Biofilms and intracellular infection in otitis media
Ruth Thornton A B * , Elke Seppanen B and Sharon Clark B CA Centre for Child Health Research, University of Western Australia, Perth, WA, Australia.
B Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, WA, Australia.
C School of Medicine, University of Western Australia, Perth, WA, Australia.

Dr Ruth Thornton is a Passe and Williams Foundation Mid-Career Research Fellow, Senior Research Fellow in the Centre for Child Health Research at the University of Western Australia and an Honorary Research Fellow at the Telethon Kids Institute. She co-leads the Bacterial Respiratory Infectious Disease Group. Dr Thornton is a translational microbiologist and immunologist with considerable experience in host–pathogen interactions. She currently leads the ATOMIC ears clinical trial at Perth Children’s Hospital, investigating the safety and effectiveness of using an anti-biofilm agent to treat recurrent and chronic otitis media. |

Dr Elke Seppanen is Program Manager of the Bacterial Respiratory Disease Group, Telethon Kids Institute. Dr Seppanen has extensive experience in immunology, cell and molecular biology and clinical lab coordination and supports the team’s vision to reduce the global burden of ear and lung disease through discovery, translation and collaboration. |

Ms Sharon Clark is a PhD student in the Bacterial Respiratory Disease Group, Wesfarmers Centre of Vaccine and Infectious Diseases, Telethon Kids Institute and University of Western Australia. Ms Clark’s research focusses on understanding the immunology and biofilm biology of chronic and recurrent otitis media, to find better ways to treat and prevent otitis media in children. |
Microbiology Australia 44(2) 88-91 https://doi.org/10.1071/MA23025
Submitted: 21 February 2023 Accepted: 1 May 2023 Published: 12 May 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Otitis media (OM), middle ear infection, represents a significant burden on children, their families, and the healthcare system. OM is the major cause of hearing loss in children and if left untreated in children who suffer chronic and recurrent forms of OM, this disease can have serious life-long sequelae. Chronic and recurrent OM are recalcitrant to current therapies due to the formation of biofilms and intracellular biofilm pods by otopathogens on the middle ear mucosa and within the middle ear fluid. These pathogens actively hijack the children’s own immune response and persist in the neutrophil extracellular trap-derived DNA in the middle ear. Children who suffer from chronic and recurrent forms of OM have also been shown to have reduced antibody levels to important anti-biofilm protein antigens. These both represent potential targets for treatment or prevention and are under investigation.
Keywords: biofilms, glue ear, intracellular infection, neutrophil extracellular traps, otitis media.
Otitis media in Australia
Globally, there are estimated to be more than 709 million cases of acute OM annually, with more than 51% occurring in children under 5 years of age.1 In Australia alone, OM affects up to 365 000 children each year, representing a massive burden on families and the healthcare system.2 The conductive hearing loss associated with OM can cause difficulties in language development, learning behaviour and has long term impacts on a child’s life course.3 Although most children will experience at least one episode of OM by their second birthday, up to one-third of children suffer from recurrent acute OM (rAOM), which is characterised by the presence of a reddened bulging ear drum, ear pain and fever (defined as having three episodes of AOM in 6 months or four or more in 12 months), or chronic otitis media with effusion (cOME also known as ‘glue ear’), which is characterised by the presence of bulging ear drum without inflammation, and fluid in the middle ear in the absence of systemic symptoms (defined as the presence of fluid for 3 months or longer).4 Children with cOME are usually unresponsive to medical treatment, and those with rAOM often experience repeat infection within a month of antibiotic use.5 Although ~33% of non-Indigenous children develop rAOM or cOME, 50–90% of all Australian First Nations children will have developed recurrent or chronic OM by 12 months old and this often persists to school age.6,7 Australian Aboriginal children suffer the highest prevalence of chronic OM in the world including chronic suppurative otitis media (CSOM), which is defined as the presence of otorrhoea (ear discharge) through an ear drum perforation for 2–6 weeks or more.4 CSOM is the most severe form of OM and the rates among Australian Aboriginal children are the highest globally (15%), being declared by the WHO as a ‘public health emergency requiring urgent attention’.8
Current treatments for OM
Pain and fever management (i.e. paracetamol), and antibiotics are the most common treatments for OM.3 Pneumococcal conjugate vaccines have also reduced OM from pneumococcal serotypes covered in the vaccine; however, their overall effect on OM has been limited with an increased incidence of disease from non-vaccine serotypes and other pathogens, such as non-typeable Haemophilus influenzae (NTHi).9 Analgesics, antibiotics and currently available vaccines have no effect on the chronicity or recurrence of OM and the final treatment is usually surgical through grommet insertion. Although grommets are a moderately effective short-term intervention,10 up to 43% of children undergoing grommet surgery will require repeat surgeries for the recurrence of cOME or rAOM following extrusion or blockage of the grommet.11 Importantly, the current treatments available for OM do not target the underlying mechanisms of microbial persistence and thus have limited efficacy. These treatments need to be improved to target persistence mechanisms to be more effective, minimise sequelae and reduce hospital wait-list times.
