Changes in environmental salinity during the life of Pangasius krempfi in the Mekong Delta (Vietnam) estimated from otolith Sr : Ca ratios
Ngan T. Tran A C , Maylis Labonne B , Huy D. Hoang A and Jacques Panfili A BA Vietnam National University Ho Chi Minh City–University of Science, 227 Nguyen Van Cu, District 5, Ho Chi Minh City, Vietnam.
B Marine Biodiversity, Exploitation and Conservation (MARBEC), Institut de Recherche pour le Développement (IRD), Institut Français de Recherche pour l’Exploitation de la Mer (Ifremer), Université de Montpellier (UM), Centre national de la recherche scientifique (CNRS), cc093, Place E. Bataillon, F-34095 Montpellier, France.
C Corresponding author. Email: ttngan@hcmus.edu.vn
Marine and Freshwater Research 70(12) 1734-1746 https://doi.org/10.1071/MF18269
Submitted: 27 July 2018 Accepted: 1 May 2019 Published: 13 August 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Pangasius krempfi is a commercially important catfish in the Mekong River and is believed to migrate along the Mekong River basin. To verify this migration, elemental concentrations were measured in the water and in otoliths to infer the salinity of the water through the fish’s lifetime. In 2017, eight element concentrations were measured along the Mekong Delta using solution-based inductively coupled plasma mass spectrometry (ICP-MS). Concentrations of Sr, Li and Rb were strongly and positively correlated with salinity. Otoliths were taken from P. krempfi caught in the brackish waters of the lower Mekong Delta and seven element : Ca ratios were measured from the core to the otolith edge using laser ablation ICP-MS. The Sr : Ca, Ba : Ca, P : Ca and Mn : Ca ratios varied through the lifetime of the fish, but only Sr : Ca was suitable for estimating ambient salinity. The Sr : Ca profiles in otoliths were analysed and significantly correlated between individuals, with all fish hatched in water with very low levels of salinity, indicating a single freshwater spawning ground, and then living in waters with higher salinity, with two types of migration behaviour. Some individuals may return to low-salinity waters when older. These conclusions were supported by the Ba : Ca and Mn : Ca ratios. These migration patterns may have implications for fishery management.
Additional keywords: behaviour, climate change, diadromous fish, microchemistry, Pangasiidae.
Introduction
Understanding the movement patterns of migratory fish is essential for fishery management and conservation across large spatial scales (Gillanders 2005a; Carlson et al. 2017). In some major rivers, knowledge of these movements has become a priority for management because fish caught in one country may have migrated across several other countries (Hogan et al. 2007; Fukushima et al. 2014). Migratory fish in fresh water are particularly vulnerable because the water properties and the connectivity of different habitats may change (Poulsen et al. 2004). Although migration across national boundaries has an effect on fisheries, it is also necessary to understand the life history traits in the various habitats because human activities and climate change may have different effects on different life history stages (Poulsen et al. 2004; Dugan et al. 2010).
Catfish species (Siluriformes) are very important target species for fisheries and aquaculture in South-east Asia. Catfish aquaculture is one of the most developed socioeconomic sectors in this area, relying upon only two freshwater species from the Pangasiidae family: Pangasianodon hypophthalmus, the striped catfish or sutchi catfish, and Pangasius bocourti, the basa catfish (Halls and Johns 2013; Quyen et al. 2017). In the Mekong River basin, wild catfish are an important resource for income and food for local populations. Most wild catfish caught in the Mekong River are migratory (Baird et al. 2004; Poulsen et al. 2004). One of these, Pangasius krempfi Fang & Chaux, 1949 (Pangasiidae), is a diadromous, large-bodied catfish classified as ‘Vulnerable’ in the International Union for Conservation of Nature (IUCN) Red List, and commands a higher economic value in local markets than cultivated catfish or other wild catfish species (Poulsen et al. 2004; Hogan et al. 2007; Baird 2013). P. krempfi is one of 13 Pangasiidae species known to be present in the Mekong River and is commercially important in the Mekong Delta, Cambodia and Laos (Poulsen et al. 2004; Rainboth et al. 2012). P. krempfi has a wide geographic distribution in the Mekong River basin, from China to Thailand, Laos, Cambodia and Vietnam, but is primarily found in the middle and lower Mekong basin (Rainboth 1996; Poulsen et al. 2004; Vidthayanon 2008). In the Mekong Delta, P. krempfi has been found and caught in estuarine zones in both the main branches and in the Vam Nao Canal (Poulsen et al. 2004; Vu et al. 2009). This species could be a candidate for shifting catfish aquaculture from freshwater to brackish water in the Mekong Delta affected by climate change and recent saline intrusion (Joint Assessment Teams 2016; Southern Institute of Water Resources Research 2016). Although P. krempfi is commercially important, its migration patterns have received little attention. Studies of P. krempfi collected in Laos, more than 20 years ago, provided some evidence of possible long-distance migrations and a marine life stage for P. krempfi using a combination of catches, otolith Sr : Ca ratios and the stable isotope ratios of carbon and nitrogen (Baird et al. 2004; Hogan et al. 2007). However, these studies were restricted to a specific area and fish caught in estuarine and marine areas were not included.
Fish considered to be P. krempfi may be two different species in the Mekong (Rainboth 1996) or there may be two contingents of P. krempfi with different migration patterns (Poulsen et al. 2004). However, neither of these hypotheses has been confirmed. The spawning grounds, migration patterns and the timing of spawning and migration of P. krempfi are not fully known (Poulsen et al. 2004; Baird 2013), although P. krempfi is caught in the Khone Falls area (Laos) from May to July and in the Mekong Delta almost all year around (Poulsen et al. 2004). Therefore, the spawning grounds are thought to be upstream in the Mekong River, around the Khone Falls area, which has rapids and deep pools, and the young fish migrate rapidly downstream, spending most of their life in estuarine areas or at sea (Poulsen et al. 2004; Hogan et al. 2007). However, the P. krempfi population in the wild is declining and it has become vulnerable due to threats from overexploitation and habitat degradation. Dams and water management are changing migration behaviour by modifying the water flow and habitats, potentially reducing genetic diversity and connectivity, and the increased mortality is reducing the sustainable population size (Hogan 2011a; Baird 2013; Junge et al. 2014). Understanding P. krempfi migration patterns has become essential for monitoring and managing the population(s), as well as for exploiting this species for aquaculture (Baird 2013).
