Are spatial closures better than size limits for halting the decline of the North Sea thornback ray, Raja clavata?
Jessica Wiegand A , Ewan Hunter B and Nicholas K. Dulvy C DA Department for Environment, University of York, York, YO10 5DD, UK.
B Centre for Environment, Fisheries and Aquaculture Science, Lowestoft, Suffolk, NR33 OHT, UK.
C Earth to Ocean Research Group, Department of Biological Sciences, Simon Fraser University, Burnaby, V5A 1S6, Canada.
D Corresponding author. Email: dulvy@sfu.ca
Marine and Freshwater Research 62(6) 722-733 https://doi.org/10.1071/MF10141
Submitted: 16 June 2010 Accepted: 25 February 2011 Published: 24 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
A key challenge of the ecosystem approach to fisheries management is to sustain viable populations of large-bodied less-productive vulnerable elasmobranchs that are the by-catch of fisheries that target more productive species. The North Sea population of the thornback ray (Raja clavata) is now mainly confined to the Thames Estuary and surrounding SW North Sea, which is subject to a flatfish trawl fishery. We explored the relative effectiveness of seasonal closures versus size-based landing restrictions using a four-season age-structured model. More than a third of adult thornback rays are currently removed by fishing each year, and without effective management, a further 90% decline within 30 years is likely. A three-season closure of the Thames Estuary was the shortest closure that ensured thornback ray recovery and minimal loss of fishery yield. Minimum and maximum landing size restrictions are nearly as effective at recovering thornback rays but less so at improving yield. While long seasonal closures and full marine protected areas are more effective at ensuring the recovery of thornback rays, length restrictions may be simpler to implement under the current institutional framework and may have less impact on the multispecies trawl fisheries operating in the area.
Additional keywords: by-catch, discard, elasmobranch, length restrictions, management strategy evaluation, marine reserve.
Introduction
The emerging challenge for fisheries management is to ensure continued fisheries for the most productive species while minimising risk to and allowing recovery of less productive species, such as chondrichthyans (Walker 1998b; Smith et al. 2007). There have been increasing calls for spatial management, such as the use of spatial zones and marine protected areas, to solve the problem of balancing both fisheries and biodiversity targets (Gell and Roberts 2003; Gaylord et al. 2005). However, marine protected areas may not be the simple effective solution they are often portrayed to be; they are rarely effective on their own and there are often transition costs and unintended ecological surprises associated with their use (Hilborn et al. 2004; Kaiser 2005). Marine protected areas are but one of a suite of tools available to managers and their relative utility is rarely compared with other more traditional fisheries management measures such as fishing effort controls, catch quotas and landing size restrictions (Le Quesne 2009).
Here we explore the degree to which one form of marine protected area, seasonal spatial fisheries closures, can halt declines of the thornback ray (Raja clavata) in the North Sea compared with landing size restrictions – a more traditional and widely used management tool. Many target fishes are overexploited in the North Sea; only a small proportion (10–25%) are safe within fisheries limit-reference points and some target and non-target populations are threatened (Piet and Rice 2004; Dulvy et al. 2005). Arguably the greatest impact of fishing in the North Sea has been the historic depletion and near local extinction of large skates (Rajidae), particularly the common or flapper skate, Dipturus intermedia (Walker and Heessen 1996; Walker and Hislop 1998; Rogers et al. 1999). Approximately half of the standing biomass of elasmobranchs is removed annually by fisheries, and skates in particular are subject to some of the highest levels of fishing mortalities (Piet et al. 2009). Skates are mainly taken as a retained by-catch of beam and otter trawl fleets targeting flatfish (plaice Pleuronectes platessa and sole Solea solea). The smallest skate, the starry ray (Amblyraja radiata), faces relatively low fishing mortality – around 38% of the standing biomass is removed each year. In contrast, 98% and 71% of the biomass of the two largest remaining skates, the blonde ray (Raja brachyura) and thornback ray, is removed annually (Piet et al. 2009).
The thornback ray (Raja clavata) is the most commonly landed skate and comprises ~95% of all ray catches in the North Sea (Walker and Heessen 1996). There has been a 50% decrease in thornback ray catches in the North Sea since the 1950s and this has been accompanied by a substantial contraction in the extent of occurrence and presumably a decline in abundance also (Walker and Heessen 1996). The remaining thornback ray population is now centred on the Thames Estuary in the south-west North Sea (Fig. 1). The North Sea population of thornback ray matures at 77 cm total length (TL) at around 7.3 years, and reaches a maximum size of around 90+ cm at a maximum age of 12 years (Ellis et al. 2005a, 2005b). Once mature, the thornback ray lays around two benthic eggs every 2 days during the breeding season, producing somewhere between 50 to 150 eggs per female each year depending on the environmental temperature, and the breeding season is typically from spring through summer (March to September) (Holden 1971; Ellis and Shackley 1995). The juveniles hatch out after 4–5 months at a size of 12 cm TL and are immediately catchable by North Sea flatfish trawl fisheries, which use an 80-mm codend mesh size. Their relatively large body size, later maturation and slow rates of population increase render them intrinsically sensitive to overexploitation, which, when coupled with high catchability and intense fishing mortality, may explain the highly depleted and shrunken stock distribution (Walker and Hislop 1998; Dulvy et al. 2000). The thornback ray is listed as Near Threatened by the International Union for the Conservation of Nature (IUCN) Red List (Ellis 2005).

