |
Ryan, T., and O’Mahony, J. (2005). Movement of golden perch and Murray cod in the Nagambie lakes system. Department of Sustainability and Environment, Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Ryan, T., Lennie, R., Lyon, J., and O’Brien, T. (2003). Thermal rehabilitation of the southern Murray–Darling Basin. Final Report to Agriculture, Forestry, Fisheries Australia, MD 2001 FishRehab Program. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Ryan, K. A., Lintermans, M., Ebner, B. C., and Norris, R. (2013). Using fine-scale overlap in predator–prey distribution to assess avian predation risk to a reservoir population of threatened Macquarie Perch. Freshwater Science 32, 1057–1072.
| Using fine-scale overlap in predator–prey distribution to assess avian predation risk to a reservoir population of threatened Macquarie Perch.Crossref | GoogleScholarGoogle Scholar |
Ryder, D. S., Tomlinson, M., Gawne, B., and Likens, G. E. (2010). Defining and using best available science: a policy conundrum for the management of aquatic ecosystems. Marine and Freshwater Research 61, 821–828.
| Defining and using best available science: a policy conundrum for the management of aquatic ecosystems.Crossref | GoogleScholarGoogle Scholar |
Saddlier, S. R., Crowther, D., and Papas, P. (2002). Aquatic fauna and habitat structure of Seven Creeks: status of pre- and post-rehabilitation works condition. A consultant’s report for the Goulburn–Broken Catchment Management Authority and the Natural Heritage Trust, Freshwater Ecology Section, Department of Natural Resources and Environment, Melbourne, Vic., Australia.
Saddlier, S., O’Mahony, J., and Ramsey, D. (2008). Protection and enhancement of Murray cod populations. Arthur Rylah Institute for Environmental Research Technical Report Series number 172, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Saddlier, S., Koehn, J. D., and Hammer, M. P. (2013). Let’s not forget the small fishes – conservation of two threatened species of pygmy perch in south-eastern Australia. Marine and Freshwater Research 64, 874–886.
| Let’s not forget the small fishes – conservation of two threatened species of pygmy perch in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Sarac, Z., Sewell, H., Baker, L., and Ringwood, G. (2011). ‘Paroo: Talking Fish – Making Connections with the Rivers of the Murray–Darling Basin.’ (Murray–Darling Basin Authority: Canberra, ACT, Australia.)
Scheele, B. C., Legge, S., Blanchard, W., Garnett, S. T., Geyle, H. M., Gillespie, G. R., Harrison, P. L., Lindenmayer, D. B., Lintermans, M., Robinson, N. M., and Woinarski, J. C. Z. (2019). Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country. Biological Conservation 235, 273–278.
| Continental-scale assessment reveals inadequate monitoring for threatened vertebrates in a megadiverse country.Crossref | GoogleScholarGoogle Scholar |
Sharpe, C. P. (2011). Spawning and recruitment ecology of golden perch (Macquaria ambigua Richardson 1845) in the Murray and Darling Rivers. Ph.D. Thesis, Griffith University, Brisbane, Qld, Australia.
Sharpe, C., and Stuart, I. (2018). Environmental flows in the Darling River to support native fish populations 2016–17. CPS Enviro report to the Commonwealth Environmental Water Office. CPS Enviro, Mildura, Vic., Australia.
Sharpe, C., Conallin, A., Meredith, S., and Roennfeldt, B. (2003). Status of the Cardross Lakes fish community. Technical report 9/2003, Murray–Darling Freshwater Research Centre, Mildura, Vic., Australia.
Shaw, J. L. A., Weyrich, L., and Cooper, A. (2017). Using environmental (e)DNA sequencing for aquatic biodiversity surveys: a beginner’s guide. Marine and Freshwater Research 68, 20–33.
| Using environmental (e)DNA sequencing for aquatic biodiversity surveys: a beginner’s guide.Crossref | GoogleScholarGoogle Scholar |
Sherman, B., Todd, C. R., Koehn, J. D., and Ryan, T. (2007). Modelling the impact and potential mitigation of cold water pollution on Murray cod populations downstream of Hume Dam, Australia. River Research and Applications 23, 377–389.
| Modelling the impact and potential mitigation of cold water pollution on Murray cod populations downstream of Hume Dam, Australia.Crossref | GoogleScholarGoogle Scholar |
Silva, A. T., Lucas, M. C., Castro-Santos, T., Katopodis, C., Baumgartner, L. J., Thiem, J. D., Aarestrup, K., Pompeu, P. S., O’Brien, G. C., Braun, D. C., Burnett, N. J., Zhu, D. Z., Fjeldstad, H.-P., Forseth, T., Rajaratnam, N., Williams, J. G., and Cooke, S. J. (2018). The future of fish passage science, engineering and practice. Fish and Fisheries 19, 340–362.
| The future of fish passage science, engineering and practice.Crossref | GoogleScholarGoogle Scholar |
Small, K., Kopf, R. K., Watts, R. J., and Howitt, J. (2014). Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes. PLoS One 9, e94524.
| Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes.Crossref | GoogleScholarGoogle Scholar | 24728094PubMed |
Starrs, D., Ebner, B. C., and Fulton, C. J. (2016). All in the ears: unlocking the early life history biology and spatial ecology of fishes. Biological Reviews of the Cambridge Philosophical Society 91, 86–105.
