Importance of refractory ligands and their photodegradation for iron oceanic inventories and cycling
Christel Hassler A E , Damien Cabanes A , Sonia Blanco-Ameijeiras A , Sylvia G. Sander B D and Ronald Benner CA University of Geneva, Department F.-A. Forel for Environmental and Aquatic Sciences, 66 Boulevard Carl-Vogt, CH-1205 Geneva, Switzerland.
B University of Otago, Department of Chemistry, NIWA/UO Research Centre for Oceanography, PO Box 56, Dunedin, 9054, New Zealand.
C University of South Carolina, Department of Biological Sciences and School of the Earth, Ocean, and Environment, Columbia,1521 Green Street, SC 29208, USA.
D Present address: Marine Environmental Study Laboratory, IAEA-NAEL, 4 Quai Antoine 1er, Monaco 98000, Principality of Monaco.
E Corresponding author. Present address: Ecole polytechique fédérale de Lausanne (EPFL), Swiss Polar Institute, GR C2 505, Station 2, CH-1015 Lausanne, Switzerland. Email: christel.hassler@epfl.ch
Marine and Freshwater Research 71(3) 311-320 https://doi.org/10.1071/MF19213
Submitted: 12 June 2019 Accepted: 15 August 2019 Published: 2 December 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Iron is an essential micronutrient that limits primary production in up to 40% of the surface ocean and influences carbon dioxide uptake and climate change. Dissolved iron is mostly associated with loosely characterised organic molecules, called ligands, which define key aspects of the iron cycle such as its residence time, distribution and bioavailability to plankton. Models based on in situ ligand distributions and the behaviour of purified compounds include long-lived ligands in the deep ocean, bioreactive ligands in the surface ocean and photochemical processes as important components of the iron cycle. Herein, we further characterise biologically refractory ligands in dissolved organic matter (DOM) from the deep ocean and labile ligands in DOM from the surface ocean, and their photochemical and biological reactivities. Experimental results indicated that photodegradation of upwelled refractory iron-binding ligands can fuel iron remineralisation and its association with labile organic ligands, thus enhancing iron bioavailability in surface waters. These observations better elucidate the roles of biologically refractory and labile molecules and global overturning circulation in the ocean iron cycle, with implications for the initiation and sustainment of biological activity in iron-limited regions and the residence time of iron in the ocean.
Additional keywords: DOC, organic matter, primary productivity.
Introduction
Each year, ~80 petagrams (Pg) of atmospheric CO2 is exchanged with the ocean, representing a key process in the global carbon cycle. Processes in the biological and microbial carbon pumps govern the fate of carbon during its passage through the water column (Legendre et al. 2015). Photosynthetic fixation of carbon by phytoplankton in the surface ocean sustains food webs and biogeochemical processes, leading to nutrient regeneration and the export of particulate and dissolved organic matter to the deep ocean (Legendre et al. 2015). Most of the organic carbon (C) in the deep ocean resides in small refractory molecules that are highly altered, aged and very resistant to microbial degradation (Benner and Amon 2015). Refractory dissolved organic C (DOC) comprises one of the largest global reservoirs of reduced C (630 Pg; Hansell 2013) and associated elements, such as nitrogen (N), phosphorus (P) and trace metals, that play a major role in regulating ocean productivity.
Iron is a pivotal element in the regulation of microbial activity (Van Wambeke et al. 2008) and the efficiency of the biological pump (Coale et al. 1996) in nearly half of the global oceans. Sparingly soluble in seawater in its ionic forms, iron can be found throughout the water column owing to its association with organic ligands (>99%; e.g. Gledhill and Buck 2012). These iron-binding ligands control iron solubility, reactivity and bioavailability, hence regulating the biologically mediated carbon pumps. The study of purified ligands likely to be present in seawater provided major advances in the field, namely with respect to ligand photochemistry (Barbeau et al. 2001) and bioavailability (Hutchins et al. 1999; Hassler et al. 2011; Lis et al. 2015). However, natural dissolved organic matter (DOM) includes a very diverse and complex mixture of molecules (Benner and Amon 2015), and, despite natural DOM isolates from seawater being recently studied in depth (Lechtenfeld et al. 2014; Hassler et al. 2015; Norman et al. 2015, Hawkes et al. 2016; Zhang et al. 2019), the molecular composition and structure for most iron-binding ligands remain unknown. Humic substances have been proposed to account for a large fraction of dissolved iron-binding ligands in coastal and deep Pacific waters (Laglera and van den Berg 2009). Available standard terrestrial humic substances from the Suwannee River have been shown to bind iron, but terrestrial humics, such as lignin phenols, are in very low abundance in the deep ocean (Hernes and Benner 2006). The chemical nature and reactivity of iron-reactive molecules in marine humic substances might be different from the model terrestrial humics studied so far. The chemical reactivity of organically bound iron is often related to conditional binding affinity, but evidence of linkages between chemical lability and bioavailability is lacking at the molecular level, likely because of the complex feedbacks between biology and chemistry (Hudson 1998; Hutchins et al. 1999; Hassler et al. 2011, 2017; Gledhill and Buck 2012).
The biogeochemical cycles of carbon and iron are tightly intertwined. The reactivity and fate of both elements are modulated by intrinsic factors, such as source, molecular composition and structure, and extrinsic factors, such as the structure of microbial community, nutrient availability and exposure to sunlight. Photosynthetically driven food webs provide a fresh supply of labile organic matter that is rapidly utilised in the upper ocean where significant correlations between iron-binding ligands and biological activity have been reported (Gledhill and Buck 2012; Hassler et al. 2015; Boiteau et al. 2016). Several studies have demonstrated high bioavailability of saccharides and biologically produced exopolymeric substances (Hassler and Schoemann 2009; Hassler et al. 2011, 2015; Lis et al. 2015). At depth, reactive forms of DOC are less abundant, biological activity is diminished, and refractory forms of DOC with radiocarbon ages of several millennia become dominant (Benner and Amon 2015; Hansell and Carlson 2015). Despite similar iron-binding affinities having been detected throughout the water column (Buck et al. 2015; Gerringa et al. 2015; Caprara et al. 2016), differences in the reactivity and bioavailability of dissolved molecules suggest that the nature and reactivity of iron-binding ligands might differ with depth.
Here, we investigated iron-binding properties and bioavailability of labile dissolved organic molecules collected from surface water, and refractory molecules collected from deep water. To place our results in the context of oceanic cycling, we explored the impact of photodegradation for iron-binding properties of these refractory molecules. Results are discussed in the framework of the global iron-binding ligands reported in the open ocean as well as the consequences for primary producers.
