Pneumococcal conjugate vaccines in children
Sanjay Jayasinghe A B *A
B

Dr Sanjay Jayasinghe is a medical epidemiologist with extensive experience in research on pneumococcal disease and vaccines. He is a senior research fellow at the National Centre for Immunisation Research and Surveillance (NCIRS) and a senior lecturer (conjoint) in the Children’s Hospital at Westmead Clinical School of the Faculty of Medicine, The University of Sydney. He is also a National Health and Medical Research Council (NHMRC) emerging leadership fellow. He currently chairs the Enhanced Invasive Pneumococcal Disease (IPD) Surveillance (EIPDSWG) working group that oversees national IPD surveillance. |
Abstract
Pneumococcal conjugate vaccines (PCVs), which have been in population-wide use in children for over two decades now, are highly efficacious in preventing life threatening pneumococcal infections. The strong herd effect of PCVs through reduction in pneumococcal nasopharyngeal carriage in vaccinated children prevents disease in adults as well. Since 7vPCV, which was the first PCV used widely, several new PCVs with each adding more serotypes have been developed. These new PCVs have been approved using immune-bridging criteria based on an aggregate correlate of protection (Cp) derived from vaccine efficacy and antibody responses data from early PCV trials. This Cp that the World Health Organization (WHO) accepts for assessing new PCVs for which it is impracticable to undertake placebo-controlled trials with clinical outcomes is 0.35 μg mL–1 of enzyme-linked immunosorbent assay (ELISA) immunoglobulin G (IgG). Effectiveness and antibody response data more recently of 13vPCV has led to developing Cp for each individual vaccine serotype, that, for some, varies considerably to 0.35 μg mL–1. In trials of newest PCVs, such as 15vPCV and 20vPCV, the comparator used is 13vPCV that has, in turn, been licensed using immune bridging, which leads to potential ‘downward-drift’ risk of protection from the new PCVs. This and the data that have emerged on serotype replacement disease and dosing schedules makes it important to review and rethink how new PCVs are assessed, their clinical benefits are inferred and vaccination programs are designed.
Keywords: Australia, children, correlate of protection, invasive pneumococcal disease, PCV schedule, pneumococcal conjugate vaccine, pneumococcal disease, pneumococcal serotypes, vaccine efficacy, vaccine impact.
Streptococcus pneumoniae and pneumococcal diseases
Streptococcus pneumoniae (pneumococcus) causes a spectrum of illnesses in children with the most severe forms leading to major complications and even death.1 This reflects the ability of pneumococcus to survive in various ecological niches in the human host.2 Pneumococcus resides in the nasopharynx in children most commonly without causing illness.3,4 From the nasopharynx pneumococci may spread locally to cause mucosal infections such as otitis media and pneumonia. The more severe end of the pneumococcal disease spectrum occurs when the organism enters normally sterile body sites to cause invasive disease (known as invasive pneumococcal disease, IPD) such as septicaemia and meningitis.5
Pneumococci typically produce a polysaccharide capsule that determines the antigenic properties of the organism with over 100 serotypes so far identified.6,7 Serotypes differ in their prevalence in carriage and disease, the tendency to cause severe disease (i.e. invasive potential) and the degree of antimicrobial resistance.8–10 Some serotypes seldom cause disease in humans. Serotypes chosen to be included in pneumococcal vaccines developed over the years are the ones with a greater tendency to cause disease.11
Pneumococcal vaccines for children
The initial pneumococcal vaccines developed in early 1900s had pure capsular polysaccharide only of selected serotypes as the antigen.12 A key limitation of these pneumococcal polysaccharide vaccine (PPV) formulations was that they were poorly immunogenic in young children.13 Responses generated by these pure polysaccharide vaccines are T-cell independent so do not provide immune memory and therefore provide only short-term protection. PPV does not have any observable effectiveness against pneumococcal nasopharyngeal carriage and therefore does not provide appreciable herd benefit either. Pneumococcal conjugate vaccines (PCVs) where the polysaccharide is covalently bonded to a carrier protein provides superior and robust immune responses.14 A PCV that had seven of the commonest serotypes that cause disease (7vPCV) in the USA showed remarkable efficacy in clinical trials among children in early 2000.15 The 7vPCV became the first pneumococcal vaccine that was used in infants at population-level programs globally across many countries.