Otolith microchemistry can be used as an effective method to understand fish movements across different habitats. Fish otoliths (ear stones) are calcified structures that grow in regular cycles (daily and seasonal) and contain and reflect detailed changes in temperature and element concentrations in an individual fish’s environment (Campana 1999). When combining microchemistry with age estimation, otoliths can provide valuable information about an individual fish’s life history, including potential migrations for species that pass through different habitats (Wells et al. 2003; Panfili et al. 2009, 2012; Fukushima et al. 2014). Environmental conditions during a fish’s life can be indicated by the composition of the otoliths, such as Sr, Zn, Pb, Mn, Ba and Fe concentrations, which can show differences, for example, between freshwater and marine environments (Campana 1999; Wells et al. 2003). Sr, Ca and Mg concentrations in the water have been found to be linearly correlated with salinity, whereas the Ba concentration may show segmented linear (negative then positive) relationships with salinity (Gillanders and Munro 2012). Element incorporation into otoliths is affected by temperature (Webb et al. 2012) and by salinity (Labonne et al. 2009), and, in some species, is subject to strong incorporation regulation (Panfili et al. 2015). Most of the time, studies use element to Ca ratios in otoliths to investigate variations in element incorporation. Changes in Sr : Ca and Ba : Ca ratios through the lifetime are related to the salinity of the ambient water and have proved to be a useful means of inferring the movements between freshwater and estuarine habitats with different salinity levels (Campana 1999; Gillanders 2005a; Zimmerman 2005). Therefore, element concentrations in otoliths may be useful in assessing the movements of P. krempfi in the Mekong Delta. Based on Sr : Ca profiles, Hogan et al. (2007) found evidence of migrations for P. krempfi in both marine and brackish environments for fish caught in the Lao reaches of the Mekong River Basin. This was based on the assumption that otolith Sr : Ca was linearly related to ambient salinity.
In the present study we hypothesised that P. krempfi is an anadromous catfish that undertakes long-distance migrations and spends a part of its life in the estuarine waters of the lower Mekong, as proposed by Poulsen et al. (2004) and Hogan et al. (2007). These previous studies were extended and improved by collecting fish in the lower Mekong, by using advanced methods for otolith elemental microchemistry (solution-based and laser ablation inductively coupled plasma mass spectrometry (ICP-MS)), combined with age estimation, and by determining the relationships between salinity and element concentrations in ambient water at different points in the river. The aim of the study was to evaluate the possibility that P. krempfi caught in the Mekong Delta had moved between environments with different salinities during its lifetime. The specific objectives of the study were to: (1) estimate the concentrations of specific elements in the water at various points in the delta; (2) assess the changes in otolith chemistry (element : Ca ratios) during the life of P. krempfi caught in the lower Mekong Delta; and (3) determine the effective movements between habitats with different salinities during the lifetime of the fish. The elements Mg, P, Ca, Mn, Zn Sr, Ba and Pb were selected as key elements in otoliths to investigate relationships between otolith composition and salinity.
Materials and methods
Study area
The Mekong is a major river in South-east Asia, rising in the Tibetan Plateau and crossing six countries (China, Myanmar, Laos, Thailand, Cambodia and Vietnam). After crossing the Tonle Sap Lake in Cambodia, the river divides into two distributaries, the Mekong (Tien River) and the Bassac (Hau River), which create a major delta in Vietnam that drains into the South China Sea through nine river mouths (Fig. 1). The Mekong Delta in Vietnam covers ~39 000 km2 (Vo 2012) and its hydrology depends strongly on upstream water flows, rainfall and tidal movements. The rainy season is from May to November, and the dry season is from December to April. At the end of the dry season, the Mekong Delta coastal areas are usually strongly affected by saline intrusions from the sea. Saline waters can reach up to 90 km inland from the river mouths (Southern Institute of Water Resources Research 2016).

|
Both water and fish were sampled from March to June 2017, mainly targeting the lower Mekong Delta near the sea, and the two main branches (the Tien River and the Hau River; Fig. 1). The sites in the Tien River were selected along the gradient of abnormally high salinity in April 2016 (Southern Institute of Water Resources Research 2016). The aim was to capture only individuals close to brackish waters (in the delta) and to determine their potential migrations to other parts of the Mekong River basin with higher or lower salinity.
Water sampling and element concentration analyses
Water samples were collected at four main sites in the main branches (Fig. 1). First, 15-mL tubes were cleaned with pure nitric acid, rinsed and then filled with Milli-Q water until sampling. At each sampling site, 10 mL of surface water was collected in the tube and fixed with 0.2 mL of 2% ultrapure nitric acid. Microchemical analyses were performed using solution-based ICP-MS (7700x; Agilent, Santa Clara, CA, USA) at OSU OREME (Observatoire des Sciences de l’Univers – Observatoire de REcherche Méditerranéen de l’Environnement, University of Montpellier, Montpellier, France). Samples were filtered to 0.22 µm to remove suspended particles and diluted using milli-Q water by a factor between two and four depending on the salinity. Indium (115In) and Bismuth (209Bi) were used as internal standards. Concentrations were determined by external calibration using multielement solutions with concentrations in the range 0.25–5 ppb. We measured concentrations (ppb) of 7Li, 55Mn, 66Zn, 85Rb, 86Sr, 118Sn, 138Ba and 208Pb. Polyatomic interference was limited by keeping the oxide level below 1%. The certified material SLRS-6 (National Research Council Canada, Ottawa, ON, Canada) was used and typical analytical precisions reached by this technique are generally between 1 and 3% relative standard deviation. Ca concentrations could not be measured in the water, but the Sr : Ba ratios were calculated for comparison with the otoliths (see below).
Fish and otolith sampling
P. krempfi were caught in the Tien River in Binh Dai (Ben Tre Province) and My Tho (Tien Giang Province), as well as in the Hau River in Dinh An (Tra Vinh Province; Fig. 1). All individuals were collected on fish landing or in markets after confirming the provenance. Targeted fishing could not be used because the species is rare and caught by night fishing. Most of the collected fish were from Binh Dai because it was difficult to find the fish in the other areas during the sampling period. In all, 30 individuals were collected randomly with a wide range of lengths and weights. Specimens were measured (total length, TL, and fork length, FL, mm) and weighed (g). The right largest otolith (the lapillus; Fig. 2) was extracted using plastic forceps from a sagittal head section (because the skull is very thick), cleaned in 99.9% ethanol and stored dry in 1.5-mL microtube vials.

|
Otolith preparation and element concentration analyses
Whole otoliths were first photographed on both sides (internal and external) using a binocular microscope and reflected light against a dark background (Motic 2.0 video camera, Wetzlar, Germany). Seasonal increments were not clearly visible on the whole otoliths. These images were then used for determination of the transverse sectioning planes. Each otolith was then embedded in epoxy resin (polymerised in an oven at 40°C for 24 h) and sliced transversally using a low-speed cutting machine (Isomet saw Buehler, Esslingen am Neckar, Germany). A cut was made either side of the otolith core to obtain 1-mm sections including the core. The posterior face of each otolith section was then polished using 1200-, 2400- and 4000-grit dry abrasive paper until the core was reached. The sections were then sonicated for 5 min in ultrapure water, dried under a Class 100 laminar flow hood and attached to a clean microscope slide for further processing. The otolith sections were photographed using a binocular microscope with reflected light against a dark background (Motic 2.0 video camera). The seasonal growth increments were interpreted on the section from the core to the internal face of the otolith as alternate opaque and translucent bands (Fig. 2). The translucent bands were counted to estimate the age, in seasons, and their distance from the otolith core was measured (for age verification and growth modelling, see Fig. S1 of the Supplementary material).