Microbiology of chronic OM
Although preceding viral upper respiratory tract infections are associated with the development of AOM in children, most chronic disease is caused by bacterial infection with NTHi, Streptococcus pneumoniae and Moraxella catarrhalis.12 NTHi is now the predominant otopathogen isolated from the nasopharynx and middle ear of children with OM globally.9 Colonisation of the nasopharynx with at least one of these otopathogens is a prerequisite for developing OM. Although transition from colonisation to disease is not well understood, increased density of otopathogens in the nasopharynx is seen to correlate with OM onset.13–15
Biofilms in otitis media
Historically, the different presentations of OM in children, particularly cOME and rAOM, were believed to be due to different aetiologies. Many effusions from children with cOME were sterile on culture, which suggest to clinicians that this condition was related to inflammation and allergy. With the development of molecular based techniques, researchers were able to demonstrate this condition was due to the presence of non-culturable otopathogenic bacteria (likely persisting in biofilms).
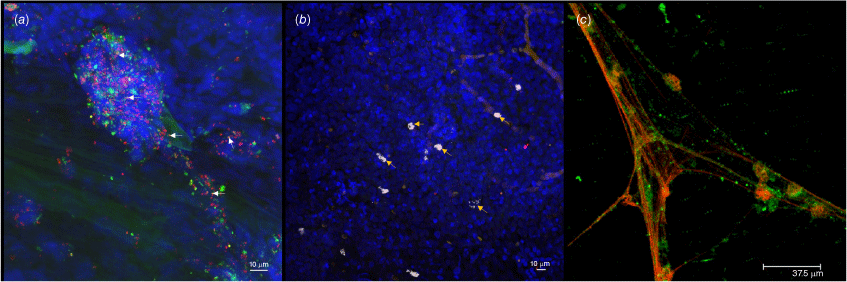
Biofilms and intracellular infection play a major role in chronic and recurrent disease processes and are implicated in more than 80% of all human microbial infections. The presence of biofilm clinically is characterised by an inability to culture the bacteria using standard culture methods, but by being able to determine their presence using molecular methodologies. Biofilms are defined as clusters of bacteria embedded in a polymeric matrix, which have enhanced resistance to both antimicrobials and host defences compared to their planktonic state.16 In 2004, our team demonstrated for the first time that the otopathogens were present in biofilms on the middle ear mucosa of children with CSOM.17 Since then, we and others have demonstrated both multi-species (polymicrobial) bacterial biofilms and single-species intracellular biofilms on the middle ear mucosa and within the middle ear effusion of children with rAOM, cOME and CSOM (Fig. 1).17–21
Biofilms, both extracellular and intracellular, are present on the middle ear mucosa and in the middle ear effusion of children undergoing surgery for recurrent acute otitis media. Maximum projection images of (a) a polymicrobial biofilm containing S. pneumoniae (green), NTHi (orange, indicated by white arrows) and other non-identified species (red) in extracellular DNA (blue cloud) on the middle ear mucosa (blue nuclei), (b) single species intracellular NTHi biofilm pods (light pink indicated by yellow arrows) on the middle ear mucosa (blue nuclei), (c) live bacteria (green) both singularly and in biofilms associated with neutrophil extracellular trap-derived DNA (red) in the middle ear effusion.
