Where do elements bind within the otoliths of fish?
Christopher Izzo A B C , Zoë A. Doubleday A B and Bronwyn M. Gillanders A BA Southern Seas Ecology Laboratories, Darling Building DX 650 418, School of Biological Sciences, The University of Adelaide, SA 5005, Australia.
B The Environment Institute, The University of Adelaide, SA 5005, Australia.
C Corresponding author. Email: c.izzo@adelaide.edu.au
Marine and Freshwater Research 67(7) 1072-1076 https://doi.org/10.1071/MF15064
Submitted: 19 February 2015 Accepted: 14 September 2015 Published: 6 November 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Otolith element analyses are used extensively to reconstruct environmental histories of fish based on the assumption that elements substitute for calcium within the CaCO3 otolith structure. However, elements may also be incorporated within the protein component of the otolith in addition to the direct substitution for calcium in the mineral component, and this could introduce errors in environmental reconstructions. The aim of the present study was to determine whether elements were incorporated into the protein or mineral components of otoliths and the relative proportion of each element in each component. Element concentrations from whole ground otoliths and the isolated protein component were quantified using solution inductively coupled plasma mass spectrometry (ICP-MS). Of the 12 elements investigated, most were found in both the proteinaceous and mineral components, but always in greater concentrations in the latter. Elements considered ‘non-essential’ to fish physiology with Ca-like properties (i.e. alkaline metals) were present in the mineral component in relatively high concentrations. Elements essential to fish physiology with smaller atomic radii than Ca (i.e. transition metals) were distributed throughout the protein and mineral components of the otolith. These findings enhance our understanding of element incorporation in the otolith and, ultimately, improve interpretations of otolith-based environmental reconstructions.
Additional keywords: CaCO3, calcium carbonate, element incorporation, ICP-MS, inductively coupled plasma mass spectrometry, protein.
Introduction
Otoliths (ear bones) of teleost fishes are a series of paired calcium carbonate (CaCO3) structures, which crystallise around an organic matrix with daily periodicity (Campana 1999; Sturrock et al. 2012). The organic otolith component constitutes between 2 and 10% of the total otolith mass and is primarily comprised of proteins, including lipoproteins and glycoproteins, glycosaminoglycans and polysaccharides; ~50% of these are water soluble (Kalish 1991; Campana 1999; Dauphin and Dufour 2003). Otoliths are acellular and metabolically inert, meaning any chemicals elements incorporated onto the precipitating surface are permanently retained (Campana 1999; Campana and Thorrold 2001). Elemental concentrations in otoliths may reflect the ambient properties of the surrounding water, such as temperature, salinity, ambient elements and potentially pH, which are mediated by physiological factors (Campana and Thorrold 2001; Elsdon et al. 2008; Sturrock et al. 2012). The continued and predictable growth of otoliths throughout the lifetime of the fish allows time-resolved environmental histories to be reconstructed (Campana and Thorrold 2001).
Determining where chemicals are incorporated within otoliths is imperative because concentrations of elements may reflect relative concentrations in the external medium (i.e. endolymph via the blood) or physiological effects on blood chemistry. Multiple element binding sites within otoliths have been identified (Campana 1999): (1) substitution for Ca within the CaCO3 crystalline lattice; (2) as inclusions within the interstitial spaces of the crystal lattice; or (3) in association with the protein component of the otolith. Some generalities about the binding fates of some groups of metals can be inferred based on specific environmental, physiological and chemical properties. Transition metals, with relatively small atomic radii, are more likely protein associated (e.g. Mugiya et al. 1991; Campana 1999; Sturrock et al. 2012) and alkaline earth metals and larger non-essential transition metals (e.g. Pb) likely substitute for Ca because they possess Ca-like properties (e.g. Mugiya et al. 1991; Radtke and Shafer 1992; Campana 1999). To date, only two studies have directly tested these generalities. Using synchrotron X-ray absorption spectrometry, Doubleday et al. (2014) validated the substitution of Sr for Ca within otolith aragonite for a range of fish species, from different environments and ambient Sr concentrations. Miller et al. (2006) extracted proteins from the otoliths of Atlantic cod (Gadus morhua L.) and determined that >70% of copper (Cu) and >40% of zinc (Zn) are incorporated within the protein matrix, regardless of surrounding ambient environmental concentrations (Brophy et al. 2004).
