Evaluation of the 137Ba mass-marking technique and potential effects in the early life history stages of Sepioteuthis lessoniana
Chun-I Chiang A B , Ming-Tsung Chung C , Tung-Wei Shih D , Tin-Yam Chan A E , Atsuko Yamaguchi B and Chia-Hui Wang E F GA Institute of Marine Biology, National Taiwan Ocean University, 2 Beining Road, Keelung, 20224, Taiwan.
B Graduate School of Fisheries and Environmental Sciences, Nagasaki University, 1–14 Bunkyo, Nagasaki, 852-8521, Japan.
C Department of Bioscience, Aquatic Biology, Aarhus University, Ole Worms Allé 1, DK-8000 Aarhus, Denmark.
D National Museum of Marine Science and Technology, 367 Beining Road, Keelung, 20224, Taiwan.
E Center of Excellence of the Oceans, National Taiwan Ocean University, 2 Beining Road, Keelung, 20224, Taiwan.
F Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, 2 Beining Road, Keelung, 20224, Taiwan.
G Corresponding author. Email: chwang99@ntou.edu.tw
Marine and Freshwater Research 70(12) 1698-1707 https://doi.org/10.1071/MF18325
Submitted: 3 September 2018 Accepted: 30 July 2019 Published: 22 October 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
The use of mass-marking techniques of enriched stable isotopes has increased in studies of ecology, movement patterns and the dispersal of marine organisms. However, the efficacy of this technique and its potential effects on hatchling size and statolith chemistry of cephalopods are yet to be investigated. Sepioteuthis lessoniana egg capsules were collected from northern Taiwan and assigned randomly to 137Ba-spiking experimental groups at 0.2, 0.5 and 1 ppm and three immersion durations (1, 3 and 7 days). Immersion duration >3 days produced significantly lower 138Ba : 137Ba ratios, with 100% marking success, indicating that it is a reliable marking technique. The 137Ba mass marking had a positive effect on size at hatch and was likely to affect statolith trace element incorporation, including Cu, Zn and Pb. These findings highlight that it is necessary to consider the species-specific effects on hatchling size and physiological responses in when using stable isotopes mass-marking techniques.
Additional keywords: barium isotopes, hatchling size, statolith chemistry.
Introduction
Cephalopods are crucial in commercial fisheries (Hunsicker et al. 2010; Jereb and Roper 2010) and are a vital component in marine food webs as a dietary source for numerous marine organisms (Clarke 1996; Klages 1996). The growth and population dynamics of cephalopods respond to environmental conditions, such as food abundance, temperature and water (Jackson and Moltschaniwskyj 2002; Forsythe 2004). Similar to most marine organisms, the degree of larval dispersal and distributional range of cephalopods determine connectivity of the population and are related to conservation of the ecosystem (Swearer et al. 1999; Cowen and Sponaugle 2009). Several methods for investigating dispersal patterns and migratory behaviours of marine animals in their early life history stages have been developed, but are not always applicable. For example, external tags may harm organisms and increase mortality rates (Sauer et al. 2000; Replinger and Wood 2007; Barry et al. 2011). In addition, the large tag size is impossible to use on squid paralarvae or juveniles (Nagasawa et al. 1993; Semmens et al. 2007). Although alternative biomarkers, like parasite communities and molecular markers, are not restricted by larval size, these methods do not provide detailed information on larval dispersal and movement patterns (Bower and Margolis 1991; Buresch et al. 2006). Traditional marking methods clearly have their limitations, and advanced marking techniques for early life history stages are required.
Recently, enriched stable isotope-marking techniques have been used, such as injecting enriched stable isotopes into mature females (Thorrold et al. 2006; Almany et al. 2007) or immersing offspring in water with enriched stable isotopes (Munro et al. 2008; Smith and Whitledge 2011; Woodcock et al. 2011a). Marked individuals with unique isotopic signatures in their biogenic carbonates are distinguishable from natural populations (Munro et al. 2008; Smith and Whitledge 2011). Stable isotopes of barium and strontium are commonly used in the marking experiments. Both elements have similarities in their ionic radius to Ca2+ and will likely be a substitute for Ca2+ in biogenic carbonates (Speer 1983). It is easier to perform this technique with barium for marine organisms because barium concentrations are low in natural seawater (varying in the range 0.007–15 ppm in seawater and fresh water; Bernat et al. 1972; Kresse et al. 2007). In addition, marking by feeding Ba-enriched dietary items has been suggested as a more effective method in marine systems (Woodcock and Walther 2014). The 137Ba isotope is stable in lower abundance (11.23%) and is not the major barium isotope (71.1% for 138Ba; Rosman and Taylor 1998). Because enrichment with 137Ba in calcified structures is greater than environmental levels, the mark is easily detected and shows a difference from natural seawater signatures (Thorrold et al. 2006). Therefore, 137Ba mass-marking techniques are likely suitable for tracking larval dispersal and movement patterns of cephalopods in the natural environment.
To date, only two studies have evaluated the enriched stable isotope mass-marking technique in cephalopod early life stages (Pecl et al. 2010; Payne et al. 2011). Maternal injection of stable isotopes in cephalopods may not mark offspring because there is difficulty in determining the oocyte maturity stage in cephalopod ovaries (Pecl et al. 2010). By contrast, Payne et al. (2011) marked Sepia apama hatchlings by immersing their spawning eggs in solutions spiked with different 137Ba concentrations (0.3 and 1 ppb) and for different durations (2, 5 and 8 days). These authors demonstrated the potential of using stable isotope-marking techniques to assess the population dynamics of cephalopods in the field (Payne et al. 2011). However, the efficacy of marking techniques varies among species. Thus, which method produces high-quality marks with lower costs in target species must be assessed (Warren-Myers et al. 2018).
Compared with fluorescent dyes (e.g. alizarin complexone), techniques for mass marking with enriched stable isotopes are usually seen as non-toxic to experimental offspring (Williamson et al. 2009; Woodcock et al. 2011a; Warren-Myers et al. 2018). Yet, there is evidence to suggest that enriched stable isotope mass-marking techniques may affect hatchling size (Williamson et al. 2009; Starrs et al. 2014a, 2014b). The size at hatch is crucially related to swimming and foraging abilities, which subsequently affect survival rate and reproduction (Sogard 1997). The effect of marking method on the hatchling size of cephalopod species is unclear. Moreover, the process of enriched stable isotope marking may alter physiological regulation, which may cause erroneous interpretations of cephalopod behaviours (de Vries et al. 2005). Such effects need to be carefully evaluated and considered in enriched stable isotope mass-marking experiments.