|
The thornback ray is caught predominantly as a valuable by-catch of multispecies otter and beam trawl fisheries that target flatfishes, particularly sole (Solea solea, Pleuronectiformes) (Hunter et al. 2006). The UK catch is taken mainly by inshore small-scale longline vessels and larger otter trawl vessels around the Thames Estuary in spring and summer (April–July) and the Dutch catch is caught mainly with beam trawls from the central and eastern part of the southern North Sea in winter (October–January) (Hunter et al. 2006). Sole, the main target catch in this part of the North Sea, is largely sustainably exploited within precautionary limit reference points (ICES 2009).
Both seasonal fisheries closures and gear restrictions have been suggested as possible approaches to maximising protection for the thornback ray while minimising the reduction in the target species catch (Walker and Hislop 1998; Hunter et al. 2006). However, there is concern that spatial closures may not be sufficient to reduce fishing mortality on thornback rays and may adversely impact the landings of the target flatfishes. Hence, the relative performance of alternate management options, such as length restrictions, should be considered also (Hunter et al. 2006). In the European Union, minimum landing size restrictions, allowing the landing of species above a size threshold, are used for many target and non-target species. However, the use of a maximum landing size threshold – capture of juveniles up to a maximum landing size threshold – has not yet been explored. There are several examples of sustainable and well managed shark fisheries, notably gummy shark (Mustelus antarcticus) and school shark (Galeorhinus galeus), and a key feature of their management is that the fishery focusses on juveniles. Such ‘gauntlet’ fisheries have been suggested as a key feature for sustainable elasmobranch fisheries (Prince 2005). We explore the degree to which seasonal closures of the Thames Estuary can improve the population status of thornback rays compared with landing size restrictions.
We evaluate the long-term (≥15 years) effects of seasonal spatial closures and size limits on thornback ray population trajectory and fisheries yield using a four-season deterministic age-based matrix population model. Specifically, we estimate the potential impact of (1) seasonal closures of the Thames Estuary and (2) minimum and maximum landing size restrictions on both population growth rate (λ) and short- (<15 years) and long-term changes in potential fisheries yield relative to the status quo scenario. Our ultimate aim was to find a management option that would halt thornback ray population declines and minimise the loss of thornback ray yield.
Materials and methods
Seasonal model structure
An aged-based matrix model was developed to assess the changes in thornback ray population dynamics and yield under two different management strategies: seasonal and full area closures and minimum and maximum landing size restrictions. We assume a closed population with no emigration or immigration and assume that the model encapsulates the whole of the remaining ray population in the North Sea (Hunter et al. 2006). The first management option considered here is seasonal area closure, which requires a seasonal time step. The model consisted of a four-matrix string to represent quarter-year time steps with one matrix representing each quarter, termed Aspring [March to May], Asummer [June to August], Aautumn [September to November] and Awinter [December to February], where A is the formal notation for a matrix of survivorship and fertility. The spring matrix (March to May) coincides with the beginning of the breeding and egg-laying season, which allowed us to implement fecundity at the appropriate time. An annual population model takes the form An(t) = n(t + 1), where ‘n’ is the number of individuals in the population, and ‘t’ represents time in annual steps, and a single iteration gives the population after 1 year. In this seasonal model,
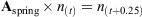
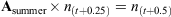
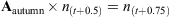
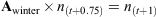
No variation in gear selectivity or catchability among age classes was assumed.
Choice of model parameters
We preferred to use parameters specific to the Thames Estuary (ICES statistical divisions 31F0, 32F0, 31F1, 32F1 and 33 F1) but if these were not available we used parameters estimated from the wider North Sea population. This age-structured approach required estimates of longevity, fecundity, natural mortality, discard mortality and fishing mortality (Table 1). Longevity, the maximum age that rays reach within this model, was assumed to be 12 years, the age of the oldest fish sampled by the Centre for Environment, Fisheries and Aquaculture Sciences, Lowestoft, Suffolk (CEFAS) and we caution that this is likely to be an underestimate of the historic longevity. Fecundity at length was calculated from Holden (1972) (Table 1). As population matrices account for females only, fecundity at length was divided by two, assuming a 1 : 1 sex ratio, and then multiplied by the proportion of mature females at age based on maturation ogives (Walker 1998a) (Table 1). Thornback rays lay eggs in spring and egg-laying was implemented by entering fecundity only in the spring matrix Aspring.

|
We assume total annual mortality Z is the sum of both natural mortality M and fishing mortality F, and these were calculated separately for neonates, juveniles and fish fully recruited to the fishery (the length at which all fish caught are retained). Total Z for fish fully recruited to the fishery at 5 years old and above was estimated from a catch curve based on the population age distribution within the Thames estuary (Fig. 2). The population length frequencies were obtained from CEFAS fisheries observers sampling catches from fishing vessels within the Thames Estuary between 2000 and 2006. Catch length frequencies were ‘sliced’ into catch numbers in each age class using the von Bertalanffy growth equation and the following parameter estimates: L∞ = 118 cm, k = –0.155 and t0 = –0.655 (CEFAS, unpubl. data). The catch curve was calculated using both sexes because single sex curves were not significantly different from each other, as estimated by least-squares regression, ln(catch) = –0.734·age + 10.7, (R2 = 0.95, F2,7 = 150, P <0.001), which resulted in a total mortality estimate of Z = 0.734. The proportion of fish surviving at each age class from 5 and above was then calculated by S = e(–z), S = 48%.

|
Natural mortality was estimated using Pauly’s (1980) method, which requires an estimate of the mean annual sea surface temperature (SST) over the population range. The mean SST for 2004 within the Thames estuary (Grid reference 52°N 1.42°E) was 12.4°C (Cefas 2006), which resulted in a natural mortality estimate M = 0.252 (78% survival) assumed to be constant for ages 1.25 and above in all simulations (Table 1). We assumed the natural mortality of eggs and 1-year-old juveniles to be M = 0.372 (69% survival) (Walker 1998a). Little is known about the natural mortality of juvenile rays, therefore the above value of M (for the rest of the population) was applied with 0.12 added to account for the potentially higher predation rates of juveniles up to age 1. One of the few estimates of this early life mortality comes from the mortality imposed by predatory gastropods upon the eggs of the starry ray, Amblyraja radiata (Cox et al. 1999).