| All in the ears: unlocking the early life history biology and spatial ecology of fishes.Crossref | GoogleScholarGoogle Scholar | 25424431PubMed |
Sternberg, D., Balcombe, S., Marshall, J., Lobegeiger, J., and Arthington, A. (2012). Subtle ‘boom and bust’ response of Macquaria ambigua to flooding in an Australian dryland river. Environmental Biology of Fishes 93, 95–104.
| Subtle ‘boom and bust’ response of Macquaria ambigua to flooding in an Australian dryland river.Crossref | GoogleScholarGoogle Scholar |
Stewardson, M. J., and Guarino, F. (2018). Basin-scale environmental water delivery in the Murray–Darling, Australia: a hydrological perspective. Freshwater Biology 63, 969–985.
| Basin-scale environmental water delivery in the Murray–Darling, Australia: a hydrological perspective.Crossref | GoogleScholarGoogle Scholar |
Stewart-Koster, B., Olden, J. D., and Gido, K. B. (2014). Quantifying flow–ecology relationships with functional linear models. Hydrological Sciences Journal 59, 629–644.
| Quantifying flow–ecology relationships with functional linear models.Crossref | GoogleScholarGoogle Scholar |
Stoessel, D. (2007). Assessment of the status of Murray hardyhead (Craterocephalus fluviatilis) populations within Round, Woorinen North and Elizabeth lakes – interim report. Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Stoessel, D. (2010). Review of Murray hardyhead (Craterocephalus fluviatilis) biology and ecology, and the environmental data for two key populations in the Kerang region. Report number 2010/30, Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Stoessel, D., Ellis, I. M., Whiterod, N., Gilligan, D., Wedderburn, S. D., and Bice, C. (2019). Craterocephalus fluviatilis. In ‘The IUCN Red List of Threatened Species 2019’. e.T40692A123379212. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/40692/123379212 [Verified 13 April 2020].
Stoessel, D. J., Fairbrother, P. S., Fanson, B. G., Raymond, S. M. C., Raadik, T. A., Nicol, M. D., and Johnson, L. A. (2020). Salinity tolerance during early development of threatened Murray hardyhead (Craterocephalus fluviatilis) to guide environmental watering. Aquatic Conservation 30, 173–182.
| Salinity tolerance during early development of threatened Murray hardyhead (Craterocephalus fluviatilis) to guide environmental watering.Crossref | GoogleScholarGoogle Scholar |
Stoffels, R. J., Clarke, K. R., Rehwinkel, R. A., and McCarthy, B. J. (2014). Response of a floodplain fish community to river–floodplain connectivity: natural versus managed reconnection. Canadian Journal of Fisheries and Aquatic Sciences 71, 236–245.
| Response of a floodplain fish community to river–floodplain connectivity: natural versus managed reconnection.Crossref | GoogleScholarGoogle Scholar |
Stoffels, R. J., Rehwinkel, R. A., Price, A. E., and Fagan, W. F. (2016). Dynamics of fish dispersal during river–floodplain connectivity and its implications for community assembly. Aquatic Sciences 78, 355–365.
| Dynamics of fish dispersal during river–floodplain connectivity and its implications for community assembly.Crossref | GoogleScholarGoogle Scholar |
Stoffels, R. J., Bond, N. R., and Nicol, S. (2018). Science to support the management of riverine flows. Freshwater Biology 63, 996–1010.
| Science to support the management of riverine flows.Crossref | GoogleScholarGoogle Scholar |
Stoffels, R., Weatherman, K., Thiem, J. D., Butler, G., Morrongiello, J., Kopf, R. K., McCasker, N., Zampatti, B., Ye, Q., and Broadhurst, B. T. (2019). Effects of river flows and temperature on the growth dynamics of Murray cod and golden perch. NIWA report, National Institute of Water and Atmospheric Research Ltd, Christchurch, New Zealand.
Stuart, I. G., and Berghuis, A. P. (2002). Upstream passage of fish through a vertical-slot fishway in an Australian subtropical river. Fisheries Management and Ecology 9, 111–122.
| Upstream passage of fish through a vertical-slot fishway in an Australian subtropical river.Crossref | GoogleScholarGoogle Scholar |
Stuart, I., and Koehn, J. (2007). Big cod do breed. Freshwater Fishing 85, 62–64.
Stuart, I. G., and Mallen‐Cooper, M. (1999). An assessment of the effectiveness of a vertical-slot fishway for non-salmonid fish at a tidal barrier on a large tropical/subtropical river. Regulated Rivers: Research and Management 15, 575–590.
| An assessment of the effectiveness of a vertical-slot fishway for non-salmonid fish at a tidal barrier on a large tropical/subtropical river.Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., and Marsden, T. J. (2019). Evaluation of cone fishways to facilitate passage of small-bodied fish. Aquaculture and Fisheries. , .
| Evaluation of cone fishways to facilitate passage of small-bodied fish.Crossref | GoogleScholarGoogle Scholar |
Stuart, I., and Sharpe, C. (2017). Towards a southern connected basin flow plan: connecting rivers to recover native fish communities. Kingfisher Research and CPS Enviro report to the Murray–Darling Basin Authority. Kingfisher Research, Melbourne, Vic., Australia; and CPS Enviro, Mildura, Vic., Australia.