Element concentrations (ppb) in the otoliths were measured using laser ablation ICP-MS (FINNIGAN- Element2 XR, Waltham, MA, USA, coupled to a Lambda Physik 193-nm laser, Göttingen, Germany) at AETE-OSU OREME (Analyse des Eléments en Trace dans l’Environnement & Isotopes – Observatoire des Sciences de l’Univers – Observatoire de REcherche Méditerranéen de l’Environnement, University of Montpellier, Montpellier, France). For each otolith, concentrations were measured along a transect from the core to the edge (as for age estimation; Fig. 2). The transect was ablated in two passes. The first pass (pulse rate 4 Hz, energy 15 J cm–2, spot diameter 77 µm) removed residual surface contamination before the measurement pass (pulse rate 7 Hz, speed 20 µm s–1, energy 15 J cm–2, spot diameter 51 µm). For calibration and machine drift correction, a glass reference material (NIST 612; National Institute of Standards and Technology, Gaithersburg, MD, USA) was analysed at the start of each session, every five samples and at the end of each session. A second reference material (Multi Axis Crystal Spectrometer, MACS, National Institute of Standards and Technology; Rodriguez et al. 2008; Strnad et al. 2009) was also analysed during the session for quality control. To remove residual sample gas that may have interfered with the analysis, the laser chamber was purged for 30 s before the analysis of each sample. Seven elements were chosen as the most commonly used in the literature: 24Mg, 31P, 55Mn, 66Zn, 86Sr, 138Ba and 208Pb. Most of these were above the detection limit in each otolith, except 66Zn and 208Pb. 43Ca was used as the internal standard and concentrations are expressed as ratios to 43Ca (ppb/ppb). For each chemical element, the counts per second (CPS) were divided by the 43Ca CPS and corrected using the ratios measured for the NIST 612 and the reference values (ppb/ppb). All raw data were processed using the elementR package for R (see https://cran.r-project.org/web/packages/elementR/index.html, https://github.com/charlottesirot/elementR, accessed 11 June 2019; Sirot et al. 2017), including blank subtraction, limit of detection correction, reference material and sample reduction, machine drift correction and outlier detection (for details, see Sirot et al. 2017). Of these elements, only Sr, Ba and Mn were also measured in the river water; other elements were used to confirm the change in fish environment. The Sr : Ca and Ba : Ca ratios were selected as the main target for studying the relationship with salinity. In addition, otolith Sr : Ba was examined as a direct comparison to water concentrations.
Statistical analysis
Relationships between element concentrations in the water and salinity were established using linear regression. The element : Ca profiles for each otolith were established using moving means of three adjacent values at a given distance from the core to the edge. The overall variations in the element : Ca mean profile was tested by comparing the average profile to each individual’s profile. Correlations of element : Ca ratios between individuals were tested using a correlation matrix for the profiles with pairwise comparisons. To test for similarity of the Sr : Ca ratio profiles between individuals, we used a hierarchical cluster analysis (HCA) based on the Euclidian distances between their Sr : Ca profiles and Ward’s method for clustering. Differences between the mean ratios of the clusters identified by HCA were tested by one-way analysis of variance (ANOVA). The statistical analyses were performed using R (ver. 3.6.0, R Foundation for Statistical Computing, Vienna, Austria) and Statistica (ver. 13.5, StatSoft, Hamburg, Germany).
Unless indicated otherwise, data are presented as the mean ± s.d.
Results
Water element signatures
Fourteen water samples from the four main sites were collected, with salinities ranging from 0 to 22. Linear regressions between element concentration and salinity showed different patterns for Sr, Ba and Mn. The regression was highly significant and positive for Sr, as well as for Li and Rb (Fig. 3; Table 1; R2 > 0.80, P < 0.05). For Mn and Pb, the regressions were less significant (R2 = 0.55 and 0.58 respectively, P < 0.05), whereas for Ba, Sn and Zn the regression was not significant (R2 < 0.49, P > 0.05; Table 1 and Fig. 3). The Sr : Ba ratio was significantly correlated with salinity, as indicated by a positive linear regression (R2 = 0.78; Fig. 3).

|
Otolith element concentrations throughout the lifetime
The largest of the 30 fish was 845 mm TL and the smallest was 166 mm TL. The translucent increments on the otolith sections could not be clearly identified for 5 of the 30 fishes (17%), and so they were discarded from further analysis. The remaining 25 otoliths were then analysed successfully for element concentrations and element : Ca ratios throughout the life of the fish. The maximum number of translucent increments was 5 and the minimum was 1, so the ages were taken to be between 1 and 5 years (see Fig. S1 and S2 of the Supplementary material).
Of the seven element : Ca ratios in P. krempfi otoliths, Sr : Ca, Ba : Ca, Mn : Ca and P : Ca varied greatly throughout the lifetime, whereas Mg : Ca was fairly constant (Fig. 4). Zn : Ca and Pb : Ca were below the limits of detection and therefore not considered further. Individual Ba : Ca profiles showed a consistent pattern for all individuals: a very high Ba : Ca at the core (77.4 ± 2.4 × 10–6) with a fast decrease to a low plateau before the end of the first year, with an average ratio of 9.8 ± 5.0 × 10–6 until the end of life (Fig. 4). In some otoliths, the Ba : Ca ratio also exhibited a small peak after the first year, but this did not correspond to any visible feature in the otolith structure. Mn : Ca and P : Ca profiles showed similar patterns to that of the Ba : Ca profile, but more variable at the end of the life, with a strong increase in the P : Ca ratio. The Mg : Ca profiles looked fairly constant, with a very small peak at the core and becoming stable after that.
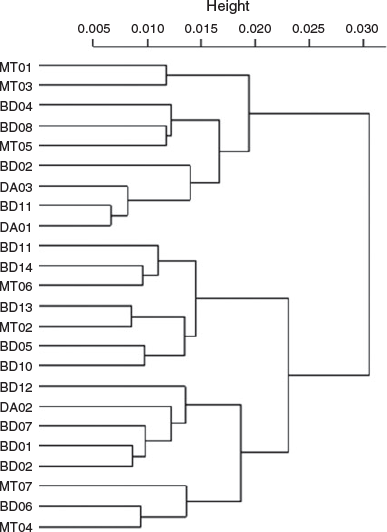
Because the Sr concentration in the water was significantly positively correlated with salinity, Sr : Ca was selected for inferring the migration patterns between different environmental salinities. The Sr : Ca profiles showed a similar pattern for all individuals at the beginning of their life, with very low Sr : Ca ratios close to the core (2.16 ± 0.26 × 10–3) and rising to the first peak (7.62 ± 1.14 × 10–3) at 142 ± 40 µm from the core (Fig. 4, 5, and Fig. S3 of the Supplementary material). This distance was not associated with a specific growth mark (or region) on the otolith. Most profiles showed a peak Sr : Ca of 8.3 ± 2.4 × 10–3 at ~860 µm from the core before decreasing. The Sr : Ca profile for five large individuals decreased strongly towards the edge, with a final value close to that of the core (2.68 ± 0.75 × 10–3; Fig. 5a, b). The Sr : Ba ratios throughout fish life (i.e. the otolith profile) showed very small variations at the beginning of life, followed by an increase in this variation and maximal variation at the end of life (Fig. 4). This implies a similar, possibly common, habitat at birth and during early life stages, and different migratory behaviours, with different salinities crossed, until the end of life. The intercorrelations between individual Sr : Ca profiles were high (mean = 0.57), except for two individuals collected at Binh Dai (BD10 and BD13; Fig. 6). The overall variations in the Sr : Ca mean profile (Fig. 4) were not significant. The mean values of Sr : Ca varied with age and were significantly different between years (Table 2; P < 0.05), except between the second and third years (P = 0.06) and the first and fifth years (P = 0.08). HCA of the general form of each individual Sr : Ca profile (Fig. 7) showed two main groups: one group with highly variable Sr : Ca (36% of individuals; Group 1) with high peaks (Fig. 5a, c, e), and another group with less variable Sr : Ca (64% of individuals; Group 2) and smaller peaks (Fig. 5b, d, f). The two groups showed significant differences in Sr : Ca ratios between each pair of the same year, except for the fifth year (ANOVA, P < 0.05). The intercorrelations between individual Sr : Ca profiles were higher in Group 1 than Group 2 (0.72 v. 0.49 respectively). In Group 1, all individuals showed complex differences in Sr : Ca ratios between years (Table 2). In contrast, the Sr : Ca ratios of Group 2 showed similar values between years, except between the first and fifth years, and between the second and third years (Table 2).

