SnotWatch: data collaboration informing disease impact
Jim P. Buttery A B C D * , Aaron L. Weinman B C , Rana Sawires B C E , Eric Zhao B C , Tu Quan Nguyen A B C , Hazel J. Clothier A B C F and on behalf of the SnotWatch Collaboration GroupA
B
C
D
E
F

Jim Buttery is professor of child health informatics at The University of Melbourne, head of the Epidemiology–Informatics Research Group at Murdoch Children’s Research Institute, chief research information officer at the Royal Children’s Hospital and Research team lead, at the Centre for Health Analytics, The Melbourne Children’s Campus. He has a well-established research profile in paediatric infectious diseases and vaccine safety. |

Aaron Weinman is the post-doctoral study coordinator for the SnotWatch platform within the Epidemiology–Informatics group at Murdoch Children’s Research Institute. He is an infectious diseases epidemiologist. |

Rana Sawires is a recent graduate of the Doctor of Philosophy and Doctor of Medicine at Monash University. She completed her PhD at Murdoch Children’s Research Institute and has continued in her role as a research associate with the Institute, expanding on her work in paediatric infectious diseases and public health. She is currently also a doctor at St Vincent’s Hospital, Melbourne. |

Eric Zhao completed his Doctor of Philosophy in statistics at Australian National University. He is a statistical data analyst within the Epidemiology–Informatics group at Murdoch Children’s Research Institute. |

Tu Nguyen is a PhD candidate in the Centre for Health Analytics, Murdoch Children’s Research Institute and Department of Paediatrics, The University of Melbourne. She is an epidemiologist with experience in public health research, analysing large datasets and COVID-19 surveillance. |

Hazel Clothier is the epidemiology team lead within the Epidemiology Informatics group at Murdoch Children’s Research Institute. She serves as an academic supervisor for the Masters of Applied Epidemiology program. |

The SnotWatch Collaboration Group includes Monash Health (Tony Korman), Royal Children’s Hospital (Andrew Daley and Vanessa Clifford), Alfred Health (Adam Jenney), Barwon Health (Eugene Athan), Australian Clinical Labs (Benjamin Benc), Melbourne Health (Katherine Bond), Eastern Health (Roy Chean), Northern Health (Yvonne Hersusianto and Craig Aboltins), Outcome Health (Chris Pearce) and the Victorian Department of Health (Jim Black). |
Abstract
The spectrum of health conditions associated with the circulation of respiratory viruses remains poorly quantified. The SnotWatch platform has established a databank containing test results for respiratory polymerase chain reaction tests conducted by eight major Victorian pathology laboratories and has obtained access to major healthcare presentation databases, such as the Victorian Admitted Episodes Dataset, Victorian Emergency Minimum Dataset and POLAR GP. By establishing a statistical approach to describe the associations between healthcare presentations and circulating viruses in space and time at a population level, SnotWatch is uncovering the health conditions associated with virus circulation. SnotWatch’s methods have been validated by demonstrating the capacity to describe well-known associations, but have also uncovered novel associations. Methods used have also been able to incorporate environmental exposures. By elucidating the associations between viruses and health conditions, SnotWatch allows clinicians to make better informed diagnoses, anticipate healthcare presentations and outcomes, optimises resource management by healthcare providers and allows the benefits of vaccination to be more fully appreciated. SnotWatch’s future includes establishing methods for attributable burden, developing models for nowcasting and deepening our understanding of the economic impact of viruses.
Keywords: data analytics, epidemiology, public health, surveillance, viruses.