Stuart, I. G., and Sharpe, C. P. (2020). Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia. Aquatic Conservation 30, 675–690.
| Riverine spawning, long distance larval drift, and floodplain recruitment of a pelagophilic fish: a case study of golden perch (Macquaria ambigua) in the arid Darling River, Australia.Crossref | GoogleScholarGoogle Scholar |
Stuart, I., Baumgartner, L., and Zampatti, B. (2008). Can a low slope fishway provide passage for a lowland river fish community? Marine and Freshwater Research 59, 332–346.
| Can a low slope fishway provide passage for a lowland river fish community?Crossref | GoogleScholarGoogle Scholar |
Stuart, I. G., Koehn, J. D., O’Brien, T. A., McKenzie, J. A., and Quinn, G. P. (2010). Too close for comfort: a fishway exit and a hydro power station inlet. Marine and Freshwater Research 61, 23–33.
| Too close for comfort: a fishway exit and a hydro power station inlet.Crossref | GoogleScholarGoogle Scholar |
Stuart, I., Sharpe, C., Stanislawski, K., Parker, A., and Mallen-Cooper, M. (2019). From an irrigation system to an ecological asset: adding environmental flows establishes recovery of a threatened fish species. Marine and Freshwater Research 70, 1295–1306.
| From an irrigation system to an ecological asset: adding environmental flows establishes recovery of a threatened fish species.Crossref | GoogleScholarGoogle Scholar |
Swirepik, J. L., Burns, I. C., Dyer, F. J., Neave, I. A., O’Brien, M. G., Pryde, G. M., and Thompson, R. M. (2016). Establishing environmental water requirements for the Murray–Darling Basin, Australia’s largest developed river system. River Research and Applications 32, 1153–1165.
| Establishing environmental water requirements for the Murray–Darling Basin, Australia’s largest developed river system.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Ebner, B. C., and Broadhurst, B. T. (2008). Diel activity of the endangered trout cod (Maccullochella macquariensis) in the Murrumbidgee River. Proceedings of the Linnean Society of New South Wales 129, 167–173.
Thiem, J. D., Broadhurst, B. T., Lintermans, M., Ebner, B. C., Clear, R. C., and Wright, D. (2013). Seasonal differences in the diel movements of Macquarie perch (Macquaria australasica) in an upland reservoir. Ecology Freshwater Fish 22, 145–156.
| Seasonal differences in the diel movements of Macquarie perch (Macquaria australasica) in an upland reservoir.Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J. P., and Conallin, J. (2017). Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes? Austral Ecology 42, 218–226.
| Recovery from a fish kill in a semi-arid Australian river: can stocking augment natural recruitment processes?Crossref | GoogleScholarGoogle Scholar |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Forbes, J., Taylor, M. D., and Watts, R. J. (2018). Abiotic drivers of activity in a large, free-ranging, freshwater teleost, Murray cod (Maccullochella peelii). PLoS One 13, e0198972.
| Abiotic drivers of activity in a large, free-ranging, freshwater teleost, Murray cod (Maccullochella peelii).Crossref | GoogleScholarGoogle Scholar | 29883481PubMed |
Thiem, J. D., Wooden, I. J., Baumgartner, L. J., Butler, G. L., Taylor, M. D., and Watts, R. J. (2020). Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes. Ecohydrology 13, e2197.
| Hypoxic conditions interrupt flood-response movements of three lowland river fish species: implications for flow restoration in modified landscapes.Crossref | GoogleScholarGoogle Scholar |
Thoms, M., Suter, P., Roberts, J., Koehn, J., Jones, G., Hillman, T., and Close, A. (2000). River Murray Scientific Panel report on environmental flows. River Murray – Dartmouth to Wellington and the lower Darling River. Murray–Darling Basin Commission, Canberra, ACT, Australia.
Thoms, M. C., Southwell, M., and McGinness, H. M. (2005). Floodplain–river ecosystems: fragmentation and water resources development. Geomorphology 71, 126–138.
| Floodplain–river ecosystems: fragmentation and water resources development.Crossref | GoogleScholarGoogle Scholar |
Thurstan, S. J. (1992). Commercial extensive larval rearing of Australian freshwater native fish. In ‘Larval Biology. Australian Society for Fish Biology Workshop, Hobart, 20 August 1991, Proceedings Number 15’. (Ed. D. A. Hancock.) pp. 71–75. (Bureau of Rural Resources: Canberra, ACT, Australia.)
Tockner, K., Pusch, M., Borchardt, D., and Lorang, M. S. (2010). Multiple stressors in coupled river–floodplain ecosystems. Freshwater Biology 55, 135–151.
| Multiple stressors in coupled river–floodplain ecosystems.Crossref | GoogleScholarGoogle Scholar |
Todd, C. R., and Koehn, J. D. (2009). Murray cod modelling to address key management actions. Final report for project MD745, report to the Murray–Darling Basin Commission. Arthur Rylah Institute for Environmental Research, Melbourne, Vic., Australia.