|

|
|
Discussion
Climate change, sea level rise, the construction of upstream dams and habitat changes have affected and will continue to affect fish migration in the Mekong River and its delta. P. krempfi is a commercially valuable species living in the brackish waters of the Mekong Delta and, being diadromous, it could be affected by major changes in its habitats (Poulsen et al. 2004; Hogan et al. 2007; Baird 2013). A study of its behaviour is then a precondition for evaluating the level of the effects of global changes in the Mekong. This study used elemental concentration profiles in otoliths, and primarily Sr : Ca ratios, together with an analysis of elemental concentrations in the water to infer the movements of the catfish P. krempfi throughout its lifetime (Campana 1999).
Relationships between water element concentrations and salinity in the Mekong Delta
The Sr concentration in the water was strongly correlated with salinity in the Mekong Delta. This agrees with numerous previous studies that have shown positive correlations between Sr concentrations in natural waters and salinity up to hypersaline conditions (Gillanders 2005a; Diouf et al. 2006; Gillanders and Munro 2012). Sr incorporated into the otolith could then be a good indicator of the salinity of the fish’s environment in the Mekong Delta, and therefore useful for identifying migrations between waters with different salinities. Previous studies have indicated that Ba concentrations in the water are negatively correlated with salinity (Nozaki et al. 2001) and are independent of temperature (Webb et al. 2012). However, the correlation between Ba concentration and salinity in the present study was positive but not significant (R2 = 0.315, P < 0.05). This may be due to flood events during the sampling period (beginning of the rainy season), which could have brought Ba from upstream tributaries to the higher salinity zones downstream, or from groundwater or intertidal sediments (Nozaki et al. 2001; Gillanders 2005a; Gillanders and Munro 2012). The relationship between salinity and Ba concentration in the Mekong River could be clarified by increasing the number of samples along a salinity gradient. The correlation between the Sr : Ba ratio and water salinity was linear, positive and significant, but strongly affected by the relationship between Sr concentration in the water and salinity. Thus, Ba concentration in the otolith is not a good candidate for estimating the salinity of a fish’s environment. Li and Rb concentrations in the water have a positive relationship with water salinity, and this has also been reported by others (Hicks et al. 2010). Nevertheless, although Li and Rb were not measured in otoliths in the present study, they could be variables that could be added in future research if other elements are not powerful enough. Finally, the concentrations of other elements (Mn, Pb, Sn and Zn) showed weak or no correlation with salinity in the Mekong Delta.
Element concentrations in the otolith
The concentrations of Sr, Ba, Mn, Mg, P, Ca, Zn and Pb were measured along the otolith growth profile and the element : Ca ratios (ppb/ppb) were used to infer the movement history of each individual. Combining the element : Ca profiles with age estimation can indicate the migration history as a function of age (Campana 1999). The Sr : Ca, Ba : Ca, P : Ca and Mn : Ca ratios in otoliths varied significantly along the transects. The Sr : Ca ratio was selected as the best candidate for inferring the migration through habitats with different salinities in this study, as found in previous studies (Gillanders 2005b; Correia et al. 2014), first because the Sr concentration showed a high positive correlation with ambient water salinity (Lin et al. 2007; Hicks et al. 2010) and second because the Sr : Ca ratio is not strongly affected by ontogeny, metamorphosis, growth or diet (Elsdon and Gillanders 2005; Ruttenberg et al. 2005; Lin et al. 2007). However, Sr incorporation in the otoliths is sometimes regulated in some species (Panfili et al. 2015), which may limit its usefulness for inferring environmental Sr concentration. The variations in the Sr : Ca ratio with different peaks in the otolith life history profile suggest different ambient water salinities during the fish’s lifetime. Campana (1999) and Hogan et al. (2007) reported that the Sr : Ca ratio in otoliths formed in marine saltwater ranges from 4.0 to 4.8 × 10–3, much higher than in fresh water, and so the high maximum Sr : Ca ratio in the present study (more than 10 × 10–3) indicated a marine stage during the fish’s lifetime. The low Sr : Ca ratio at the beginning of life (2.16 ± 0.26 × 10–3) suggested that P. krempfi hatches in fresh water, migrates from the spawning grounds to higher salinity habitats after hatching and then lives in brackish or marine water for most of its life, but neither the spawning grounds nor the element concentrations in the original waters were known in the present study.
Hogan et al. (2007) reported that P. krempfi otoliths collected in Laos had peak Sr : Ca ratios of at least 7.68 × 10–3 (maximum 26.14 × 10–3), near the maximum values of the present study, but it should be noted that the analytical methods differed between the studies (X-ray wavelength dispersive electron microscopy v. laser ICP-MS). This could indicate that P. krempfi caught in the Mekong in Laos had lived in comparable salinity levels and could imply a common population or could suggest a common movement pattern among spatially segregated contingents (e.g. anadromy). The different analytical techniques could explain the differences in the range of Sr : Ca ratios along the transect between the present study (from 1.60 × 10–3 to 16.65 × 10–3) and that of Hogan et al. (2007; from 0.00 × 10–3 to 26.14 × 10–3). Alternatively, there could be different contingents present in Laos, described by Hogan et al. (2007), and in the Mekong Delta, as in the present study, with different migration patterns (Poulsen et al. 2004). It is also possible that the migration patterns changed between 1999 and 2017 owing to changes in environmental conditions along the Mekong River, especially with the development of hydropower dams along the river. None of these hypotheses can be excluded given our current state of knowledge, and a population genetic analysis should be conducted to identify the number of separate populations along the Lower Mekong basin. Nevertheless, the very low level of Sr : Ba ratios at the beginning of the fish lifetime, with a very small dispersion around the mean, could indicate a single population that breeds in a single habitat after individual anadromous migrations.