Pneumococcal vaccination program in Australia
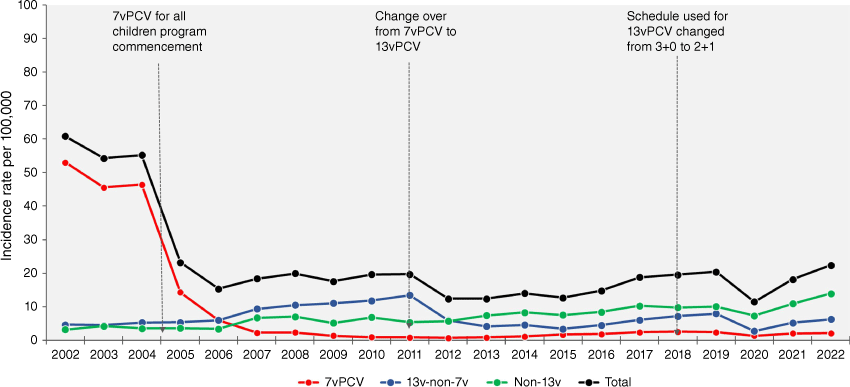
In Australia, 7vPCV was fast-tracked for use in Aboriginal and Torres Strait Islander children and children with underlying medical at-risk conditions due to their high incidence of IPD in 2001.16 The program was extended to all children in 2005. Consistent with strong efficacy in clinical trials 7vPCV led to a dramatic reduction in IPD of vaccine serotypes among Australian children17,18 (Fig. 1). In parallel, there were impressive reductions in disease also in unvaccinated populations through herd impact. On the back of this remarkable success story of 7vPCV, new PCVs with more serotypes covered were developed during 2009–2010.19,20 These new higher valency vaccines were also to mitigate the emergence of some non-vaccine serotypes following 7vPCV use (‘serotype replacement’). In 2011, 7vPCV was replaced by new 13vPCV in the Australian pneumococcal vaccination program.16 Reductions in some of the vaccine serotypes being offset by serotype replacement became a constant feature of PCV programs.21 However, there continued to be an overall net decline in IPD as a result of PCV programs despite this serotype replacement. In the two decades following advent of 7vPCV, several other PCVs have also been developed sequentially adding more serotypes that were causing residual pneumococcal disease and those emerging to cause serotype replacement disease.22 Few of the new PCVs in the pipeline use novel vaccine platforms. Table 1 summarises the composition of these existing and new PCVs.
IPD Incidence rates (per 100,000) in children aged <5 years in Australia by vaccine serotype categories, 2002–2022. Data source: national enhanced IPD surveillance data, National Notifiable Disease Surveillance System (NNDSS), Enhanced IPD surveillance working group (EIPDSWG), Communicable Diseases Network Australia (CDNA). Data request ID# 547 (Nov)/2022, May 2023 data extract. Denominator for rate calculations: Australian Bureau of Statistics population estimates (September 2022 release).