Todd, C. R., and Lintermans, M. (2015). Who do you move? A stochastic population model to guide translocation strategies for an endangered freshwater fish in south-eastern Australia. Ecological Modelling 311, 63–72.
| Who do you move? A stochastic population model to guide translocation strategies for an endangered freshwater fish in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Todd, C. R., Nicol, S. J., and Koehn, J. D. (2004). Density-dependence uncertainty in population models for the conservation management of trout cod, Maccullochella macquariensis. Ecological Modelling 171, 359–380.
| Density-dependence uncertainty in population models for the conservation management of trout cod, Maccullochella macquariensis.Crossref | GoogleScholarGoogle Scholar |
Todd, C. R., Ryan, T., Nicol, S. J., and Bearlin, A. R. (2005). The impact of cold water releases on the critical period of post-spawning survival and its implications for Murray cod (Maccullochella peelii peelii): a case study of the Mitta Mitta River, southeastern Australia. River Research and Applications 21, 1035–1052.
| The impact of cold water releases on the critical period of post-spawning survival and its implications for Murray cod (Maccullochella peelii peelii): a case study of the Mitta Mitta River, southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Todd, C. R., Koehn, J. D., Pearce, L., Dodd, L., Humphries, P., and Morrongiello, J. R. (2017). Forgotten fishes: what is the future for small threatened freshwater fish? Population risk assessment for southern pygmy perch, Nannoperca australis. Aquatic Conservation 27, 1290–1300.
| Forgotten fishes: what is the future for small threatened freshwater fish? Population risk assessment for southern pygmy perch, Nannoperca australis.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Humphries, P., and Pridmore, P. A. (2006). Ontogeny of feeding in two native and one alien fish species from the Murray–Darling Basin, Australia. Environmental Biology of Fishes 76, 303–315.
| Ontogeny of feeding in two native and one alien fish species from the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., King, A., Mahoney, J., and Morrongiello, J. (2007). Diel and spatial drifting patterns of silver perch Bidyanus bidyanus eggs in an Australian lowland river. Journal of Fish Biology 70, 313–317.
| Diel and spatial drifting patterns of silver perch Bidyanus bidyanus eggs in an Australian lowland river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., King, A. J., and Mahoney, J. (2008). Effects of flooding on recruitment and dispersal of the southern pygmy perch (Nannoperca australis) at a Murray River floodplain wetland. Ecological Management & Restoration 9, 196–201.
| Effects of flooding on recruitment and dispersal of the southern pygmy perch (Nannoperca australis) at a Murray River floodplain wetland.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Lyon, J., and Pickworth, A. (2009). An assessment of spawning stocks, reproductive behaviour and habitat use of Macquarie perch Macquaria australasica in Lake Dartmouth, Victoria. Technical Report Series number 188, Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment, Melbourne, Vic., Australia.
Tonkin, Z., Lyon, J., and Pickworth, A. (2010). Spawning behaviour of the endangered Macquarie perch Macquaria australasica in an upland Australian river. Ecological Management & Restoration 11, 223–226.
| Spawning behaviour of the endangered Macquarie perch Macquaria australasica in an upland Australian river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Lyon, J., Ramsey, D. S., Bond, N. R., Hackett, G., Krusic-Golub, K., Ingram, B., and Balcombe, S. R. (2014). Reservoir refilling enhances growth and recruitment of an endangered remnant riverine fish. Canadian Journal of Fisheries and Aquatic Sciences 71, 1888–1899.
| Reservoir refilling enhances growth and recruitment of an endangered remnant riverine fish.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kearns, J., O’Mahony, J., and Mahoney, J. (2016). Spatio-temporal spawning patterns of two riverine populations of the threatened Macquarie perch (Macquaria australasica). Marine and Freshwater Research 67, 1762–1770.
| Spatio-temporal spawning patterns of two riverine populations of the threatened Macquarie perch (Macquaria australasica).Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kearns, J., Lyon, J., Balcombe, S. R., King, A. J., and Bond, N. R. (2017a). Regional-scale extremes in river discharge and localised spawning stock abundance influence recruitment dynamics of a threatened freshwater fish. Ecohydrology 10, e1842.
| Regional-scale extremes in river discharge and localised spawning stock abundance influence recruitment dynamics of a threatened freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kitchingman, A., Lyon, J., Kearns, J., Hackett, G., O’Mahony, J., Moloney, P. D., Krusic-Golub, K., and Bird, T. (2017b). Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river. Hydrobiologia 797, 289–301.
| Flow magnitude and variability influence growth of two freshwater fish species in a large regulated floodplain river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Stuart, I., Kitchingman, A., Jones, M., Thiem, J., Zampatti, B., Hackett, G., Koster, W., and Koehn, J. (2017c). The effects of flow on silver perch population dynamics in the Murray River. Technical Report number 282, Arthur Rylah Institute for Environmental Research, Department of Environment, Land, Water and Planning, Melbourne, Vic., Australia.