The present study used the method of immersing eggs of Sepioteuthis lessoniana with the enriched isotope of 137Ba and aimed to evaluate the optimum spiked concentration and immersion duration for successful marking of their statoliths. In addition, we examined hatchling size (mantle length (ML) and bodyweight) and growth condition factor (Fulton’s condition factor K) after marking, in addition to analysing elemental concentrations (24Mg, 25Mn, 63Cu, 64Zn, 88Sr and 208Pb) in hatchling statoliths. Changes to statolith chemistry may be induced by physiological regulation and varying accretion rates of calcium carbonate (Hamer and Jenkins 2007; Sturrock et al. 2015). This study looked at the potential effects of the enriched stable isotope mass-marking technique on cephalopod size at hatch and statolith chemistry.
Material and methods
Bigfin reef squid S. lessoniana are widely distributed in the neritic waters of the Indo-Pacific Ocean (Okutani 2015). They spawn almost throughout the entire year, with the embryo development period being ~23–26 days when the temperature is ~25°C (Segawa 1987). S. lessoniana egg capsules were collected by SCUBA diving from artificial bamboo reefs (depth ~20–25 m) at Wanghaixiang Bay in northern Taiwan in August 2015. The egg capsules were put in an opaque plastic bucket with natural seawater and immediately transported (<2 h) to the aquaculture station of the National Museum of Marine Science and Technology (Keelung, Taiwan). Before the experiments, all the egg capsules were suspended on nylon threads in a 200-L tank for initial acclimation. Natural seawater was collected from Wanghaixiang Bay and pumped through a filter bed to supply the rearing system. During the experiment, the seawater temperature was maintained at a mean (±s.d.) temperature of 25 ± 1°C, salinity was maintained at 34.1–34.7 PSU and experiments were conducted under a 12-h light–dark cycle.
In all, 150 eggs with visible embryos at 23–25 developmental stages, which were classified according to Segawa (1987), were randomly selected for each group and reared in a 10-L tank. There were nine experimental groups in total: three 137Ba spike concentrations (0.2, 0.5 and 1 ppm) and three immersion durations (1, 3 and 7 days). These groups were compared against a control group with no spiking. Different 137Ba concentrations were prepared by dissolving 137Ba-enriched BaCO3 (≥91% 137Ba and 8% 138Ba; Trace Sciences International, Richmond Hill, ON, Canada) in ultrapure water. For groups immersed for >1 day, half the rearing seawater was replaced daily and extra 137Ba spike was added to maintain the concentration of the 137Ba spike. After immersion, eggs were returned to the natural seawater until they hatched. The ML (mm) and bodyweight (mg) of the hatchlings were measured. Individuals were then killed by exposure to a high concentration of ethyl alcohol and their statoliths extracted. The experimental procedures followed the Guidelines for the Care and Welfare of Cephalopods in Research – A Consensus Based on an Initiative by CephRes, FELASA and the Boyd Group (Fiorito et al. 2015). The growth condition factor of hatchlings was estimated based on Fulton’s condition factor K, calculated as follows (Ricker 1975):
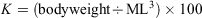
Statoliths were extracted under a stereomicroscope (SteREO Discovery, V12; Carl Ziess Microscopy GmbH, Jena, Germany), cleaned ultrasonically with 70% hydrogen peroxide to remove adhering tissue, rinsed three times in ultrapure water, placed into acid-washed Eppendorf microcentrifuge tubes and oven dried overnight. The statoliths were then transferred to 1.5-mL acid-washed high-density polyethylene vials and weighed on a microbalance to the nearest 10 μg. Individual pairs of statoliths were dissolved in 0.5 mL of 0.3 M ultrapure nitric acid. Solutions were analysed using inductively coupled plasma–mass spectrometry (ICP-MS; ELEMENT XR ICP-MS; Thermo Scientific, Bremen, Germany) at the Institute of Earth Science, Academia Sinica, Taipei, Taiwan. Nine isotopes (25Mg, 43Ca, 55Mn, 88Sr, 137Ba, 138Ba and 208Pb) were analysed in a low-resolution mode and two isotopes (63Cu and 64Zn) were evaluated in a medium-resolution mode. Element concentration is shown as a ratio relative to the concentration of calcium (mean element (Me) : Ca ratio). The carbonate (otolith)-certified reference material FEBS-1 (National Research Council, Ottawa, ON, Canada) was used to determine the Me : Ca ratio of samples and analysed every fifth sample to instrument drift. In regard to the matrix effect, statolith solutions in various calcium concentrations (0.5, 1, 5, 25 and 50 ppm) were tested and the Me : Ca ratios of every sample were normalised at the same level of matrix concentration. The relative standard deviations of the Me : Ca ratio measurements of FEBS-1 were lower than 4% for most elements expect Mn : Ca (Mg : Ca 3.37%; Mn : Ca 5.16%; Sr : Ca 1.79%; Ba : Ca 3.44%; Pb : Ca 2.46%; Cu : Ca 1.85%; Zn : Ca 3.06%), and the percentage accuracy of the Me : Ca ratios was better for Mg : Ca, Sr : Ca, Ba : Ca and Pb : Ca (1.04, 0.36; 0.60 and 1.21% respectively) than for Mn : Ca, Cu : Ca and Zn : Ca (7.20, 7.91 and 6.76% respectively). However, Mn concentrations detected were close to the background level and were excluded from further analyses.
All statistical analyses were performed using SPSS (ver. 20, IBM Corp., Armonk, NY, USA), as described below. A Shapiro–Wilk was used to assess the normality of the data, and Ba stable isotope ratios were found to be non-normally distributed. Therefore, a non-parametric Scheirer Ray Hare extension of the Kruskal–Wallis test was used to examine the effects of spiked concentration and immersion duration on 138Ba : 137Ba ratios. In addition, the effect of 137Ba spikes on the size and condition of marked hatchlings were analysed by two-way analysis of variance (ANOVA). If significant differences were detected, Tukey’s post hoc test was used to evaluate the difference between groups. For statolith chemistry, a forward stepwise canonical discriminant analysis was used to evaluate variations in element composition (Mg : Ca, Sr : Ca, Zn : Ca, Cu : Ca and Pb : Ca) among the control and all treatment groups, and cross-validation was further conducted to assess the percentage of successful classifications. In addition, Spearman’s ρ test was used to assess correlations between barium stable isotopes (137Ba : Ca and 138Ba : Ca) and other trace elements.