Fishing mortality (F) on rays aged 5 and above was then calculated from Z – M = 0.482, which is slightly conservative compared with a recent independent estimate of 0.59 derived from a spatially-explicit abundance model with gear-, species- and size-dependent catch efficiency (Piet et al. 2009). Fishing mortality rates on juveniles aged 1 to 4 were determined from virtual population analysis (VPA) (Pope 1972). We applied the estimates of F and M for the oldest age class and back-calculated the values of F and the population numbers required to sustain the observed yield across other age classes. The observed yield at age was taken from the CEFAS observer database (Enever et al. 2007) and resulted in F values of 0, 0.002, 0.02 and 0.15 for ages 1 to 4 years respectively.
To assess the likely effects of size-based catch or landing restrictions on this thornback ray population, we accounted for the higher mortality of fish discarded due to being outside the landing length range. This discard mortality (D) was added to the current fishing mortality rate (F) by assuming that 38% of all rays are caught, as estimated from the catch curve where fishing mortality = 1 – e–0.482, or 38%. All adults longer than 47 cm, or older than 4 years, were assumed to be retained when caught and hence discard mortality (D) was set to zero (F = 0.482; D = 0; Z = 0.734). For juveniles aged 0–4 with no market value, or captured fish outside the landing length restrictions, the survival rate on the proportion that was discarded was arbitrarily set at 70%, which is comparable to a 7-h in-tank survival estimate that suggests thornback ray survival ranges from 55% to 87% (Enever et al. 2009). All age classes that were discarded therefore suffered a discard survival rate of 88.5%, or D = 0.122 (62% not caught + (70% survival rate of the 38% caught)). The fishing, discard and total mortality parameters for fish aged 1–4 years are hence: Age 1: F = 0, D = 0.122, Z = 0.122; Age 2: F = 0.002, D = 0.122, Z = 0.124; Age 3: F = 0.02, D = 0.122, Z = 0.142; Age 4: F = 0.15, D = 0.122, Z = 0.272.
The total annual mortality rates, Z, were divided by four to give quarterly survival rate. Mortality rates expressed in this manner are additive. For example, a population of 1000 individuals encountering annual mortality Z = 0.8 would result in the same number of individuals after a year (1000·e(–0.8) = 449) as 1000 individuals encountering a quarterly rate of mortality of Z = 0.2 (1000·e(–0.2)·e(–0.2)·e(–0.2)·e(–0.2) = 449).
Implementation of spatial and size-based management options
Seasonal closures were modelled by setting fishing and discard mortality to zero over all age classes for the corresponding closed season. Combinations of one-, two- and three-season and a full closure (Marine Protected Area) were simulated. The similarity of these seasonal matrices meant that our findings are insensitive to the timing of closure and instead we were interested only in the duration of closure. We assumed that when the area was reopened for fishing, effort would remain the same as under current fishing and that all rays longer than 47 cm TL would be retained. This is the current length for which a market exists and was calculated from the current length at which rays are retained (CEFAS discard database, unpubl. data). A discard mortality of 30% was applied to rays below 47 cm once the area was reopened. It was assumed that no discarding mortality would occur during closed periods as no fishing would occur.
We considered the effects of protecting mainly juveniles only with a minimum landing size and mainly adults only with a maximum landing size. Minimum landing sizes were modelled by starting with current F on all age classes (apart from those under 47 cm, which suffer 30% discard mortality but no fishing mortality). This is the equivalent of no landing size restrictions. Fishing mortality F was then removed from age 12, thus modelling the equivalent of introducing a minimum landing size of 94 cm (the length of rays at age 12). The reduction in the minimum landing size was then continued by removing F from the age class below one at a time (replacing it with discard mortality at 30% as the rays would still be caught but have to be discarded) thus reducing the minimum landing size in length increments corresponding to average lengths at age, until the minimum landing size was 47 cm or 3 years of age.
Gauntlet maximum landing sizes, to protect adults and subadults, were modelled by initially setting fishing mortality F to zero over all ages and seasons. We assumed that all rays are caught at the current level of adult fishing mortality, but are discarded if they are below 47 cm (the smallest size to have market value, D = 0.122). F at the current level (F = 0.482) was then added to each age group, starting with F occurring only on age 3, hence a maximum landing size of 47 cm. The maximum landing size was then increased to 56 cm by adding F to age class 4 as well, then to 64 cm by adding it to age class 5 and so on, until all age classes were fished. We assumed ‘knife-edge’ fishery retention of all thornback rays once they reached 47 cm, as compared with some increasing proportion of retained fish with increasing size centred on 47 cm.
Comparison of the relative performance of seasonal closure and size restrictions
We compared the performance of the spatial closures and size-based landing restriction using the population growth rate (recovery) and the loss of thornback ray yield. First, we calculated the numerical population difference between the population after 1 year under natural and discard mortality with and without fishing mortality (i.e. Δn = Nt [N, D] – Nt [N, D, F]), thus giving the number of fish dying due to fishing. Second, yield was calculated as the product of the change in numbers due to fishing and the average weight-at-age and standardised relative to the current yield. Simulations of each management scenario were run over a period of 100 years, starting with the initial population age distribution calculated by VPA using Poptools version 2.7 (Hood 2005).