Tonkin, Z., Lyon, J., Moloney, P., Balcombe, S. R., and Hackett, G. (2018). Spawning-stock characteristics and migration of a lake-bound population of the endangered Macquarie perch Macquaria australasica. Journal of Fish Biology 93, 630–640.
| Spawning-stock characteristics and migration of a lake-bound population of the endangered Macquarie perch Macquaria australasica.Crossref | GoogleScholarGoogle Scholar | 29956321PubMed |
Tonkin, Z., Stuart, I., Kitchingman, A., Thiem, J. D., Zampatti, B., Hackett, G., Koster, W., Koehn, J., Morrongiello, J., Mallen-Cooper, M., and Lyon, J. (2019a). Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river. Marine and Freshwater Research 70, 1333–1344.
| Hydrology and water temperature influence recruitment dynamics of the threatened silver perch Bidyanus bidyanus in a regulated lowland river.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Sharley, J., Fanson, B., Raymond, S., Ayres, R., Lyon, J., Balcombe, S., and Bond, N. (2019b). Climate variability regulates population dynamics of a threatened freshwater fish. Endangered Species Research 40, 257–270.
| Climate variability regulates population dynamics of a threatened freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Tonkin, Z., Kitchingman, A., Fanson, B., Lyon, J., Ayres, R., Sharley, J., Koster, W., O’Mahony, J., Hackett, G., Reich, P., and Hale, R. (2020). Quantifying links between instream woody habitat and freshwater fish species in south eastern Australia to inform waterway restoration. Aquatic Conservation 30, 1385–1396.
| Quantifying links between instream woody habitat and freshwater fish species in south eastern Australia to inform waterway restoration.Crossref | GoogleScholarGoogle Scholar |
Trout Cod Recovery Team (2008a). Background and implementation information for the trout cod Maccullochella macquariensis national recovery plan. (Department of Sustainability and Environment: Melbourne, Vic., Australia.) Available at http://www.environment.gov.au/system/files/resources/a3331850-8f2a-4662-b0ef-f73a7da89852/files/trout-cod-background.pdf [Verified 31 March 2020].
Trout Cod Recovery Team (2008b). National recovery plan for the trout cod Maccullochella macquariensis. (Department of Sustainability and Environment: Melbourne, Vic., Australia.) Available at http://www.environment.gov.au/system/files/resources/a3331850-8f2a-4662-b0ef-f73a7da89852/files/trout-cod.pdf [Verified 31 March 2020].
Trueman, W. (2012a). ‘True Tales of the Trout Cod: River Histories of the Murray–Darling Basin.’ (Murray–Darling Basin Authority: Canberra, ACT, Australia.)
Trueman, W. (2012b). Early Aborigines active managers of fish. RipRap 34, 40–41.
Twining, C. W., Taipale, S. J., Ruess, L., Bec, A., Martin-Creuzburg, D., and Kainz, M. J. (2020). Stable isotopes of fatty acids: current and future perspectives for advancing trophic ecology. Philosophical Transactions of the Royal Society – B. Biological Sciences 375, 2019.0641.
| Stable isotopes of fatty acids: current and future perspectives for advancing trophic ecology.Crossref | GoogleScholarGoogle Scholar |
Tyler, S., Jensen, O. P., Hogan, Z., Chandra, S., Galland, L. M., and Simmons, J. (2018). Perspectives on the application of unmanned aircraft for freshwater fisheries census. Fisheries 43, 510–516.
| Perspectives on the application of unmanned aircraft for freshwater fisheries census.Crossref | GoogleScholarGoogle Scholar |
Unmack, P. J. (2001). Biogeography of Australian freshwater fishes. Journal of Biogeography 28, 1053–1089.
| Biogeography of Australian freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
Unmack, P. J. (2013). Biogeography. Ecology of Australian freshwater fishes. In ‘Ecology of Australian Freshwater Fishes’. (Eds P. Humphries and K. Walker.) pp. 25–48. (CSIRO Publishing: Melbourne, Vic., Australia.)
van Dijk, A. I. J. M., Beck, H. E., Crosbie, R. S., de Jeu, R. A. M., Liu, Y. Y., Podger, G. M., Timbal, B., and Viney, N. R. (2013). The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resources Research 49, 1040–1057.
| The Millennium Drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society.Crossref | GoogleScholarGoogle Scholar |
Vertessy, R., Barma, D., Baumgartner, L., Mitrovic, S., Sheldon, F., and Bond, N. (2019). Independent assessment of the 2018–19 fish deaths in the lower Darling. Final report. (MDBA: Canberra, ACT, Australia.) Available at https://www.mdba.gov.au/sites/default/files/pubs/Final-Report-Independent-Panel-fish-deaths-lower%20Darling_4.pdf [Verified 11 May 2020].
Victorian Environmental Water Holder (2020). Seasonal watering plan 2020–21. https://www.vewh.vic.gov.au/watering-program/seasonal-watering-plan/swp-2020-flipbook/seasonal-watering-plan-2020-flipbook [Verified 11 July 2020].
Vietz, G. J., Sammonds, M. J., and Stewardson, M. J. (2013). Impacts of flow regulation on slackwaters in river channels. Water Resources Research 49, 1797–1811.
| Impacts of flow regulation on slackwaters in river channels.Crossref | GoogleScholarGoogle Scholar |
Wajon, S. (1983). Hybridisation between Murray cod and trout cod in Cataract Dam, NSW. B.Sc.(Hons) Thesis, University of New South Wales, Sydney, NSW, Australia.
Walker, K. F. (2006). Serial weirs, cumulative effects: the lower River Murray, Australia. In ‘Ecology of Desert Rivers’. (Ed. R. Kingsford.) pp. 248–79. (Cambridge University Press: Cambridge, UK.)