Results
Barium isotope ratios and mark success
The 137Ba spike was successfully marked in statoliths because 138Ba : 137Ba values decreased with increasing spike concentration or immersion duration. The mean (±s.d.) 138Ba : 137Ba ratio in statoliths in the control group was 6.28 ± 0.17, which decreased to 3.50 ± 0.22 after 7 days of immersion in 1-ppm 137Ba-spiked solution (Fig. 1). Significant interactions were found between immersion duration and the concentration of the 137Ba spike on 138Ba : 137Ba ratios in hatchling statoliths (Scheirer–Ray–Hare extension of the Kruskal–Wallis test, d.f. = 9, SS = 166601.9, H = 34.577, P < 0.001), so separate Dunn’s tests were used to compare the mean 138Ba : 137Ba ratios within groups. Overall, 7 days of immersion produced significantly lower mean 138Ba : 137Ba ratios than 1 day immersion for the same spiked concentration (Z > 4.057, P < 0.001), and the mean 138Ba : 137Ba ratios of the 1-ppm treatment were significantly lower than those of the 0.2-ppm treatment for the same immersion duration (Z > 3.510, P < 0.01). Longer immersion durations (3 and 7 days) with higher spiked concentrations (0.5 and 1 ppm) produced significantly lower 138Ba : 137Ba ratios than seen in the control group (Z > 4.564, P < 0.001). An additional significant difference was detected between 3- and 1-day immersions in the 0.2-ppm 137Ba-spiked group (Z > 3.510, P = 0.015).
|
Following the criteria of Payne et al. (2011), the critical value of successfully marked squid was set at 5.78, which was the mean ratio of the control group minus 3 s.d. for 138Ba : 137Ba. A successfully marked statolith was defined as a 138Ba : 137Ba ratio in the hatchling statolith that was lower than this value. Higher spiked concentrations and longer immersion duration both increased the success rate of statolith marking (Fig. 1). For example, no mark was found after 1 day of immersion with a 0.2-ppm 137Ba spike, but the success rate increased to 40% after 3 days of immersion with the same concentration. In total, 100% of squid were successfully marked after 3 days of immersion with the 0.5- and 1-ppm concentrations and after 7 days of immersion with all concentrations.
Hatchling size and growth condition factor
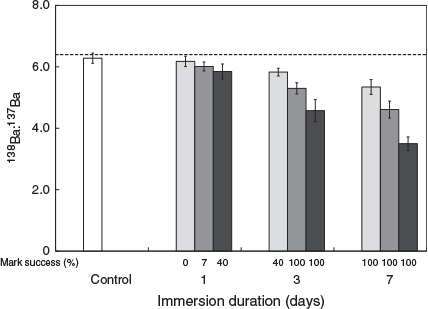
All eggs hatched 1–5 days after marking. The mean ML of the hatchlings in each group ranged from 5.54 to 5.99 mm, the mean bodyweight ranged from 24.4 to 31.3 mg and mean Fulton’s condition factor K ranged from 12.98 to 16.43 (Fig. 2). No interaction between spike concentration and immersion duration was found for ML (F = 0.795, P = 0.622), bodyweight (F = 1.162, P = 0.321) or Fulton’s condition factor K (F = 0.821, P = 0.597) of hatchlings. The spiked concentration of 137Ba significantly affected ML (F = 5.789, P = 0.001) and bodyweight (F = 6.687, P < 0.001) of hatchlings, but not Fulton’s condition factor K (F = 2.530, P = 0.058). Hatchlings exposed to spike concentrations of 0.2 and 1 ppm were significantly longer than those in the control group (Tukey’s honest significant difference (HSD), P = 0.001 and 0.008 respectively; Fig. 2a). The bodyweight of hatchlings in the control group was significantly lower than that of hatchlings in all spiked groups (P < 0.01, Fig. 2b). Conversely, the ML (F = 5.190, P = 0.002), bodyweight (F = 8.222, P < 0.001) and Fulton’s condition factor K (F = 3.214, P = 0.024) of hatchlings differed significantly among immersion duration treatments. Individuals in most immersion duration groups had a larger size in terms of ML and bodyweight than those in the control group, except for bodyweight observed after 1 day immersion (P = 0.063). In addition, there was a significant difference in Fulton’s condition factor K between 1 and 3 days of immersion (P = 0.013; Fig. 2c).
|
Element discrimination and correlation
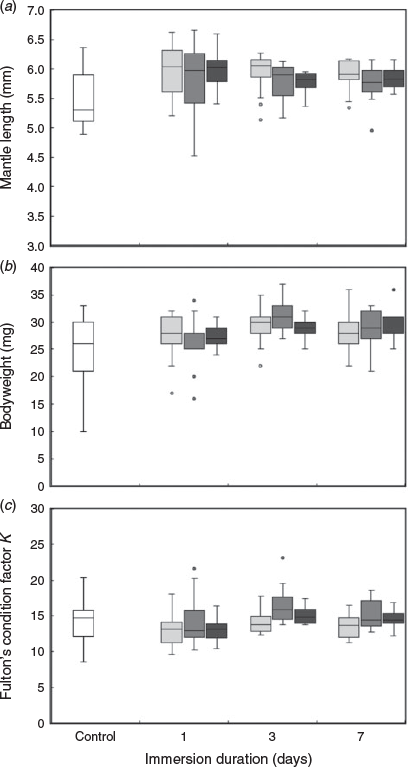
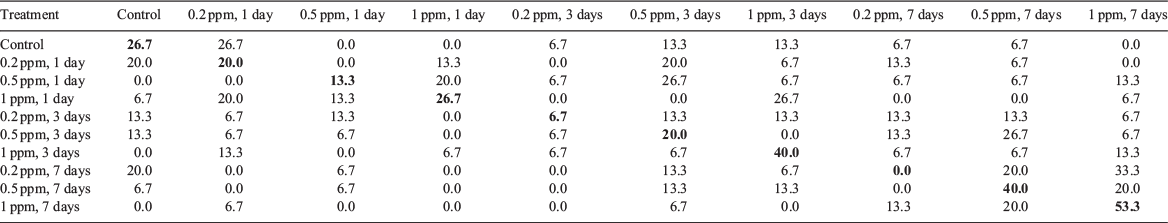
According to canonical discriminant analysis, hatchling statolith element composition did not show a clear pattern of discrimination between the control and all experimental groups (Fig. 3). The variations explained by Functions 1 and 2 were 53.9 and 24.0% respectively. Cu primarily contributed to Function 1 and Zn contributed to Function 2 (Table 1). The cross-validated classification success for all hatchlings was 24.7%, and ranged from 0% (7 days of immersion with 0.2 ppm of 137Ba) to 53.3% (7 days of immersion with 1 ppm of 137Ba) (Appendix 1).