Materials and methods
Collection of refractory and labile DOC
Deep water (1000 m) was collected from the North Atlantic Ocean (35°39.847′N, 74°30.792′W) by using Niskin bottles mounted on a CTD rosette. Water samples were collected in acid-rinsed Nalgene HDPE carboys (20 L) and filtered through 0.2-µm Nuclepore polycarbonate cartridge filters (VWR Scientific Inc., Bridgeport, NJ, USA) by using a peristaltic pump with silicone tubing. The filtered water was acidified to pH 2.5 with 6 mol L–1 sulfuric acid and passed through C-18 cartridges (Agilent Bond Elut, Agilent, Santa Clara, CA, USA; 10 g) at a flow rate of 50 mL min–1. The DOC was eluted from the cartridge with 30 mL of methanol, dried under a gentle stream of N2 and dissolved in Milli-Q water. The C-18 extraction recovered 30% of deep-water DOC and over half of the chromophoric dissolved organic matter (Shen and Benner 2018). Bioassay experiments conducted with parallel C-18-extracted DOC samples from this site demonstrated that the DOC was refractory to microbial degradation (i.e. rDOC), and photodegradation experiments indicated extensive photobleaching and alteration of the chromophoric components of the extracted DOC (Shen and Benner 2018). Labile DOC was collected by filtering (Whatman GF/F, Fisher Scientific, Waltham, MA, USA) material recovered in a plankton tow (50-µm mesh) that passed through a diatom bloom. Previous short-term (≤12 days) experiments with this plankton DOC determined that the DOC was rapidly and thoroughly utilised when added to seawater and, therefore, was labile DOC (lDOC; Shen et al. 2016). The rDOC and lDOC samples were stored frozen and thawed immediately before use in experiments.
Trace metal contents
Total dissolved Fe, valium (V), chromium (Cr), manganese (Mn), nickel (Ni), copper (Cu), arsenic (As), cobalt (Co), zinc (Zn) and Se concentrations were determined in duplicate by an Agilent 7500ce quadrupole inductively coupled plasma spectrometer (q-ICP–MS) with an octopole collision cell and autosampler (Agilent Technologies, Wellington, New Zealand). Prior to analysis, DOC concentrates were thawed, transferred into acid-cleaned ICP–MS vials and diluted with 2% v/v quartz-distilled nitric acid (q-HNO3) to final salinities of <2. Quantification was performed by spiking with a multi-element high-purity standard (NIST traceable). External calibration standards were prepared by a serial dilution of a SPEX CertiPrep multi-element standard (NIST traceable, ThermoFisher Scientific, Auckland, New Zealand) in 2% v/v q-HNO3. All data were corrected for dilution and blank. Accuracy and precision of the analytical technique was within ±5% relative standard deviations. Seawater spiked with two biological certified samples was also analysed; recoveries of spiked seawater analyses together with certified samples are given in Table S1, available as Supplementary material to this paper.
Photochemical transformation
Refractory DOC stock solution was exposed to full sunlight spectra by using a solar simulator (ABET Technologies Sun 2000, Milford, CT, USA) with atmosphere filter (Fig. S1, available as Supplementary material to this paper). Organic material was placed in a quartz cuvette with Teflon cap to a light intensity of 690 W m–2 for 23 h, so as to mimic a seasonal exposure in the surface mixed layer (Shen and Benner 2018). During exposure, temperature was maintained at 20°C by using a thermostatic bath. Samples were recovered and diluted in synthetic seawater (Aquil, major salt only, Cabanes et al. 2017) for further analyses.
Characterisation of iron chemistry
Voltammetric analyses
All voltammetric measurements were conducted using a μAutolab-II potentiostat (Ecochemie, Utrecht, Netherlands) connected to VA STAND 663 with a hanging mercury drop electrode (HMDE, Metrohm 663VA, Herisau, Switzerland), a platinum counter and an Ag|AgCl|3MKCl reference electrode. Iron-speciation analyses of labile and refractory DOC were performed following the established competitive ligand exchange–adsorptive cathodic stripping voltammetry (CLE–AdCSV) technique with salicylaldoxime (SA) as the competing ligand, optimised by Abualhaija and van den Berg (2014). Labile and refractory DOC aliquots (30 µM C) were diluted in inorganic artificial seawater (AQUIL major salt only). Briefly, 10-mL aliquots were pipetted into acid-cleaned and pre-conditioned low-density polyethylene 15-mL bottles (Nalgene, Fisher Scientific AG, Reinach, Switzerland). Then, each aliquot was buffered to pH 8.2 with a borate buffer (1 mol L–1 boric acid/0.35 mol L–1 NH4OH; Sigma, Buchs, Switzerland/VWR, Nyon, Switzerland) to which 0.4–10 nmol L–1 Fe was added (ICP–MS standard solution, Sigma). Three tubes were kept with no Fe addition. Following a 1-h equilibration time at room temperature, 5 µmol L–1 SA (98% Acros Organics, Fisher Scientific AG) solution was then added in all the tubes, which were left to equilibrate overnight before analysis. Finally, Fe organic speciation parameters such as the total concentration of ligand (LT) and the conditional stability constant of their complexes with Fe (log K′Fe′L) were calculated using proMCC software (ver. 3, see https://sites.google.com/site/mccprosece/download, accessed 10 July 2019; Omanović et al. 2015) and method was validated by analysis of certified materials (Cabanes et al. 2017). So as to confirm our results, we also performed back-calculations by using proMCC software to simulate titration curves and compare them to the experimental ones. The full dataset is presented in Table S2, which is available as Supplementary material to this paper.
Determination of humic substances (HS-like) was realised using the voltammetric method described by Laglera et al. (2007). This method is based on CSV, and makes use of the adsorptive properties of Fe–HS complexes on the mercury drop electrode. From each voltammogram, the intensity current induced by the reduction of the Fe–HS complexes was determined. HS-like material concentration of each sample was determined following Suwannee River fulvic acid (SRFA) standard addition. The limit of detection was 1.9 µg L–1 SRFA (Cabanes et al. 2017).
Characterisation of iron bioavailability
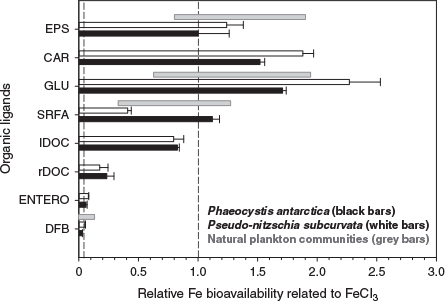
Iron bioavailability was measured using 55Fe (Perkin Elmer, Schwerzenbach, Switzerland, specific activity 44.66 mCi mg–1 as FeCl3 in 0.5 mol L–1 HCl). Briefly, cultures were grown at 4°C under 50 µmol quanta s–1 m–2 and a 16 : 8 light : dark cycle in natural seawater from the Polar Front region in the Drake Passage (RV Polarstern PS97, 25-m depth, 60°1.08′S, 66°21.85′W, 2–3 March 2016). Iron limitation was confirmed with a maximum effective quantum yield of <0.3 (Fast Ocean PTX coupled to a FastAct base unit, Chelsea Technologies, West Molesey, UK) and axenicity was verified using DAPI staining and microscopic observations. The 55Fe was let to equilibrate with stock solutions of organic ligands for 48 h up to 7 days. After addition of 55Fe-organic ligands to the inorganic synthetic seawater (AQUIL, major salt only, pH 8.1), solutions were left to equilibrate overnight prior to the addition of phytoplankton. Following 24-h incubation in the dark at 4°C (to avoid photodegradation during bioaccumulation experiment), solutions were filtered to collect phytoplankton (MicroAnalytix glass-fibre filters, 1.2 µm, Sydney, NSW, Australia) and rinsed for 2 min with 3 × 5 mL of Ti-EDTA solution to determine intracellular iron (Tang and Morel 2006). In addition to iron bioavailability associated with refractory and labile DOC (at concentration of 7 and 14 µmol L–1 C respectively), bioavailability of mono- and poly-saccharides (Hassler and Schoemann 2009; glucuronic acid, GLU, and carrageenan, CAR, 0.2 mg L–1), bacterial exopolymeric substances (EPS, 0.2 mg L–1), bacterial siderophores (Enterobactin, ENTERO, and desferrioxamine B, DFB, 15 nmol L–1), and Suwanne River fulvic acid (SRFA std 1, 0.1 mg L–1) were determined. Relative bioavailability to Pseudo-nitzschia subcurvata and Phaeocystis antarctica was determined by comparison of the Fe-uptake rates measured and the uptake predicted from the internalisation-rate constant (kint) at the corresponding dFe concentration (Hassler et al. 2011; Table S3, available as Supplementary material to this paper). Kint was determined in parallel for each strain in synthetic inorganic seawater with an increasing 55Fe concentration up to 0.9 nM, which represents inorganic-iron solubility limit at 4°C (Liu and Millero 2002; Fig. S2, available as Supplementary material to this paper). Dissolved inorganic iron is considered as fully bioavailable (Shaked et al. 2005; Lis et al. 2015). Experiments in presence of organic compounds were designed so that 55Fe additions did not saturate the iron-binding ligands associated with the compounds studied, and were performed in triplicate. Procedural blank, to account for Fe retention on filters not associated with phytoplankton, were run in duplicate. Calculations were performed considering specific activity and background Fe concentrations (nmol L–1) contained in synthetic seawater (0.2) and in the organic compounds added (Table 1, GLU: 0.6, CAR: 0.2, EPS: 0.1, SRFA: 0.4, DFB and ENTERO: 0.3). Quenching was determined using a calibration curve in the presence of acetonitrile (Sigma).
