New antibiotics to combat One Health AMR
Abiodun David Ogunniyi A , Henrietta Venter
A , Henrietta Venter  B and Mark A. T. Blaskovich
B and Mark A. T. Blaskovich  C *
C *
A
B
C

Assoc. Prof. David Ogunniyi is a Fellow of the Australian Society for Microbiology and novel antibacterial pre-clinical experimental lead at the Australian Centre for Antimicrobial Resistance Ecology, The University of Adelaide. He currently focuses on developing bioluminescent mouse infection models for testing new antimicrobial drug classes. He has published 2 book chapters, >110 manuscripts and >25 scientific reports for Industry. At CEAStAR, Assoc. Prof Ogunniyi co-ordinates the novel antibacterial pre-clinical experimental strategy, industry engagement and training of HDRs and postdocs in bioluminescent mouse infection models. |

Assoc. Prof. Rietie Venter is the Head of Microbiology in Clinical and Health Sciences at the University of South Australia. After obtaining PhD degree at the University of Leeds, Rietie spent 12 years at the University of Cambridge doing research on multidrug transporters, first as a postdoc and later leading her research group as a Dorothy Hodgkin Royal Society Fellow. She joined UniSA in 2012 where she is heading an internationally acclaimed research group on understanding and combating AMR. |

Prof. Mark Blaskovich is an ‘antibiotic hunter’ and Director of Translation for the Institute for Molecular Bioscience at The University of Queensland, as well as Director of the ARC Industrial Transformation Training Centre, CEAStAR, and the antibiotic crowdsourcing initiative CO-ADD. A medicinal chemist with 15 years of industrial drug development experience, since 2010 he has been developing new antibiotics, non-antibiotic therapies and diagnostics to detect and treat resistant bacterial and fungal infections, including multiple industry collaborations focused on AMR. |
Abstract
The rise of antimicrobial resistance has been accompanied by a decline in the development of new antibiotics. In this article, we explore the current state of affairs and trends in both human- and animal-related antibiotic development activity, with distinct differences between the two sectors.
Keywords: AMR, antimicrobial drug development, antimicrobial pipeline, antimicrobial resistance, One Health.
Introduction
The discovery of antibiotics marked the beginning of a new era in modern medicine, but now, 1 century later, we are witnessing their demise. Antimicrobial use is the single biggest driver of resistance development. Antimicrobial consumption is increasing across the One Health space including the use of antibiotics as growth promotors in food producing animals and for prophylaxis in intensive farming in some countries.1 Unfortunately, the extensive use of antibiotics is combined with a decline in antibiotic research, contributing to a rapid rise in untreatable infections by antimicrobial-resistant bacteria. Infections caused by these resistant bacteria cost the medical and veterinary industries billions of dollars per year and are increasingly affecting global human and animal health, as well as food stability and production. In support, the World Health Organization (WHO) has declared that antimicrobial resistance (AMR) is one of the top ten global public health threats facing humanity.2
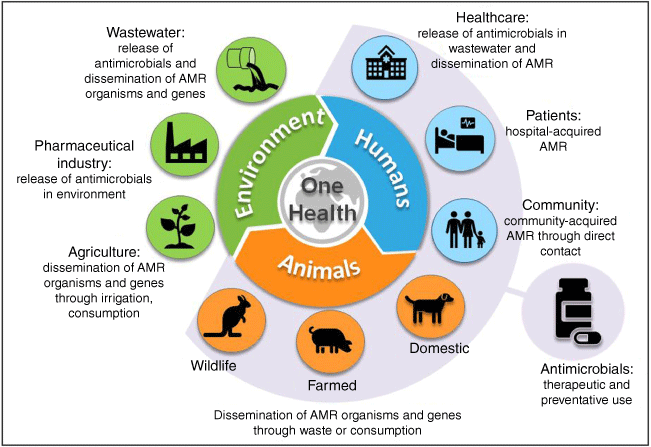
Several organisms on the WHO list of pathogens that are deemed to be the most critical priority in need of new drug development are opportunistic pathogens of relevance to both human- and veterinary health (e.g. carbapenem-resistant Acinetobacter baumannii, CRAB; carbapenem-resistant Pseudomonas aeruginosa; and carbapenem-resistant or third generation cephalosporin-resistant Enterobacterales), as well as agriculture and the environment, highlighting the One Health dimensions of this problem (Fig. 1).3 Drug discovery efforts are not able to keep pace with the increase in resistance, hence antibacterials with novel mechanisms of action, not subject to existing resistance mechanisms, are needed.