|
|
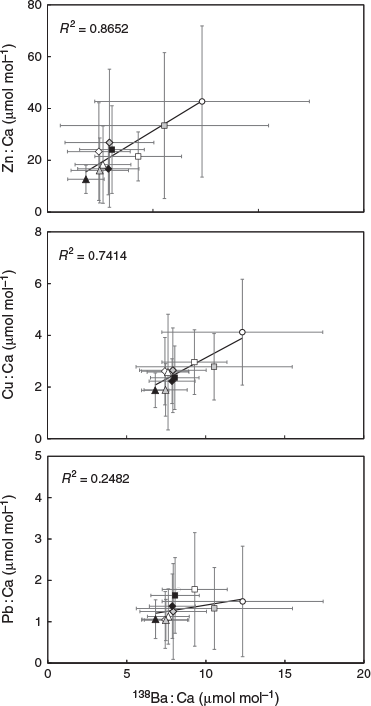
Although statoliths were enriched with 137Ba, their elemental : Ca ratios (Cu : Ca, Zn : Ca and Pb : Ca) positively correlated to 138Ba but not to 137Ba (Table 2; Fig. 4). The regressions of 138Ba : Ca with Cu : Ca, Zn : Ca and Pb : Ca were significant (P < 0.01), with determination coefficients (R2) of 0.865, 0.741 and 0.248 respectively.

|
|
Discussion
Because of its crucial role in marine ecosystems and being a highly attractive fishery target, effective ecological monitoring and resource management of S. lessoniana are needed. In particular, larval dispersal patterns and demographic population connectivity have significant effects on marine organism resources (Cowen et al. 2000; Thorrold et al. 2001; Jones et al. 2005; Cowen and Sponaugle 2009). There are many factors influencing the success rate of mass marking (e.g. spike concentration or developmental stage; Payne et al. 2011; Woodcock and Walther 2014). Consistent achievement of 100% mark success is a vital goal for any mass-marking technique (Warren-Myers et al. 2018). For fish larvae or eggs, concentrations of ≥0.1 ppm of 137Ba have been used to achieve 100% mark success by immersion (Woodcock et al. 2011a, 2011b; de Braux et al. 2014, Warren-Myers et al. 2015). However, the eggs of many cephalopod species (e.g. myopsid squid and sepioidea cuttlefish) are coated with encapsulation substances (i.e. a capsule) that are effective barriers against metal uptake into the embryo (Rosa et al. 2015). Therefore, the present study examined higher 137Ba spike concentrations to mark large numbers of S. lessoniana hatchlings and found that 100% mark success was achieved steadily after 3 days of immersion with concentrations >0.5 ppm of the enriched barium stable isotope.
This study revealed that 7 days of immersion with lower spike concentrations could also achieve 100% mark success, indicating that immersion duration is a critical factor for marking S. lessoniana statolith through egg immersion. This may be because the perivitelline fluid, which is in the capsule and encasing the embryo, is conducive to ambient seawater influx and swells gradually during the late development stage (Cronin and Seymour 2000). Extension of immersion during egg swelling results in the uptake of the spiked water, decreasing the 138Ba : 137Ba ratio within eggs. A similar effect of immersion duration on Ba stable isotope ratios in otoliths of fish species has been reported (Munro et al. 2008; de Braux et al. 2014). Yet, this is inconsistent with the results reported for S. apama by Payne et al. (2011), who found a significant interaction between the concentration of enriched 137Ba and immersion duration, but no significant differences among immersion durations for the lower concentration tested (0.3 ppb). Species and physiological differences may explain these different results. For example, egg swelling time varies according to embryo development period, thus the longer embryo development of S. apama (3–5 months; Hall and Fowler 2003) would dilute the contribution of immersion time to the 138Ba : 137Ba ratios in S. apama statoliths. Moreover, the low enriched 137Ba concentration may need a longer time of immersion, and the effect of immersion duration would become significant. In the study of Payne et al. (2011), extension of immersion duration from 2 to 8 days did decrease 138Ba : 137Ba ratios for the higher-concentration (1 ppb) treatment group. Therefore, determining the appropriate concentration and corresponding time of immersion before using this technique on a species of interest is important, because life history characteristics (e.g. developmental stage) and habitats (e.g. seawater or fresh water) may affect the effectiveness and the costs for mass marking.
The ML of hatchlings in this study was consistent with that reported by Lee et al. (1994), who continuously cultured S. lessoniana through three successive generations and whose hatchlings averaged 5.3 mm ML, ranging from 3.5 to 6.4 mm ML. The bodyweight of hatchlings in past studies varies, from a range of 4.3–12.0 mg (mean 8.2 mg; Lee et al. 1994) to 50 mg (Segawa 1987); the bodyweight of hatchlings in the present study fell between values published in the literature. In the present study, 137Ba mass marking slightly increased the ML and bodyweight of marked hatchlings in some of the experimental groups. Larger hatchling size may benefit from an increased attack speed (Sugimoto and Ikeda 2013) and a reduction in the distance required to capture prey accurately (Chen et al. 1996). In addition, hatchling size is linked to vulnerability to predators (Blaxter 1986; Sogard 1997), so that larger size hatchlings would have a greater survival rate in the early life history stages. Moreover, the growth condition (K) is related to embryo development and environmental variables, and individuals in a better condition (K) have higher survivorship and greater growth rate (Bolger and Connolly 1989). However, the K values of hatchlings in the present study only differed significantly between two immersion duration groups, indicating that 137Ba mass marking did not affect hatchling growth condition. Previous experimental results of the effects of transgenerational marking (i.e. injection method) on the condition of larval fish were species specific. Positive (Starrs et al. 2014a, 2014b), negative (Williamson et al. 2009) and no significant effects (Zitek et al. 2013; Warren-Myers et al. 2015) on size at hatch, yolk sac area, oil globule area and eyeball diameter were found among species. The findings of the present study provide additional information on cephalopod species marked using the immersion method. As noted by Starrs et al. (2014b), the effects of such mass marking with stable isotopes on hatchling morphology require additional research, as does the roles of barium during the developmental of S. lessoniana embryos.