| Vaccine | Brand name | Company | Serotypes | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7v | 10v-non-7v | 13v-non-10v | 15v-non13v | 20v-non-15v | 24v-non-20v | ||||||||||||||||||||||
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 2 | 9N | 17F | 20 | ||||
| PCV7 | Prevenar | Pfizer | 4 | 6B | 9V | 14 | 18C | 19F | 23F | ||||||||||||||||||
| PCV10 | Synflorix | GSK | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | |||||||||||||||
| PCV10 | Pneumosil | SII | 6B | 9V | 14 | 23F | 1 | 3 | 5 | 6A | 7F | 19A | |||||||||||||||
| PCV13 | Prevenar-13 | Pfizer | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | ||||||||||||
| PCV15 | Vaxneuvance | MSD | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | ||||||||||
| PCV20 | Prevenar-20 | Pfizer | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | |||||
| PCV21 | NA (GBP410) | Sanofi | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 9N | ||||
| PCV24 | Vax-24 | Vaxcyte | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 2 | 9N | 17F | 20 | |
| PCV24 | AFX3772 | Affinivax/GSK | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 2 | 9N | 17F | 20 | |
| PCV25 | IVT PCV-25 | Inventprise | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 7F | 19A | 22F | 33F | 8 | 10A | 12F | 15B | 2 | 9N | |||||
| PCV31 | Vax-31 | Vaxcyte | 4 | 6B | 9V | 14 | 18C | 19F | 23F | 1 | 3 | 5 | 6A | 7F | 19A | 22F | 33F | 8 | 10A | 11A | 12F | 15B | 2 | 9N | 17F | 20 | |
PCV25 also includes serotypes 6C, 15A, 16F, 24F and 35B; PCV31 includes serotypes 7C, 15A, 16F, 23A, 23B, 31 and 35B.
Assessing PCVs for use in children and predicting protective efficacy from immune responses
The licensing of 7vPCV for use in children was based on several randomised placebo controlled clinical trials (RCTs) with IPD as the outcome assessed.23 Owing to the remarkably high effectiveness of 7vPCV, undertaking similar RCTs where clinical outcomes were assessed became neither ethical nor feasible for PCVs developed subsequently. To circumvent this, the strategy of inferring clinical efficacy from comparative immune responses elicited by new PCVs was developed.24 An immune correlate of protection (Cp) threshold that could predict clinical protection was determined for this bridging of antibody responses to clinical efficacy. This threshold Cp antibody level was derived from aggregate immunoglobulin G (IgG) responses and efficacy against all vaccine type IPD from combined data of three PCV trials (i.e. two 7vPCV trials in First Nations children in the USA and one 9vPCV trial in South African children24). A pneumococcal anticapsular polysaccharide IgG antibody concentration of 0.35 μg mL–1 measured by enzyme-linked immunosorbent assay (ELISA) was the threshold correlate thus established, and subsequently recognised by the World Health Organization (WHO) as criterion for use in assessing PCV developed post-7vPCV where vaccine efficacy data on clinical endpoints are not available.25 This Cp was the antibody level in reverse cumulative distributions plot of PCV vaccinated children and placebo controls corresponding to proportions with disease risk that generate the observed vaccine efficacy.26,27
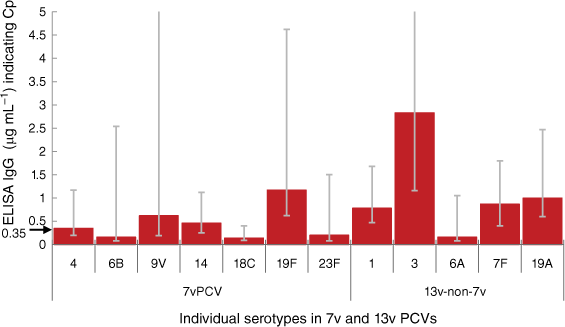
The first two new PCVs post-7vPCV, 10vPCV and 13vPCV, were licensed based on immune non-inferiority using the Cp criterion from data in head-to-head trials between those respective PCVs and 7vPCV. The inference therein was, if the new PCV product is non-inferior to the already licensed product, then that equates to similar clinical efficacy.22,26,27 Although overall the reductions of vaccine-type IPD in vaccinated children was consistent with that found in clinical trials, the real-world post-licensing effectiveness of PCVs showed considerable variation across individual serotypes.22,26–28 This led to further research to estimate Cps for each individual pneumococcal serotype included in PCVs, using observed serotype-specific vaccine effectiveness from observational studies and immune responses, applying the same methodology utilised for deriving the initial Cp from 7v/9vPCV trial data.24,26,27,29 This calculated serotype-specific Cp IgG levels varied considerably from the 0.35 μg mL–1 value for most serotypes in 7vPCV and 13vPCV (Fig. 2).26 For few serotypes this estimates Cp is lower than 0.35 μg mL–1 but for majority of serotypes in 13vPCV the thresholds IgG antibody level required for protection against IPD were considerably higher. For example, for serotype 3 it is approximately eight-fold higher and this is reflected in almost no effect of 13vPCV on IPD caused by this serotype observed.21,26,30,31
Correlate of protection of PCVs against IPD by serotype based on estimates in Andrews et al.26 compared to the accepted 0.35 μg mL–1 level.