Human antibiotics
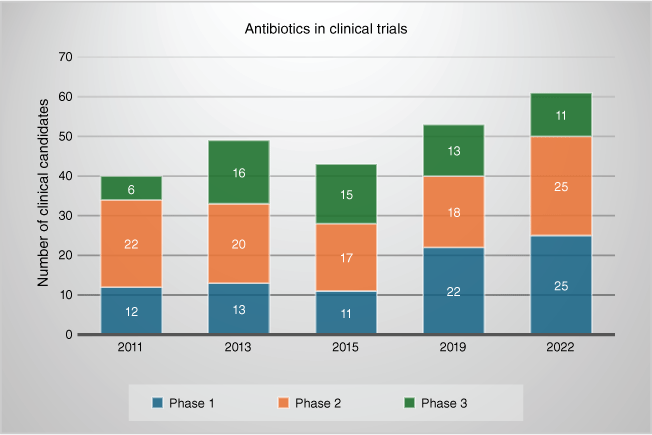
The number of antibiotics in the clinical pipeline has been regularly reviewed in a consistent format since 2011,4–8 providing an opportunity to assess changes in the pipeline over the past decade. The most recent review, along with others of both clinical9 and preclinical10 candidates, highlight that there are only ~60 in clinical development, substantially less than other therapeutic areas such as oncology, or even the specific disease of COVID-19. Critical analyses11,12 of these clinical candidates highlights the lack of novelty: most are derivatives of established antibiotic classes, albeit generally designed to overcome at least some of the known resistance mechanisms present at the time of their initial development. However, given that initial development is generally more than a decade before approval, resistance often continues to evolve beyond what the new drugs can cope with. New β-lactamase and β-lactamase inhibitor combinations dominate the most advanced candidates, reflected by the recent (European Medicines Agency in January 202413 and US Food and Drug Administration in February 202414) approval of Exblifep (cefepime and enmetazobactam). However, there is hope, both in an increase in the number of candidates entering clinical trials, and in innovation of new chemical scaffolds acting on novel targets. The former is highlighted by the number of candidates in the first stage of human testing (Phase 1), which has doubled from 12 in 2011 to 25 in 2022 (see Fig. 2).7 This positive trend potentially reflects the effect of initiatives designed to prime the pipeline, such as the antibiotic funding agency CARB-X.15 One example of the latter is the advanced preclinical development of a new macrocyclic peptide, zosurabalpin, that inhibits a lipopolysaccharide transporter essential for moving lipopolysaccharide to the bacterial surface, disrupting formation of the outer-membrane of Gram-negative bacteria.16–18 It is specific to A. baumannii, and retains activity against one of the most dangerous strains, CRAB. In the past, a species-selective antibiotic would not have been progressed due to the difficulty in rapidly diagnosing an infecting strain and the reduction in market size, but advances in diagnostics and antibiotic market incentives mean that a viable pathway may now be possible.
Another example of pathogen-specific antibiotic candidates with novel mechanisms of action, is inhibitors of FtsZ, a critical protein in the bacterial cell-division machinery. The 3-methoxybenzamides (‘benzamides’), exemplified by the early progenitor PC190723, are well-characterised FtsZ inhibitors that bind into the interdomain cleft of FtsZ to disrupt GTPase activity.19 An important trait of the benzamide class of FtsZ inhibitors is their ability to synergise with beta-lactam antibiotics, thereby reversing beta-lactam resistance at concentrations much below their own inhibitory concentrations. Significant efforts by Taxis Pharmaceuticals identified TXA709, a prodrug form of the benzamide PC190723. This orally active anti-MRSA agent, designed to be used as an adjunctive therapy in combination with ‘obsolete antibiotics’, successfully completed Phase I clinical trials, representing a major advancement in anti-FtsZ drug discovery. In 2021, the WHO named TXA709 as one of only two (out of twenty-six) antibacterials targeting WHO priority pathogens that meet all the WHO innovation criteria.20 New analogues of TXA709 in early development have been designed to target an extended spectrum of Gram-positive pathogens, including vancomycin resistant E. faecium.21
Animal antibiotics
The global magnitude and pace of resistance development to existing drug classes in veterinary medicine22 is exacerbated by the bond between humans and animals – particularly with companion animals,23 the limited range of registered drug classes, the risk of transfer of resistance genes through the food chain24 and the rapid development of pan-resistance in one of the most important animal pathogens, enterotoxigenic E. coli (ETEC).25 However, in Australia, antimicrobial stewardship measures are in place to prevent and manage AMR in food producing animals. The Expert Advisory Group on Antimicrobial Resistance (AGAR) tests and collects data on the level of AMR, whereas The Australian Pesticides and Veterinary Medicines for Agricultural and Veterinary Chemicals (APVMA) assesses the risks and registers the supply, distribution and sale of veterinary antibiotic products. Such coordinated information gathering and strict regulation of antimicrobial use together with ongoing surveillance by the Department of Agriculture, Fisheries and Forestry (DAFF) accounts for the low level of AMR in food animals in Australia.
