Neuroangiostrongyliasis: accidental zoonosis caused by the rat lungworm
Phoebe Rivory A and Jan Šlapeta A B *A
B

Phoebe Rivory is a PhD candidate at The University of Sydney. Her primary research focus is Angiostrongylus in Australia. |

Jan Šlapeta is a professor at The University of Sydney. He applies traditional and state of the art molecular approaches to understand parasite identity, origin and role in disease and pathogenesis. |
Abstract
The genus Angiostrongylus comprises parasitic nematodes that primarily infect the respiratory and circulatory systems of vertebrates, posing significant medical and veterinary concerns. The life cycle involves an indirect transmission between definitive and intermediate mollusc hosts, such as slugs or snails. Three species, A. cantonensis, A. mackerrasae and A. malaysiensis, are notable for their migration into the central nervous system (CNS) of their definitive rat hosts. Angiostrongylus cantonensis is particularly concerning, because it initiates CNS migration in various vertebrates, including accidental hosts like humans and dogs, leading to parasitic meningitis called neuroangiostrongyliasis (NAS). Angiostrongylus cantonensis, first identified in China and causing eosinophilic meningitis in humans and dogs, has expanded globally. Transmission to accidental hosts often occurs through ingestion of raw or undercooked snails, contaminated produce or water. Human cases in eastern Australia are rare but can be severe, whereas canine cases are more frequent. Diagnostic challenges persist, with advanced imaging and serological tests offering limited utility. Recent developments in hypersensitive quantitative polymerase chain reaction (qPCR) assays show promise for improved detection. Understanding the transmission dynamics and enhancing diagnostic capabilities are crucial for managing this zoonotic threat.
Keywords: Angiostrongylus cantonensis, Australia, diagnostic, ELISA, epidemiology, meningitis, neural angiostrongyliasis, parasitology, PCR, veterinary.
Introduction
The parasitic nematodes in the genus Angiostrongylus primarily infect the respiratory and circulatory systems of vertebrates. To medical and veterinary professionals, Angiostrongylus species are of particular interest due to their potential to cause severe disease. With over 20 described species within the genus Angiostrongylus, only three species (A. cantonensis, A. mackerrasae and A. malaysiensis) undergo a remarkable active and compulsory migration into the central nervous system (CNS) and from there into the lungs of their definitive hosts, which are rats1 (Fig. 1). The life cycle of these nematodes is indirect, so it circulates between the definitive host, the rat and the intermediate host, which is usually a mollusc, such as slug or snail, where infective larvae develop.2 When the rat ingests the infected molluscs, the life cycle continues, and the nematode starts its journey towards the rat’s lungs. Yet there is a twist, and, at least for A. cantonensis, the infective larvae are rather indisciminate and will happily initiate the migration towards the CNS in many vertebrates other than rats.3 Until the infective larvae reach the CNS, there seems to be little to no resistance from the accidental host, whether that is a human, a dog or other mammals and birds. Once A. cantonensis enters the CNS of an accidental host, the host immune response reacts strongly, leading to eosinophilic meningitis (neuroangiostrongyliasis, NAS).4
The life cycle of Angiostrongylus cantonensis. Stages of the parasite are indicated by different coloured circles (green, larval stage 1 (L1), 4 (L4) and 5 (L5); yellow, infective larval stage 3 (L3); maroon, adult). The natural life cycle involves definitive rat hosts and intermediate gastropod hosts (pink cycle); and the migration and development within the rat is detailed in the pink bubble. Alternative pathways exist involving paratenic hosts, where the L3s remain quiescent; and accidental hosts (such as humans, dogs, horses and birds), where the larvae typically do not develop and may cause NAS.