|
Results and discussion
Labile DOC was collected by filtration of material recovered from a plankton tow in a diatom bloom. The plankton-derived DOM had a C : N ratio of 6.4 (Table 1) and was enriched in amino acids and carbohydrates (Shen et al. 2016). Additions of plankton-derived DOM to seawater bioassay experiments were rapidly consumed by microorganisms, demonstrating that it is labile DOM (Shen et al. 2016). Refractory DOC was isolated by solid-phase extraction (C-18) from deep water (1000 m) in the North Atlantic Ocean (Shen and Benner 2018). Solid-phase extraction preferentially isolates non-polar, hydrophobic components that are depleted in nitrogen, enriched in carboxylated aliphatic molecules, and commonly referred to as humic substances (Hedges et al. 1992). The C : N ratio of the C-18 extract from the deep Atlantic Ocean was 36.6 (Shen and Benner 2018), which is similar to the C : N ratio (43.4) of marine humic substances extracted from the deep Pacific Ocean (Hedges et al. 1992). Low concentrations of dissolved lignin phenols and stable carbon isotopic ratios of DOC in deep Atlantic and Pacific waters indicated a predominantly marine origin of the humic substances (Hernes and Benner 2006). Detailed molecular characterisation using nuclear magnetic-resonance spectroscopy and ultrahigh-resolution mass spectrometry indicated that carboxyl-rich alicyclic molecules (CRAM), which are among the most refractory components of DOC in the ocean (Lechtenfeld et al. 2014), account for ~8% of marine DOC (Hertkorn et al. 2006). These refractory molecules appear to be decomposition products of terpenoids, and the abundance of carboxyl groups in CRAM makes these molecules likely candidates for metal–ligand binding (Hertkorn et al. 2006).
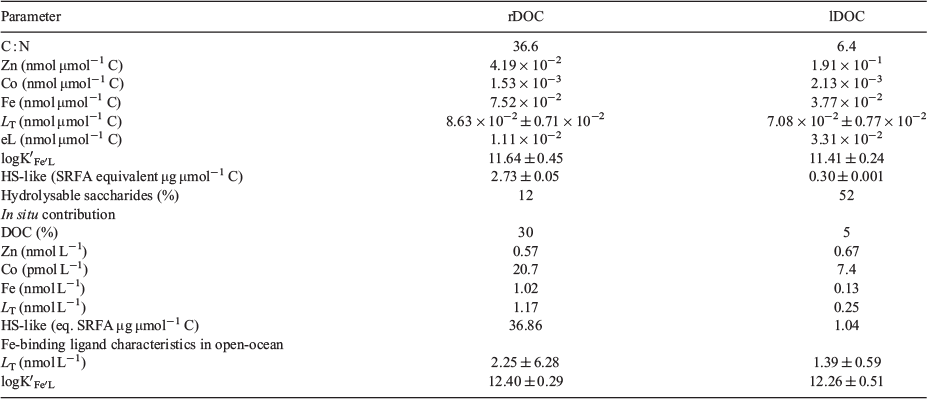
Iron and iron-binding ligands were detected in both refractory and labile DOC (Tables 1, S1, S2), demonstrating their potential impact on the biogeochemical cycling of iron. The conditional stability constants measured were similar and within the range of values reported in the open ocean (Caprara et al. 2016), suggesting that these dissolved organic molecules are representative of some ligands detected in situ. Seawater bioassay experiments in the North Atlantic Ocean indicated microorganisms re-mineralised ~5% of the total DOC (~70 µmol L–1) in surface water within 20 days (Shen and Benner 2018); so, we estimate that 3.5 µmol L–1 of the DOC is labile. The C-18-isolated refractory DOC accounted for 30% of the DOC (~45 µmol L–1) in deep waters of the North Atlantic (Shen and Benner 2018); so, we estimate that 13.5 µM C of the DOC is refractory. Considering that the concentrations of iron-binding ligands and HS-like material equal 3.5 µmol L–1 labile DOC in our experiment, one can calculate a contribution of iron-binding ligands of 0.25 nmol L–1 and HS-like material of 1.04 µg SRFA eq. L–1 respectively. Similarly, refractory DOC accounts for 13.5 µmol C L–1, 1.17 nmol L–1 iron-binding ligands, and 36.86 µg SRFA eq. L–1 (Table 1). To go further, comparing these estimated in situ ligand concentrations with the average ligand concentration from the global ocean (Caprara et al. 2016), we calculated that labile and refractory DOC contribute 18 and 51% of the iron-binding ligands reported in surface and deep water respectively (Table 1). Although 18% is a low contribution, labile DOC is rapidly utilised by microorganisms. Considering that 100 µg L–1 SRFA can bind 1.69 nmol L–1 iron (Laglera and van den Berg 2009), humic substances could account for 7% (0.02 of 0.25 nmol L–1 associated with labile DOC in situ) and 41% (0.81 of 1.17 nmol L–1 associated with refractory DOC in situ) of the iron-binding ligands associated with labile DOC and refractory DOC in surface and deep waters respectively (Table 1, Fig. 1).