One other point to note regarding this antibody responses-based assessment of PCVs to infer clinical protection benefits is that currently it is only available for IPD. Other pneumococcal disease phenotypes such as otitis media and non-bacteraemic pneumonia are considerably more common than IPD therefore PCV benefit assessments ideally need to be able to estimate predictions regarding preventing those outcomes as well.29,32 Also important for assessing PCV benefits is the magnitude of expected reduction in nasopharyngeal carriage as it is an essential precursor of disease and plays a key role in disease transmission and therefore inducing herd protection. For protection against these mucosal infections however, rather than serum IgG, immunoglobulin A (IgA) produced by mucosally associated B-cells and IgG that leaks from circulation would be what is relevant.32 Nevertheless, serum IgG levels could serve as a proxy for determining the level of immune responses required for protection against these mucosal infections. There have been attempts to establish Cp for these other pneumococcal infection outcomes and, as would be expected, they are much higher than the level for IPD.29,32
The latest PCVs that have been registered for use in Australia and several other countries are 15vPCV and 20vPCV.22,29 The basis for their licensing was non-inferior immunogenicity, compared to 13vPCV, in trials, for the shared serotypes and superior immunogenicity for the respective additional serotypes in these two vaccines. The 13vPCV was in turn approved based on immune bridging to 7vPCV that had efficacy data. Therefore, for 15vPCV and 20vPCV the approval was based on bridge-to-bridge inferences. The risk in this approach of approving vaccines based on non-inferiority to a prior vaccine, which itself was justified based on non-inferiority is that subsequent vaccines could be accepted with less than equal immunogenicity to the originally licensed – downward drift of efficacy. These latest higher valency vaccines while having definite advantages of broader serotype coverage actually show somewhat lower immune responses to the shared serotypes with existing PCVs.33
Dosage schedule of PCVs for children
One other aspect of PCV programs in children that has developed over the years is the dosing schedules. The pivotal trial that established remarkable efficacy of 7vPCV used a 4 dose ‘3 + 1’ schedule (doses at age 2, 4, 6 and 12–15 months).15 Most countries that subsequently implemented 7vPCV programs adopted one of two abridged three-dose schedules, with all three doses provided in infancy (3 + 0 schedule), or two doses in infancy and one dose (booster) at 12 months or after (2 + 1 schedule). The 7vPCV program in Australia adopted the 3 + 0 schedule.16 With subsequent PCVs such as 13vPCV, considerable waning of protection was observed when children entered toddler age groups, highlighting the importance of the booster dose for sufficient vaccine immunity persistence.28 In Australia, the schedule was changed to address this in 2018 by moving the third dose to be a booster (2 + 1 schedule).34 There are countries like the UK that have dropped one dose in their PCV schedule to have a 1 + 1 schedule with the rationale being that with decades of PCV use with high coverage a single primary and a booster dose are sufficient to maintain the gains achieved35. Their premise was that the 3rd dose did not lead to much further gain that commensurate the cost incurred.