Although there are effective steps to monitor, prevent and manage AMR in veterinary medicine in Australia, the available literature indicates that combination therapy, development of new drug classes, phage therapy and novel efflux pump inhibitors are critically important new approaches for treating multidrug-resistant Gram-negative veterinary pathogens in the global context.26 Given that the discovery and development of new antibiotics is very slow, drug combination can be a promising approach to extend the life of existing antibiotics in veterinary practice. Indeed, combination antibiotic therapy is now frequently used to treat severe Gram-negative (especially carbapenem-resistant Enterobacterales, CRE) infections, particularly in the absence of evidence-based treatment options.27 Nonetheless, the justification for the best approach would necessarily require consideration of several factors including time to drug discovery, cost and propensity to overcome rapid resistance development. Additionally, new veterinary antibiotics against intracellular veterinary pathogens are urgently needed. For example, brucellosis is a worldwide zoonotic disease caused by Brucella spp.28,29 and failure of regular treatment with the usual antimicrobials (quinolones, doxycycline, rifampicin, streptomycin and aminoglycoside alone or in combination) have been reported recently with emergence of AMR.30
Although the risk of AMR transmission of anthropogenic or zoonotic origin is low,31–35 the overarching guiding principles on antimicrobial use in veterinary practice will necessarily involve reduction in antimicrobial use, preserving the lifespan of currently registered animal antibiotics and finding new ways of managing microbial infections or by developing animal species-only drugs. Such activities will include regular AMR surveillance, avoiding the use of critically important antimicrobials (e.g. third generation cephalosporins), using safe alternatives to antibiotics (e.g. probiotics, vaccines, electrolysed oxidising water), development of new, rapid diagnostics to reduce unnecessary and inappropriate use of antimicrobials as well as registration of animal and pathogen-specific only drugs.
The recently established (2023) Australian Research Council (ARC) Industrial Transformation Training Centre, CEAStAR (the Centre for Environmental and Agricultural Solutions to Antimicrobial Resistance, see www.ceastar.org.au), was assembled to build Australia’s capability in addressing AMR by training the next generation of antimicrobial researchers. Led by two universities (The University of Queensland and The University of Adelaide), it focuses on industry-driven research projects that advance the development of potential commercial products provided by seven industry partners. These include new antimicrobials, antibiotic potentiators, non-antibiotic alternatives, and methods for environmental and microbiome monitoring. Notably, the awareness of the dangers of using antibiotics in agriculture is reflected by the types of projects: only one of the partners is developing what could be considered a traditional small-molecule direct acting antimicrobial, and this is a novel class, not used for humans.
Looking forward
For both human and veterinary antibiotics, a promising avenue for antimicrobial drug discovery is development of adjuvants that do not necessarily have antimicrobial action by themselves, but can, for example, act as resistance breakers to overcome resistance mechanisms and restore the activity of existing antibiotics.36 This strategy can increase the lifetime of currently used antibiotics as well as restore activity of obsolete antibiotics. Notably, nearly half of the 28 small molecules active against Gram-negative bacilli that are currently in clinical trials are new beta-lactamase inhibitors (the progenitor adjuvant class) combined with existing beta-lactam antibiotics.37 Other types of resistance breakers include efflux pump inhibitors. Drug efflux pumps are proteins that expel many different classes of antibiotics from bacterial cells thereby conferring multidrug resistance to these bacteria hence efflux pump inhibitors have the potential to restore the activity of multiple classes of antibiotics.38 Despite the obvious advantages of efflux pump inhibitors and efforts by many research groups including ours,39 efflux pump inhibitors are yet to progress to clinical trials.
Conclusion
The rapid rise in antimicrobial resistance development has coincided with a sharp decline in new antibiotic development due to the departure of most pharmaceutical companies from the field, a logical response to the poor return on investments. Concerns that the antimicrobial pipeline will run dry portend a future where there will be few or no treatment options for infections caused by resistant pathogens. Concerted international efforts funded by public and private investment, such as the AMR Action Fund, CARB-X and GARDP, have started to pay off and the number of antimicrobials in the clinical pipeline has slowly increased over the last few years. Encouragingly, the pipeline also now contains an increasing number of antimicrobials against new targets or with novel mechanisms of action.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
The ARC Industrial Transformation Training Centre, CEAStAR, is funded by its partners and the Australian Government (IC220100050).