Although the Ba concentration in the otolith probably depends on both the salinity and temperature of the ambient water (Gillanders and Munro 2012; Webb et al. 2012), its concentration has been widely used as a better indicator of fish migration patterns than other elements. Although some authors have reported that the otolith Ba : Ca ratio varied linearly with Ba : Ca in the water (Wells et al. 2003), in the present study there was no clear relationship between Ba concentration in the water and salinity. This makes it difficult to relate otolith Ba : Ca ratios to salinity changes and movements between different habitats. The most interesting result concerning the Ba : Ca ratio is the uniform pattern for all individuals in the Mekong Delta, with a significant peak at the beginning of the lifetime and a clear decrease to a stable low value. Peaks in the otolith Ba : Ca ratio may be due to flood events diluting the saltwater with fresh water or to transient movements into freshwater environments (Alibert et al. 2003; Gillanders 2005a). Ba concentrations are higher in the core region of the otolith in some species (Ruttenberg et al. 2005). Given that the concentration of Ba in the water is not related to salinity in the Mekong Delta, Ba : Ca is higher at the core for reasons other than salinity and could be attributed to an ontogenetic process or metamorphosis (Hicks et al. 2010), or because of a strong accumulation of elements in the core zone (Ruttenberg et al. 2005). Together with constantly low levels for the rest of the lifetime, this indicates that Ba : Ca profiles are not useful for inferring migration through different salinities in the Mekong Delta.
The Mn : Ca ratio in fish otoliths is not affected by salinity (Elsdon and Gillanders 2002; Dorval et al. 2007), but varies with geographical range, and may be influenced by temperature (Elsdon and Gillanders 2002), and more especially by ontogeny, which can cause higher Mn concentrations at the core region (Wells et al. 2003; Ruttenberg et al. 2005; Correia et al. 2014; Javor and Dorval 2016). The Mg : Ca ratio has also been reported as being dependent on salinity (Gillanders and Munro 2009; Sarimin and Mohamed 2014), ontogeny, sex or growth rate (Sturrock et al. 2014), as has the P : Ca ratio (Javor and Dorval 2016). Although the changes in these ratios could indicate changes in the habitat of P. krempfi in the early life stages, previous studies have found that Mn : Ca, Mg : Ca and P : Ca ratios are not very useful for reconstructing fish habitats because of possible effects of many factors other than the environment. The present study showed that the concentration of Mn in the water was not strongly correlated with salinity and that the Mn : Ca ratio in the otoliths of P. krempfi showed strong variations only at the beginning and end of the lifetime. Therefore, the profiles for elements other than Sr were not used in this study as indicators for studying the migration patterns of P. krempfi between different ambient water salinities.
Movements of P. krempfi in the Mekong River basin
Sr was the only element used in this study for inferring P. krempfi migration patterns assuming that the otolith Sr : Ca profiles reflected changes in ambient salinity. These indicated that P. krempfi moved from lower-salinity water after hatching to higher-salinity water during its life in the Mekong Delta. Combining the Sr : Ca profiles in the otoliths and age estimation (see Fig. S1), a general pattern of P. krempfi movements emerged for each year. All individuals seemed to hatch at the same low salinity level, but it is not known whether the young come from a single or from multiple spawning grounds, or even from a single region. In the first year, all individuals showed the same migratory behaviour, moving to higher-salinity environments and reaching brackish water while growing rapidly. During the second year, the fish seemed to move between different habitats with different salinities and continued to grow fast. At this time, there were two different contingents of fish. One part of the population moved between lower-salinity habitats, possibly between fresh water and brackish water, whereas the other moved between higher-salinity habitats, which could be brackish water and marine waters. This could be due to the wide range of habitat available, especially in terms of food availability, or to an adaption to different environments that can reduce the competition for the food. Growth was slower in the third year, whereas changes in ambient salinity were more frequent than in the first 2 years. Some 3-year-old individuals, longer than 67 cm TL, moved back to lower-salinity environments, perhaps to where they hatched, after growing in brackish water and then returned to higher-salinity water. However, a few individuals had peaks in the otolith Sr : Ca ratio between the second and fourth years, which could indicate movements to more saline habitats or that their habitats were affected by saline intrusions. These results support the hypothesis that P. krempfi captured in the Mekong Delta are anadromous, hatching in fresh water and moving to higher-salinity waters quickly to grow (<1 year), living in more saline habitats for most of their lifetime and probably returning to the area where they hatched for spawning (Hogan et al. 2007). However, the present study could not find any evidence to support the hypothesis that P. krempfi return to their hatching location for spawning.
Knowledge of migration patterns from early life to adult plays an important role in identifying nursery habitats for fish and conservation (Beck et al. 2001). A study of P. krempfi in Khone Falls (Laos) suggested that this species migrates up to 720 km from the Lower Mekong to the middle river near Khone Falls for spawning during the rainy season (Hogan et al. 2007) and that this migration is closely associated with the first seasonal increase in river flow (Baran et al. 2005). The spawning site may be Khone Falls, where there are rapids and deep pools (Poulsen et al. 2002, 2004; Gupta 2012). During the dry season, this species shelters in deep pools in the Lower Mekong basin, especially in Vam Nao deep pools in An Giang Province, Vietnam (Vu et al. 2009), or in the estuarine waters, and is then an important target for fisheries in these areas. Although many studies have reported that P. krempfi was only present in the Mekong Delta during the dry season from December to April, with no fish available in local markets during the rainy season (Baird et al. 2004; Vu et al. 2009), we found that there were many P. krempfi for sale in the local markets in Ben Tre province during the rainy season, from April to September. These fish may come from non-migratory populations or may have been migrating when caught.
In the Mekong River, between 40 and 70% of the fish species are believed to migrate between different habitats (Hogan 2011b). Therefore, migratory fishes are an important resource for the livelihoods of fishermen along the Mekong River in Laos, Cambodia and Vietnam. However, the migrations of Mekong fish, including P. krempfi, are threatened by habitat degradation, pollution, overexploitation, changes in hydrological triggers and, in particular, the increasing number of hydropower dams along the river (Baran et al. 2005; Strayer and Dudgeon 2010; Ferguson et al. 2011). Dams, which cause changes in habitat and hydrological flow, can reduce populations of migratory fish by blocking the migration pathway, increasing the mortality of adults and juveniles migrating upstream for breeding or downstream after spawning, affecting spawning habitats downstream by altering river flow patterns and reducing connectivity, all of which reduce the effective population size and genetic diversity (Ferguson et al. 2011; Junge et al. 2014). Dams have already had, and will continue to have, a tremendous effect on fish populations and fisheries (Stone 2016). Climate change, causing more floods upstream or saline intrusions downstream, could change the distribution of P. krempfi by changing their spawning grounds or feeding areas (Reist et al. 2006). To conserve migratory fish, protection of the hatching habitat, nursery environment and the environment along the migration route becomes important. It is therefore difficult to find a balance between economic development and fish conservation. Furthermore, the management and conservation of migrating fish such as P. krempfi require close cooperation between nations in managing the fisheries and ensuring the continuity of the migrations.