Introduction
The ubiquity of viral infections and broad acceptance of their impact upon the community and health system contrasts with our understanding of the breadth and quantum of their impact upon human health. Owing to the frequency of carriage, multiple agents causing similar clinical syndromes, co-infection and the complexity of their interaction with other infectious disease, infection-related presentations and non-communicable diseases, our understanding is surprisingly limited. Most studies are limited by incomplete testing, resulting in under-ascertainment, predominantly hospital testing informing estimates, and reliance upon discharge coding. Even large, well-conducted randomised controlled trials of viral vaccines often inform on the most common expected outcomes. The 2007 implementation of infant rotavirus vaccines was the first ecologic opportunity to appreciate that rotavirus caused 20% of febrile convulsions.1
The era of routine sensitive multiplex polymerase chain reaction (PCR), big data and advanced ecologic statistical techniques, offers more opportunities to understand the organism-specific impact of common viral infections on human health. SnotWatch is a collaborative spatiotemporal ecologic platform utilising de-identified routine microbiologic testing and healthcare utilisation data to understand the clinical and health system impact of common respiratory and gastrointestinal viruses.
Burden of respiratory and gastrointestinal viruses
Common respiratory and gastrointestinal viruses have a devastating effect on Australians, their health system and economy. This has been underlined by the COVID-19 pandemic, with more than 11.8 million cases, 25,000 deaths (to March 2024) and a cumulative loss of A$158 billion from projected gross domestic product (to June 2022) in Australia.2,3 Viral infections are pervasive, with at least nine new non-SARS-CoV-2 respiratory viral infections per child-year for the first 2 years of life in US and Australian cohorts.4,5 An additional average 3.3 new non-rotaviral gastrointestinal viral infections per infant were also detected in the first 41 weeks of life in the rotavirus vaccinated Australian ORChID cohort subset.6 There were an estimated 12,835 annual excess hospitalisations due to influenza between 2007 and 2015, with the highest risk in adults >65 years and children <5 years.7
The need to understand the impact of common respiratory and gastrointestinal viruses was illustrated starkly by the SARS-CoV-2 virus and resultant COVID-19 pandemic, and vaccine implementation. It is also made increasingly important by the burgeoning development of new vaccines against pathogens such as respiratory syncytial virus (RSV), human metapneumovirus (hMPV) and norovirus.8,9
The gap in knowledge that SnotWatch fulfils
Utilising the power of millions of datapoints, SnotWatch has been established to provide insights into acute respiratory and gastrointestinal infections:
Their association with important paediatric and adult health conditions, e.g. asthma,10 febrile seizures,11 Kawasaki Disease,12 chilblains,13 invasive bacterial infections and acute cardiovascular outcomes.
Understanding the proportion of disease impact caused by each pathogen.
Inform on the organism-specific health and economic burden of each pathogen, informing policy decisions.
Using historical patterns of viral spread and disease to predict short term future patterns of transmission (nowcasting).
Provide visual real-time local insights for communities and health care workers.
The concept for SnotWatch grew out of the need for large scale, near real-time capable data analysis to inform decision-making and policy. The privacy implications of data linkage, while powerful and more statistically robust, reduce the availability of data and increase data lags and costs. By not requiring personally identifiable data, but common spatial and temporal elements, SnotWatch overcomes these barriers. This has allowed the creation of a population-level spatiotemporal map of viral exposures, in conjunction with a wide range of health outcome datasets.
Because of the Victoria-wide reach of SnotWatch, with more than a decade of data, SnotWatch is able to detect associations with even rare conditions, including Kawasaki disease12 and paediatric hepatitis,14 that have proven difficult to investigate using more traditional methods due to small data volumes. The addition of the spatial component to traditional ecologic temporal study designs also increases the clinical significance of our results.