Model sensitivity to parameter variation
We explored the sensitivity of the modelled population growth rate (λ = N(t+1)/Nt) after 10 years to variation in juvenile survival, fecundity, ogive length (the number of years between the size at first maturity and 100% maturity within a cohort), age at 50% maturity and discard mortality by altering parameters across the range of published values (see Table 2 for parameters modelled). Sensitivity was measured as the difference in population growth rate occurring when the simulations were run using the highest and lowest levels of each parameter. The least restrictive management scenario for each simulation under the range of possible parameter values was also measured to assess how uncertainty in the biological parameters influenced the management option.
Results
Based on catch curve analysis of catches taken from 2000 to 2006, 38% of thornback rays >64 cm in length (>5 years) are removed through fishing each year (F ≈0.48). If current fishing patterns continue, this would result in a deterministic projected population decline of ~90% within 30 years, or within 25 years under an assumption that the mortality of discarded individuals is ~30% (Fig. 3). The current population size relative to the unexploited baseline is unknown, but the population is already likely to be in a depleted state. Assuming a generation length of around 10 years based on the average reproductive age (average maximum age, 12 and age at first reproduction, 7), then this gives a modelled percentage decline of 90% over three generations, or 94% if discard mortality is taken into account, which would be sufficient to result in a Critically Endangered listing using IUCN Red List A criteria.

|
Changes in population growth rate resulting from spatial and size restrictions
One- and two- season closures are insufficient to halt or reverse the projected decline. This finding is not sensitive to which seasons the closure was implemented. Only longer three-season and full closures resulted in population increases and sustained population growth rate within approximately 5 years (Fig. 4). Discarding of thornback rays also resulted in population growth. Under current fishing effort, population recovery occurred only if juveniles, subadults and adults were protected under a minimum landing size by allowing the capture and landing of individuals >85 cm or where adults were protected by a maximum landing size that allowed fish to be retained up to 56 cm (Fig. 5). It should be noted that the size at 50% maturity is around 77 cm and the maximum size captured in the North Sea is rarely above 90 cm TL. Thornback ray recovery is likely only through the implementation of highly-restrictive spatial closures, size restrictions and discard reduction.

|

|
Changes in yield resulting from spatial and size restrictions
In the very short-term (5 years), the current fishing effort maintains the highest annual yield of rays compared with the other management options considered here. The benefits of seasonal closures or size restrictions were only realised if management was in place for at least 10 years (Fig. 6). Over the longer term, spatial closure yielded the greatest returns (Fig. 6), with a three-season closure resulting in a 70-fold increase in yield within 30 years over that projected to occur under current fishing levels (Fig. 6). By comparison, both landing size restrictions resulted in a more modest 10-fold increase in yield within the same time period. The difference in yield between both landing size restrictions is small and takes longer to be realised. Protecting juveniles and subadults up to a minimum landing size of 85 cm generates greater yield (27-fold increase) compared with a 20-fold increase in yield from protecting adults with a maximum landing length of 56 cm.

|
The management measures that resulted in recovery while minimising loss of thornback ray yield were: (1) a three-season closure; (2) a minimum landing size of 85 cm (allowing capture only of adults that had bred at least once); and (3) a maximum landing size of 56 cm (allowing capture only of small juveniles <4 years). The greatest increase in population growth rate was achieved by implementing a three-season closure (>6% year–1 within 10 years), compared with the size restrictions which resulted in a population growth rate of between 2 and 3% year–1 within 40 years (Fig. 7).

|
Sensitivity to variation in biological parameters
The model was most sensitive to the age at 50% maturity parameter; the range of estimates in the literature resulted in a 23% variation in population growth rate (Table 2). The range of maturation estimates had consequences for the future population trajectory under current fishing effort. The lowest reported age at 50% maturity results in population increase, whereas the highest reported age of 50% maturity increased the rate of population decline. The reported range of estimates of juvenile survival, fecundity and discard survival all resulted in differences of 10–14% in the population growth rate. The population continued to decline under current fishing pressure, across the reported range of parameter estimates. Population growth rate was insensitive to the breadth of the maturation ogive, with less than 4% change in the population growth rate between the published extremes after 10 years under current fishing mortality.
This sensitivity of the simulated population growth rate to variation in the biological parameters affects the length restrictions or seasonal closures that result in a stable or increasing population (mean lambda > 1) (Table 3). Altering the age at 50% maturity showed the biggest variation in the management strategy that would result in positive population growth rate. The results from simulations using the lower age at 50% maturity indicated that the population could sustain current fishing, while using the higher age indicated that no length restriction could prevent the population from decline. Running the simulations using low juvenile survival, low fecundity or low discard survival also resulted in length restriction being ineffective at reversing the population decline, with only a full closure of the estuary resulting in population increases using the low estimate of juvenile survival.
Discussion
In the absence of management action, using the best available data and this deterministic age-structured model, we show that the remaining North Sea thornback ray population will inevitably decline further from its already depleted state. To halt the decline of the North Sea thornback ray population requires the successful implementation of one or a combination of the following management strategies: a three-season closure to fishing; protection of juveniles and subadults with a minimum landing size of 85 cm; or protection of adults with a maximum landing size of 56 cm.