This study was limited by sampling only at the beginning of the rainy season, and only near the regions affected by human activities, but also by not sampling fish and water or salinity at the same time. Rainfall and river flow can bring matter from upstream or from land to the sampling sites, and waste water from human activities is discharged into the river near the sampling sites. Both these factors make it more difficult to interpret the element concentrations in the water. Although the present study provides powerful evidence that P. krempfi moves from fresh water to brackish water in the Mekong Delta, the life history of this species in the whole Mekong basin is still incomplete. Although Rainboth (1996) suggested that there may be two separate species of P. krempfi in the Upper and Lower Mekong, Poulsen et al. (2004) suggested that there may be two different migration patterns: one population spawning in the Upper Mekong and migrating to the Lower Mekong, spending most of its life in the Mekong Delta or at sea, and one population that has the same spawning and migration pattern but does not reach marine habitats. The number of populations in the upper river and their migration patterns remain unknown. Further research into the populations in the middle and Upper Mekong should be conducted, using genetic fingerprinting and Sr isotopes in the otoliths and the river water in order to understand the whole life history of P. krempfi in the whole of the Mekong River basin.
In conclusion, the Sr concentrations in the water showed a significant linear relationship with salinity in the Mekong Delta, strengthening the possibility of using this element to reflect the environmental conditions throughout a fish’s lifetime in the Mekong Delta. Sr was the only element used in this study for inferring P. krempfi migration patterns assuming that the otolith Sr : Ca profiles reflected changes in ambient salinity. The Sr : Ca profiles indicated that P. krempfi is anadromous, hatching in fresh water with two contingents migrating very quickly through habitats with higher salinities, from fresh water to areas of brackish water in the Lower Mekong. These data agree with those reported by Hogan et al. (2007), namely that there are multiple migratory contingents in a single population. This may provide some benefit to the species, probably in terms of reducing competition for food and habitat, but at the moment this is unknown and needs further investigation. Nevertheless, the identification of two movement types has implications for the management and conservation of the species, especially given that it is classified as ‘Vulnerable’. For example, fish populations will become very sensitive (e.g. reduce in population size, the connection between populations) if more dams are constructed along the Mekong River in the near future.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was financed by the SEDES International Joint Laboratory (small project), the Mixed Research Unit MARBEC Marine Biodiversity, Exploitation and Conservation (IRD, Institut de Recherche pour le Développement; Ifremer, Institut Français de Recherche pour l’Exploitation de la Mer; CNRS, Centre national de la recherche scientifique; UM, Université de Montpellier) and the Institut de Recherche pour le Développement (IRD).
Acknowledgements
The authors thank Phong Hoai Trinh for her assistance with the field work and Truong Nguyen Le Khoa, Pham Thi Thu Hong, Huynh Thi Ngoc Mai and Phong Hoai Trinh for their assistance with the laboratory work. The authors also thank Olivier Bruguier, University of Montpellier, for his assistance with laser ablation and solution-based ICP-MS analyses and Monique Simier (MARBEC Montpellier) for her help with statistical analyses. The authors are grateful to the four reviewers for their major contributions that improved this manuscript.
References
Alibert, C., Kinsley, L., Fallon, S. J., McCulloch, M. T., Berkelmans, R., and McAllister, F. (2003). Source of trace element variability in Great Barrier Reef corals affected by the Burdekin flood plumes. Geochimica et Cosmochimica Acta 67, 231–246.| Source of trace element variability in Great Barrier Reef corals affected by the Burdekin flood plumes.Crossref | GoogleScholarGoogle Scholar |
Baird, I. (2013). Pa Souay Hang Leuang Pangasius krempfi. In ‘The IUCN Red List of Threatened Species 2011’. e.T181328A7668262. (International Union for Conservation of Nature and Natural Resources.) Available at https://www.iucnredlist.org/species/181328/7668262 [Verified 27 November 2018].
Baird, I. G., Flaherty, M. S., and Phylavanh, B. (2004). Mekong River Pangasiidae catfish migration and the Khone falls wing trap fishery in Southern Laos. Natural History Bulletin of Siam Society 52, 81–109.
Baran, E., Baird, I., and Cans, G. (2005). ‘Fisheries Bioecology at the Khone Falls (Mekong River, Southern Laos).’ (WorldFish Center: Penang, Malaysia.)
Beck, M. W., Heck, K. L., Able, K. W., Childers, D. L., Eggleston, D. B., Gillanders, B. M., Halpern, B., Hays, C. G., Hoshino, K., Minello, T. J., Orth, R. J., Sheridan, P. F., and Weinstein, M. P. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas. Bioscience 51, 633–641.
| The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates: a better understanding of the habitats that serve as nurseries for marine species and the factors that create site-specific variability in nursery quality will improve conservation and management of these areas.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar |
Carlson, A. K., Phelps, Q. E., and Graeb, B. D. S. (2017). Chemistry to conservation: using otoliths to advance recreational and commercial fisheries management. Journal of Fish Biology 90, 505–527.
| Chemistry to conservation: using otoliths to advance recreational and commercial fisheries management.Crossref | GoogleScholarGoogle Scholar | 27704556PubMed |
Correia, A., Hamer, P., Carocinho, B., and Silva, A. (2014). Evidence for meta-population structure of Sardina pilchardus in the Atlantic Iberian waters from otolith elemental signatures of a strong cohort. Fisheries Research 149, 76–85.
| Evidence for meta-population structure of Sardina pilchardus in the Atlantic Iberian waters from otolith elemental signatures of a strong cohort.Crossref | GoogleScholarGoogle Scholar |
Diouf, K., Panfili, J., Labonne, M., Aliaume, C., Tomás, J., and Do, C. T. (2006). Effects of salinity on strontium:calcium ratios in the otoliths of the West African black-chinned tilapia Sarotherodon melanotheron in a hypersaline estuary. Environmental Biology of Fishes 77, 9–20.
| Effects of salinity on strontium:calcium ratios in the otoliths of the West African black-chinned tilapia Sarotherodon melanotheron in a hypersaline estuary.Crossref | GoogleScholarGoogle Scholar |
Dorval, E., Jones, C. M., Hannigan, R., and Montfrans, J. v. (2007). Relating otolith chemistry to surface water chemistry in a coastal plain estuary. Canadian Journal of Fisheries and Aquatic Sciences 64, 411–424.
| Relating otolith chemistry to surface water chemistry in a coastal plain estuary.Crossref | GoogleScholarGoogle Scholar |
Dugan, P. J., Barlow, C., Agostinho, A. A., Baran, E., Cada, G. F., Chen, D., Cowx, I. G., Ferguson, J. W., Jutagate, T., Mallen-Cooper, M., Marmulla, G., Nestler, J., Petrere, M., Welcomme, R. L., and Winemiller, K. O. (2010). Fish migration, dams, and loss of ecosystem services in the Mekong basin. Ambio 39, 344–348.
| Fish migration, dams, and loss of ecosystem services in the Mekong basin.Crossref | GoogleScholarGoogle Scholar | 20799685PubMed |
Elsdon, T. S., and Gillanders, B. M. (2002). Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59, 1796–1808.
| Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2005). Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions. Marine and Freshwater Research 56, 609–617.
| Consistency of patterns between laboratory experiments and field collected fish in otolith chemistry: an example and applications for salinity reconstructions.Crossref | GoogleScholarGoogle Scholar |
Ferguson, J. W., Healey, M., Dugan, P., and Barlow, C. (2011). Potential effects of dams on migratory fish in the Mekong river: lessons from Salmon in the Fraser and Columbia rivers. Environmental Management 47, 141–159.