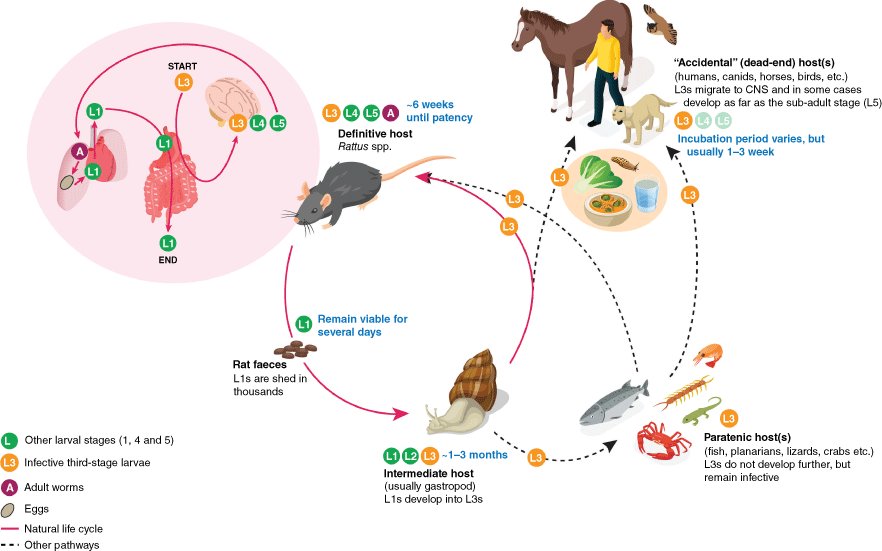
In Australia, A. cantonensis and A. mackerrasae are now endemic on the east coast. The brown rat (Rattus norvegicus) hosts both species, black rats (R. rattus) only harbour A. cantonensis, and Australian bush rat (R. fuscipes) almost exclusively hosts A. mackerrasae.3,5 Angiostrongylus mackerrasae represents a unique member of the genus due to its geographical isolation and host preferences, and is considered to be native only to Australia.
The invasive species of rat lungworm, Angiostrongylus cantonensis
Angiostrongylus cantonensis, commonly known as the rat lungworm, was first described from the pulmonary arteries of rats in Guangzhou (formerly Canton), China. This species has since gained significant attention due to its expanding distribution and its ability to cause meningitis in humans and other vertebrate hosts.6
Eosinophilic meningitis, the hallmark condition of A. cantonensis infection in accidental hosts, is an inflammation of the meninges, characterised by an increased number of eosinophils in the cerebrospinal fluid (CSF) triggered by the larvae migrating to the CNS.7 This condition can lead to severe neurological symptoms and, in some cases, can be life threatening.8
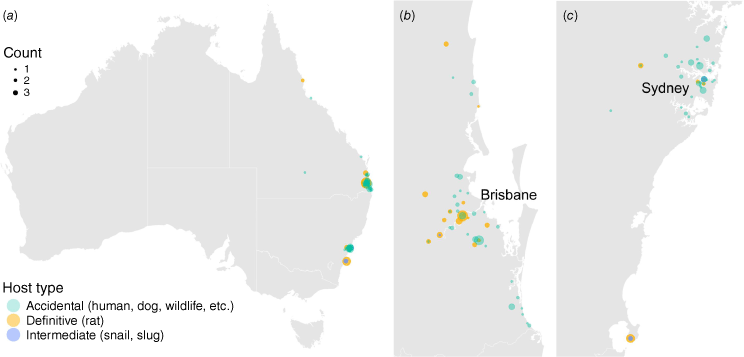
The parasite’s presence has been confirmed in over 30 countries across all inhabited continents, posing ongoing challenges for public and veterinary health globally. Today, it is considered endemic in many tropical and subtropical areas worldwide, with its range continually expanding due to factors such as invasive species proliferation, climate change and globalisation. The global spread of Angiostrongylus cantonensis is largely attributed to increased global trade, travel, and the inadvertent transportation of infected rats and intermediate hosts.9 The parasite’s range extended from its native South-east Asian distribution to Pacific islands, including Hawai’i, in the 1960s. By the late 20th and early 21st Centuries, A. cantonensis had been reported in diverse regions, including eastern Australia, the Caribbean, southern USA and South America, southern Europe and even parts of Africa.10,11 In Australia, A. cantonensis is mainly found near Sydney and Brisbane, but its spread has not been fully researched (Fig. 2).
Known locations of Angiostrongylus cantonensis in Australia. The maps show Australia (a), Brisbane and surrounding areas in Queensland (b), and Sydney to Jervis Bay in New South Wales (c). These maps are based on published records from 1955 to 2024. The data, representing counts of detected A. cantonensis cases, are shown on the same scale across all three maps.
Transmission to accidental hosts: not just eating slugs
In most documented cases, people become infected with A. cantonensis by deliberately consuming raw or undercooked snails, especially in cultures where certain species of snail are considered delicacies (Fig. 1). Cases have also occurred through dare-based and accidental consumption of raw snails or slugs in young men and in small children respectively.12,13 Additionally, transport hosts (e.g. freshwater prawns, crabs, frogs, monitor lizards and planarians), which effectively store quiescent infective larvae, contribute to infections in humans.9
Inadvertent ingestion of intermediate hosts, such as semi-slugs in Hawai’i, can also occur. Exotic to Australia, the semi-slug Parmarion martensi is an invasive species that was first documented in Hawai’i in 2004. This miniscule semi-slug is often found on lettuce and appears to be a highly successful intermediate host, capable of supporting large burdens of infective A. cantonensis larvae.14 Fresh produce, such as leafy vegetables, can easily conceal snails and slugs, including semi-slugs, especially if they are small. One of the early human NAS cases occurred in the early 1970s in Brisbane, Australia, presumably after the patient ate contaminated lettuce.15
Contaminated water presents another potential infection source, as studies show larvae can survive in water when released from damaged or drowning gastropods.16 This route of transmission has been well documented in a case series in Hawai’i, where a group of adults were infected from home-made kava.17 This transmission pathway has not yet been observed in Australia, but it remains plausible for dogs to become infected by drinking contaminated water, such as from puddles or contaminated water bowls.