Targeting biofilms in OM
Clinically, biofilms prove difficult to treat particularly when associated with tissues that cannot be removed or physically debrided. Therefore, efforts need to be targeted towards preventing initial formation (or re-establishment) and destabilising biofilms present through physical or immune-mediated means. When bacteria are forced into their planktonic phase, they are more susceptible to antimicrobials and host immune mechanisms than when in their pre-biofilm state.22
Destabilisation of biofilms by targeting structural components
Biofilms require a DNA component, which may be bacterial or host derived, to maintain their infectious reservoir and provide increased resistance to host defences and antimicrobials.22 The middle ear effusion we see in OM largely consists of host-derived DNA from neutrophil extracellular traps (NETs) (Fig. 1).21,23 NETs are an active immune mechanism where neutrophils release DNA embedded with antimicrobial peptides and enzymes to confine, bind and kill bacteria.24 In OM, otopathogenic bacteria have been demonstrated to induce and hijack NETs to form a DNA scaffold in which they can reside, proliferate and evade antibacterial attack.21,25,26 In vitro, the application of a clinically used recombinant DNAse, Dornase alfa, to middle ear effusion from children with OM resulted in complete degradation of NET-derived DNA.21 This observation led to a Phase 1B, clinical trial (CTN#2011/0635) that demonstrated that Dornase alfa was safe and well tolerated (R. B. Thornton, S. Jeffares, E. Seppanen et al., unpubl. data) and subsequently a Phase 2B, extended-dosing randomised control trial (ACTRN12619001306101) is currently underway.
Immune mediated prevention or treatment of OM biofilms
Traditionally, vaccines have been developed targeting acute or systemic infections with animal sepsis model testing performed to demonstrate potential effectiveness in humans. Candidate vaccine identification is often based on antigen conservation across strains and protection against acute, often fatal diseases. Historically, for conditions such as OM, development or testing of these antigens rarely if ever considers the chronicity of infection, often due to a lack of suitable animal models or understanding of the microbial persistence mechanisms being targeted.
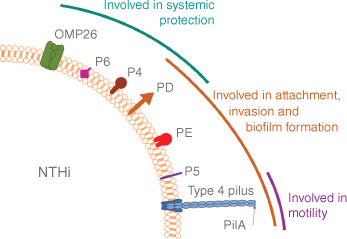
Our early data assessed whether there were deficiencies in antibody responses to candidate vaccine proteins for the common OM pathogens S. pneumoniae and NTHi, in otitis-prone children compared to non-otitis-prone children. These proteins largely consisted of known candidate antigens to protect against invasive disease and included pneumolysin, pneumococcal surface proteins A 1 and 2, and choline binding protein for S. pneumoniae, and outer membrane proteins 4, 6 and 26, and protein D from NTHi. Using these, we demonstrated that overall there were no differences in antibody titres between otitis-prone and non-otitis-prone children except for NTHi protein D, which facilitates adhesion and is included as a carrier protein in the 10 valent pneumococcal conjugate vaccine and was subsequently shown to have an effect on OM in children.27,28 Subsequently, we assessed responses in these same children to other proteins that have been demonstrated to play important roles in adherence, biofilm formation and maintenance, PilA (Fig. 2) and a chimeric version of PilA linked to outer membrane protein 5, ChimV4.29–31 Assessing natural responses in children to these highly conserved, but clinically relevant proteins, we saw that otitis-prone children, and in particular Australian Aboriginal otitis-prone children, had reduced antibody responses when compared to non-otitis-prone children.28,32 Together these data highlight the need to better understand the differences in protein expression and develop appropriate disease models to identify suitable vaccine candidates to both prevent and resolve chronic diseases such as OM. These future strategies also need to take into account the polymicrobial nature of this disease.
Intracellular biofilm pods
Otopathogens are able to form single species biofilm pods in the middle ear mucosal cells of children with OM.18–21 Intracellular biofilm pods enable the added protection of an intracellular niche that is unable to be accessed by many antimicrobials or host immune mechanisms allowing these bacteria to persist to re-seed infection at a later time. Understanding this phenomenon and developing appropriate treatments for these biofilms require further research.
Conclusions
Chronic and recurrent OM are biofilm-related conditions that result in major impacts on children, their families, the healthcare system and have the potential to affect a child’s entire life course. Currently no effective treatments or preventative therapies exist; however, several therapeutics are currently being developed and evaluated.