Walker, K. F., Sheldon, F., and Puckeridge, J. T. (1995). A perspective on dryland river ecosystems. Regulated Rivers: Research and Management 11, 85–104.
| A perspective on dryland river ecosystems.Crossref | GoogleScholarGoogle Scholar |
Ward, D. L., Casper, A. F., Counihan, T. D., Bayer, J. M., Waite, I. R., Kosovich, J. J., Chapman, C. G., Irwin, E. R., Sauer, J. S., and Ickes, B. S. (2017). Long-term fish monitoring in large rivers: utility of ‘benchmarking’ across basins. Fisheries 42, 100–114.
| Long-term fish monitoring in large rivers: utility of ‘benchmarking’ across basins.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D. (2009). Population fragmentation in the Murray hardyhead Craterocephalus fluviatilis McCulloch, 1912 (Teleostei: Atherinidae): ecology, genetics and osmoregulation. Ph.D. Thesis, University of Adelaide, Adelaide, SA, Australia.
Wedderburn, S. D. (2018). Multi-species monitoring of rare wetland fishes should account for imperfect detection of sampling devices. Wetlands Ecology and Management 26, 1107–1120.
| Multi-species monitoring of rare wetland fishes should account for imperfect detection of sampling devices.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., and Barnes, T. C. (2016). Piscivory by alien redfin perch (Perca fluviatilis) begins earlier than anticipated in two contrasting habitats of Lake Alexandrina, South Australia. Australian Journal of Zoology 64, 1–7.
| Piscivory by alien redfin perch (Perca fluviatilis) begins earlier than anticipated in two contrasting habitats of Lake Alexandrina, South Australia.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S., and Hammer, M. (2003). The lower lakes fish inventory: distribution and conservation of freshwater fishes of the Ramsar Convention wetland at the terminus of the Murray–Darling Basin, South Australia. Native Fish Australia (SA), Adelaide, SA, Australia.
Wedderburn, S. D., Walker, K. F., and Zampatti, B. P. (2007). Habitat separation of Craterocephalus (Atherinidae) species and populations in off-channel areas of the lower River Murray, Australia. Ecology Freshwater Fish 16, 442–449.
| Habitat separation of Craterocephalus (Atherinidae) species and populations in off-channel areas of the lower River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S., Walker, K., and Zampatti, B. (2008). Salinity may cause fragmentation of hardyhead (Teleostei: Atherinidae) populations in the River Murray, Australia. Marine and Freshwater Research 59, 254–258.
| Salinity may cause fragmentation of hardyhead (Teleostei: Atherinidae) populations in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Hammer, M. P., and Bice, C. M. (2012). Shifts in small-bodied fish assemblages resulting from drought-induced water level recession in terminating lakes of the Murray–Darling Basin, Australia. Hydrobiologia 691, 35–46.
| Shifts in small-bodied fish assemblages resulting from drought-induced water level recession in terminating lakes of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Hillyard, K. A., and Shiel, R. J. (2013). Zooplankton response to flooding of a drought refuge and implications for the endangered fish species Craterocephalus fluviatilis cohabiting with alien Gambusia holbrooki. Aquatic Ecology 47, 263–275.
| Zooplankton response to flooding of a drought refuge and implications for the endangered fish species Craterocephalus fluviatilis cohabiting with alien Gambusia holbrooki.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Barnes, T. C., and Hillyard, K. A. (2014a). Shifts in fish assemblages indicate failed recovery of threatened species following prolonged drought in terminating lakes of the Murray–Darling Basin, Australia. Hydrobiologia 730, 179–190.
| Shifts in fish assemblages indicate failed recovery of threatened species following prolonged drought in terminating lakes of the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Wedderburn, S. D., Bice, C. M., and Barnes, T. C. (2014b). Prey selection and diet overlap of native golden perch and alien redfin perch under contrasting hydrological conditions. Australian Journal of Zoology 62, 374–381.
| Prey selection and diet overlap of native golden perch and alien redfin perch under contrasting hydrological conditions.Crossref | GoogleScholarGoogle Scholar |
Weeks, A. R., Sgro, C. M., Young, A. G., Frankham, R., Mitchell, N. J., Miller, K. A., Byrne, M., Coates, D. J., Eldridge, M. D., Sunnucks, P., Breed, M. F., James, E. A., and Hoffmann, A. A. (2011). Assessing the benefits and risks of translocations in changing environments: a genetic perspective. Evolutionary Applications 4, 709–725.
| Assessing the benefits and risks of translocations in changing environments: a genetic perspective.Crossref | GoogleScholarGoogle Scholar | 22287981PubMed |
Welsh, A. B., Carlson, D. M., Schlueter, S. L., and Jackson, J. R. (2020). Tracking stocking success in a long-lived species through genetics and demographics: evidence of natural reproduction in Lake Sturgeon after twenty-two years. Transactions of the American Fisheries Society 149, 121–130.
| Tracking stocking success in a long-lived species through genetics and demographics: evidence of natural reproduction in Lake Sturgeon after twenty-two years.Crossref | GoogleScholarGoogle Scholar |
Wharton, J. C. (1973). Spawning induction, artificial fertilization and pond culture of the Macquarie Perch (Macquaria australasica [Cuvier, 1830]). Australian Society for Limnology Bulletin 5, 43–65.