The aim of the present study was to further test these generalities by directly relating element concentration of the protein otolith component to corresponding elemental composition of whole otoliths (both the protein + mineral components) of Lutjanus malabaricus (Bloch & Schneider, 1801).
Material and methods
Otolith collection
Paired sagittal otoliths were extracted from specimens of saddletail snapper (L. malabaricus, Family Lutjanidae), a pelagic marine species. Otoliths were rinsed twice in ultrapure water to remove excess tissue and sonicated in ultrapure water for 5 min to remove fine particulate matter. Otoliths were then immersed in 5% H2O2 solution for 10 min, followed by rapid immersion in 2% HNO3 solution to remove superficial contamination before being finally rinsed three times in ultrapure water (Rooker et al. 2001). Ten left sagittal otoliths were hand-ground with an agate mortar and pestle. It has been shown that grinding the otoliths of Lutjanus species does not contaminate their chemical signatures (Barnett and Patterson 2010). For each fish, ~100 mg (range 99.17–101.73 mg) ground otolith mass was used for each of the nitric acid dissolutions of protein + mineral components, and a similar amount was used for the protein extraction assay. All otolith preparations were performed in a laminar flow hood and all contact surfaces were cleaned with dilute (~2%) HNO3. Similarly, all labware was acid washed and rinsed three times in ultrapure water before use.
Nitric acid dissolution of whole otoliths: protein + mineral components
Ground otoliths were dissolved in ultrapure HNO3 overnight. Upon complete dissolution of the ground otolith mass, ultrapure water was added to bring the solution to a final dilution of 2% HNO3 for analysis (Dove et al. 1996). The resultant solution reflects the combined protein + mineral components of the otoliths.
Extraction of the protein component of the otolith
Ground otoliths were decalcified with 0.125 M EDTA and the solution adjusted to pH 6 with ammonium hydroxide (Miller et al. 2006). The vials were mixed, vented and placed in a sonicating bath for 5 min (Miller et al. 2006). Aliquots of the decalcified solution were passed through two serially connected 0.45-µm polyvinylidene difluoride (PVDF) syringe filters to remove any undissolved material and then passed through DP-10 desalting columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and eluted with a 250 mM ammonium acetate buffer (pH 7). Elution fractions were collected in microcentrifuge tubes and stored at 2°C. Successful protein extraction was verified using a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA).
Solution inductively coupled plasma mass spectrometry (ICP-MS) analysis requires total dissolved solids to be <0.1% of the aqueous solution, requiring the removal of free organic particulates. The protein extracts were acid boiled at 95°C for 30 min in 2% HNO3 to denature the proteins, initiating the decoupling of the bound trace elements. Samples were mixed periodically during the incubation stage. The supernatant was collected by centrifugation at 14 000g for 30 min (McDevitt et al. 2011).
Elemental analysis
Aliquots of the protein + mineral samples, as well as the protein samples, were diluted to a final 2% HNO3 solution before being filtered through 0.45-μm PVDF syringe filters before analysis. Aliquots were randomised and analysed for a suite of elements (7Li, 27Al, 39K, 55Mn, 56Fe, 63Cu, 66Zn, 85Rb, 88Sr, 111Cd, 138Ba, 208Pb) using an Agilent Technologies (Palo Alto, CA, USA) 7500cs solution-based ICP-MS. The protein + mineral and protein samples were analysed separately using matrix-matched blanks and standards. A multi-element stock standard was run across a concentration gradient of 0, 1, 50, 100 and 500 μg L–1 and an indium spike was also used to measure element recoveries. Standards and blanks were analysed periodically throughout the session. In addition, three randomly selected samples were run in duplicate per session to test for precision. The mean CVs of repeated measures of the standards for all elements were 5.67 and 5.83% for the whole and protein duplicate samples respectively. Agilent Mass Hunter software was used to collect raw counts per second, which were calibrated against the elemental standards to correct for machine drift. Overall, standard recovery (99–101%) and precision (<5%) were within acceptable levels.
Element concentrations of the ~100-mg ground otolith material (parts per million, ppm) were calculated from raw counts per second. These values were corrected for intersample differences in the masses of the ground otolith material used in the whole otolith dissolution and protein extraction assays. Finally, the relative proportions of protein-bound elements for each otolith were calculated by dividing the concentration of individual elements in the whole otolith (both the protein + mineral component) by the corresponding concentration values of the protein component.