References
1 O’Neill J (2016) Tackling drug-resistant infections globally: final report and recommendations. The Wellcome Trust and Her Majesty’s Government, London, UK. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf
2 World Health Organization (2020) 10 global health issues to track in 2021. 24 December 2020. WHO. www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021
3 Willyard C (2017) The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15.
| Crossref | Google Scholar | PubMed |
4 Butler MS et al. (2013) Antibiotics in the clinical pipeline in 2013. J Antibiot 66, 571-591.
| Crossref | Google Scholar | PubMed |
5 Butler MS et al. (2017) Antibiotics in the clinical pipeline at the end of 2015. J Antibiot 70, 3-24.
| Crossref | Google Scholar | PubMed |
6 Butler MS, Cooper MA (2011) Antibiotics in the clinical pipeline in 2011. J Antibiot 64, 413-425.
| Crossref | Google Scholar | PubMed |
7 Butler MS et al. (2023) Antibiotics in the clinical pipeline as of December 2022. J Antibiot 76, 431-473.
| Crossref | Google Scholar | PubMed |
8 Butler MS, Paterson DL (2020) Antibiotics in the clinical pipeline in October 2019. J Antibiot 73, 329-364.
| Crossref | Google Scholar | PubMed |
9 Butler MS et al. (2022) Analysis of the clinical pipeline of treatments for drug-resistant bacterial infections: despite progress, more action is needed. Antimicrob Agents Chemother 66, e0199121.
| Crossref | Google Scholar | PubMed |
10 Theuretzbacher U et al. (2020) The global preclinical antibacterial pipeline. Nat Rev Microbiol 18, 275-285.
| Crossref | Google Scholar | PubMed |
11 Theuretzbacher U et al. (2020) Critical analysis of antibacterial agents in clinical development. Nat Rev Microbiol 18, 286-298.
| Crossref | Google Scholar | PubMed |
12 Theuretzbacher U (2023) Evaluating the innovative potential of the global antibacterial pipeline. Clin Microbiol Infect
| Crossref | Google Scholar | PubMed |
13 European Medicines Agency (2024) Exblifep: cefepime / enmetazobactam. EMA, Amsterdam, Netherlands. https://www.ema.europa.eu/en/medicines/human/EPAR/exblifep#:~:text=The%20European%20Medicines%20Agency%20decided,for%20use%20in%20the%20EU (last updated 22 March 2024)
14 Stewart J (2024) Exblifep FDA approval history. Drugs.com. https://www.drugs.com/history/exblifep.html (last updated 12 March 2024)
15 Alm RA, Gallant K (2020) Innovation in antimicrobial resistance: the CARB-X perspective. ACS Infect Dis 6, 1317-1322.
| Crossref | Google Scholar | PubMed |
16 Pahil KS et al. (2024) A new antibiotic traps lipopolysaccharide in its intermembrane transporter. Nature 625, 572-577.
| Crossref | Google Scholar | PubMed |
17 Zampaloni C et al. (2024) A novel antibiotic class targeting the lipopolysaccharide transporter. Nature 625, 566-571.
| Crossref | Google Scholar | PubMed |
18 Gugger MK, Hergenrother PJ (2024) A new type of antibiotic targets a drug-resistant bacterium. Nature 625, 451-452.
| Crossref | Google Scholar | PubMed |
19 Casiraghi A et al. (2020) Targeting bacterial cell division: a binding site-centered approach to the most promising inhibitors of the essential protein FtsZ. Antibiotics 9, 69.
| Crossref | Google Scholar | PubMed |
20 Antimicrobial Resistance Division, Global Coordination and Partnership (2022) Antibacterial agents in clinical and preclinical development: an overview and analysis. Technical document, 27 May 2022. World Health Organization. www.who.int/publications/i/item/9789240047655
21 Chai WC et al. (2020) Antimicrobial action and reversal of resistance in MRSA by difluorobenzamide derivatives targeted at FtsZ. Antibiotics 9, 873.
| Crossref | Google Scholar | PubMed |
22 Palma E et al. (2020) Antimicrobial resistance in veterinary medicine: an overview. Int J Mol Sci 21, 1914.
| Crossref | Google Scholar | PubMed |
23 Walther B et al. (2017) Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet Microbiol 200, 71-78.
| Crossref | Google Scholar | PubMed |
24 Trott D (2013) β-lactam resistance in Gram-negative pathogens isolated from animals. Curr Pharm Des 19, 239-249.
| Google Scholar | PubMed |
25 Abraham S et al. (2014) Phylogenetic and molecular insights into the evolution of multidrug-resistant porcine enterotoxigenic Escherichia coli in Australia. Int J Antimicrob Agents 44, 105-111.