References
[1] Monasta, L et al. (2012) Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 7, e36226.| Burden of disease caused by otitis media: systematic review and global estimates.Crossref | GoogleScholarGoogle Scholar |
[2] Access Economics (2008) The cost burden of OM in Australia. Access Economics.
[3] Schilder, AGM et al. (2016) Otitis media. Nat Rev Dis Primers 2, 16063.
| Otitis media.Crossref | GoogleScholarGoogle Scholar |
[4] Leach, AJ et al. (2021) Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations. Med J Aust 214, 228–233.
| Otitis media guidelines for Australian Aboriginal and Torres Strait Islander children: summary of recommendations.Crossref | GoogleScholarGoogle Scholar |
[5] Vlastarakos, PV et al. (2007) Biofilms in ear, nose, and throat infections: how important are they? Laryngoscope 117, 668–673.
| Biofilms in ear, nose, and throat infections: how important are they?Crossref | GoogleScholarGoogle Scholar |
[6] Leach, AJ et al. (2014) Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules. BMC Pediatr 14, 200.
| Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules.Crossref | GoogleScholarGoogle Scholar |
[7] Richmond, HJ et al. (2023) Early onset of otitis media is a strong predictor of subsequent disease in urban Aboriginal infants: Djaalinj Waakinj cohort study. J Paediatr Child Health 59, 729–734.
| Early onset of otitis media is a strong predictor of subsequent disease in urban Aboriginal infants: Djaalinj Waakinj cohort study.Crossref | GoogleScholarGoogle Scholar |
[8] Mackenzie, GA et al. (2009) Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination. BMC Pediatr 9, 14.
| Pneumococcal vaccination and otitis media in Australian Aboriginal infants: comparison of two birth cohorts before and after introduction of vaccination.Crossref | GoogleScholarGoogle Scholar |
[9] Ngo, CC et al. (2016) Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic Review. PLoS One 11, e0150949.
| Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic Review.Crossref | GoogleScholarGoogle Scholar |
[10] Venekamp, RP et al. (2018) Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database Syst Rev 5, CD012017.
| Grommets (ventilation tubes) for recurrent acute otitis media in children.Crossref | GoogleScholarGoogle Scholar |
[11] Seppanen, EJ et al. (2020) Bacterial reservoirs in the middle ear of otitis-prone children are associated with repeat ventilation tube insertion. Pediatr Infect Dis J 39, 91–96.
| Bacterial reservoirs in the middle ear of otitis-prone children are associated with repeat ventilation tube insertion.Crossref | GoogleScholarGoogle Scholar |
[12] Chonmaitree, T et al. (2008) Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis 46, 815–823.
| Viral upper respiratory tract infection and otitis media complication in young children.Crossref | GoogleScholarGoogle Scholar |
[13] Leach, AJ et al. (1994) Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr Infect Dis J 13, 983–989.
| Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants.Crossref | GoogleScholarGoogle Scholar |
[14] Smith-Vaughan, H et al. (2006) Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 6, 10.
| Measuring nasal bacterial load and its association with otitis media.Crossref | GoogleScholarGoogle Scholar |
[15] Sun, W et al. (2012) Association between early bacterial carriage and otitis media in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia: a cohort study. BMC Infect Dis 12, 366.
| Association between early bacterial carriage and otitis media in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia: a cohort study.Crossref | GoogleScholarGoogle Scholar |
[16] Burmølle, M et al. (2010) Biofilms in chronic infections – a matter of opportunity – monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 59, 324–336.
| Biofilms in chronic infections – a matter of opportunity – monospecies biofilms in multispecies infections.Crossref | GoogleScholarGoogle Scholar |
[17] Coates H (2004) Chronic suppurative OM without cholesteatoma. In Advanced Therapy of OM. pp. 299–305. B.C. Decker Incorporated.