Human cases in Australia are rare, but can be fatal
Human cases of NAS in Australia are fortunately reasonably rare. However, this disease can have drastic impacts on people’s lives, as it can lead to temporary and permanent disability and even death. The first case was reported in 1971 near Brisbane, although case records suggest infections may have occurred as early as 1959.15,18 Since then, at least 34 severe cases have been documented in Australia, some of them occurring in young children.13,19
Common presentations in adults include severe headache, neck stiffness, and paraesthesia. Neurological manifestations in humans include eosinophilic meningitis (usually ≥10 eosinophils mm–3 CSF), encephalitis, radiculitis, cranial nerve abnormalities and ataxia.20,21 Rarely, cases can result in an ocular form of the disease. Initial symptoms are non-specific and include enteritis, respiratory issues, fever and malaise as larvae migrate through organs in the digestive tract, lungs and trachea. Eosinophilic meningitis typically develops after 2 weeks, when the larvae have migrated into the CNS, though incubation periods can vary from 1 day to months. Necropsy studies of human NAS, of which there are very few, revealed meningeal infiltration by inflammatory cells, brain parenchymal tracks with cell debris and microthrombi, and eosinophilic granulomas around dead larvae. Although larval migration causes physical damage, the main pathology stems from host immune responses to dead larvae, as living larvae trigger minimal inflammation. The response involves T-helper 2 cytokines and eosinophil recruitment,20 with resulting cellular infiltration increasing CSF pressure and worsening neurological symptoms.
Although eosinophilic meningitis is typically self limiting, the increase in cases requiring intervention in Hawai’i over the past 20 years have led to publication of ‘Guidelines for the diagnosis and treatment of neuroangiostrongyliasis: updated recommendations’ in 2021.22 The current recommendations are the use of corticosteroids and the anthelmintic drug albendazole in humans. Anecdotal experience in Hawai’i has shown that many patients develop chronic neurological sequelae affecting functional status and quality of life in those infected. The mortality rates are reported to be <1%.21
Canine cases are relatively frequent in Australia
In Australia, canine NAS (also known as canine neural angiostrongylosis) has been reported frequently along the eastern coastal areas of Queensland and New South Wales since 1972. A more recent update revealed a concerning increase in the number of cases over a 10-year period.23
Dogs affected by NAS typically present with hindlimb and tail paresis or paralysis, muscle wasting, often coupled with urinary incontinence, and a distinctive hyperaesthesia. In dogs, clinical signs typically appear 10 days after ingestion of infective larvae. In Australia, the disease has a seasonal pattern, with peak incidence between April and June in Queensland and April and May in New South Wales. This is thought to occur because wetter weather encourages the proliferation of intermediate gastropod hosts. Young male dogs seem particularly susceptible.24 The mortality rate in dogs is alarmingly high, ranging from 14 to 58%, and both diagnosis and treatment remain challenging, invasive and costly. Undoubtedly there are many dogs with NAS that are only mildly affected or show no obvious signs and so are unlikely to be brought to a veterinarian or are misdiagnosed.