| Potential effects of dams on migratory fish in the Mekong river: lessons from Salmon in the Fraser and Columbia rivers.Crossref | GoogleScholarGoogle Scholar | 20924582PubMed |
Fukushima, M., Jutagate, T., Grudpan, C., Phomikong, P., and Nohara, S. (2014). Potential effects of hydroelectric dam development in the Mekong river basin on the migration of Siamese mud carp (Henicorhynchus siamensis and H. lobatus) elucidated by otolith microchemistry. PLoS One 9, e103722.
| Potential effects of hydroelectric dam development in the Mekong river basin on the migration of Siamese mud carp (Henicorhynchus siamensis and H. lobatus) elucidated by otolith microchemistry.Crossref | GoogleScholarGoogle Scholar | 25099147PubMed |
Gillanders, B. M. (2005a). Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources 18, 291–300.
| Otolith chemistry to determine movements of diadromous and freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (2005b). Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuarine, Coastal and Shelf Science 64, 47–57.
| Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., and Munro, A. R. (2009). Relations between water chemistry, otolith chemistry and salinity of hypersaline system: implications for determining past environmental history of fish. (CSIRO Water for a Healthy Country National Research Flagship: Adelaide, SA, Australia.) Available at http://www.clw.csiro.au/publications/waterforahealthycountry/cllamm/CLLAMM-environmental-history-of-fish.pdf [Verified 7 August 2019].
Gillanders, B. M., and Munro, A. R. (2012). Hypersaline waters pose new challenges for reconstructing environmental histories of fish based on otolith chemistry. Limnology and Oceanography 57, 1136–1148.
| Hypersaline waters pose new challenges for reconstructing environmental histories of fish based on otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Gupta, A. (2012). Geology and landforms of the Mekong Basin. In ‘The Mekong: Biophysical Environment of an International River Basin’. (Ed. I. Campbell.) pp. 29–51. (Academic Press: Oxford, UK.)
Halls, A.S., and Johns, M. (2013). Assessment of the vulnerability of the Mekong Delta Pangasius catfish industry to development and climate change in the Lower Mekong Basin. Report prepared for the Sustainable Fisheries Partnership. Johns Associates Limited, Wiltshire, UK.
Hicks, A., Closs, G., and Swearer, S. (2010). Otolith microchemistry of two amphidromous galaxiids across an experimental salinity gradient: a multi-element approach for tracking diadromous migrations. Journal of Experimental Marine Biology and Ecology 394, 86–97.
| Otolith microchemistry of two amphidromous galaxiids across an experimental salinity gradient: a multi-element approach for tracking diadromous migrations.Crossref | GoogleScholarGoogle Scholar |
Hogan, Z. S. (2011a). Ecology and conservation of large-bodied freshwater catfish: a global perspective. In ‘Conservation, Ecology, and Management of Catfish’. (Eds P. H. Michaletz and V. H. Travnichek.) Vol. 77, pp. 39–53. (American Fisheries Society: Bethesda, MD, USA.)
Hogan, Z. S. (2011b). Review of migratory freshwater fish. In ‘Convention on the Conservation of Migratory Species of Wild Animals. Tenth Meeting of the Conference of the Parties’, 20–25 November 2011, Bergen, Norway. UNEP/CMS/Inf.10.33. (Convention on the Conservation of Migratory Species of Wild Animals: Bonn, Germany.) Available at https://www.cms.int/sites/default/files/document/inf_33_freshwater_fish_eonly_0.pdf [Verified 11 June 2019].
Hogan, Z., Baird, I. G., Radtkek, R., and Zanden, M. J. V. (2007). Long distance migration and marine habitation in the tropical Asian catfish, Pangasius krempfi. Journal of Fish Biology 71, 818–832.
| Long distance migration and marine habitation in the tropical Asian catfish, Pangasius krempfi.Crossref | GoogleScholarGoogle Scholar |
Javor, B. J., and Dorval, E. (2016). Sarability of trace elements in otolith of juvenile Pacific sardine Sardinops Sagax. California Cooperative Oceanic Fisheries Investigations Reports 57, 109–123.
Joint Assessment Teams (2016). Viet Nam drought and saltwater intrusion rapid assessment report. Ministry of Agriculture Rural Development (MARD), Ministry of Health (MoH), People’s Aid Coordinating Committee (PACCOM), UN and INGOs. Available at https://reliefweb.int/sites/reliefweb.int/files/resources/Viet%20Nam%20Drought%20Assessment%20Report.pdf [Verified 11 June 2019].
Junge, C., Museth, J., Hindar, K., Kraabøl, M., and Vøllestad, L. A. (2014). Assessing the consequences of habitat fragmentation for two migratory salmonid fishes. Aquatic Conservation 24, 297–311.
| Assessing the consequences of habitat fragmentation for two migratory salmonid fishes.Crossref | GoogleScholarGoogle Scholar |
Labonne, M., Morize, E., Scolan, P., Lae, R., Dabas, E., and Bohn, M. (2009). Impact of salinity on early life history traits of three estuarine fish species in Senegal. Estuarine, Coastal and Shelf Science 82, 673–681.
| Impact of salinity on early life history traits of three estuarine fish species in Senegal.Crossref | GoogleScholarGoogle Scholar |
Lin, S.-H., Chang, C.-W., Iizuka, Y., and Tzeng, W.-N. (2007). Salinities, not diets, affect strontium/calcium ratios in otoliths of Anguilla japonica. Journal of Experimental Marine Biology and Ecology 341, 254–263.
| Salinities, not diets, affect strontium/calcium ratios in otoliths of Anguilla japonica.Crossref | GoogleScholarGoogle Scholar |
Nozaki, Y., Yamamoto, Y., Manaka, T., Amakawa, H., and Snidvongs, A. (2001). Dissolved barium and radium isotopes in the Chao Phraya River estuarine mixing zone in Thailand. Continental Shelf Research 21, 1435–1448.
| Dissolved barium and radium isotopes in the Chao Phraya River estuarine mixing zone in Thailand.Crossref | GoogleScholarGoogle Scholar |
Panfili, J., Tom, Á. S. J., and Morales-Nin, B. (2009). Otolith microstructure in tropical fish. In ‘Tropical Fish Otoliths: Information for Assessment, Management and Ecology’. (Eds B. S. Green, B. D. Mapstone, G. Carlos, and G. A. Begg.) pp. 212–248. (Springer: Dordrecht, Netherlands.)
Panfili, J., Darnaude, A. M., Lin, Y. J., Chevalley, M., Iizuka, Y., Tzeng, W. N., and Crivelli, A. J. (2012). Habitat residence during continental life of the European eel Anguilla anguilla investigated using linear discriminant analysis applied to otolith Sr : Ca ratios. Aquatic Biology 15, 175–185.
| Habitat residence during continental life of the European eel Anguilla anguilla investigated using linear discriminant analysis applied to otolith Sr : Ca ratios.Crossref | GoogleScholarGoogle Scholar |
Panfili, J., Darnaude, A. M., Vigliola, L., Jacquart, A., Labonne, M., and Gilles, S. (2015). Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. Journal of Experimental Marine Biology and Ecology 467, 65–70.
| Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Poulsen, A. F., Poeu, O., Viravong, S., Suntornratana, U., and Tung, N. T. (2002). Fish migrations of the Lower Mekong River Basin: implications for development, planning and environmental management. MRC Technical Paper number 8, Mekong River Commission, Phnom Penh, Cambodia.