We found different element compositions of statoliths in hatchlings that were related to size at hatch, and significant correlations were found between Me : Ca ratios (Cu : Ca, Zn : Ca and Pb : Ca) and 138Ba : Ca. The effects on element composition of statoliths are not often mentioned in the literature when marking cephalopod offspring with enriched stable isotopes. Element uptake in cephalopod statoliths is presumably similar to the observations in fish otoliths (Gillanders et al. 2013) and is primarily associated with environmental changes, such as water chemistry composition (Arkhipkin et al. 2004) and ambient temperature (Ikeda et al. 2002; Zumholz et al. 2007). However, in this study the rearing seawater was maintained at consistent conditions and egg capsules in the same cluster were used to eliminate any possible affects from the maternal yolk (e.g. Lloyd et al. 2008). The difference in growth rate between control and experimental groups was a potential explanation for variations in element incorporation. Growth rate has been confirmed to be negatively correlated with the elemental partition coefficient in otoliths of teleost species (Walther et al. 2010). A faster growth rate could result in more calcium-binding proteins, altering relative ion concentrations in the calcifying fluid (Kalish 1989). Therefore, trace elements such as Cu and Zn have a greater likelihood of being associated with organic matrix protein (Miller et al. 2006). In addition, a fast growth rate usually occurs with higher calcium carbonate accretion rate (Ikeda et al. 1999), which raises the pH of the calcifying fluid and reduces trace element concentrations in the endolymph, resulting in a negative relationship between the Me : Ca ratio and accretion rate (Sinclair 2005; Sinclair and Risk 2006; Hamer and Jenkins 2007). The lower hatchling size in the control group could simultaneously lead to elevated patterns of Me : Ca in statoliths. Although the effects of growth rate on the element composition of cephalopod statoliths have not been adequately clarified, the physiological processes do significantly affect the microchemistry of biogenic carbonates. We emphasise that the mechanisms of trace element incorporation into statoliths should be carefully considered to avoid confounding environmental signatures with artificial marking.
Understanding the population connectivity and migration of cephalopods is critical in developing approaches for the resource management and conservation of marine ecosystems. Stable isotope mass-marking techniques can be successfully used in fishes. This study demonstrated unique signatures in S. lessoniana statoliths with 100% marking success after 3 days of immersion in 137Ba and provides a method to unravel the questions regarding dispersal mechanisms and movement patterns in cephalopods. However, we also found potential effects of stable isotope mass marking on offspring size at hatch that are consistent with those reported by an increasing number of studies. The effects on embryo development and growth may induce variations in element composition in statoliths, probably reflecting physiological processes and statolith accretion, which affect statolith chemistry and may subsequently affect the accuracy of interpreting an individual’s environmental history. The present study defined the successful marking conditions for use with stable isotope-marking techniques on S. lessoniana and their potential effects on cephalopod statoliths. We highlight that the effects of this technique need to be taken into consideration in field applications. Additional research investigating the relationships among multiple elements and physiological responses to enriched stable isotope incorporation will advance our knowledge for the application of these techniques to wild cephalopods.
Conflicts of interest
Chia-Hui Wang declares that she is a guest editor of the Otoliths Symposium special issue for Marine and Freshwater Research but took no part in the review and acceptance of this manuscript. The authors declare that they have no further conflicts of interest.
Declaration of funding
This study was supported by the Ministry of Science and Technology, Taiwan (grant number 106-2611-M-019-004).
Acknowledgements
The authors extend particular thanks to Ming-Hsiang Wang and Ruei-Yuan Li for egg collections in the field. The authors also thank Ming-Ren Yang and Yu-Hung Tsai from the National Museum of Marine Science and Technology for experimental support at the aquaculture station, Kuo-Fang Huang from the Institute of Earth Science, Academia Sinica, for assisting with sample analysis and Nan-Jay Su from the Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, for his suggestions for statistical analyses.
References
Almany, G. R., Berumen, M. L., Thorrold, S. R., Planes, S., and Jones, G. P. (2007). Local replenishment of coral reef fish populations in a marine reserve. Science 316, 742–744.| Local replenishment of coral reef fish populations in a marine reserve.Crossref | GoogleScholarGoogle Scholar | 17478720PubMed |
Arkhipkin, A. I., Campana, S. E., FitzGerald, J., and Thorrold, S. R. (2004). Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Canadian Journal of Fisheries and Aquatic Sciences 61, 1212–1224.
| Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi).Crossref | GoogleScholarGoogle Scholar |
Barry, P. D., Tamone, S. L., and Tallmon, D. A. (2011). A comparison of tagging methodology for North Pacific giant octopus Enteroctopus dofleini. Fisheries Research 109, 370–372.
| A comparison of tagging methodology for North Pacific giant octopus Enteroctopus dofleini.Crossref | GoogleScholarGoogle Scholar |
Bernat, M., Church, T., and Allegre, C. J. (1972). Barium and strontium concentrations in Pacific and Mediterranean Sea water profiles by direct isotope dilution mass spectrometry. Earth and Planetary Science Letters 16, 75–80.
| Barium and strontium concentrations in Pacific and Mediterranean Sea water profiles by direct isotope dilution mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Blaxter, J. H. S. (1986). Development of sense organs and behavior of teleost larvae with special reference to feeding and predator avoidance. Transactions of the American Fisheries Society 115, 98–114.
| Development of sense organs and behavior of teleost larvae with special reference to feeding and predator avoidance.Crossref | GoogleScholarGoogle Scholar |
Bolger, T., and Connolly, P. L. (1989). The selection of suitable indices for the measurement and analysis of fish condition. Journal of Fish Biology 34, 171–182.
| The selection of suitable indices for the measurement and analysis of fish condition.Crossref | GoogleScholarGoogle Scholar |
Bower, S. M., and Margolis, L. (1991). Potential use of helminth parasites in stock identification of flying squid, Ommastrephes bartrami, in North Pacific Waters. Canadian Journal of Zoology 69, 1124–1126.
| Potential use of helminth parasites in stock identification of flying squid, Ommastrephes bartrami, in North Pacific Waters.Crossref | GoogleScholarGoogle Scholar |
Buresch, K. C., Gerlach, G., and Hanlon, R. T. (2006). Multiple genetic stocks of longfin squid Loligo pealeii in the NW Atlantic: stocks segregate inshore in summer, but aggregate offshore in winter. Marine Ecology Progress Series 310, 263–270.
| Multiple genetic stocks of longfin squid Loligo pealeii in the NW Atlantic: stocks segregate inshore in summer, but aggregate offshore in winter.Crossref | GoogleScholarGoogle Scholar |
Chen, D. S., Dykhuizen, G. V., Hodge, J., and Gilly, W. F. (1996). Ontogeny of copepod predation in juvenile squid (Loligo opalescens). The Biological Bulletin 190, 69–81.
| Ontogeny of copepod predation in juvenile squid (Loligo opalescens).Crossref | GoogleScholarGoogle Scholar | 8852631PubMed |
Clarke, M. R. (1996). Cephalopods in the world’s oceans: cephalopods as prey. III Cetaceans. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 351, 1053–1065.