At present, the only management for North Sea skates is an ‘aggregate’ total allowable catch (TAC) for all North Sea skates and rays, which effectively provides no protection to thornback rays in the North Sea. The combined landings of skates and rays have always been less than the TAC in any year. The European Commission TAC in 2004 was set at 3503 tonnes, of which the uptake was only 58% (2044 tonnes). Hence, the current quota offers no protection or limit to the level of fishing mortality on any one species. Our findings suggest that the remaining North Sea thornback ray population is declining at a rate that would be sufficient to qualify for ‘threatened’ status under the IUCN ‘A’ decline criterion and this is consistent with the regional IUCN Red List Assessment and empirical population trajectory. The thornback ray is listed as ‘Near Threatened’ in the north-east Atlantic based on the ‘A’ decline criterion, or past population declines (Gibson et al. 2008). Within the North Sea, thornback rays have declined sufficiently since 1982–4 to be consistently categorised as either Vulnerable or Endangered in each year from 1993 to 2003, based on their catch rate in the English Groundfish Survey (Dulvy et al. 2006). In light of the historic decline and the results presented, the current TAC has not been effective at reducing the level of threat faced by thornback rays. There is a pressing need for additional effective management to halt and reverse the rate of decline in the remaining fragment of the North Sea thornback ray population.
Effects of seasonal closures
Partial closures will not be enough; neither one- or two-season closures resulted in recovery. Two-season closures of the estuary in spring and summer have been proposed to result in a reduction in ray yield even when accounting for fishing effort redistribution (Hunter et al. 2006). The current model indicates that annual three-season closures would be required to halt declines and allow population increase. Our key assumption is that the whole North Sea thornback ray population is contained within the area of closure throughout the year – and efficacy of any proposed marine protected area will be diluted by the degree to which the population extends beyond its boundary (Fig. 1). Conventional tagging experiments suggest thornback rays are highly localised; most (80 to 96%) recaptures occurred within the Thames Estuary typically within 20 km of the release site (Walker 1998a; Hunter et al. 2006). Tagging with electronic data storage tags has revealed that although the thornback ray population is centred on the Thames Estuary, there is a clear annual inshore-offshore migration cycle. Thornback rays disperse eastwards away from the estuary into slightly deeper water (>35 m) in winter, presumably to feed and avoid the intense cooling of the shallow waters of the southern North Sea, and in spring and summer move westwards into the estuary to breed (Holden 1975; Hunter et al. 2005). While most recaptures are close to the release site, a large proportion of tagged adults (77%) spend some time outside the Thames Estuary. This has two implications for management: (1) the Thames Estuary is the area where catchability by the fishing fleet is greatest and hence closures should result in greatest population protection; but (2) spatial closures only in this area are unlikely to protect the whole population throughout the year depending on the degree of leakage (Hunter et al. 2006).
Effects of size restrictions
Size restrictions are a longstanding, relatively well understood form of fisheries management and hence are more attractive, particularly within the Common Fisheries Policy, where minimum landing sizes are routinely implemented to minimise juvenile mortality. However, maximum landings size restrictions that aim to protect mature fish, allowing only juvenile and subadult fishes to run the gauntlet of fishing mortality, to the best of our knowledge, have not been widely used within Europe. The broad aim of size measures is to reduce mortality on life stages that contribute most significantly to the population growth rate (Crouse et al. 1987; Heppell et al. 2000). A common misperception is that the best way to guarantee continued population growth is to protect juvenile stages and their habitats (Heupel et al. 2007; Kinney and Simpfendorfer 2009; Knip et al. 2010). However, the relative contribution of the first-year survival to population growth rate may be smaller than expected (<10%) compared with the contribution of subadult and adult stages (>30%), particularly for longer-lived species (Cortés 2002; Kinney and Simpfendorfer 2009).
A proposed maximum landing size of 85 cm (Department for Food Agriculture and Rural Affairs, UK, unpublished report) was modelled in the current study, but was assumed to result in the retention of rays smaller than is currently practised by the fishery. Here, the underlying assumption is that fishers would act to maximise their economic potential and retain all rays caught of marketable value. The thornback ray population could sustain exploitation only up to a maximum landing size of 56 cm. However, this size limit resulted in lower yield than introducing a minimum landing size of 85 cm, which is the smallest minimum landing size that would sustain the population. This result is not unique to the thornback ray. The increase in survival necessary to sustain the loggerhead turtle population was smaller for large juveniles than for adults (Crouse et al. 1987). The juvenile survival of elasmobranchs before maturity exhibits the greatest variation in contribution to population growth rate (Cortés 2002; Frisk et al. 2005). While mature females have higher overall reproductive value, relatively few individuals survive to maturity. Increasing juvenile and subadult survival boosts the number of individuals surviving to maturity and amplifies reproductive output.
Implications for management and recovery
Population recovery and long-term economic returns can only occur by moving towards some form of effective management, which requires consideration of the ability to detect recovery and balance the consequences for the thornback ray and other affected fisheries. We warn that detecting recovery can be masked by the implementation of length restrictions, which are likely to alter the population size structure, resulting in initial fluctuations in population growth rate before stabilisation over the longer term (as seen in the sinusoidal population trajectory; Fig. 3). The inherent uncertainty in the population trajectory will hamper the confident detection of recovery and indicates the need for long-term strategic planning and goal-setting for long-lived species requiring recovery. The short-term reduction in yield is likely to result in lower economic returns from the ray fishery: a 50% decrease in thornback yield within 15 years. Only a longer-term multispecies management vision can counter these short-term costs against the benefits of population recovery and a more secure long-term yield. This problem is more acute since other valuable fisheries, most notably the flatfish trawl fishery, would also be impacted through seasonal closures (Hunter et al. 2006). We have not been able to consider the effect of seasonal closure on all fleets fishing the Thames Estuary area and this is worth exploring in future. Hence, the development of management options requires careful consideration of the equitable distribution of short- and long-term costs and benefits to all fishers and stakeholders. A full closure of the Thames Estuary would result in the fastest recovery of thornback rays, with a three-season closure resulting in the highest yield over time, but both ray and sole yields would be impacted. Furthermore, the financial costs of policing the closure or of implementation of size measures have not been considered in this model. The incorporation of socioeconomics and stakeholder concerns into any management design would be more likely to result in wider acceptance of policy so further research is needed on the impact of the management strategies on other fisheries within the area (Badalamenti et al. 2000). As a first step, we recommend that the flatfish management plan explicitly consider the consequences of management changes in the sole fishery for the viability of the North Sea thornback ray population.