White, L. J., Harris, J. H., and Keller, R. J. (2011). Movement of three non-salmonid fish species through a low-gradient vertical-slot fishway. River Research and Applications 27, 499–510.
| Movement of three non-salmonid fish species through a low-gradient vertical-slot fishway.Crossref | GoogleScholarGoogle Scholar |
Whiterod, N. S. (2010). Calibration of a rapid non-lethal method to measure energetic status of a freshwater fish (Murray cod, Maccullochella peelii peelii). Marine and Freshwater Research 61, 527–531.
| Calibration of a rapid non-lethal method to measure energetic status of a freshwater fish (Murray cod, Maccullochella peelii peelii).Crossref | GoogleScholarGoogle Scholar |
Whiterod, N. S. (2013). The swimming capacity of juvenile Murray cod (Maccullochella peelii): an ambush predator endemic to the Murray–Darling Basin, Australia. Ecology Freshwater Fish 22, 117–126.
| The swimming capacity of juvenile Murray cod (Maccullochella peelii): an ambush predator endemic to the Murray–Darling Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Whiterod, N. (2019). A translocation strategy to ensure the long-term future of threatened small-bodied freshwater fishes in the South Australian section of the Murray–Darling Basin. A report to Natural Resources, South Australian Murray–Darling Basin and the Riverine Recovery Project. Aquasave–Nature Glenelg River Trust, Goolwa Beach, SA, Australia.
Whiterod, N. S., Meredith, S. N., and Humphries, P. (2013). Refining the activity component of a juvenile fish bioenergetics model to account for swimming costs. Marine and Freshwater Behaviour and Physiology 46, 201–210.
| Refining the activity component of a juvenile fish bioenergetics model to account for swimming costs.Crossref | GoogleScholarGoogle Scholar |
Whiterod, N. S., Meredith, S. N., Humphries, P., Sherman, B. S., Koehn, J. D., Watts, R. J., Ingram, B. A., and Ryan, T. (2018). Flow alteration and thermal pollution depress modelled growth rates of an iconic riverine fish. Ecology Freshwater Fish 27, 686–698.
| Flow alteration and thermal pollution depress modelled growth rates of an iconic riverine fish.Crossref | GoogleScholarGoogle Scholar |
Wilson, P., Leigh, S., Bice, C., and Zampatti, B. (2012). Chowilla icon site fish assemblage condition monitoring 2012. SARDI Publication number F2008/000907-4, SARDI Research Report Series number 674, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Winemiller, K. O., and Rose, K. A. (1992). Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences 49, 2196–2218.
| Patterns of life-history diversification in North American fishes: implications for population regulation.Crossref | GoogleScholarGoogle Scholar |
Winemiller, K. O., Fitzgerald, D. B., Bower, L. M., and Pianka, E. R. (2015). Functional traits, convergent evolution, and periodic tables of niches. Ecology Letters 18, 737–751.
| Functional traits, convergent evolution, and periodic tables of niches.Crossref | GoogleScholarGoogle Scholar | 26096695PubMed |
Wong, C. M., Williams, C. E., Pittock, J., Collier, U., and Schelle, P. (2007). ‘World’s Top 10 Rivers at Risk.’ (WWF International: Gland, Switzerland.)
Woodcock, S. H., Gillanders, B. M., Munro, A. R., McGovern, F., Crook, D. A., and Sanger, A. C. (2011). Using enriched stable isotopes of barium and magnesium to batch mark otoliths of larval golden perch (Macquaria ambigua, Richardson). Ecology Freshwater Fish 20, 157–165.
| Using enriched stable isotopes of barium and magnesium to batch mark otoliths of larval golden perch (Macquaria ambigua, Richardson).Crossref | GoogleScholarGoogle Scholar |
Woodward, G. M. A. (2005). Factors influencing the distribution and abundance of Nannoperca obscura (Yarra pygmy perch) and Nannoperca australis (southern pygmy perch). Ph.D. Thesis, LaTrobe University, Melbourne, Vic., Australia.
Woodward, G. M. A., and Malone, B. S. (2002). Patterns of abundance and habitat use by Nannoperca obscura (Yarra pygmy perch) and Nannoperca australis (southern pygmy perch). Proceedings of the Royal Society of Victoria 114, 61–72.
Wright, D. W., Zampatti, B. P., Baumgartner, L. J., Brooks, S., Butler, G. L., Crook, D. A., Fanson, B. G., Koster, W., Lyon, J., Strawbridge, A., Tonkin, Z., and Thiem, J. D. (2020). Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient. Marine and Freshwater Research. , .
| Size, growth and mortality of riverine golden perch (Macquaria ambigua) across a latitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Ye, Q. (2005). Golden perch (Macquaria ambigua). Fishery assessment report to PIRSA Fisheries 2004, SARDI Research Report Series number 71, Publication number RD04/0167, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ye, Q., and Zampatti, B. (2007). Murray Cod stock status: the Lower River Murray, South Australia. Stock Status Report to PIRSA Fisheries, Publication number F2007-000211-1, SARDI Research Report Series number 208. (South Australian Research and Development Institute (Aquatic Sciences): Adelaide, SA, Australia.)