|
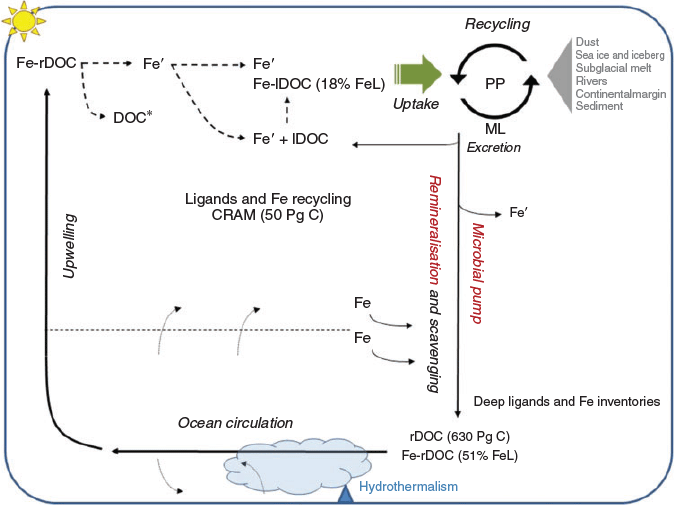
Refractory DOC was more saturated with iron than was labile DOC from the surface water, which is consistent with the increased ligand saturation reported in the deep West Atlantic Ocean (Gerringa et al. 2015). Concentrations of labile DOC decline rapidly with depth in the upper ocean, whereas refractory DOC remains fairly constant throughout the water column (Hansell 2013), thereby maintaining a high binding capacity for iron. Indeed, these molecules are very resistant to microbial degradation (Shen and Benner 2018) and play a critical role in maintaining the dissolved-iron inventory as well as its sequestration in the deep ocean (Fig. 1).
The concentrations of iron-binding humics were nine-fold greater in refractory DOC than in labile DOC, which is consistent with the strong humic signatures reported in the deep ocean (Hedges et al. 1992). Therefore, it is not surprising that the conditional stability constant of refractory DOC (Table 1) was close to those reported for standard terrestrial humics (log K′Fe′L = 11.1 and 10.6 for Suwannee River humic and fulvic acids respectively; Laglera and van den Berg 2009), suggesting that carboxyl groups could be involved in iron binding by terrestrial and marine humic substances. Cyclic terpenoids are biosynthesised by terrestrial and marine organisms, and oxidised decomposition products derived from these compounds are thought to be the major source of CRAM in freshwater (Lam et al. 2007) as well as marine DOC (Hertkorn et al. 2006). Therefore, it seems that CRAM could be an important ligand class for binding metals in terrestrial as well as marine environments. It is important to acknowledge that there is tremendous molecular diversity within CRAM (Hertkorn et al. 2006; Lam et al. 2007), and it appears that these degraded and altered molecules are acting as ligands by chance rather than by design.
Iron bioavailability was assessed using two axenic, iron-limited phytoplankton cultures from the Southern Ocean (the diatom Pseudo-nitzschia subcurvata and the prymnesiophyte Phaeocystis antarctica). To put these results in the context of in situ iron bioavailability, measurements for labile and refractory DOC were compared with organic compounds that are likely to be present in seawater, such as siderophores, saccharides, exopolymeric and humics substances (Fig. 2; Hutchins et al. 1999; Hassler et al. 2011). Iron equilibrated with labile DOC was highly bioavailable to both phytoplankton species, whereas its bioavailability was four-fold more than iron equilibrated with refractory DOC (Fig. 2). The similarity of the results for both taxonomic groups suggests that this trend could be transposed to most eukaryotic primary producers. The high bioavailability of iron associated with labile DOC in the present study was similar to that of labile compounds released by plankton, such as saccharides and exopolymeric substances (Hassler et al. 2011, 2015), and it fell in the upper limit of the bioavailability envelope (Lis et al. 2015), defined by Fe′, which is considered as fully bioavailable (Fig. 2). Heterotrophic microorganisms rapidly utilise these labile forms of organic matter in seawater, and it seems microbial utilisation of labile DOC could be coupled with iron utilisation by algal phytoplankton. Note that labile DOC showed an intermediate bioavailability between EPS (and saccharides) and SRFA, illustrating that labile DOC behaviour cannot solely be predicted by any of these compounds. Although less bioavailable than labile DOC, iron associated with refractory DOC is more bioavailable to algal phytoplankton than iron bound to the bacterially produced siderophores (strong ligands), which represents the lower limit of bioavailability (Fig. 2). The higher bioavailability reported for iron bound to terrestrial humic substances (SRFA) than to marine humics associated with refractory DOC (Fig. 2) infers differences in the composition and structure of ligands between terrestrial and marine humics (Hertkorn et al. 2006; Lam et al. 2007). This limits the applicability of terrestrial humics as model ligands for the open ocean. A mixture of refractory and labile DOC, and siderophores in the open ocean (Hassler et al. 2017), could reconcile the averaged bioavailability reported for natural communities in the presence of in situ ligands within the Tasman Sea and the Sub-Antarctic Zone (Hassler et al. 2015), as well as the Southern Ocean (Hassler and Schoemann 2009). Labile and refractory organic molecules are, thus, paramount for the iron cycle in the ocean, sustaining primary producers in iron-limited regions (Fig. 1).
|
The major iron sources in surface waters of the ocean are dust at lower latitudes and deep water upwelling at higher latitudes (Tagliabue et al. 2017). In the Southern Ocean, upwelled iron sources can be from refractory compounds (this study), hydrothermal vents and continental margins (Tagliabue et al. 2010, 2017). The organic nature of hydrothermal vents is not well constrained yet; however, it appears that hydrothermal-vent inputs of refractory DOC are minor (Lang et al. 2006; Hawkes et al. 2016). Ambient ligands at depth (Fitzsimmons et al. 2016), and possibly refractory DOC, are important to spread iron away from the venting site (Sander and Koschinsky 2011), whether ligands are originating from the vents, or the surrounding water, has not yet been determined. Ocean ventilation and deep winter mixing are critical to replenish dissolved iron in surface water and set up the spring growing season for primary producers (Tagliabue et al. 2014), especially in the Southern Ocean, a region prone to iron limitation. Upwelling and diapycnal diffusion (Tagliabue et al. 2014) of both iron and ligands associated with refractory DOC could play an important role, because photochemical alterations of marine siderophores releases reactive iron species (Barbeau et al. 2001). Upwelling exposes refractory DOC and iron to solar radiation, resulting in extensive photobleaching, the production of bioavailable photoproducts and remineralisation of refractory DOC (Shen and Benner 2018). Here, we tested, for the first time, the impact of photochemistry on refractory marine DOC and iron by exposure to a simulated solar spectrum mimicking seasonal light exposure in the mixed layer, as in Shen and Benner (2018). Electrochemical data demonstrated that both humic material and iron-binding ligands could not be measured (Table S2), indicating a significant alteration of organic ligands because of photodegradation. It is to be noted that refractory ligands might be degraded into ligands with much weaker conditional stabilities, out of our analytical window, as has previously been observed for siderophores (Barbeau et al. 2001; Maldonado et al. 2005; Amin et al. 2009). Given that refractory marine DOC with a similar sunlight exposure resulted in an enhanced bacterial carbon utilisation by only 2–3%, one can conclude a comparatively high sensitivity of iron-binding ligands to photodegradation. Minimal photodegradation of Antarctic diatom-aged DOC (Thomas and Lara 1995) was reported following an exposure to 40 W m–2 (~200 µmol photons m–2 s–1 in the visible spectrum; Woodward and Sheehey 1983). This lower light level was representative of average diatom light exposure in the mixed layer rather than surface-water exposure. In our study, because only the atmosphere filter was applied, light intensity of >700 nm was significant (see Fig. S1) and light exposure within the visible range was 1130 µmol photon m–2 s–1, a light intensity close to what is generally observed at midday in the temperate regions (~350 Wm–2 or 1500 µmol photons m–2 s–1; Woodward and Sheehey 1983).