The implementation of length restrictions has two potential advantages over spatial closures. First, they would be less likely to impact on the yield of the sole fishery. Second, size-based technical measures have been widely used and the framework for their implementation is already in place. However, the efficacy of any length restrictions depends on the survival of discarded rays. Here, consistent with recent findings, we assumed discard survival to be 70% (Enever et al. 2009). However, we found that a scenario in which the fishing of other species was allowed but all ray by-catch had to be discarded at a rate of no more than 30% discard mortality had the potential to increase the likelihood of recovery. Further research is required on methods for reducing discard mortality of the thornback ray.
Scope for further model development
All models are restricted by data availability and the trade-off between accuracy and complexity. The temporal dynamics of this model are more accurate than a conventional annual matrix model, as changes in seasonal fishing mortality, the spawning to hatching time delay, and a season-specific birth pulse have all been incorporated. Density-dependence was not incorporated but if it were, it would only serve to reduce the rate of recovery and hence our estimates of recovery times are likely to be conservative. Age at maturity profoundly influences the population trajectory. The observed population decline cannot be reversed by any of the length restrictions if using the highest observed age of maturity of 9.5 years (Walker 1998a). However, using the lowest estimate of age at maturity (6 years) results in a population increase under current fishing effort. The model was also sensitive to estimates of juvenile survival, fecundity and discard mortality; population growth rate varied by more than 10% when running simulations of current fishing effort using a plausible range of these parameters. Accurate measurement of these biological parameters and/or the inclusion and propagation of uncertainty would improve the capacity of these models to inform management options.
Conclusions
Based on our model and the available parameter estimates, reversing and halting declines of North Sea thornback rays could be achieved indirectly by reducing overall effort in the sole fishery, or more directly through (1) a seasonal closure (as rays are principally caught as a by-catch of the sole fishery), (2) through landing length restrictions or (3) reduction in discard mortality. Our results demonstrate that annual, three-season closures of the Thames Estuary have the potential to improve population growth rate and fisheries yield of the thornback ray, assuming fisher behaviour and fleet distributions remain unchanged following closures. The concentration of fishing effort on the boundaries of any closed area could impact heavily on the population as they migrate out of the estuary following egg-laying. A seasonal closure would also impact on the sole fishery, and the nature and degree of these impacts and opportunities would need further consideration. Minimum length restriction results in an increasing population (even when taking into account the decreasing effect that discard mortality has), would be easier to implement under the current institutional framework, and may have less impact on the sole fishery. The implementation of any management strategy will result in a loss of thornback yield in the short-term, but this needs to be traded-off against the consequences of longer-term yield and biodiversity losses under current fishing versus the long-term ongoing benefits of the recovery of thornback rays.
Acknowledgements
The authors thank Mike Pawson for his invaluable advice throughout, and Panayiota Apostolaki, Ainsley Buckley, John Cotter and Jim Ellis for providing data and advice on model structure. The authors thank Andrew Boulton, the Guest Editor, Colin Simpfendorfer and an anonymous referee for their comments and insights which greatly improved this work. This work was part-funded by the Natural Environment Research Council (NERC) through a Masters studentship award to J.W. and Defra projects MF0154, MF1004, MF1101 and MF1102 and by the Natural Environment Science and Engineering Research Council of Canada.
References
Badalamenti, F., Ramos, A. A., Voultsiadou, E., Lizaso, L. J. S., D’Anna, G., et al. (2000). Cultural and socio-economic impacts of Mediterranean marine protected areas. Environmental Conservation 27, 110–125.| Cultural and socio-economic impacts of Mediterranean marine protected areas.Crossref | GoogleScholarGoogle Scholar |
CEFAS (2006). Sea temperature and salinity trends. Available at http://www.cefas.co.uk/data/seatempandsal/default.htm [Accessed 4 August 2006].
Cortés, E. (2002). Incorporating uncertainty into demographic modelling: application to shark populations and their conservation. Conservation Biology 16, 1048–1062.
| Incorporating uncertainty into demographic modelling: application to shark populations and their conservation.Crossref | GoogleScholarGoogle Scholar |
Cox, D. L., Walker, P., and Koob, T. J. (1999). Predation on eggs of the thorny skate. Transactions of the American Fisheries Society 128, 380–384.
| Predation on eggs of the thorny skate.Crossref | GoogleScholarGoogle Scholar |
Crouse, D. T., Crowder, L. B., and Caswell, H. (1987). A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423.
| A stage-based population model for loggerhead sea turtles and implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Metcalfe, J. D., Glanville, J., Pawson, M. G., and Reynolds, J. D. (2000). Fishery stability, local extinctions and shifts in community structure in skates. Conservation Biology 14, 283–293.
| Fishery stability, local extinctions and shifts in community structure in skates.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Jennings, S. J., Goodwin, N. B., Grant, A., and Reynolds, J. D. (2005). Comparison of threat and exploitation status in Northeast Atlantic marine populations. Journal of Applied Ecology 42, 883–891.
| Comparison of threat and exploitation status in Northeast Atlantic marine populations.Crossref | GoogleScholarGoogle Scholar |
Dulvy, N. K., Jennings, S., Rogers, S. I., and Maxwell, D. L. (2006). Threat and decline in fishes: an indicator of marine biodiversity. Canadian Journal of Fisheries and Aquatic Sciences 63, 1267–1275.
| Threat and decline in fishes: an indicator of marine biodiversity.Crossref | GoogleScholarGoogle Scholar |
Ellis, J. R. (2005). Raja clavata Linnaeus 1758. Available at http://www.iucnredlist.org [Accessed 24 March 2011].