Future of PCV programs for children
Over the last two decades, highly efficacious PCVs have been responsible for preventing thousands of life-threatening pneumococcal diseases in children globally. In countries like Australia that have had a long-term childhood PCV program with very high coverage the residual IPD burden is now low. The new PCVs emerging cover some of the key serotypes responsible for this residual disease but with serotype replacement the nett gains of those new PCVs are much less than the initial impact of PCVs. The benefits prediction assessments of these new PCVs need to take into account the variability of immune Cp by serotype. New vaccine platforms for PCVs seen developed may provide solutions to address seemingly vaccine resistant serotypes such as serotype 3. With the potential downward drift in these new PCVs the booster dose would be essential to achieve sufficient protection. This all points to a new era of PCVs and pneumococcal disease control.
Data availability
For the data used in Fig. 1, the author acknowledges the Enhanced IPD Surveillance Working Group of the Communicable Diseases Network Australia (CDNA) and all public health officers involved in the collection of IPD surveillance data; state and territory public health communicable disease and surveillance units; and all laboratories that support national IPD surveillance in Australia. (Data access approval #547 [November]/2022.)
Declaration of funding
Sanjay Jayasinghe is supported by a National Health and Medical Research Council of Australia Investigator Grant (ID number 2010091).
References
1 Tuomanen EI et al. (1995) Pathogenesis of pneumococcal infection. N Engl J Med 332, 1280-4.
| Crossref | Google Scholar | PubMed |
2 Klein JO (1981) The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis 3, 246-53.
| Crossref | Google Scholar | PubMed |
3 Austrian R (1986) Some aspects of the pneumococcal carrier state. J Antimicrob Chemother 18, 35-45.
| Crossref | Google Scholar | PubMed |
4 Bogaert D et al. (2004) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4, 144-54.
| Crossref | Google Scholar | PubMed |
6 Li Y et al. (2013) Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infect Immun 81, 4519-24.
| Crossref | Google Scholar | PubMed |
7 Blacklock CB et al. (2014) Streptococcus pneumoniae serotypes. 106 serotypes. https://pneumococcalcapsules.github.io/serotypes/ (accessed 25 September 2024)
8 Austrian R (1953) Morphologic variation in pneumococcus. I. An analysis of the bases for morphologic variation in pneumococcus and description of a hitherto undefined morphologic variant. J Exp Med 98, 21-34.
| Crossref | Google Scholar | PubMed |
9 Hausdorff WP et al. (2005) Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 5, 83-93.
| Crossref | Google Scholar | PubMed |
10 Kalin M (1998) Pneumococcal serotypes and their clinical relevance. Thorax 53, 159-62.
| Crossref | Google Scholar | PubMed |
11 Malley R (2010) Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med 88, 135-42.
| Crossref | Google Scholar | PubMed |
12 Musher DM et al. (2022) The remarkable history of pneumococcal vaccination: an ongoing challenge. Pneumonia 14, 5.
| Crossref | Google Scholar | PubMed |
13 Daniels CC et al. (2016) A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther 21, 27-35.
| Crossref | Google Scholar | PubMed |
14 Pichichero ME (2013) Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother 9, 2505-23.
| Crossref | Google Scholar | PubMed |
15 Black S et al. (2000) Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 19, 187-95.
| Crossref | Google Scholar | PubMed |
16 National Centre for Immunisation Research and Surveillance (2023) Significant events in pneumococcal vaccination practice in Australia. NCIRS. https://ncirs.org.au/sites/default/files/2023-04/Pneumococcal-history-April%202023.pdf (accessed 31 October 2023)
17 Barry C et al. (2012) Invasive pneumococcal disease in Australia 2007 and 2008. Commun Dis Intell 36, E151-65.
| Google Scholar | PubMed |
18 Jayasinghe S et al. (2015) Evaluation of impact of 23 valent pneumococcal polysaccharide vaccine following 7 valent pneumococcal conjugate vaccine in Australian Indigenous children. Vaccine 33, 6666-74.