The apparent high sensitivity of iron-binding ligands to photoalteration in the context of global overturning circulation (England 1995) is consistent with the estimated residence time (779–1039 years) of ligands in North Atlantic deep waters (Gerringa et al. 2015). Changes in deep iron-binding ligands imply a drastic modification in iron chemical lability, with bioavailable inorganic iron (Fe′) (Lis et al. 2015) changing from 0.055 × 10–2 up to 7.52 × 10–2 nmol µmol–1 C as a result of photodegradation. The Fe′ resulting from photodegradation is not likely to remain as inorganic iron but will either be directly taken up by phytoplankton or associated with organic ligands present in surface water (Fig. 1), some of which, such as labile DOC and EPS, have a high level of bioavailability (Fig. 2). This, thus, magnifies the potential biological impact of upwelled iron. Indeed, not only does the dissolved iron stock increase, but also its bioavailability (Lis et al. 2015), which will be critical for sustaining phytoplankton growth and iron recycling in iron-limited or co-limited regions (Rafter et al. 2017) subjected to upwelling, cold-core eddies and following winter mixing (Browning et al. 2017; Tagliabue et al. 2017).
Global overturning circulation transports refractory ligands to surface waters on a millennial time scale (England 1995; Hansell 2013), where they are exposed to solar radiation and photochemical transformation. The photodegradation products, including iron, could be directly utilised by plankton or exchanged with bioavailable ligands in surface waters (such as labile DOC; Fig. 1). Because labile DOC is short-lived molecules, produced rapidly and forming highly bioavailable iron-complexes, they could play a central role for iron cycling in surface waters. Dissolved iron cycling in surface waters is recognised to be rapid and able to fuel up to 90% of the iron requirement for primary producers in iron-limited water (Tagliabue et al. 2017). In the Southern Ocean, this requirement is then completed with other sustained iron inputs such as diapycnal diffusion (1%; Tagliabue et al. 2014) and hydrothermal vents (5% and up to 30% in specific regions; Tagliabue et al. 2010). However, winter mixing is the major input to initiate phytoplankton growth in the spring (Tagliabue et al. 2014, 2017). On the basis of the iron content of refractory DOC in this work, upwelled refractory DOC of marine origin could be the dominant contributor to the mean dissolved-iron vertical flux reported in the Southern Ocean (21.1 µmol m–2 year–1; Tagliabue et al. 2014). Considering that other annual iron inputs equal 20 µmol Fe m–2 year–1 (Tagliabue et al. 2014), iron associated with refractory DOC could contribute up to 50% of the iron input within the mixed layer of the Southern Ocean. Given that 20% of the iron associated with refractory DOC is readily bioavailable to sustain growth (a flux up to 4.2 µM m–2 year–1), with the rest potentially being degraded by photochemical processes that liberate highly bioavailable inorganic iron (this study), these organic molecules are essential to satisfy phytoplankton iron utilisation, especially at times of high demand, such as, for example, during spring blooms. Other iron sources such as hydrothermal vents and continental margin (Tagliabue et al. 2010, 2017) will contribute in initiating and sustaining primary producers. These will be complemented by localised iron sources such as sea ice, icebergs and subglacial melt (Death et al. 2014; Lannuzel et al. 2016). Biological activities of primary producers, zooplankton and the microbial loop maintain rapid iron cycling (Gledhill and Buck 2012; Tagliabue et al. 2017), which is critical for sustaining phytoplankton growth throughout the year.
Conclusions
Labile and refractory dissolved organic molecules appear to play critical roles in the distributions, concentrations and cycling of iron and other trace elements in the ocean. Because of limited bioavailability, iron-binding ligands associated with refractory DOC are expected to be distributed throughout the ocean water column. Photochemical processes in the surface ocean transform refractory ligands and associated iron into bioavailable forms (Shen and Benner 2018), with global overturning circulation and cold-core eddies regulating the delivery of refractory ligands to surface waters and, thereby, influencing ocean productivity. This study has shown a strong linkage between the cycling of marine DOC and iron, which supports the recent model proposed to predict iron cycling and its role in regulating primary productivity in iron-limited regions (Völker and Tagliabue 2015). Refractory DOC and its CRAM components are abundant and globally distributed, and it appears that these altered biomolecules play an important role in maintaining the ocean iron inventory. These iron-binding ligands and their photochemical degradation should be acknowledged as a regenerated iron source in surface waters in addition to other sources, such as atmospheric dust, continental margins, icebergs and subglacial melt. The marine refractory DOC studied here had an iron-binding affinity and an electrochemical humic ‘signature’ close to terrestrial humic and fulvic compounds; however, differences in bioavailability and aromatic content indicate molecular differences between marine and terrestrial humics (Hedges et al. 1992). In contrast, data on labile marine DOC are important for understanding iron and ligand cycling in the upper ocean as well as the magnitude of the response to local input, such as iron-laden dust at lower latitudes (Tagliabue et al. 2017). The subducted compounds in the Southern Ocean travelling to lower latitudes as refractory DOC can affect ocean biogeochemistry in remote surface waters. Moreover, because refractory DOC contains other micronutrients such as Cu, Co and Zn (Browning et al. 2017; Vance et al. 2017) (at levels representing ~30% of their in situ dissolved concentrations on the GEOTRACES GA03 expedition (Mawji et al. 2015; Table 1), it may affect ocean biogeochemistry and biological activity in numerous ways, for which our study has provided a mechanistic hypothesis.
Author contributions
C. Hassler, S. G. Sander and R. Benner contributed to experimental design and funding, R. Benner contributed to DOC isolation and chemical characterisation, S. G. Sander, C. Hassler and D. Cabanes contributed to trace metal analyses, C. Hassler and S. Blanco-Ameijeiras contributed to bioavailability analyses, all authors contributed to writing.
Data availability
Data and ancillary dataset are made available as Supplementary material.
Conflicts of interest
C. Hassler is an Associate Editor with Marine and Freshwater Research. Despite this relationship, she did not at any stage have editorial-level access to the manuscript while it was in peer review. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declarations of funding
C. Hassler, D. Cabanes and S. Blanco-Ameijeiras were supported by the Swiss National Science Foundation (PP00P2_138955 and PP00P2_166197). S. G. Sander was supported by Marsden Grant UOO1117 and through a collaborative agreement with the National Institute of Atmosphere and Water (NIWA). The IAEA is grateful to the Government of the Principality of Monaco for the support provided to its Environment Laboratories. R. Benner was supported by a grant (1504137) from the US National Science Foundation.
Acknowledgements
With this study, the authors and specifically I, Sylvia Sander, pay tribute to Keith Hunter, to whom the special issue is dedicated. Keith, who passed away sadly in October last 2018, was a pioneer and expert in the field of trace-metal speciation in marine and freshwater. Keith took me on as a postdoc in 2001 to boost his research group in the voltammetric determination of trace elements and their species. Although I had been working on redox speciation before, the ligand titrations were new to me and I still remember Keith explaining the speciation calculations to me for the first time. His trust in my abilities, support and friendship throughout my 16 years at the University of Otago were more than once the reason to keep doing what I was doing. I hope he knew how grateful I was for what he did for me, because we never say ‘thank you’ often enough to those who give a lot. We have lost, too early, a great scientist and ambassador for the environment, a wonderful person, mentor and good friend. But if it is true that we live as long as we are not forgotten, then Keith will probably outlive many us. We thank Y. Shen for collection of the deep refractory DOC.