Ellis, J. R., and Shackley, S. E. (1995). Observations on egg-laying in the thornback ray. Journal of Fish Biology 46, 903–904.
| Observations on egg-laying in the thornback ray.Crossref | GoogleScholarGoogle Scholar |
Ellis, J. R., Cruz-Martinez, A., Rackham, B. D., and Rogers, S. I. (2005). The distribution of chondrichthyan fishes around the British Isles and implications for conservation. Journal of North Atlantic Fisheries Organisation 35, 195–214.
Ellis, J. R., Dulvy, N. K., Jennings, S., Parker-Humphreys, M., and Rogers, S. I. (2005). Assessing the status of demersal elasmobranchs in UK waters: a review. Journal of the Marine Biological Association of the United Kingdom 85, 1025–1047.
| Assessing the status of demersal elasmobranchs in UK waters: a review.Crossref | GoogleScholarGoogle Scholar |
Enever, R., Revill, A., and Grant, A. (2007). Discarding in the English Channel, Western approaches, Celtic and Irish seas (ICES subarea VII). Fisheries Research 86, 143–152.
| Discarding in the English Channel, Western approaches, Celtic and Irish seas (ICES subarea VII).Crossref | GoogleScholarGoogle Scholar |
Enever, R., Catchpole, T. L., Ellis, J. R., and Grant, A. (2009). The survival of skates (Rajidae) caught by demersal trawlers fishing in UK waters. Fisheries Research 97, 72–76.
| The survival of skates (Rajidae) caught by demersal trawlers fishing in UK waters.Crossref | GoogleScholarGoogle Scholar |
Frisk, M. G., Miller, T. J., and Fogarty, M. J. (2002). The population dynamics of little skate Leucoraja erinacea, winter skate Leucoraja ocellata, and barndoor skate Dipturus laevis: predicting exploitation limits using matrix analyses. ICES Journal of Marine Science 59, 576–586.
| The population dynamics of little skate Leucoraja erinacea, winter skate Leucoraja ocellata, and barndoor skate Dipturus laevis: predicting exploitation limits using matrix analyses.Crossref | GoogleScholarGoogle Scholar |
Frisk, M. G., Miller, T. J., and Dulvy, N. K. (2005). Life histories and vulnerability to exploitation of elasmobranchs: Inferences from elasticity, perturbation and phylogenetic analyses. Journal of the North Atlantic Fisheries Organisation 35, 27–45.
| Life histories and vulnerability to exploitation of elasmobranchs: Inferences from elasticity, perturbation and phylogenetic analyses.Crossref | GoogleScholarGoogle Scholar |
Gallagher, M. J., Nolan, C. P., and Jeal, F. (2005). Age, growth and maturity of the commercial ray species from the Irish Sea. Journal of Northwest Atlantic Fishery Science 35, 47–66.
Gaylord, B., Gaines, S. D., Siegel, D. A., and Carr, M. H. (2005). Marine reserves exploit population structure and life history in potentially improving fisheries yields. Ecological Applications 15, 2180–2191.
| Marine reserves exploit population structure and life history in potentially improving fisheries yields.Crossref | GoogleScholarGoogle Scholar |
Gell, F. R., and Roberts, C. M. (2003). Benefits beyond boundaries: the fishery effects of marine reserves. Trends in Ecology & Evolution 18, 448–455.
| Benefits beyond boundaries: the fishery effects of marine reserves.Crossref | GoogleScholarGoogle Scholar |
Gibson, C., Valenti, S. V., Fowler, S. L., and Fordham, S. V. (2008). ‘The Conservation Status of Northeast Atlantic Chondrichthyans.’ (IUCN Shark Specialist Group: Newbury, UK.)
Heppell, S. S., Crouse, D. T., and Crowder, L. B. (2000). Using matrix models to focus research and management efforts in conservation. In ‘Quantitative Methods for Conservation Biology’. (Eds S. Ferson and M. A. Burgman.) pp. 148–168. (Springer: New York.)
Heupel, M. R., Carlson, J. K., and Simpfendorfer, C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Marine Ecology Progress Series 337, 287–297.
| Shark nursery areas: concepts, definition, characterization and assumptions.Crossref | GoogleScholarGoogle Scholar |
Hilborn, R., Stokes, K., Maguire, J. J., Smith, T., Botsford, L. W., et al. (2004). When can marine reserves improve fisheries management? Ocean and Coastal Management 47, 197–205.
| When can marine reserves improve fisheries management?Crossref | GoogleScholarGoogle Scholar |
Holden, M. J. (1971). The rate of egg laying by three species of ray. Journal du Conseil International de l’Exploration de la Mer 33, 335–339.
Holden, M. J. (1972). The growth rates of R. brachyura, R. clavata and R. montegui as determined by tagging data. Journal du Conseil International pour l’Exploration de la Mer 34, 161–168.
Holden, M. J. (1975). The fecundity of Raja clavata in British waters. Journal du Conseil International pour l’Exploration de la Mer 36, 110–118.
Hood, G. (2005). PopTools, version 2.7.1. Available at http://www.poptools.org [Accessed 31 July 2005].