Poulsen, A. F., Hortle, K. G., Valbo-Jorgensen, J. S., Chan, C. K. C., Viravong, S., Bouakhamvongsa, K., Suntornratana, U., Yoorong, N., Nguyen, T. T., and Tran, B. Q. (2004). Distribution and ecology of some important riverine fish species of the Mekong River Basin. MRC Technical Paper number 10, Mekong River Commission, Phnom Penh, Cambodia.
Quyen, N. T. K., Berg, H., Gallardo, W., and Da, C. T. (2017). Stakeholders’ perceptions of ecosystem services and Pangasius catfish farming development along the Hau River in the Mekong Delta, Vietnam. Ecosystem Services 25, 2–14.
| Stakeholders’ perceptions of ecosystem services and Pangasius catfish farming development along the Hau River in the Mekong Delta, Vietnam.Crossref | GoogleScholarGoogle Scholar |
Rainboth, W. J. (1996). ‘Fishes of the Cambodian Mekong. FAO Species Identification Field Guide for Fishery Purposes.’ (FAO: Rome, Italy.)
Rainboth, W. J., Vidthayanon, C., and Yên, M. Đ. (2012). ‘Fishes of the Greater Mekong Ecosystem with Species List and Photographic Atlas.’ (Museum of Zoology, University of Michigan: Ann Arbor, MI, USA.)
Reist, J. D., Wrona, F. J., Prowse, T. D., Power, M., Dempson, J. B., King, J. R., and Beamish, R. J. (2006). An overview of effects of climate change on selected Arctic freshwater and anadromous fishes. Ambio 35, 381–387.
| An overview of effects of climate change on selected Arctic freshwater and anadromous fishes.Crossref | GoogleScholarGoogle Scholar | 17256642PubMed |
Rodriguez, J. A., Adler, D. M., Brand, P. C., Broholm, C., Cook, J. C., Brocker, C., Hammond, R., Huang, Z., Hundertmark, P., Lynn, J. W., Maliszewskyj, N. C., Moyer, J., Orndorff, J., Pierce, D., Pike, T. D., Scharfstein, G., Smee, S. A., and Vilaseca, R. (2008). MACS – a new high intensity cold neutron spectrometer at NIST. Measurement Science & Technology 19, 034023.
| MACS – a new high intensity cold neutron spectrometer at NIST.Crossref | GoogleScholarGoogle Scholar |
Ruttenberg, B., Hickford, M., Paradis, G., Sheehy, M. S., Standish, J. D., Ben-Tzvi, O., and Warner, R. (2005). Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Marine Ecology Progress Series 297, 273–281.
| Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags.Crossref | GoogleScholarGoogle Scholar |
Sarimin, A. S., and Mohamed, C. A. R. (2014). Sr/Ca, Mg/Ca and Ba/Ca ratios in the otolith of sea bass in Peninsular Malaysia as salinity influence markers. Sains Malaysiana 43, 757–766.
Sirot, C., Ferraton, F., Panfili, J., Childs, A. R., Guilhaumon, F., and Darnaude, A. M. (2017). ElementR: an R package for reducing elemental data from LA‐ICPMS analysis of biological calcified structures. Methods in Ecology and Evolution 8, 1659–1667.
| ElementR: an R package for reducing elemental data from LA‐ICPMS analysis of biological calcified structures.Crossref | GoogleScholarGoogle Scholar |
Southern Institute of Water Resources Research (2016). Report on salinity intrusion in coastal at Mekong Delta and recommendation for drought resistance. SIWRR, Ho Chi Minh City, Vietnam.
Stone, R. (2016). ‘Dam-Building Threatens Mekong Fisheries.’ (American Association for the Advancement of Science: Washington, DC, USA.)
Strayer, D. L., and Dudgeon, D. (2010). Freshwater biodiversity conservation: recent progress and future challenges. The North American Benthological Society 29, 344–358.
| Freshwater biodiversity conservation: recent progress and future challenges.Crossref | GoogleScholarGoogle Scholar |
Strnad, L., Ettler, V., Mihaljevic, M., Hladil, J., and Chrastny, V. (2009). Determination of trace elements in calcite using solution and laser ablation ICP‐MS: calibration to NIST SRM glass and USGS MACS carbonate, and application to real landfill calcite. Geostandards and Geoanalytical Research 33, 347–355.
| Determination of trace elements in calcite using solution and laser ablation ICP‐MS: calibration to NIST SRM glass and USGS MACS carbonate, and application to real landfill calcite.Crossref | GoogleScholarGoogle Scholar |
Sturrock, A. M., Trueman, C. N., Milton, J. A., Waring, C. P., Cooper, M. J., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research. Marine Ecology Progress Series 500, 245–264.
| Physiological influences can outweigh environmental signals in otolith microchemistry research.Crossref | GoogleScholarGoogle Scholar |
Vidthayanon, C. (2008). ‘Field Guide to Fishes of the Mekong Delta, Lao PDR.’ (Mekong River Commission: Vientiane, Laos.)
Vo, T. K. (2012). Hydrology and hydraulic infrastructure systems in the Mekong Delta, Vietnam. In ‘The Mekong Delta System: Interdisciplinary Analyses of a River Delta’. (Eds F. G. Renaud and C. Kuenzer.) pp. 49–81. (Springer: Dordrecht, Netherlands.)
Vu, V. A., Nguyen, N. D., Hidas, E., and Nguyen, M. N. (2009). Vam Nao deep pools: a critical habitat for Pangasius krempfi and other valuable species in the Mekong Delta, Vietnam. Asian Fisheries Science 22, 631–639.
Webb, S. D., Woodcock, S. H., and Gillanders, B. M. (2012). Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature. Marine Ecology Progress Series 453, 189–199.
| Sources of otolith barium and strontium in estuarine fish and the influence of salinity and temperature.Crossref | GoogleScholarGoogle Scholar |
Wells, B. K., Rieman, B. E., Clayton, J. L., Horan, D. L., and Jones, C. M. (2003). Relationships between water, otolith, and scale chemistries of westslope cutthroat trout from the Coeur d’Alene River, Idaho: the potential application of hard-part chemistry to describe movements in freshwater. Transactions of the American Fisheries Society 132, 409–424.
| Relationships between water, otolith, and scale chemistries of westslope cutthroat trout from the Coeur d’Alene River, Idaho: the potential application of hard-part chemistry to describe movements in freshwater.Crossref | GoogleScholarGoogle Scholar |
Zimmerman, C. E. (2005). Relationship of otolith strontium-to-calcium ratios and salinity: experimental validation for juvenile salmonids. Canadian Journal of Fisheries and Aquatic Sciences 62, 88–97.
| Relationship of otolith strontium-to-calcium ratios and salinity: experimental validation for juvenile salmonids.Crossref | GoogleScholarGoogle Scholar |