References
Abualhaija, M. M., and van den Berg, C. M. G. (2014). Chemical speciation of iron in seawater using catalytic cathodic stripping voltammetry with ligand competition against salicylaldoxime. Marine Chemistry 164, 60–74.| Chemical speciation of iron in seawater using catalytic cathodic stripping voltammetry with ligand competition against salicylaldoxime.Crossref | GoogleScholarGoogle Scholar |
Amin, S. A., Greene, D. H., Hart, M. C., Küpper, F. C., Sunda, W. G., and Carrano, C. J. (2009). Photolysis of iron, siderophore chelates promotes bacterial, algal mutualism. Proceedings of the National Academy of Sciences of the United States of America 106, 17071–17076.
| Photolysis of iron, siderophore chelates promotes bacterial, algal mutualism.Crossref | GoogleScholarGoogle Scholar | 19805106PubMed |
Azam, F., and Malfatti, F. (2007). Microbial structuring of marine ecosystems. Nature Reviews. Microbiology 5, 782–791.
| Microbial structuring of marine ecosystems.Crossref | GoogleScholarGoogle Scholar | 17853906PubMed |
Barbeau, K., Rue, E. L., Bruland, K. W., and Butler, A. (2001). Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature 413, 409–413.
| Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands.Crossref | GoogleScholarGoogle Scholar | 11574885PubMed |
Benner, R., and Amon, R. M. W. (2015). The size-reactivity continuum of major bioelements in the ocean. Annual Review of Marine Science 7, 185–205.
| The size-reactivity continuum of major bioelements in the ocean.Crossref | GoogleScholarGoogle Scholar | 25062478PubMed |
Boiteau, R. M., Mende, D. R., Hawco, N. J., McIlvin, M. R., Fitzsimmons, J. N., Saito, M. A., Sedwick, P. N., DeLong, E. F., and Repeta, D. J. (2016). Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean. Proceedings of the National Academy of Sciences of the United States of America 113, 14237–14242.
| Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 27911777PubMed |
Browning, T. J., Achterberg, E. P., Rapp, I., Engel, A., Bertrand, E. M., Tagliabue, A., and Moore, M. (2017). Nutrient co-limitation at the boundary of an oceanic gyre. Nature 551, 242–246.
| Nutrient co-limitation at the boundary of an oceanic gyre.Crossref | GoogleScholarGoogle Scholar | 29088696PubMed |
Buck, K. N., Sohst, B., and Sedwick, P. N. (2015). The organic complexation of dissolved iron along the US GEOTRACES (GA03) North Atlantic Section. Deep-sea Research – II Topical Studies in Oceanography 116, 152–165.
| The organic complexation of dissolved iron along the US GEOTRACES (GA03) North Atlantic Section.Crossref | GoogleScholarGoogle Scholar |
Cabanes, D. J. E., Norman, L., Santos-Echeandia, J., Iversen, M. H., Trimborn, S., Laglera, L. M., and Hassler, C. S. (2017). First evaluation of the role of salp fecal pellets on iron biogeochemistry. Frontiers in Marine Science 3, 289.
| First evaluation of the role of salp fecal pellets on iron biogeochemistry.Crossref | GoogleScholarGoogle Scholar |
Caprara, S., Buck, K. N., Gerringa, L. J. A., Rijkenberg, M. J. A., and Monticelli, D. A. (2016). Compilation of iron speciation data for open oceanic waters. Frontiers in Marine Science 3, 1–7.
| Compilation of iron speciation data for open oceanic waters.Crossref | GoogleScholarGoogle Scholar |
Coale, K. H., Johnson, K. S., Fitzwater, S. E., Gordon, R. M., Tanner, S., Chavez, F. P., Ferioli, L., Sakamoto, C., Rogers, P., Millero, F., Steinberg, P., Nightingale, P., Cooper, D., Cochlan, W. P., Landry, M. R., Constantinou, J., Rollwagen, G., Trasvina, A., and Kudela, R. (1996). A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383, 495–501.
| A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 18680864PubMed |
Death, R., Wadham, J. L., Monteiro, F. M., Le Brocq, A. M., Tranter, M., Ridgwell, A., Dutkiewicz, S., and Raiswell, R. (2014). Antarctic ice sheet fertilises the Southern Ocean. Biogeosciences 11, 2635–2643.
| Antarctic ice sheet fertilises the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
England, M. H. (1995). The age of water and ventilation timescales in a global ocean model. Journal of Physical Oceanography 25, 2756–2777.
| The age of water and ventilation timescales in a global ocean model.Crossref | GoogleScholarGoogle Scholar |
Fitzsimmons, J. N., Conway, T. M., Lee, J.-M., Kayser, R., Thyng, K. M., John, S. G., and Boyle, E. A. (2016). Dissolved iron and iron isotopes in the southeastern Pacific Ocean. Global Biogeochemical Cycles 30, 1372–1395.
| Dissolved iron and iron isotopes in the southeastern Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Gerringa, L. J. A., Rijkenberg, M. J. A., Schoemann, V., Laan, P., and de Baar, H. J. W. (2015). Organic complexation of iron in the West Atlantic Ocean. Marine Chemistry 177, 434–446.
| Organic complexation of iron in the West Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Gledhill, M., and Buck, K. N. (2012). The organic complexation of iron in the marine environment: a review. Frontiers in Microbiology 3, 1–17.
| The organic complexation of iron in the marine environment: a review.Crossref | GoogleScholarGoogle Scholar |
Hansell, D. A. (2013). Recalcitrant dissolved organic carbon fractions. Annual Review of Marine Science 5, 421–445.
| Recalcitrant dissolved organic carbon fractions.Crossref | GoogleScholarGoogle Scholar | 22881353PubMed |
Hansell, D. A., and Carlson, C. A. (2015). ‘Biogeochemistry of Marine Dissolved Organic Matter’, 2nd edn. (Academic Press: London, UK.)
Hassler, C. S., and Schoemann, V. (2009). Bioavailability of organically bound Fe to model phytoplankton of the Southern Ocean. Biogeosciences 6, 1677–1712.
| Bioavailability of organically bound Fe to model phytoplankton of the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Hassler, C. S., Schoemann, V., Nichols, C. M., Butler, E. C. V., and Boyd, P. W. (2011). Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proceedings of the National Academy of Sciences of the United States of America 108, 1076–1081.
| Saccharides enhance iron bioavailability to Southern Ocean phytoplankton.Crossref | GoogleScholarGoogle Scholar | 21169217PubMed |
Hassler, C. S., Norman, L., Mancuso-Nichols, C. A., Clementson, L. A., Robinson, C., Schoemann, V., Watson, R. J., and Doblin, M. A. (2015). Iron associated with exopolymeric substances is highly bioavailable to oceanic phytoplankton. Marine Chemistry 173, 136–147.
| Iron associated with exopolymeric substances is highly bioavailable to oceanic phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Hassler, C. S., van den Berg, C. M. G., and Boyd, P. W. (2017). Toward a regional classification to provide a more inclusive examination of the ocean biogeochemistry of iron-binding ligands. Frontiers in Marine Science 4, 19.