Results and discussion
Element concentrations
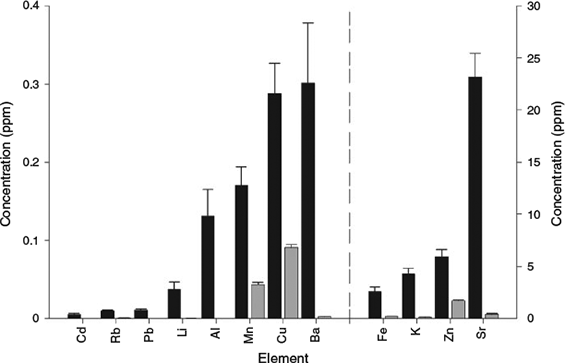
Elements in the dissolved whole otolith samples were present at either minor (>1 ppm) or trace (<1 ppm) levels (Fig. 1). Concentrations of protein-bound elements were considerably less than the corresponding whole otolith values (Fig. 1). Of the elements surveyed, Al, Pb and Cd were not detected in the protein extracts (Fig. 1).
|
Proportion of elements
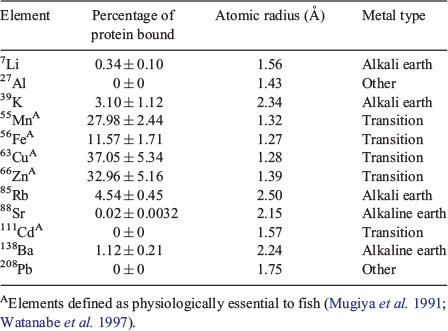
The relative proportion of elements bound to the protein matrix varied among the metals surveyed (Table 1). Overall, the relative proportion of elements in the proteinaceous otolith component relative to the whole otoliths largely conformed to the proposed patterns of otolith elemental incorporation in the literature. Transition metals (Cu, Zn, Mn and Fe), which are regarded as physiologically essential in teleosts (Mugiya et al. 1991; Watanabe et al. 1997) and have relatively smaller atomic radii than Ca (Å = 1.97; Table 1), showed an affinity for protein–metal complexes (with the exception of Cd). Among the protein-bound metals, Cu, Zn and Mn were incorporated into the otolith protein component at relatively high levels (>27%; Table 1), although in considerably lower quantities than seen in the otoliths of Gadus morhua (Miller et al. 2006). This disparity may be due to interspecific differences in otolith protein content (values ranging from 1 to 8% protein composition; Campana 1999), which can alter the availability of metal–protein binding sites within the otolith.
|
The alkaline metals surveyed (Sr and Ba) were predominantly bound within the mineral crystalline matrix of the otolith, with minor quantities of concentrations associated (<1%) with the protein component of the otolith (Fig. 1; Table 1). These elements are considered non-essential to physiological processes and are readily transported through the blood plasma (Campana 1999; Elsdon et al. 2008). Strontium directly substitutes for Ca in aragonitic CaCO3 (Doubleday et al. 2014) and other elements, with similar atomic radii to Ca, may also substitute or coprecipitate with Ca in otoliths (e.g. Ba (Å = 2.24); Campana 1999). The detection of these metal–protein bonds likely reflects the non-specific binding to the acidic soluble proteins that control the biomineralisation of aragonitic CaCO3 (Sturrock et al. 2012). Elements with Ca-like properties may become bound to Ca-binding proteins, albeit at considerably lower affinities. This is indirectly supported by the heterogeneous incorporation of some elements in otoliths to protein-rich growth increments (refer to otolith element maps in Arai et al. 2003; Limburg et al. 2007; McGowan et al. 2014).
The three alkali metals examined (Li, Rb and K) were incorporated at minor levels in the otolith protein component (Fig. 1; Table 1). However, these findings do not indicate the proportion of elements taken up in the interstitial spaces of the otolith. Otolith dissolution, as part of the protein extraction methodology, releases elements from both the crystalline and interstitial spaces of the otolith matrix (Miller et al. 2006). Elements such as Li and K, which are prone to post-mortem leaching from otoliths (Proctor and Thresher 1998), or Rb, which can be lost through DNA extraction protocols (Therkildsen et al. 2010), may also be bound within the interstitial regions of otoliths (Radtke and Shafer 1992). These findings suggest that alkali metals may be distributed across all three potential binding sites within the otolith: (1) substituting for Ca; (2) as an inclusion into the interstitial regions; or (3) bound to the protein matrix (Campana 1999).