| Cephalopods in the world’s oceans: cephalopods as prey. III Cetaceans.Crossref | GoogleScholarGoogle Scholar |
Cowen, R. K., and Sponaugle, S. (2009). Larval dispersal and marine population connectivity. Annual Review of Marine Science 1, 443–466.
| Larval dispersal and marine population connectivity.Crossref | GoogleScholarGoogle Scholar | 21141044PubMed |
Cowen, R. K., Lwiza, K. M. M., Sponaugle, S., Paris, C. B., and Olson, D. B. (2000). Connectivity of marine populations: open or closed? Science 287, 857–859.
| Connectivity of marine populations: open or closed?Crossref | GoogleScholarGoogle Scholar | 10657300PubMed |
Cronin, E. R., and Seymour, R. S. (2000). Respiration of the eggs of the giant cuttlefish Sepia apama. Marine Biology 136, 863–870.
| Respiration of the eggs of the giant cuttlefish Sepia apama.Crossref | GoogleScholarGoogle Scholar |
de Braux, E., Warren-Myers, F., Dempster, T., Fjelldal, P. G., Hansen, T., and Swearer, S. E. (2014). Osmotic induction improves batch marking of larval fish otoliths with enriched stable isotopes. ICES Journal of Marine Science 71, 2530–2538.
| Osmotic induction improves batch marking of larval fish otoliths with enriched stable isotopes.Crossref | GoogleScholarGoogle Scholar |
de Vries, M. C., Gillanders, B. M., and Elsdon, T. S. (2005). Facilitation of barium uptake into fish otoliths: influence of strontium concentration and salinity. Geochimica et Cosmochimica Acta 69, 4061–4072.
| Facilitation of barium uptake into fish otoliths: influence of strontium concentration and salinity.Crossref | GoogleScholarGoogle Scholar |
Fiorito, G., Affuso, A., Basil, J., Cole, A., de Girolamo, P., D’Angelo, L., Dickel, L., Gestal, C., Grasso, F., Kuba, M., Mark, F., Melillo, D., Osorio, D., Perkins, K., Ponte, G., Shashar, N., Smith, D., Smith, J., and Andrews, P. L. R. (2015). Guidelines for the care and welfare of cephalpods in research – a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Laboratory Animals 49, 1–90.
| Guidelines for the care and welfare of cephalpods in research – a consensus based on an initiative by CephRes, FELASA and the Boyd Group.Crossref | GoogleScholarGoogle Scholar | 26354955PubMed |
Forsythe, J. W. (2004). Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice. Marine and Freshwater Research 55, 331–339.
| Accounting for the effect of temperature on squid growth in nature: from hypothesis to practice.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., Wilkinson, L. M., Monro, A. R., and de Vries, M. C. (2013). Statolith chemistry of two life history stages of cuttlefish: effects of temperature and seawater trace element concentration. Geochimica et Cosmochimica Acta 101, 12–23.
| Statolith chemistry of two life history stages of cuttlefish: effects of temperature and seawater trace element concentration.Crossref | GoogleScholarGoogle Scholar |
Hall, K. C., and Fowler, A. J. (2003). The fisheries biology of the cuttlefish, Sepia apama Gray, in South Australian waters. FRDC final report, Project number 98/151, South Australian Research and Development Institute, Adelaide, SA, Australia.
Hamer, P. A., and Jenkins, G. P. (2007). Comparison of spatial variation in otolith chemistry of two fish species and relationships with water chemistry and otolith growth. Journal of Fish Biology 71, 1035–1055.
| Comparison of spatial variation in otolith chemistry of two fish species and relationships with water chemistry and otolith growth.Crossref | GoogleScholarGoogle Scholar |
Hunsicker, M. E., Essingtion, T. E., Watson, R., and Sumaila, U. R. (2010). The contribution of cephalopods to global marine fisheries: can we have our squid and eat them too? Fish and Fisheries 11, 421–438.
| The contribution of cephalopods to global marine fisheries: can we have our squid and eat them too?Crossref | GoogleScholarGoogle Scholar |
Ikeda, Y., Wada, Y., Arai, N., and Sakamoto, W. (1999). Note on size variation of body and statoliths in the oval squid Sepioteuthis lessoniana hatchlings. Journal of the Marine Biological Association of the United Kingdom 79, 757–759.
| Note on size variation of body and statoliths in the oval squid Sepioteuthis lessoniana hatchlings.Crossref | GoogleScholarGoogle Scholar |
Ikeda, Y., Yatsu, A., Arai, N., and Sakamoto, W. (2002). Concentration of statolith trace elements in the jumbo flying squid during El Niño and non-El Niño years in the eastern Pacific. Journal of the Marine Biological Association of the United Kingdom 82, 863–866.
| Concentration of statolith trace elements in the jumbo flying squid during El Niño and non-El Niño years in the eastern Pacific.Crossref | GoogleScholarGoogle Scholar |
Jackson, G. D., and Moltschaniwskyj, N. A. (2002). Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae). Marine Biology 140, 747–754.
| Spatial and temporal variation in growth rates and maturity in the Indo-Pacific squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae).Crossref | GoogleScholarGoogle Scholar |
Jereb, P., and Roper, C. F. E. (2010). Sepioteuthis lessoniana Férussac in Lesson, 1831. In ‘Cephalopods of the World: an Annotated and Illustrated Catalogue of Cephalopod Species Known to Date, Volume 2. Myopisd and Oegopsid Squids’. (Eds P. Jereb and C. F. E. Roper.) FAO Species Catalogue for Fishery Purposes 4, pp. 95–97. (FAO: Rome, Italy.) Available at http://www.fao.org/3/i1920e/i1920e.pdf [Verified 5 September 2019].
Jones, G. P., Planes, S., and Thorrold, S. R. (2005). Coral reef fish larvae settle close to home. Current Biology 15, 1314–1318.
| Coral reef fish larvae settle close to home.Crossref | GoogleScholarGoogle Scholar | 16051176PubMed |
Kalish, J. M. (1989). Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. Journal of Experimental Marine Biology and Ecology 132, 151–178.
| Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition.Crossref | GoogleScholarGoogle Scholar |
Klages, N. T. W. (1996). Cephalopods in the world’s oceans: cephalopods as prey. II Seals. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 351, 1045–1052.
| Cephalopods in the world’s oceans: cephalopods as prey. II Seals.Crossref | GoogleScholarGoogle Scholar |
Kresse, R., Baudis, U., Jäger, P., Riechers, H. H., Wagner, H., Winkler, J., and Wolf, H. U. (2007). Barium and barium compounds. In ‘Ullmann’s Encyclopedia of Industrial Chemistry’. (Ed. H. Pelc.) pp 621–640. (Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany.)