| Crossref | Google Scholar | PubMed |
26 Bhandari V, Suresh A (2022) Next-generation approaches needed to tackle antimicrobial resistance for the development of novel therapies against the deadly pathogens. Front Pharmacol 13, 838092.
| Crossref | Google Scholar | PubMed |
27 Tängdén T (2014) Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci 119, 149-153.
| Crossref | Google Scholar | PubMed |
28 Johansen TB et al. (2018) Whole-genome sequencing and antimicrobial resistance in Brucella melitensis from a Norwegian perspective. Sci Rep 8, 8538.
| Crossref | Google Scholar | PubMed |
29 Głowacka P et al. (2018) Brucella – virulence factors, pathogenesis and treatment. Pol J Microbiol 67, 151-161.
| Crossref | Google Scholar | PubMed |
30 Khan AU et al. (2019) Identification, genotyping and antimicrobial susceptibility testing of Brucella spp. isolated from livestock in Egypt. Microorganisms 7, 603.
| Crossref | Google Scholar | PubMed |
31 Swift BMC et al. (2019) Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci Total Environ 649, 12-20.
| Crossref | Google Scholar | PubMed |
32 Lee T et al. (2021) Antimicrobial resistance in porcine enterococci in Australia and the ramifications for human health. Appl Environ Microbiol 87, e03037-20.
| Crossref | Google Scholar | PubMed |
33 Lee T et al. (2020) A three-year whole genome sequencing perspective of Enterococcus faecium sepsis in Australia. PLoS One 15, e0228781.
| Crossref | Google Scholar | PubMed |
34 Messele YE et al. (2022) Phenotypic and genotypic analysis of antimicrobial resistance in Escherichia coli recovered from feedlot beef cattle in Australia. Animals (Basel) 12, 2256.
| Crossref | Google Scholar | PubMed |
35 Messele YE et al. (2023) Phylogeny, virulence, and antimicrobial resistance gene profiles of Enterococcus faecium isolated from Australian feedlot cattle and their significance to public and environmental health. Antibiotics 12, 1122.
| Crossref | Google Scholar | PubMed |
36 Venter H (2019) Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci Rep 39, BSR20180474.
| Crossref | Google Scholar | PubMed |
37 Paterson DL (2024) Antibacterial agents active against Gram negative bacilli in phase I, II, or III clinical trials. Expert Opin Investig Drugs 33, 371-387.
| Crossref | Google Scholar | PubMed |
38 Venter H et al. (2015) RND-type drug efflux pumps from Gram-negative bacteria: molecular mechanism and inhibition. Front Microbiol 6, 377.
| Crossref | Google Scholar | PubMed |
39 Pisoni LA et al. (2023) Combined structure- and ligand-based approach for the identification of inhibitors of AcrAB-TolC in Escherichia coli. ACS Infect Dis 9(12), 2504-2522.
| Crossref | Google Scholar | PubMed |
 Assoc. Prof. David Ogunniyi is a Fellow of the Australian Society for Microbiology and novel antibacterial pre-clinical experimental lead at the Australian Centre for Antimicrobial Resistance Ecology, The University of Adelaide. He currently focuses on developing bioluminescent mouse infection models for testing new antimicrobial drug classes. He has published 2 book chapters, >110 manuscripts and >25 scientific reports for Industry. At CEAStAR, Assoc. Prof Ogunniyi co-ordinates the novel antibacterial pre-clinical experimental strategy, industry engagement and training of HDRs and postdocs in bioluminescent mouse infection models. |
 Assoc. Prof. Rietie Venter is the Head of Microbiology in Clinical and Health Sciences at the University of South Australia. After obtaining PhD degree at the University of Leeds, Rietie spent 12 years at the University of Cambridge doing research on multidrug transporters, first as a postdoc and later leading her research group as a Dorothy Hodgkin Royal Society Fellow. She joined UniSA in 2012 where she is heading an internationally acclaimed research group on understanding and combating AMR. |
 Prof. Mark Blaskovich is an ‘antibiotic hunter’ and Director of Translation for the Institute for Molecular Bioscience at The University of Queensland, as well as Director of the ARC Industrial Transformation Training Centre, CEAStAR, and the antibiotic crowdsourcing initiative CO-ADD. A medicinal chemist with 15 years of industrial drug development experience, since 2010 he has been developing new antibiotics, non-antibiotic therapies and diagnostics to detect and treat resistant bacterial and fungal infections, including multiple industry collaborations focused on AMR. |