Although metal–protein complexes were detected in the otoliths of L. malabaricus, the methodology only extracted the soluble protein component, which comprises ~50% of the protein component of otoliths (Asano and Mugiya 1993; Miller et al. 2006). As such, additional metal binding sites associated with the insoluble protein component are likely to be under-represented. Furthermore, the inadvertent loss of some protein-bound metals during the extraction method is also likely to have occurred (see Miller et al. 2006). The mechanical grinding of the otolith generated a homogeneous otolith sample, averaging over the lifetime of each fish. The relative proportion of otolith proteins is higher in the inner part of the otolith (e.g. Kalish 1989; Dove et al. 1996; Zhang et al. 2008), and some elements are detected at high concentrations in the otolith primordium, suggesting an affinity for incorporation to the protein component (e.g. Mn; Brophy et al. 2004; Macdonald et al. 2008). Similarly, heterogeneity in the protein content of opaque and translucent regions of the otoliths (Hüssy et al. 2004; Fablet et al. 2011) will likely result in seasonally variable elemental concentrations (e.g. Kalish 1989; Sturrock et al. 2015). Subsampling discrete regions of the otolith (e.g. the otolith primordium v. the marginal edge, opaque v. translucent regions) would facilitate finer-scale assessments of metal–protein complex distribution, thus allowing ontogenetic and temporal effects on element incorporation to be assessed.
Ecological implications
This study provides greater insight into where elements bind within otoliths and has implications for the application of otolith chemistry to trace the environmental and movement histories of fish. For example, the preferential binding of metals to either the protein or mineral otolith component will result in the heterogeneous distribution of elements throughout the otolith structure, even if a fish is exposed to a homogeneous environment. This has implications for high-resolution spatial analyses of otoliths using laser- or ion probe-based approaches, and requires consideration when comparing relative concentrations of elements among regions of an otolith (e.g. opaque v. translucent regions). Elements with Ca-like properties, such as Sr and Ba, which preferentially bind within the crystalline otolith matrix, appear suitable candidates for retrospective analyses because they are less prone to post-depositional alteration. However, to improve the accuracy of retrospective otolith-based analyses, further research is required to better elucidate the relative effects of the external environment and physiological processes that regulate element incorporation into otoliths.
Acknowledgements
The authors thank Aoife McFadden and Angus Netting (Adelaide Microscopy, The University of Adelaide) for discussion and technical support in the use of ICP-MS. Thanks also to Michael Miller (Forest Research Institute, Inc., Commack, NY, USA), Christopher McDevitt (Research Centre for Infectious Diseases, The University of Adelaide) and Tony Fowler (SARDI Aquatic Sciences, West Beach, SA, Australia) for insights into extraction methodologies. The authors thank Joseph Kocian (Angelakis Brothers Pty Ltd Seafood Wholesalers, Adelaide, SA) for providing the fish samples used in this study. The authors thank Audrey Geffen and the anonymous referees for detailed comments that helped improve this paper. This research was funded by an Australian Research Council (ARC) Discovery Grant (ARC DP110100716) and Future Fellowship (ARC FT100100767), both awarded to B. M. Gillanders.
References
Arai, T., Sato, H., Ishii, T., and Tsukamoto, K. (2003). Alkaline earth metal and Mn distribution in otoliths of Anguilla spp. glass eels and elvers. Fisheries Science 69, 421–423.| Alkaline earth metal and Mn distribution in otoliths of Anguilla spp. glass eels and elvers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjs1artrY%3D&md5=72f8ad99cdfe85aafdc14bd67278b444CAS |
Asano, M., and Mugiya, Y. (1993). Biochemical and calcium-binding properties of water-soluble proteins isolated from otoliths of the tilapia, Orecchromis niloticus. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 104, 201–205.
| Biochemical and calcium-binding properties of water-soluble proteins isolated from otoliths of the tilapia, Orecchromis niloticus.Crossref | GoogleScholarGoogle Scholar |
Barnett, B. K., and Patterson, W. F. (2010). The effect of coring and pulverizing juvenile red snapper, Lutjanus campechanus, otoliths on their chemical signatures. Environmental Biology of Fishes 89, 463–471.