| Crossref | Google Scholar | PubMed |
19 Vesikari T et al. (2009) Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 28, S66-76.
| Crossref | Google Scholar | PubMed |
20 Yeh SH et al. (2010) Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 126, 493-505.
| Crossref | Google Scholar | PubMed |
21 Jayasinghe S et al. (2017) Long-term impact of a “3+0” schedule for 7- and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in Australia, 2002-2014. Clin Infect Dis 64, 175-83 Epub 21 October 2016.
| Crossref | Google Scholar | PubMed |
22 Feemster K et al. (2024) Immunogenicity of current and next-generation pneumococcal conjugate vaccines in children: current challenges and upcoming opportunities. Open Forum Infect Dis 11, ofae220.
| Crossref | Google Scholar | PubMed |
23 Therapeutic Goods Administration (2003) Product Information: Prevenar. TGA. https://www.tga.gov.au/sites/default/files/foi-025-1718-09.pdf
24 Siber GR et al. (2007) Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 25, 3816-26.
| Crossref | Google Scholar | PubMed |
25 World Health Organization (2009) WHO Expert Committee on Biological Standardization, sixtieth report. (WHO technical report series; number 977). WHO, Geneva, Switzerland. https://iris.who.int/bitstream/handle/10665/89142/9789241209779_eng.pdf?sequence=1
26 Andrews NJ et al. (2014) Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 14, 839-46.
| Crossref | Google Scholar | PubMed |
27 Ryman J et al. (2022) Predicting vaccine effectiveness against invasive pneumococcal disease in children using immunogenicity data. NPJ Vaccines 7, 140.
| Crossref | Google Scholar | PubMed |
28 Jayasinghe S et al. (2018) Effectiveness of 7- and 13-valent pneumococcal conjugate vaccines in a schedule without a booster dose: a 10-year observational study. Clin Infect Dis 67, 367-74.
| Crossref | Google Scholar | PubMed |
29 Ryman J et al. (2024) Predicted serotype-specific effectiveness of pneumococcal conjugate vaccines V114 and PCV20 against invasive pneumococcal disease in children. Expert Rev Vaccines 23, 60-8.
| Crossref | Google Scholar | PubMed |
30 Domínguez Á et al. (2017) Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7–59 months. A matched case–control study. PLoS ONE 12, e0183191.
| Crossref | Google Scholar | PubMed |
31 Jayasinghe S et al. (2024) Assessing the impact of pneumococcal conjugate vaccine immunization schedule change from 3+0 to 2+1 in Australian children: a retrospective observational study. Clin Infect Dis ciae377.
| Crossref | Google Scholar | PubMed |
32 Voysey M et al. (2018) Serotype-specific correlates of protection for pneumococcal carriage: an analysis of immunity in 19 countries. Clin Infect Dis 66, 913-20.
| Crossref | Google Scholar | PubMed |
33 De Wals P (2024) PCV13, PCV15 or PCV20: which vaccine is best for children in terms of immunogenicity? Can Commun Dis Rep 50, 35-9.
| Crossref | Google Scholar | PubMed |
34 Blyth CC et al. (2020) A rationale for change: an increase in invasive pneumococcal disease in fully vaccinated children. Clin Infect Dis 70, 680-3.
| Crossref | Google Scholar | PubMed |
35 Bertran M et al. (2024) Invasive pneumococcal disease 3 years after introduction of a reduced 1+1 infant 13-valent pneumococcal conjugate vaccine immunisation schedule in England: a prospective national observational surveillance study. Lancet Infect Dis 24, 546-56.
| Crossref | Google Scholar | PubMed |
 Dr Sanjay Jayasinghe is a medical epidemiologist with extensive experience in research on pneumococcal disease and vaccines. He is a senior research fellow at the National Centre for Immunisation Research and Surveillance (NCIRS) and a senior lecturer (conjoint) in the Children’s Hospital at Westmead Clinical School of the Faculty of Medicine, The University of Sydney. He is also a National Health and Medical Research Council (NHMRC) emerging leadership fellow. He currently chairs the Enhanced Invasive Pneumococcal Disease (IPD) Surveillance (EIPDSWG) working group that oversees national IPD surveillance. |