Ye, Q., Jones, G. K., and Pierce, B. E. (2000). Murray Cod (Maccullochella peelii peelii). Fishery Assessment Report to PIRSA for the Inland Waters Fishery Management Committee, South Australian Fisheries Assessment Series 2000/17, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ye, Q., Cheshire, K., and Fleer, D. (2008). Recruitment of golden perch and selected large-bodied fish species following the weir pool manipulation in the River Murray, South Australia. SARDI Aquatic Sciences Publication number F2008/000606-1, SARDI Research Report Series number 303. (SARDI Aquatic Sciences: Adelaide, SA, Australia.)
Ye, Q., Bucater, L., Zampatti, B., Bice, C., Wilson, P., Suitor, L., Wegener, I., Short, D., and Fleer, D. (2015). Population dynamics and status of freshwater catfish (Tandanus tandanus) in the lower River Murray, South Australia. Report to PIRSA Fisheries and Aquaculture, SARDI Publication number F2014/000903-1, SARDI Research Report Series number 841, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Ye, Q., Butler, G., Giatas, G., Ingram, B., Kind, P., Koehn, J., Lintermans, M., Beitzel, M., Zampatti, B., Brooks, S., Forbes, J., Gilligan, D., Hunt, T., and Todd, C. (2018). Murray cod Maccullochella peelii. In ‘Status of Key Australian Fish Stocks Reports 2018’. (Eds C. Stewardson, J. Andrews, C. Ashby, M. Haddon, K. Hartmann, P. Hone, P. Horvat, S. Mayfield, A. Roelofs, K. Sainsbury, T. Saunders, J. Stewart, S. Nicol, and B. Wise.) (Fisheries Research and Development Corporation: Canberra, ACT, Australia.) Available at https://www.fish.gov.au/report/207-Murray-Cod-2018?jurisdictionId=5 [Verified 11 May 2020].
Yen, J. D. L., Bond, N. R., Shenton, W., Spring, D. A., and Mac Nally, R. (2013). Identifying effective water-management strategies in variable climates using population dynamics models. Journal of Applied Ecology 50, 691–701.
| Identifying effective water-management strategies in variable climates using population dynamics models.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B., and Leigh, S. (2013a). Effects of flooding on recruitment and abundance of golden perch (Macquaria ambigua ambigua) in the lower River Murray. Ecological Management & Restoration 14, 135–143.
| Effects of flooding on recruitment and abundance of golden perch (Macquaria ambigua ambigua) in the lower River Murray.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., and Leigh, S. J. (2013b). Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua – implications for environmental flow management in the River Murray, Australia. Marine and Freshwater Research 64, 618–630.
| Within-channel flows promote spawning and recruitment of golden perch, Macquaria ambigua ambigua – implications for environmental flow management in the River Murray, Australia.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., Bice, C. M., and Jennings, P. R. (2010). Temporal variability in fish assemblage structure and recruitment in a freshwater-deprived estuary: The Coorong, Australia. Marine and Freshwater Research 61, 1298–1312.
| Temporal variability in fish assemblage structure and recruitment in a freshwater-deprived estuary: The Coorong, Australia.Crossref | GoogleScholarGoogle Scholar |
Zampatti, B. P., Bice, C. M., Wilson, P. J., and Ye, Q. (2014). Population dynamics of Murray cod (Maccullochella peelii) in the South Australian reaches of the River Murray: a synthesis of data from 2002–2013. Report to PIRSA Fisheries and Aquaculture, SARDI Publication number F2014/000089-1, SARDI Research Report Series number 761, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B. P., Wilson, P. J., Baumgartner, L., Koster, W. M., Livore, J. P., McCasker, N. G., and Ye, Q. (2015). Reproduction and recruitment of golden perch (Macquaria ambigua ambigua) in the southern Murray–Darling Basin in 2013–2014: an exploration of river-scale response, connectivity and population dynamics. SARDI Publication number F2014/000756-1, SARDI Research Report Series number 820, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B. P., Strawbridge, A., Thiem, J., Tonkin, Z., Mass, R., Woodhead, J., and Fredberg, J. (2018). Golden perch (Macquaria ambigua) and silver perch (Bidyanus bidyanus) age demographics, natal origin and migration history in the River Murray, Australia. SARDI Publication number F2018/000116-1, SARDI Research Report Series number 993, South Australian Research and Development Institute (Aquatic Sciences), Adelaide, SA, Australia.
Zampatti, B., Fanson, B., Strawbridge, A., Tonkin, Z., Thiem, J., Butler, G., Balcombe, S., Koster, W., King, A., Crook, D., Woods, R., Brooks, S., Lyon, J., Baumgartner, L., and Doyle, K. (2019). Basin-scale population dynamics of golden perch and Murray cod: relating flow to provenance, movement and recruitment in the Murray–Darling Basin. In ‘Murray–Darling Basin Environmental Water Knowledge and Research Project – Fish Theme Research Report’. (Eds A. Price, S. Balcombe, P. Humphries, A. King, and B. Zampatti.) p. 42. (Centre for Freshwater Ecology, La Trobe University: Wodonga, Vic., Australia.)