| The effect of coring and pulverizing juvenile red snapper, Lutjanus campechanus, otoliths on their chemical signatures.Crossref | GoogleScholarGoogle Scholar |
Brophy, D., Jeffries, T. E., and Danilowicz, B. S. (2004). Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins. Marine Biology 144, 779–786.
| Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXis1Ghurw%3D&md5=79d9615b55031f37b9652826297b44a8CAS |
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtFKmtA%3D%3D&md5=6aa6bd7f37d02a8ee60835938650a0bdCAS |
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58, 30–38.
| Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?Crossref | GoogleScholarGoogle Scholar |
Dauphin, Y., and Dufour, E. (2003). Composition and properties of the soluble organic matrix of the otolith of a marine fish: Gadus morhua Linne, 1758 (Teleostei, Gadidae). Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 134, 551–561.
| Composition and properties of the soluble organic matrix of the otolith of a marine fish: Gadus morhua Linne, 1758 (Teleostei, Gadidae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s%2FovFajtA%3D%3D&md5=b192d8ec8bcc4b31f7f8e1884eeec652CAS |
Doubleday, Z. A., Harris, H. H., Izzo, C., and Gillanders, B. M. (2014). Strontium randomly substituting for calcium in fish otolith aragonite. Analytical Chemistry 86, 865–869.
| Strontium randomly substituting for calcium in fish otolith aragonite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvV2ku77M&md5=eb694917e610e6e393b90dad87646e0fCAS | 24299165PubMed |
Dove, S. G., Gillanders, B. M., and Kingsford, M. J. (1996). An investigation of chronological differences in the deposition of trace metals in the otoliths of two temperate reef fishes. Journal of Experimental Marine Biology and Ecology 205, 15–33.
| An investigation of chronological differences in the deposition of trace metals in the otoliths of two temperate reef fishes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xntl2gsrY%3D&md5=d1893e45c21efda91083fa320ad78634CAS |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillanders, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life-history parameters of fishes – hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life-history parameters of fishes – hypotheses, assumptions, limitations and inferences.Crossref | GoogleScholarGoogle Scholar |
Fablet, R., Pecquerie, L., de Pontual, H., Høie, H., Millner, R., Mosegaard, H., and Kooijman, S. A. L. M. (2011). Shedding light on fish otolith biomineralization using a bioenergetic approach. PLoS One 6, e27055.
| Shedding light on fish otolith biomineralization using a bioenergetic approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFyntbvP&md5=d2ad91ce201d756c527862c52833f28cCAS | 22110601PubMed |
Hüssy, K., Mosegaard, H., and Jessen, F. (2004). Effect of age and temperature on amino acid composition and the content of different protein types of juvenile Atlantic cod (Gadus morhua) otoliths. Canadian Journal of Fisheries and Aquatic Sciences 61, 1012–1020.
| Effect of age and temperature on amino acid composition and the content of different protein types of juvenile Atlantic cod (Gadus morhua) otoliths.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1989). Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition. Journal of Experimental Marine Biology and Ecology 132, 151–178.
| Otolith microchemistry: validation of the effects of physiology, age and environment on otolith composition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXpsFyktQ%3D%3D&md5=b6b09cc965f998f18bbaa428167c44a3CAS |
Kalish, J. M. (1991). 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects. Marine Ecology Progress Series 75, 191–203.
| 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., Huang, R., and Bilderback, D. H. (2007). Fish otolith trace element maps: new approaches with synchrotron microbeam X-ray fluorescence. X-Ray Spectrometry 36, 336–342.
| Fish otolith trace element maps: new approaches with synchrotron microbeam X-ray fluorescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFCnsLrL&md5=4b80a9fe4ba7a12c8e9ca7726c712a7cCAS |
Macdonald, J. I., Shelley, J. M. G., and Crook, D. A. (2008). A method for improving the estimation of natal chemical signatures in otoliths. Transactions of the American Fisheries Society 137, 1674–1682.
| A method for improving the estimation of natal chemical signatures in otoliths.Crossref | GoogleScholarGoogle Scholar |
McDevitt, C. A., Ogunniyi, A. D., Valkov, E., Lawrence, M. C., Kobe, B., McEwan, A. G., and Paton, J. C. (2011). A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathogens 7, e1002357.