Data sources and ethics
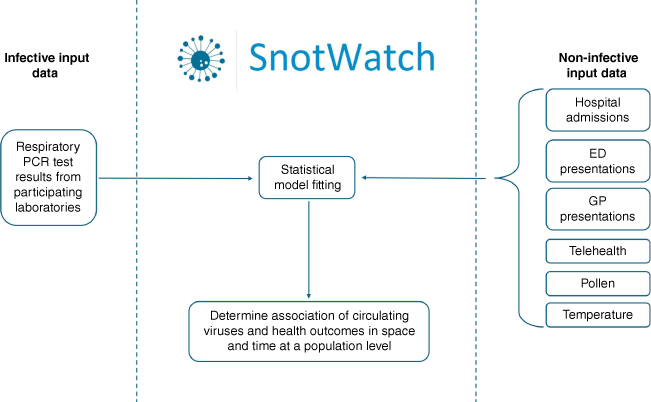
SnotWatch collects de-identified exposure data from the following collaborating Victorian sources: multiplex respiratory PCR tests from most public hospital and selected private pathology providers; weather data from The Bureau of Meteorology; and, pollen data from Melbourne Pollen (pollen levels have been shown to attenuate immune responses against respiratory viruses).15 Outcome data sources include the: Department of Health Victorian Admitted Episodes Dataset (VAED), Victorian Emergency Minimum Dataset (VEMD), SynSurv Triage text from telehealth and emergency department (ED) presentations, and Outcome Health POLAR general practice data collaborations16 (Fig. 1).
As Snotwatch data are potentially available in near real-time, the ability to use it for both nowcasting, and for informing clinical decisions by general practitioners and their patients is enticing. In the US, Germwatch, a Utah initiative by Intermountain Healthcare, provides updates for clinicians and the community regarding what is currently circulating, together with simple clinical advice.17
SnotWatch has received ethics approval from Monash Health Human Research Ethics Committee (HREC; reference number: RES-19-0000333L-53611). A waiver of consent has been approved by the reviewing HREC. The platform is governed by a steering committee, including representatives from data custodians, Department of Health Victoria, participating laboratories and consumer representatives.
Statistical methods
SnotWatch assembles each retrospective exposure and outcome dataset into temporal (time) and spatial (space) units: typically aggregating time by month or week, and space by statistical areas (SA), typically SA3 (electorate area equivalent). The statistical model was initially codeveloped with Martin Kulldorff at the Harvard School of Public Health in 2019. The peaks of both exposures (e.g. weekly positive virus proportion for each virus) and outcomes are then explored using generalised linear models, including negative binomial and Poisson models, as well as their zero-inflated variants. The multivariate model can include both infective and environmental non-infective (e.g. pollen counts) exposures. A significance level of 0.01 or lower is assumed throughout the statistical analysis. The SaTScan (Boston, MA, USA) space-time scan statistic with a Poisson model is also used, where applicable, to independently identify spatiotemporal clusters, exposures and outcomes.
Investigations and results to date
To date, investigations have examined the association between respiratory viruses and febrile seizures, chilblains, paediatric hepatitis clusters, Kawasaki disease and asthma (Table 1). The initial validation study was conducted using Monash Health pathology and ED data, and demonstrated a temporal relationship with each of hMPV, RSV and influenza circulation with febrile seizures in children <5 years and a spatiotemporal relationship with RSV and influenza.11
The ‘COVID-toes’ phenomenon was investigated using POLAR GP primary care data demonstrating a spatiotemporal association between SARS-CoV-2 and chilblains in both adults and children.13 Reports in 2022 from Northern America and Europe of clusters of paediatric hepatitis, many in association with the (non-respiratory) adenovirus, led to an analysis of historic statewide paediatric hepatitis clusters and respiratory viral exposures. After excluding hepatitis A–E admissions (5518 admissions over 11 years), the analysis revealed 16 space-time clusters of hepatitis. Multiple associations were observed between paediatric hepatitis clusters and respiratory viruses but none with respiratory adenoviruses or SARS-CoV-2.
A statewide analysis of Kawasaki disease admissions from July 2011 to November 2021 found increased rate ratios of 1.52 (99% CI: 1.27–1.82) and 1.43 (99% CI: 1.17–1.73) of Kawasaki disease presentations in association with hMPV and RSV peak circulation respectively, before the COVID-19 pandemic. No associations were observed during the COVID-19 pandemic.12
More recent studies in progress have incorporated environmental exposures including pollen counts as well as respiratory viral exposures in an assessment of triggers of acute asthma.10
Where does SnotWatch fit?