| Toward a regional classification to provide a more inclusive examination of the ocean biogeochemistry of iron-binding ligands.Crossref | GoogleScholarGoogle Scholar |
Hawkes, J. A., Hansen, C. T., Goldhammer, T., Bach, W., and Dittmar, T. (2016). Molecular alteration of marine dissolved organic matter under experimental hydrothermal conditions. Geochimica et Cosmochimica Acta 175, 68–85.
| Molecular alteration of marine dissolved organic matter under experimental hydrothermal conditions.Crossref | GoogleScholarGoogle Scholar |
Hedges, J. I., Hatcher, P. G., Ertel, J. R., and Meyersschulte, K. J. A. (1992). Comparison of dissolved humic substances from seawater with amazon river counterparts by 13C-NMR spectrometry. Geochimica et Cosmochimica Acta 56, 1753–1757.
| Comparison of dissolved humic substances from seawater with amazon river counterparts by 13C-NMR spectrometry.Crossref | GoogleScholarGoogle Scholar |
Hernes, P. J., and Benner, R. (2006). Terrigenous organic matter sources and reactivity in the North Atlantic Ocean and a comparison to the Arctic and Pacific oceans. Marine Chemistry 100, 66–79.
| Terrigenous organic matter sources and reactivity in the North Atlantic Ocean and a comparison to the Arctic and Pacific oceans.Crossref | GoogleScholarGoogle Scholar |
Hertkorn, N., Benner, R., Frommberger, M., Schmitt-Kopplin, P., Witt, M., Kaiser, K., Kettrup, A., and Hedges, J. I. (2006). Characterization of a major refractory component of marine dissolved organic matter. Geochimica et Cosmochimica Acta 70, 2990–3010.
| Characterization of a major refractory component of marine dissolved organic matter.Crossref | GoogleScholarGoogle Scholar |
Hudson, R. J. M. (1998). Which aqueous species control the rates of trace metal uptake by aquatic biota? Observation and predictions of non-equilibrium effects. The Science of the Total Environment 219, 95–115.
| Which aqueous species control the rates of trace metal uptake by aquatic biota? Observation and predictions of non-equilibrium effects.Crossref | GoogleScholarGoogle Scholar |
Hutchins, D. A., Witter, A. E., Butler, A., and Luther, G. W. (1999). Competition among marine phytoplankton for different chelated iron species. Nature 400, 858–861.
| Competition among marine phytoplankton for different chelated iron species.Crossref | GoogleScholarGoogle Scholar |
Jiao, N., Herndl, G. J., Hansell, D. A., Benner, R., Kattner, G., Wilhelm, S. W., Kirchman, D. L., Weinbauer, M. G., Tingwei, L., Chen, F., and Azam, F. (2010). Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature Reviews. Microbiology 8, 593–599.
| Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean.Crossref | GoogleScholarGoogle Scholar | 20601964PubMed |
Laglera, L. M., and van den Berg, C. M. G. (2009). Evidence for geochemical control of iron by humic substances in seawater. Limnology and Oceanography 54, 610–619.
| Evidence for geochemical control of iron by humic substances in seawater.Crossref | GoogleScholarGoogle Scholar |
Laglera, L. M., Battaglia, G., and van den Berg, C. M. G. (2007). Determination of humic substances in natural waters by cathodic stripping voltammetry of their complexes with iron. Analytica Chimica Acta 599, 58–66.
| Determination of humic substances in natural waters by cathodic stripping voltammetry of their complexes with iron.Crossref | GoogleScholarGoogle Scholar | 17765064PubMed |
Lam, B., Baer, A., Alaee, M., Lefedvre, B., Moser, A., Williams, A., and Simpson, A. J. (2007). Major structural components in freshwater dissolved organic matter. Environmental Science & Technology 41, 8240–8247.
| Major structural components in freshwater dissolved organic matter.Crossref | GoogleScholarGoogle Scholar |
Lang, S. Q., Butterfield, D. A., Lilley, M. D., Paul Johnson, H., and Hedges, J. I. (2006). Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochimica et Cosmochimica Acta 70, 3830–3842.
| Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems.Crossref | GoogleScholarGoogle Scholar |
Lannuzel, D., Vancoppenolle, M., van der Merwe, P., de Jong, J., Meiners, K. M., Nishioka, J., and Schoemann, V. (2016). Iron in sea ice: review and new insights. Elementa 4, 000130.
| Iron in sea ice: review and new insights.Crossref | GoogleScholarGoogle Scholar |
Lechtenfeld, O. J., Kattner, G., Flerus, R., McCallister, S. L., Schmitt-Kopplin, P., and Koch, B. P. (2014). Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean. Geochimica et Cosmochimica Acta 126, 321–337.
| Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Legendre, L., Rivkin, R. B., Weinbauer, M. G., Guidi, L., and Uitz, J. (2015). The microbial carbon pump concept: potential biogeochemical significance in the globally changing ocean. Progress in Oceanography 134, 432–450.
| The microbial carbon pump concept: potential biogeochemical significance in the globally changing ocean.Crossref | GoogleScholarGoogle Scholar |
Lis, H., Shaked, Y., Kranzler, C., Keren, N., and Morel, F. M. M. (2015). Iron bioavailability to phytoplankton: an empirical approach. The ISME Journal 9, 1003–1013.
| Iron bioavailability to phytoplankton: an empirical approach.Crossref | GoogleScholarGoogle Scholar | 25350155PubMed |
Liu, X., and Millero, F. J. (2002). The solubility of iron in seawater. Marine Chemistry 77, 43–54.
| The solubility of iron in seawater.Crossref | GoogleScholarGoogle Scholar |
Maldonado, M. T., Strzepek, R. F., Sander, S. G., and Boyd, P. W. (2005). Acquisition of iron bound to strong organic complexes – with different Fe binding groups and photochemical reactivities by plankton communities in Fe-limited subantarctic waters. Global Biogeochemical Cycles 19, GB4S20.
| Acquisition of iron bound to strong organic complexes – with different Fe binding groups and photochemical reactivities by plankton communities in Fe-limited subantarctic waters.Crossref | GoogleScholarGoogle Scholar |
Mawji, E., Schlitzer, R., Masferrer Dodas, E., Abadie, C., Abouchami, W., Anderson, R. F., Baars, O., Bakker, K., Baskaran, M., Bates, N. R., Bluhm, K., Bowie, A., Bown, J., Boye, M., Boyle, E. A., Branellec, P., Bruland, K. W., Brzezinski, M. A., Bucciarelli, E., Buesseler, K., Butler, E., Cai, P., Cardinal, D., Casciotti, K., Chaves, J., Cheng, H., Chever, F., Church, T. M., Colman, A. S., Conway, T. M., Croot, P. L., Cutter, G. A., de Baar, H. J. W., de Souza, G. F., Dehairs, F., Deng, F., Thi Dieu, H., Dulaquais, G., Echegoyen-Sanz, Y., Edwards, R. L., Fahrbach, E., Fitzsimmons, J., Fleisher, M., Frank, M., Friedrich, J., Fripiat, F., Galer, S. J. G., Gamo, T., Garcia Solsona, E., Gerringa, L. G. A., Marcus Godoy, J., Gonzalez, S., Grossteffan, E., Hatta, M., Hayes, C. T., Iris Heller, M., Henderson, G., Huang, K.-F., Jeandel, C., Jenkins, W. J., John, S., Kenna, T. C., Klunder, M., Kretschmer, S., Kumamoto, Y., Laan, P., Labatut, M., Lacan, F., Lam, P. J., Lannuzel, D., le Moigne, F., Lechtenfeld, O. J., Lohan, M. C., Lu, Y., Masqué, P., McClain, C. R., Measures, C., Middag, R., Moffett, J., Navidad, A., Nishioka, J., Noble, A., Obata, H., Ohnemus, D. C., Owens, S., Planchon, F., Pradoux, C., Puigcorbé, V., Quay, P., Radic, A., Rehkämper, M., Remenyi, T., Rijkenberg, M. J. A., Rintoul, S., Robinson, L. F., Roeske, T., Rosenberg, M., van der Loeff, M. R., Ryabenko, E., Saito, M. A., Roshan, S., Salt, L., Sarthou, G., Schauer, U., Scott, P., Sedwick, P. N., Sha, L., Shiller, A. M., Sigman, D. M., Smethie, W., Smith, G. J., Sohrin, Y., Speich, S., Stichel, T., Stutsman, J., Swift, J. H., Tagliabue, A., Thomas, A., Tsunogai, U., Twining, B. S., van Aken, H. M., van Heuven, S., van Ooijen, J., van Weerlee, E., Venchiarutti, C., Voelker, A. H. L., Wake, B., and Warner, M. J. (2015). The GEOTRACES intermediate data product 2014. Marine Chemistry 177, 1–8.