| A molecular mechanism for bacterial susceptibility to zinc.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFSrtLzN&md5=f8a026662174cbe8aa2ed21811ddac86CAS | 22072971PubMed |
McGowan, N., Fowler, A. M., Parkinson, K., Bishop, D. P., Ganio, K., Doble, P. A., Booth, D. J., and Hare, D. J. (2014). Beyond the transect: an alternative microchemical imaging method for fine scale analysis of trace elements in fish otoliths during early life. The Science of the Total Environment 494–495, 177–186.
| Beyond the transect: an alternative microchemical imaging method for fine scale analysis of trace elements in fish otoliths during early life.Crossref | GoogleScholarGoogle Scholar | 25046609PubMed |
Miller, M. B., Clough, A. M., Batson, J. N., and Vachet, R. W. (2006). Transition metal binding to cod otolith proteins. Journal of Experimental Marine Biology and Ecology 329, 135–143.
| Transition metal binding to cod otolith proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XotVKlsw%3D%3D&md5=02467be3911b4d785d16da4d4df8588fCAS |
Mugiya, Y., Hakomori, T., and Hatsutori, K. (1991). Trace metal incorporation into otoliths and scales in the goldfish, Carassius auratus. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 99, 327–331.
| Trace metal incorporation into otoliths and scales in the goldfish, Carassius auratus.Crossref | GoogleScholarGoogle Scholar |
Proctor, C. H., and Thresher, R. E. (1998). Effects of specimen handling and otolith preparation on concentration of elements in fish otoliths. Marine Biology 131, 681–694.
| Effects of specimen handling and otolith preparation on concentration of elements in fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Radtke, R., and Shafer, D. (1992). Environmental sensitivity of fish otolith microchemistry. Marine and Freshwater Research 43, 935–951.
| Environmental sensitivity of fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltFelu7w%3D&md5=73f377e05c091888b9c6a81d08b02ddbCAS |
Rooker, J., Zdanowicz, V., and Secor, D. (2001). Chemistry of tuna otoliths: assessment of base composition and postmortem handling effects. Marine Biology 139, 35–43.
| Chemistry of tuna otoliths: assessment of base composition and postmortem handling effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtlSmurw%3D&md5=6a2d51b43da2dad9617daa0b185a356dCAS |
Sturrock, A. M., Trueman, C. N., Darnaude, A. M., and Hunter, E. (2012). Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81, 766–795.
| Can otolith elemental chemistry retrospectively track migrations in fully marine fishes?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhtl2ltLvN&md5=356e934bf72dfe3f0d23e5f6837210b5CAS | 22803735PubMed |
Sturrock, A. M., Hunter, E., Milton, J. A., EIMF, Johnson, R. C., Waring, C. P., and Trueman, C. N. (2015). Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution 6, 806–816.
| Quantifying physiological influences on otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Therkildsen, N. O., Nielsen, E. E., Hüssy, K., Meldrup, D., and Geffen, A. J. (2010). Does DNA extraction affect the physical and chemical composition of historical cod (Gadus morhua) otoliths? ICES Journal of Marine Science 67, 1251–1259.
| Does DNA extraction affect the physical and chemical composition of historical cod (Gadus morhua) otoliths?Crossref | GoogleScholarGoogle Scholar |
Watanabe, T., Kiron, V., and Satoh, S. (1997). Trace minerals in fish nutrition. Aquaculture 151, 185–207.
| Trace minerals in fish nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslKnsbs%3D&md5=53a13c0af9b1476f319018544b5729d9CAS |
Wedepohl, K. H., Correns, C. W., Shaw, D. M., Turekian, K. K., and Zemann, J. (1969). ‘Handbook of Geochemistry.’ (Springer-Verlag: Berlin.)
Zhang, F., Cai, W., Sun, Z., and Zhang, J. (2008). Regular variations in organic matrix composition of small yellow croaker (Pseudociaena polyactis) otoliths: an in situ Raman microspectroscopy and mapping study. Analytical and Bioanalytical Chemistry 390, 777–782.
| Regular variations in organic matrix composition of small yellow croaker (Pseudociaena polyactis) otoliths: an in situ Raman microspectroscopy and mapping study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXis1Sgsw%3D%3D&md5=93bd031fb9dac31d455f83e9ac8d099eCAS | 17999055PubMed |