Hunter, E., Buckley, A. A., Stewart, C., and Metcalfe, J. D. (2005). Migratory behaviour of the thornback ray Raja clavata, in the southern North Sea. Journal of the Marine Biological Association of the United Kingdom 85, 1095–1105.
| Migratory behaviour of the thornback ray Raja clavata, in the southern North Sea.Crossref | GoogleScholarGoogle Scholar |
Hunter, E., Berry, F., Buckley, A. A., Stewart, C., and Metcalfe, J. D. (2006). Seasonal migration of thornback rays and implications for closure management. Journal of Applied Ecology 43, 710–720.
| Seasonal migration of thornback rays and implications for closure management.Crossref | GoogleScholarGoogle Scholar |
ICES (2009). Report of the working group on the assessment of demersal stocks in the North Sea and Skagerrak. International Council for the Exploration of the Seas, Copenhagen. Available from: http://www.ices.dk/workinggroups/ViewWorkingGroup.aspx?ID=31 [Accessed 30 March 2011].
Kaiser, M. J. (2005). Are marine protected areas a red herring or fisheries panacea? Canadian Journal of Fisheries and Aquatic Sciences 62, 1194–1199.
| Are marine protected areas a red herring or fisheries panacea?Crossref | GoogleScholarGoogle Scholar |
Kinney, M. J., and Simpfendorfer, C. A. (2009). Reassessing the value of nursery areas to shark conservation and management. Conservation Letters 2, 53–60.
| Reassessing the value of nursery areas to shark conservation and management.Crossref | GoogleScholarGoogle Scholar |
Knip, D. M., Heupel, M. R., and Simpfendorfer, C. A. (2010). Sharks in nearshore environments: models, importance, and consequences. Marine Ecology Progress Series 402, 1–11.
| Sharks in nearshore environments: models, importance, and consequences.Crossref | GoogleScholarGoogle Scholar |
Le Quesne, W. J. F. (2009). Are flawed MPAs any good or just a new way of making old mistakes? ICES Journal of Marine Science: Journal du Conseil 66, 132–136.
| Are flawed MPAs any good or just a new way of making old mistakes?Crossref | GoogleScholarGoogle Scholar |
Mandelman, J. W., and Farrington, M. A. (2007). The estimated short-term discard mortality of a trawled elasmobranch, the spiny dogfish (Squalus acanthias). Fisheries Research 83, 238–245.
| The estimated short-term discard mortality of a trawled elasmobranch, the spiny dogfish (Squalus acanthias).Crossref | GoogleScholarGoogle Scholar |
Pauly, D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. Journal du Conseil International pour l’Exploration de la Mer 39, 175–192.
Piet, G. J., and Rice, J. C. (2004). Performance of precautionary reference points in providing management advice on North Sea fish stocks. ICES Journal of Marine Science 61, 1305–1312.
| Performance of precautionary reference points in providing management advice on North Sea fish stocks.Crossref | GoogleScholarGoogle Scholar |
Piet, G. J., van Hal, R., and Greenstreet, S. P. R. (2009). Modelling the direct impact of bottom trawling on the North Sea fish community to derive estimates of fishing mortality for non-target fish species. ICES Journal of Marine Science 66, 1985–1998.
| Modelling the direct impact of bottom trawling on the North Sea fish community to derive estimates of fishing mortality for non-target fish species.Crossref | GoogleScholarGoogle Scholar |
Pope, J. G. (1972). An investigation of the accuracy of Virtual Population Analysis. International Commission for the Northwest Atlantic Fisheries (ICNAF) Research Bulletin 9, 65–74.
Prince, J. D. (2005). Gauntlet fisheries for elasmobranchs – the secret of sustainable shark fisheries. Journal of North Atlantic Fisheries Organisation 35, 407–416.
| Gauntlet fisheries for elasmobranchs – the secret of sustainable shark fisheries.Crossref | GoogleScholarGoogle Scholar |
Rogers, S. I., Clarke, K. R., and Reynolds, J. D. (1999). The taxonomic distinctness of coastal bottom-dwelling fish communities of the North-east Atlantic. Journal of Animal Ecology 68, 769–782.
| The taxonomic distinctness of coastal bottom-dwelling fish communities of the North-east Atlantic.Crossref | GoogleScholarGoogle Scholar |
Ryland, J. S., and Ajayi, T. O. (1984). Growth and population dynamics of three ray species in Carmarthen Bay, British Isles. Journal du Conseil International pour l’Exploration de la Mer 41, 111–120.
Smith, A. D. M., Fulton, E. J., Hobday, A. J., Smith, D. C., and Shoulder, P. (2007). Scientific tools to support the practical implementation of ecosystem-based fisheries management. International Council for Exploration of the Seas Journal of Marine Science 64, 633–639.
Stobutzki, I., Miller, M., and Brewer, D. (2001). Sustainability of fishery bycatch: a process for assessing highly diverse and numerous bycatch. Environmental Conservation 28, 167–181.
| Sustainability of fishery bycatch: a process for assessing highly diverse and numerous bycatch.Crossref | GoogleScholarGoogle Scholar |
Walker, P. A. (1998a). Fleeting images: dynamics of North Sea ray populations. Ph.D. Thesis, University of Amsterdam.
Walker, T. I. (1998). Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Marine and Freshwater Research 49, 553–572.
| Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries.Crossref | GoogleScholarGoogle Scholar |
Walker, P. A., and Heessen, H. J. L. (1996). Long-term changes in ray populations in the North Sea. International Council for Exploration of the Seas Journal of Marine Science 53, 1085–1093.
Walker, P. A., and Hislop, J. R. G. (1998). Sensitive skates or resilient rays? Spatial and temporal shifts in ray species composition in the central and north-western North Sea between 1930 and the present day. International Council for Exploration of the Seas Journal of Marine Science 55, 392–402.