Lee, P. G., Turk, P. E., Yang, W. T., and Hanlon, R. T. (1994). Biological characteristics and biomedical applications of the squid Sepioteuthis lessoniana cultured through multiple generations. The Biological Bulletin 186, 328–341.
| Biological characteristics and biomedical applications of the squid Sepioteuthis lessoniana cultured through multiple generations.Crossref | GoogleScholarGoogle Scholar | 8043657PubMed |
Lloyd, D. C., Zacherl, D. C., Walker, S., Paradis, G., Sheehy, M., and Warner, R. R. (2008). Egg source, temperature and culture seawater affect elemental signatures in Kelletia kelletii larval statoliths. Marine Ecology Progress Series 353, 115–130.
| Egg source, temperature and culture seawater affect elemental signatures in Kelletia kelletii larval statoliths.Crossref | GoogleScholarGoogle Scholar |
Miller, B. M., Clough, A. M., Batson, J. N., and Vachet, R. W. (2006). Transition metal binding to cod otolith proteins. Journal of Experimental Marine Biology and Ecology 329, 135–143.
| Transition metal binding to cod otolith proteins.Crossref | GoogleScholarGoogle Scholar |
Munro, A. R., Gillanders, B. M., Elsdon, T. S., Crook, D. A., and Sanger, A. C. (2008). Enriched stable isotope marking of juvenile golded perch (Macquaria ambigua) otoliths. Canadian Journal of Fisheries and Aquatic Sciences 65, 276–285.
| Enriched stable isotope marking of juvenile golded perch (Macquaria ambigua) otoliths.Crossref | GoogleScholarGoogle Scholar |
Nagasawa, K., Takayanagi, S., and Takami, T. (1993). Cephalopod tagging and marking in Japan, a review. In ‘Recent Advances in Cephalopod Fisheries Biology’. (Eds T. Okutani, R. K. O’Dor, and T. Kuboera.) pp. 313–330. (Tokai University Press: Tokyo, Japan.)
Okutani, T. (2015). Sepioteuthis lessoniana Férussac in Lesson, 1832. In ‘Cuttlefishes and Squids of the World’. (Tokai University Press: Tokyo, Japan.) Available at http://www.zen-ika.com/zukan/pdf/cs184.pdf?idx=1 [Verified 6 September 2019].
Payne, N. L., Semmens, J. M., and Gillanders, B. M. (2011). Elemental uptake via immersion: a mass-marking technique for early life-history stages of cephalopods. Marine Ecology Progress Series 436, 169–176.
| Elemental uptake via immersion: a mass-marking technique for early life-history stages of cephalopods.Crossref | GoogleScholarGoogle Scholar |
Pecl, G. T., Doubleday, Z. A., Danyushevsky, L., Gilbert, S., and Moltschaniwskyj, N. A. (2010). Transgenerational marking of cephalopods with an enriched barium isotope: a promising tool for empirically estimating post-hatching movement and population connectivity. ICES Journal of Marine Science 67, 1372–1380.
| Transgenerational marking of cephalopods with an enriched barium isotope: a promising tool for empirically estimating post-hatching movement and population connectivity.Crossref | GoogleScholarGoogle Scholar |
Replinger, S. E., and Wood, J. B. (2007). A preliminary investigation of the use of subcutaneous tagging in Caribbean reef squid Sepioteuthis sepioidea (Cephalopoda: Loliginidae). Fisheries Research 84, 308–313.
| A preliminary investigation of the use of subcutaneous tagging in Caribbean reef squid Sepioteuthis sepioidea (Cephalopoda: Loliginidae).Crossref | GoogleScholarGoogle Scholar |
Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish population. Bulletin – Fisheries Research Board of Canada 191, 209–210.
Rosa, I. C., Raimundo, J., Lopes, V. M., Brandão, C., Couto, A., Santos, C., Cabecinhas, A. S., Cereja, R., Calado, R., Caetano, M., and Rosa, R. (2015). Cuttlefish capsule: an effective shield against contaminants in the wild. Chemosphere 135, 7–13.
| Cuttlefish capsule: an effective shield against contaminants in the wild.Crossref | GoogleScholarGoogle Scholar | 25876030PubMed |
Rosman, K. J. R., and Taylor, P. D. P. (1998). Isotopic compositions of the elements 1997 (technical report). Pure and Applied Chemistry 70, 217–235.
| Isotopic compositions of the elements 1997 (technical report).Crossref | GoogleScholarGoogle Scholar |
Sauer, W. H. H., Lipinski, M. R., and Augustyn, C. J. (2000). Tag recapture studies of the chokka squid Loligo vulgaris reynaudii d’Orbigny, 1845 on inshore spawning grounds on the south-east coast of South Africa. Fisheries Research 45, 283–289.
| Tag recapture studies of the chokka squid Loligo vulgaris reynaudii d’Orbigny, 1845 on inshore spawning grounds on the south-east coast of South Africa.Crossref | GoogleScholarGoogle Scholar |
Segawa, S. (1987). Life history of the oval squid, Sepiothuthis lessoniana in Kominato and adjacent waters central Honshu, Japan. Journal of the Tokyo University of Fisheries 74, 67–105.
Semmens, J. M., Pecl, G. T., Gillanders, B. M., Waluda, C. M., Shea, E. K., Jouffre, D., Ichii, T., Zumholz, K., Katugin, O. N., Leporati, S. C., and Shaw, P. W. (2007). Approaches to resolving cephalopod movement and migration patterns. Reviews in Fish Biology and Fisheries 17, 401–423.
| Approaches to resolving cephalopod movement and migration patterns.Crossref | GoogleScholarGoogle Scholar |
Sinclair, D. J. (2005). Correlated trace element ‘vital effects’ in tropical corals: a new geochemical tool for probing biomineralization. Geochimica et Cosmochimica Acta 69, 3265–3284.
| Correlated trace element ‘vital effects’ in tropical corals: a new geochemical tool for probing biomineralization.Crossref | GoogleScholarGoogle Scholar |
Sinclair, D. J., and Risk, M. J. (2006). A numerical model of trace-element coprecipitation in a physicochemical calcification system: application to coral biomineralization and trace-element ‘vital effects’. Geochimica et Cosmochimica Acta 70, 3855–3868.
| A numerical model of trace-element coprecipitation in a physicochemical calcification system: application to coral biomineralization and trace-element ‘vital effects’.Crossref | GoogleScholarGoogle Scholar |
Smith, K. T., and Whitledge, G. W. (2011). Evaluation of a stable-isotope labelling technique for mass marking fin rays of age-0 lake sturgeon. Fisheries Management and Ecology 18, 168–175.
| Evaluation of a stable-isotope labelling technique for mass marking fin rays of age-0 lake sturgeon.Crossref | GoogleScholarGoogle Scholar |
Sogard, S. M. (1997). Size-selective mortality in the juvenile stage of teleost fishes: a review. Bulletin of Marine Science 60, 1129–1157.