In an era where many respiratory viruses are currently, or soon to be vaccine-preventable, understanding and quantifying the full impact of these viruses has never been more important to help policymakers decide on whether viral vaccines or medicines represent a good investment by Australian taxpayers. SnotWatch has identified previously unknown associations that can inform this process. Further development of the statistical models currently underway will allow quantification of the burden of a condition attributable to any individual virus. If disease burden can be quantified, cost-impact at many levels of the health system and subsequent cost effectiveness calculations will be revolutionised for interventions aimed at these viruses. Furthermore, the lack of identifiable data required for SnotWatch creates an extremely low risk to privacy and has led to high participation from multiple data partners, ultimately maximising SnotWatch’s power to detect new and existing associations.
Limitations of SnotWatch
As an ecologic platform, SnotWatch is largely hypothesis generating, and can be subject to ecological fallacy – bias from drawing conclusions about individual-level associations when only using group-level data. Reassuringly, SnotWatch studies have also confirmed previously known associations (such as influenza infection with febrile seizures).11
Future directions of SnotWatch
SnotWatch is a collaborative analytical platform that can incorporate many different spatiotemporal exposures and outcomes. Analyses are underway to explore the often-suggested observation that respiratory viral infections predispose to invasive bacterial disease. In cardiovascular disease, viral triggers of acute myocardial infarction and stroke are also being explored, in collaboration with validation datasets from Western Australia and Queensland. Nowcasting is also being explored. Finally, although gastrointestinal multiplex PCR panels were implemented later than respiratory panels in many laboratories, there has been sufficient uptake in recent years to begin exploring associations with enteric pathogens.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Acknowledgements
The authors express their gratitude to Deniz Akin & Joshua Osowicki for their work in driving expansion of the platform, Martin Kulldorff for his assistance with statistical methodology, our consumer engagement manager Jo Hickman and our consumer representatives (Elvin Paul Lam & Peter Newton) for helping guide development of this platform.
References
1 Weinberg GA (2014) Editorial commentary: unexpected benefits of immunization: rotavirus vaccines reduce childhood seizures. Clin. Infect. Dis. 58, 178-180.
| Crossref | Google Scholar | PubMed |
2 Australian Bureau of Statistics (2022) Economic gains and losses over the COVID-19 pandemic. Released 7/09/2022. ABS, Canberra, ACT, Australia. https://www.abs.gov.au/articles/economic-gains-and-losses-over-covid-19-pandemic
3 Australian Bureau of Statistics (2024) Deaths due to acute respiratory infections in Australia – 2022 – March 2024. Released 30/04/2024. ABS, Canberra, ACT, Australia. https://www.abs.gov.au/articles/deaths-due-acute-respiratory-infections-australia-2022-march-2024
4 Teoh Z et al. (2023) Burden of respiratory viruses in children less than 2 years old in a community-based longitudinal US birth cohort. Clin Infect Dis 77, 901-909.
| Crossref | Google Scholar | PubMed |
5 El-Heneidy A et al. (2023) Interference between enteric viruses and live–attenuated rotavirus vaccine virus in a healthy Australian birth cohort. J of Infect Dis 228, 851-856.
| Crossref | Google Scholar | PubMed |
6 Sarna M et al. (2018) Viruses causing lower respiratory symptoms in young children: findings from the ORChID birth cohort. Thorax 73, 969-979.
| Crossref | Google Scholar | PubMed |
7 Newall AT, Scuffham PA (2008) Influenza-related disease: the cost to the Australian healthcare system. Vaccine 26, 6818-6823.
| Crossref | Google Scholar | PubMed |
8 Miller RJ, Mousa JJ (2023) Structural basis for respiratory syncytial virus and human metapneumovirus neutralization. Curr Opin Virol 61, 101337.