Diagnostic options are limited
The main challenges in managing NAS are achieving early diagnosis and providing effective treatment.8,10,25 Although eosinophilia in blood and CSF are key diagnostic features, they are not definitive, and eosinophil counts can fluctuate significantly. Advanced imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI), are employed in both human and veterinary medicine to detect brain lesions, but they are unhelpful for providing a definitive diagnosis.26 Serological tests are available and are useful in some cases but are limited to only a few specialist reference laboratories worldwide. A key limitation of antibody detection for diagnosis is that serum antibody production occurs after the onset of acute signs or symptoms, sometimes with a significant delay.27 For example, during an outbreak of NAS in Jamaica in 2000, only 8% of serum samples collected during the acute phase (5–18 days after symptom onset) tested positive, whereas 83% of samples collected during the convalescent phase (31–45 days after symptom onset) were positive.8 The specificity and sensitivity of serological assays are largely unknown and cross reactivity with other nematodes occurs, therefore we do not know the extent of exposure of the public to this parasite. A huge variety of polymerase chain reaction (PCR) assays targeting Angiostrongylus DNA have been developed. Despite the development and adaptation of PCR assays which target multi-copy genes, such as internal transcribed spacer (ITS) and cytochrome c oxidase subunit I (cox1) regions, the limits of detection are hindered by the miniscule quantities of Angiostrongylus target DNA present in blood and CSF. In a recent significant development, a ‘hypersensitive’ quantitative PCR (qPCR) assay which targets a repetitive genomic target, AcanR3990, estimated to represent 1.3% of the parasite’s whole genome, was shown to detect 1 fg of DNA, or the equivalent of a 1:100,000 dilution of a single infective larva.28
The gold-standard test is finding the larvae in the CSF, or sometimes within the eye, but this is extremely rare. Because of the high specificity, PCR is the next best test to the gold standard for confirming active infection, and in particular the ‘hypersensitive’ qPCR has the potential to address these diagnostic challenges. To improve accessibility of the ‘hypersensitive’ qPCR, assays that can be performed in the field or bedside such as RPAcan3990 and Angie-LAMP have now been published.29,30 Initial laboratory diagnostics rely on CSF for the detection of the parasite DNA. Peripheral blood is not a good alternative, as the DNA concentrations are below the level of detection of these assays.
Conclusion
Rats, common urban vermin, are the definitive hosts of Angiostrongylus cantonensis. In Australia, 20–60% of urban rats are infected with 5–10 adult parasites.5 Each female A. cantonensis can lay up to 15,000 eggs per day for ~6 weeks, which hatch in the lungs. First stage larvae are coughed up and passed in the rat’s faeces, creating opportunities for molluscs to become infected and act as an infectious source. The disease affects not only humans and dogs but also Australian native wildlife, such as tawny frogmouths and possums, which often serve as initial sentinels.3 Interestingly, pet dogs in Australia are more frequently infected than in other regions where A. cantonensis has emerged. The exact transmission routes beyond ingestion of infected molluscs and the role of climate factors in increasing infection risks remain unclear. Increased rainfall due to the La Niña–Southern Oscillation may enhance the presence of infective larvae in urban areas, potentially raising the risk of accidental zoonotic infections by A. cantonensis. Diagnosis of this condition remains challenging. NAS is a major concern in those affected, leading to significant morbidity and sometimes mortality.
Data availability
The records used for Fig. 1 are available from LabArchives (https://doi.org/10.25833/e3tt-be05) compiled in Phoebe Rivory’s PhD thesis.
References
1 Watthanakulpanich D et al. (2021) Co-occurrence of Angiostrongylus malaysiensis and Angiostrongylus cantonensis DNA in cerebrospinal fluid: evidence from human eosinophilic meningitis after ingestion of raw snail dish in Thailand. Food Waterborne Parasitol 24, e00128.
| Crossref | Google Scholar | PubMed |
2 Bhaibulaya M (1975) Comparative studies on the life history of Angiostrongylus mackerrasae, Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935). Int J Parasitol 5, 7-20.
| Crossref | Google Scholar | PubMed |
3 Spratt DM (2015) Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: a review. Int J Parasitol Parasites Wildl 4, 178-189.
| Crossref | Google Scholar | PubMed |
4 Barratt J et al. (2016) Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143, 1087.
| Crossref | Google Scholar | PubMed |
5 Rivory P et al. (2024) Older urban rats are infected with the zoonotic nematode Angiostrongylus cantonensis. Curr Res Parasitol Vector Borne Dis 5, 100179.
| Crossref | Google Scholar | PubMed |
6 Morgan ER et al. (2021) Angiostrongylosis in animals and humans in Europe. Pathogens 10, 1236.
| Crossref | Google Scholar | PubMed |
7 Lv S et al. (2017) Eosinophilic meningitis caused by Angiostrongylus cantonensis. ACS Chem Neurosci 8, 1815-1816.
| Crossref | Google Scholar | PubMed |
8 Slom TJ et al. (2002) An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346, 668-675.
| Crossref | Google Scholar | PubMed |
9 Cowie RH et al. (2022) Neuroangiostrongyliasis: global spread of an emerging tropical disease. Am J Trop Med Hyg 107, 1166-1172.
| Crossref | Google Scholar | PubMed |
10 Chance MD et al. (2024) Angiostrongylus cantonensis meningoencephalitis in three pediatric patients in Florida, USA. J Pediatric Infect Dis Soc 13, 639-642.