Speer, J. A. (1983). Crystal chemistry and phase relations of orthorhombic carbonates. Reviews in Mineralogy and Geochemistry 11, 145–190.
Starrs, D., Ebner, B. C., Eggins, S. M., and Fulton, C. J. (2014a). Longevity in maternal transmission of isotopic marks. Marine and Freshwater Research 65, 400–408.
| Longevity in maternal transmission of isotopic marks.Crossref | GoogleScholarGoogle Scholar |
Starrs, D., Davis, J. T., Schlaefer, J., Ebner, B. C., Eggins, S. M., and Fulton, C. J. (2014b). Maternally transmitted isotopes and their effects on larval fish: a validation of dual isotopic marks with a meta-analysis. Canadian Journal of Fisheries and Aquatic Sciences 71, 387–397.
| Maternally transmitted isotopes and their effects on larval fish: a validation of dual isotopic marks with a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Sturrock, A. M., Hunter, E., Milton, J. A., EIMF Johnson, R. C., Waring, C. P., and Trueman, C. N. (2015). Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution 6, 806–816.
| Quantifying physiological influences on otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Sugimoto, C., and Ikeda, Y. (2013). Comparison of the ontogeny of hunting behavior in pharaoh cuttlefish (Sepia pharaonis) and oval squid (Sepioteuthis lessoniana). The Biological Bulletin 225, 50–59.
| Comparison of the ontogeny of hunting behavior in pharaoh cuttlefish (Sepia pharaonis) and oval squid (Sepioteuthis lessoniana).Crossref | GoogleScholarGoogle Scholar | 24088796PubMed |
Swearer, S. E., Caselle, J. E., Lea, D. W., and Warner, R. R. (1999). Larval retention and recruitment in an island population of a coral-reef fish. Nature 402, 799–802.
| Larval retention and recruitment in an island population of a coral-reef fish.Crossref | GoogleScholarGoogle Scholar |
Thorrold, S. R., Latkoczy, C., Swart, P. K., and Jones, C. M. (2001). Natal homing in a marine fish metapopulation. Science 291, 297–299.
| Natal homing in a marine fish metapopulation.Crossref | GoogleScholarGoogle Scholar | 11209078PubMed |
Thorrold, S. R., Jones, G. P., Planes, S., and Hare, J. A. (2006). Transgenerational marking of embryonic otoliths in marine fishes using barium stable isotopes. Canadian Journal of Fisheries and Aquatic Sciences 63, 1193–1197.
| Transgenerational marking of embryonic otoliths in marine fishes using barium stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Walther, B. D., Kingsford, M. J., O’Callaghan, M. D., and McCullonch, M. T. (2010). Interaction effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environmental Biology of Fishes 89, 441–451.
| Interaction effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish.Crossref | GoogleScholarGoogle Scholar |
Warren-Myers, F., Dempster, T., Fjelldal, P. G., Hansen, T., and Swearer, S. E. (2015). Immersion during egg swelling results in rapid uptake of stable isotope markers in salmonid otoliths. Canadian Journal of Fisheries and Aquatic Sciences 72, 722–727.
| Immersion during egg swelling results in rapid uptake of stable isotope markers in salmonid otoliths.Crossref | GoogleScholarGoogle Scholar |
Warren-Myers, F., Dempster, T., and Swearer, S. E. (2018). Otolith mass marking techniques for aquaculture and restocking: benefits and limitations. Reviews in Fish Biology and Fisheries 28, 485–501.
| Otolith mass marking techniques for aquaculture and restocking: benefits and limitations.Crossref | GoogleScholarGoogle Scholar |
Williamson, D. H., Jones, G. P., and Thorrold, S. R. (2009). An experimental evaluation of transgenerational isotope labelling in a coral reef grouper. Marine Biology 156, 2517–2525.
| An experimental evaluation of transgenerational isotope labelling in a coral reef grouper.Crossref | GoogleScholarGoogle Scholar |
Woodcock, S. H., and Walther, B. D. (2014). Concentration-dependent mixing models predict values of diet-derived stable isotope ratios in fish otoliths. Journal of Experimental Marine Biology and Ecology 454, 63–69.
| Concentration-dependent mixing models predict values of diet-derived stable isotope ratios in fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Woodcock, S. H., Gillanders, B. M., Munro, A. R., McGovern, F., Crook, D. A., and Sanger, A. C. (2011a). Using enriched stable isotopes of barium and magnesium to batch mark otoliths of larval golden perch (Macquaria ambigua, Richardson). Ecology Freshwater Fish 20, 157–165.
| Using enriched stable isotopes of barium and magnesium to batch mark otoliths of larval golden perch (Macquaria ambigua, Richardson).Crossref | GoogleScholarGoogle Scholar |
Woodcock, S. H., Gillanders, B. M., Munro, A. R., Crook, D. A., and Sanger, A. C. (2011b). Determining mark success of 15 combinations of enriched stable isotopes for the batch marking of larval otoliths. North American Journal of Fisheries Management 31, 843–851.
| Determining mark success of 15 combinations of enriched stable isotopes for the batch marking of larval otoliths.Crossref | GoogleScholarGoogle Scholar |
Zitek, A., Irrgeher, J., Kletzl, M., Weismann, T., and Prohaska, T. (2013). Transgenerational marking of brown trout Salmo trutta f.f. using an 84Sr spike. Fisheries Management and Ecology 20, 354–361.
| Transgenerational marking of brown trout Salmo trutta f.f. using an 84Sr spike.Crossref | GoogleScholarGoogle Scholar |
Zumholz, K., Hansteen, T. H., Piatkowski, U., and Crrot, P. L. (2007). Influence of temperature and salinity on the trace element incorporation into statoliths of the common cuttlefish (Sepia officinalis). Marine Biology 151, 1321–1330.
| Influence of temperature and salinity on the trace element incorporation into statoliths of the common cuttlefish (Sepia officinalis).Crossref | GoogleScholarGoogle Scholar |
Appendix 1 Cross-validated classification success for the statoliths of hatchlings in the control and experimental groups based on the discriminant function analysis scores
Correct classifications are in bold. Hatchlings in the experimental groups were immersed in water containing different concentrations of 137Ba spike (0.2, 0.5 and 1 ppm) for 1, 3 and 7 days
|