[18] Coates, H et al. (2008) The role of chronic infection in children with otitis media with effusion: evidence for intracellular persistence of bacteria. Otolaryngol Head Neck Surg 138, 778–781.
| The role of chronic infection in children with otitis media with effusion: evidence for intracellular persistence of bacteria.Crossref | GoogleScholarGoogle Scholar |
[19] Hall-Stoodley, L et al. (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296, 202–211.
| Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media.Crossref | GoogleScholarGoogle Scholar |
[20] Thornton, RB et al. (2011) Multi-species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr 11, 94.
| Multi-species bacterial biofilm and intracellular infection in otitis media.Crossref | GoogleScholarGoogle Scholar |
[21] Thornton, RB et al. (2013) Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media – a potential treatment target. PLoS One 8, e53837.
| Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media – a potential treatment target.Crossref | GoogleScholarGoogle Scholar |
[22] Jones, EA et al. (2013) Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun 5, 24–38.
| Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity.Crossref | GoogleScholarGoogle Scholar |
[23] Val, S et al. (2016) Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media. PLoS One 11, e0152865.
| Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media.Crossref | GoogleScholarGoogle Scholar |
[24] Wartha, F et al. (2007) Neutrophil extracellular traps: casting the NET over pathogenesis. Curr Opin Microbiol 10, 52–56.
| Neutrophil extracellular traps: casting the NET over pathogenesis.Crossref | GoogleScholarGoogle Scholar |
[25] Short, KR et al. (2014) Antibodies mediate formation of neutrophil extracellular traps in the middle ear and facilitate secondary pneumococcal otitis media. Infect Immun 82, 364–370.
| Antibodies mediate formation of neutrophil extracellular traps in the middle ear and facilitate secondary pneumococcal otitis media.Crossref | GoogleScholarGoogle Scholar |
[26] Thammavongsa, V et al. (2013) Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342, 863–866.
| Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death.Crossref | GoogleScholarGoogle Scholar |
[27] Thornton, RB et al. (2017) No evidence for impaired humoral immunity to pneumococcal proteins in Australian Aboriginal children with otitis media. Int J Pediatr Otorhinolaryngol 92, 119–125.
| No evidence for impaired humoral immunity to pneumococcal proteins in Australian Aboriginal children with otitis media.Crossref | GoogleScholarGoogle Scholar |
[28] Thornton, RB et al. (2017) Australian Aboriginal children with otitis media have reduced antibody titers to specific nontypeable Haemophilus influenzae vaccine antigens. Clin Vaccine Immunol 24, e00556-16.
| Australian Aboriginal children with otitis media have reduced antibody titers to specific nontypeable Haemophilus influenzae vaccine antigens.Crossref | GoogleScholarGoogle Scholar |
[29] Mokrzan, EM et al. (2020) Nontypeable Haemophilus influenzae newly released (NRel) from biofilms by antibody-mediated dispersal versus antibody-mediated disruption are phenotypically distinct. Biofilm 2, 100039.
| Nontypeable Haemophilus influenzae newly released (NRel) from biofilms by antibody-mediated dispersal versus antibody-mediated disruption are phenotypically distinct.Crossref | GoogleScholarGoogle Scholar |
[30] Mokrzan, EM et al. (2019) Expression of the nontypeable Haemophilus influenzae type IV pilus is stimulated by coculture with host respiratory tract epithelial cells. Infect Immun 87, e00704-19.
| Expression of the nontypeable Haemophilus influenzae type IV pilus is stimulated by coculture with host respiratory tract epithelial cells.Crossref | GoogleScholarGoogle Scholar |
[31] Novotny, LA and Bakaletz, LO (2020) Transcutaneous immunization with a nontypeable Haemophilus influenzae dual adhesin-directed immunogen induces durable and boostable immunity. Vaccine 38, 2378–2386.
| Transcutaneous immunization with a nontypeable Haemophilus influenzae dual adhesin-directed immunogen induces durable and boostable immunity.Crossref | GoogleScholarGoogle Scholar |
[32] Clark, SL et al. (2022) Australian Aboriginal otitis-prone children produce high-quality serum IgG to putative nontypeable Haemophilus influenzae vaccine antigens at lower titres compared to non-Aboriginal children. Front Cell Infect Microbiol 12, 767083.
| Australian Aboriginal otitis-prone children produce high-quality serum IgG to putative nontypeable Haemophilus influenzae vaccine antigens at lower titres compared to non-Aboriginal children.Crossref | GoogleScholarGoogle Scholar |