| Crossref | Google Scholar | PubMed |
11 Federspiel F et al. (2020) Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int J Infect Dis 93, 28-39.
| Crossref | Google Scholar | PubMed |
12 Senanayake SN et al. (2003) First report of human angiostrongyliasis acquired in Sydney. Med J Aust 179, 430-431.
| Crossref | Google Scholar | PubMed |
13 Hasan N et al. (2025) Angiostrongylus cantonensis meningo-encephalitis in children-heightened awareness needed during prolonged wet weather conditions. J Paediatr Child Health
| Crossref | Google Scholar | PubMed |
14 Kim JR et al. (2014) Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian Islands. PLoS ONE 9, e94969.
| Crossref | Google Scholar | PubMed |
15 Gutteridge BH et al. (1972) Human larval meningitis possibly following lettuce ingestion in Brisbane. Pathology 4, 63-64.
| Crossref | Google Scholar |
16 Modrý D et al. (2021) Alternative pathways in Angiostrongylus cantonensis (Metastrongyloidea: Angiostrongylidae) transmission. Parasitology 148, 167-173.
| Crossref | Google Scholar | PubMed |
17 Howe K et al. (2019) Water transmission potential of Angiostrongylus cantonensis: larval viability and effectiveness of rainwater catchment sediment filters. PLoS ONE 14, e0209813.
| Crossref | Google Scholar | PubMed |
18 Heaton DC, Gutteridge BH (1980) Angiostrongyliasis in Australia. Aust N Z J Med 10, 255-256.
| Crossref | Google Scholar | PubMed |
19 Blair NF et al. (2013) Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation. Med J Aust 198, 440-442.
| Crossref | Google Scholar | PubMed |
20 Gosnell WL, Kramer KJ (2013) The role of eosinophils in angiostrongyliasis: multiple roles for a versatile cell? Hawaii J Med Public Health 72, 49-51.
| Google Scholar | PubMed |
21 Tseng Y-T et al. (2011) Clinical manifestations of eosinophilic meningitis caused by Angiostrongylus cantonensis: 18 years’ experience in a medical center in southern Taiwan. J Microbiol Immunol Infect 44, 382-389.
| Crossref | Google Scholar | PubMed |
22 Ansdell V et al. (2021) Guidelines for the diagnosis and treatment of neuroangiostrongyliasis: updated recommendations. Parasitology 148, 227-233.
| Crossref | Google Scholar | PubMed |
23 Lee R et al. (2021) Further studies of neuroangiostrongyliasis (rat lungworm disease) in Australian dogs: 92 new cases (2010–2020) and results for a novel, highly sensitive qPCR assay. Parasitology 148, 178-186.
| Crossref | Google Scholar | PubMed |
24 Walker A et al. (2015) Canine neural angiostrongylosis: a case–control study in Sydney dogs. Aust Vet J 93, 195-199.
| Crossref | Google Scholar | PubMed |
25 Graeff-Teixeira C et al. (2009) Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev 22, 322-348.
| Crossref | Google Scholar | PubMed |
26 Senthong V et al. (2013) Differential diagnosis of CNS angiostrongyliasis: a short review. Hawai’i J Med Public Health 72, 52-54.
| Google Scholar | PubMed |
27 Wilkins PP et al. (2013) The current status of laboratory diagnosis of Angiostrongylus cantonensis infections in humans using serologic and molecular methods. Hawai’i J Med Public Health 72, 55-57.
| Google Scholar | PubMed |
28 Sears WJ (2021) AcanR3990 qPCR: a novel, highly sensitive, bioinformatically informed assay to detect Angiostrongylus cantonensis infections. Clin Infect Dis 73, e1594-e1600.
| Crossref | Google Scholar | PubMed |
29 Sears WJ et al. (2021) RPAcan3990: an ultrasensitive recombinase polymerase assay to detect Angiostrongylus cantonensis DNA. J Clin Microbiol 59, e0118521.
| Crossref | Google Scholar | PubMed |
30 Baláž V et al. (2023) Angie-LAMP for diagnosis of human eosinophilic meningitis using dog as proxy: a LAMP assay for Angiostrongylus cantonensis DNA in cerebrospinal fluid. PLoS Negl Trop Dis 17, e0011038.
| Crossref | Google Scholar | PubMed |
 Phoebe Rivory is a PhD candidate at The University of Sydney. Her primary research focus is Angiostrongylus in Australia. |
 Jan Šlapeta is a professor at The University of Sydney. He applies traditional and state of the art molecular approaches to understand parasite identity, origin and role in disease and pathogenesis. |