| Crossref | Google Scholar | PubMed |
9 Tan M (2021) Norovirus Vaccines: Current Clinical Development and Challenges. Pathogens 10, 1641.
| Crossref | Google Scholar | PubMed |
10 Sawires R (2023) Snotwatch: when data go viral. PhD thesis, Monash University, Clayton, Vic., Australia. https://bridges.monash.edu/articles/thesis/Snotwatch_When_Data_Go_Viral/24557218?file=43137016
11 Sawires R et al. (2022) SnotWatch: an ecological analysis of the relationship between febrile seizures and respiratory virus activity. BMC Pediatr 22, 359.
| Crossref | Google Scholar | PubMed |
12 Sawires R et al. (2024) Kawasaki disease and respiratory viruses: ecological spatiotemporal analysis. JMIR Pub Health and Surv 10, e49648.
| Crossref | Google Scholar | PubMed |
13 Sawires R et al. (2022) SnotWatch COVID-toes: an ecological study of chilblains and COVID-19 diagnoses in Victoria, Australia. PLOS Global Public Health 2, e0000488.
| Crossref | Google Scholar | PubMed |
14 Sawires R et al. (2023) Pediatric hepatitis and respiratory viruses: a spatiotemporal ecologic analysis. Pediatr Infect Dis J 42, 276-280.
| Crossref | Google Scholar | PubMed |
15 Gilles S et al. (2020) Pollen exposure weakens innate defense against respiratory viruses. Allergy 75, 576-587.
| Crossref | Google Scholar | PubMed |
16 Pearce C et al. (2019) What a comprehensive, integrated data strategy looks like: the Population Level Analysis and Reporting (POLAR) program. Stud Health Technol Inform 264, 303-307.
| Crossref | Google Scholar | PubMed |
17 Gesteland PH et al. (2007) Informing the front line about common respiratory viral epidemics. AMIA Annu Symp Proc 2007, 274-278.
| Google Scholar | PubMed |
 Jim Buttery is professor of child health informatics at The University of Melbourne, head of the Epidemiology–Informatics Research Group at Murdoch Children’s Research Institute, chief research information officer at the Royal Children’s Hospital and Research team lead, at the Centre for Health Analytics, The Melbourne Children’s Campus. He has a well-established research profile in paediatric infectious diseases and vaccine safety. |
 Aaron Weinman is the post-doctoral study coordinator for the SnotWatch platform within the Epidemiology–Informatics group at Murdoch Children’s Research Institute. He is an infectious diseases epidemiologist. |
 Rana Sawires is a recent graduate of the Doctor of Philosophy and Doctor of Medicine at Monash University. She completed her PhD at Murdoch Children’s Research Institute and has continued in her role as a research associate with the Institute, expanding on her work in paediatric infectious diseases and public health. She is currently also a doctor at St Vincent’s Hospital, Melbourne. |
 Eric Zhao completed his Doctor of Philosophy in statistics at Australian National University. He is a statistical data analyst within the Epidemiology–Informatics group at Murdoch Children’s Research Institute. |
 Tu Nguyen is a PhD candidate in the Centre for Health Analytics, Murdoch Children’s Research Institute and Department of Paediatrics, The University of Melbourne. She is an epidemiologist with experience in public health research, analysing large datasets and COVID-19 surveillance. |
 Hazel Clothier is the epidemiology team lead within the Epidemiology Informatics group at Murdoch Children’s Research Institute. She serves as an academic supervisor for the Masters of Applied Epidemiology program. |
 The SnotWatch Collaboration Group includes Monash Health (Tony Korman), Royal Children’s Hospital (Andrew Daley and Vanessa Clifford), Alfred Health (Adam Jenney), Barwon Health (Eugene Athan), Australian Clinical Labs (Benjamin Benc), Melbourne Health (Katherine Bond), Eastern Health (Roy Chean), Northern Health (Yvonne Hersusianto and Craig Aboltins), Outcome Health (Chris Pearce) and the Victorian Department of Health (Jim Black). |