| The GEOTRACES intermediate data product 2014.Crossref | GoogleScholarGoogle Scholar |
Myklestad, S. M., Skanoy, E., Hestmann, S., Skånøy, E., and Hestmann, S. A. (1997). Sensitive and rapid method for analysis of dissolved mono- and polysaccharides in seawater. Marine Chemistry 56, 279–286.
| Sensitive and rapid method for analysis of dissolved mono- and polysaccharides in seawater.Crossref | GoogleScholarGoogle Scholar |
Norman, L., Worms, I. A. M., Angles, E., Bowie, A. R., Mancuso Nichols, C., Pham, A. N., Slaveykova, V. I., Townsend, A. T., Waite, D., and Hassler, C. S. (2015). The role of bacterial and algal exopolymeric substances in iron chemistry. Marine Chemistry 173, 148–161.
| The role of bacterial and algal exopolymeric substances in iron chemistry.Crossref | GoogleScholarGoogle Scholar |
Omanović, D., Garnier, C., and Pižeta, I. (2015). ProMCC: an all-in-one tool for trace metal complexation studies. Marine Chemistry 173, 25–39.
| ProMCC: an all-in-one tool for trace metal complexation studies.Crossref | GoogleScholarGoogle Scholar |
Rafter, P. A., Sigman, D. M., and Mackey, K. R. M. (2017). Recycled iron fuels new production in the eastern equatorial Pacific Ocean. Nature Communications 8, 1100.
| Recycled iron fuels new production in the eastern equatorial Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 29062103PubMed |
Sander, S. G., and Koschinsky, A. (2011). Metal flux from hydrothermal vents increased by organic complexation. Nature Geoscience 4, 145–150.
| Metal flux from hydrothermal vents increased by organic complexation.Crossref | GoogleScholarGoogle Scholar |
Shaked, Y., Kustka, A. B., and Morel, F. M. M. (2005). A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnology and Oceanography 50, 872–882.
| A general kinetic model for iron acquisition by eukaryotic phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Shen, Y., and Benner, R. (2018). Mixing it up in the ocean carbon cycle and the removal of refractory dissolved organic carbon. Nature Scientific Reports 8, 2542.
| Mixing it up in the ocean carbon cycle and the removal of refractory dissolved organic carbon.Crossref | GoogleScholarGoogle Scholar |
Shen, Y., Fichot, C. G., Liang, S. K., and Benner, R. (2016). Biological hot spots and the accumulation of marine dissolved organic matter in a highly productive ocean margin. Limnology and Oceanography 61, 1287–1300.
| Biological hot spots and the accumulation of marine dissolved organic matter in a highly productive ocean margin.Crossref | GoogleScholarGoogle Scholar |
Tagliabue, A., Bopp, L., Dutay, J.-C., Bowie, A. R., Chever, F., Jean-Baptiste, P., Bucciarelli, E., Lannuzel, D., Remenyi, T., Sarthou, G., Aumont, O., Gehlen, M., and Jeandel, C. (2010). Hydrothermal contribution to the oceanic dissolved iron inventory. Nature Geoscience 3, 252–256.
| Hydrothermal contribution to the oceanic dissolved iron inventory.Crossref | GoogleScholarGoogle Scholar |
Tagliabue, A., Sallée, J.-B., Bowie, A. R., Lévy, M., Swart, S., and Boyd, P. W. (2014). Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing. Nature Geoscience 7, 314–320.
| Surface-water iron supplies in the Southern Ocean sustained by deep winter mixing.Crossref | GoogleScholarGoogle Scholar |
Tagliabue, A., Bowie, A. R., Boyd, P. W., Buck, K. N., Johnson, K. S., and Saito, M. A. (2017). The integral role of iron in ocean biogeochemistry. Nature 543, 51–59.
| The integral role of iron in ocean biogeochemistry.Crossref | GoogleScholarGoogle Scholar | 28252066PubMed |
Tang, D., and Morel, F. M. M. (2006). Distinguishing between cellular and Fe-oxide-associated trace elements in phytoplankton. Marine Chemistry 98, 18–30.
| Distinguishing between cellular and Fe-oxide-associated trace elements in phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Thomas, D. N., and Lara, R. J. (1995). Photodegradation of algal derived dissolved organic carbon. Marine Ecology Progress Series 116, 309–310.
| Photodegradation of algal derived dissolved organic carbon.Crossref | GoogleScholarGoogle Scholar |
Van Wambeke, F., Bonnet, S., Moutin, T., Raimbault, P., Alarcon, G., and Guieu, C. (2008). Factors limiting heterotrophic bacterial production in the southern Pacific Ocean. Biogeosciences 5, 833–845.
| Factors limiting heterotrophic bacterial production in the southern Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Vance, D., Little, S. H., de Souza, G. F., Khatiwala, S., Lohan, M. C., and Middag, R. (2017). Silicon and zinc biogeochemical cycles coupled through the Southern Ocean. Nature Geoscience 10, 202–206.
| Silicon and zinc biogeochemical cycles coupled through the Southern Ocean.Crossref | GoogleScholarGoogle Scholar |
Völker, C., and Tagliabue, A. (2015). Modeling organic iron-binding ligands in a three-dimensional biogeochemical ocean model. Marine Chemistry 173, 67–77.
| Modeling organic iron-binding ligands in a three-dimensional biogeochemical ocean model.Crossref | GoogleScholarGoogle Scholar |
Woodward, F. I., and Sheehey, J. E. (1983). ‘Principles and Measurements in Environmental Biology.’ (Butterworth: London, UK.)
Zhang, J., Kattner, G., and Koch, B. P. (2019). Interactions of trace elements and organic ligands in seawater and implications for quantifying biogeochemical dynamics: a review. Earth-Science Reviews 192, 631–649.
| Interactions of trace elements and organic ligands in seawater and implications for quantifying biogeochemical dynamics: a review.Crossref | GoogleScholarGoogle Scholar |


