Impact of photodynamic inactivation on microbial safety in foods
Maral Seididamyeh A B and Yasmina Sultanbawa A B *A Centre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovations, The University of Queensland, St Lucia, Qld 4072, Australia.
B ARC Industrial Transformation Training Centre for Uniquely Australian Foods, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Indooroopilly, Qld 4068, Australia.

Maral Seididamyeh has studied curcumin-based photosensitisation for inactivating Botrytis cinerea spores, the cause of grey mould in strawberry fruits, during her PhD at the University of Queensland. She is working as a Research Officer in Professor Sultanbawa’s lab on projects related to rapid non-destructive technologies to assess the provenance and authenticity of food products as well as to detect the chemical residues in food products. |

Yasmina Sultanbawa is a Professorial Research Fellow at the Queensland Alliance for Agriculture and Food Innovation and the Director of the ARC Training Centre for Uniquely Australian Foods at the University of Queensland. Some of her research is focussed on food safety and functional ingredients as natural additives in food products or packaging material to enhance shelf life as well as the nutritional value of foods. |
Microbiology Australia 43(2) 71-74 https://doi.org/10.1071/MA22017
Submitted: 19 March 2022 Accepted: 25 April 2022 Published: 17 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Food-borne diseases caused by contaminated food products continue to pose a threat to public health, as well as causing major economic losses and a negative impact on companies’ reputation among consumers. In the food industry, inactivation of pathogenic and spoilage microorganisms is conventionally performed through thermal- and chemical-based techniques, which can affect the nutritional and sensorial quality of food. Furthermore, the emergence of microbial resistance to conventional decontamination techniques has drawn increased attention to finding an alternative and sustainable approach for similar or higher decontamination efficiency. Over the past decade, photodynamic treatment has been introduced for inactivating food spoilage and pathogenic microorganisms as a promising cost-effective, chemical-free, environmentally friendly technique with no reports on toxic residues and microbial resistance. The application and efficiency of photodynamic treatment in various food matrices against a broad range of microorganisms demonstrates the potential of using this technology in the food industry.
Keywords: antimicrobial treatment, curcumin, food preservation, food safety, green technology, photodynamic, photosensitiser, reactive oxygen species.
Introduction
The key element in achieving good health and sustaining life is access to nutritious and safe food. According to the World Health Organization, over 200 different diseases with severity ranging from diarrhoea to cancers can be caused by unsafe food consumption, which contains harmful microorganisms or chemical substances.1 Food safety is generally influenced by the growing world population, climate change, and globalisation of food trade. Therefore, it contributes greatly to global food and nutrition security, as well as to national economies.
Food industry traditionally uses conventional thermal-based processing such as dry-heating and steam-heating to reduce the microbial contamination of foods caused by vegetative cells, spores, and biofilms. However, this practice sometimes suffers from undesirable impacts on flavour, nutritional composition, and texture of treated foods.2 Various non-thermal processing technologies such as ultraviolet light, irradiation, ultrasound, ozonation, cold plasma, pulsed electric field, and high hydrostatic pressure have been introduced to the food industry to reduce the microbial load while retaining the natural colour, flavour and nutrition. Nevertheless, the wide application of some of these non-thermal decontamination technologies is limited by strict processing conditions, expensive equipment, high energy consumption, and the emergence of microbial resistance.3
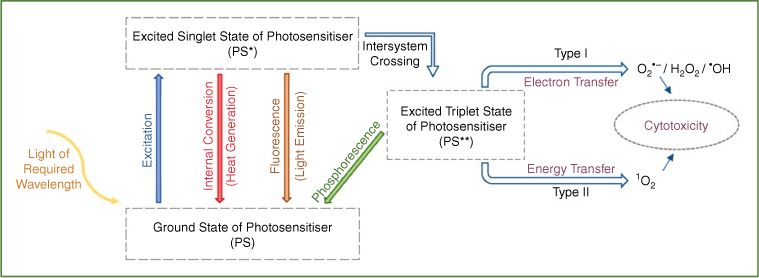
One of the recently introduced non-thermal decontamination technologies to the food industry is photodynamic treatment, which is also known as photosensitisation. For several decades, photodynamic treatment has been investigated and used for medical and dental purposes to treat tumour/cancerous cells and as antibacterial/antibiofilm treatment. Reactive oxygen species (ROS) are produced during the photodynamic treatment, which only requires the presence of oxygen, a photosensitiser, and light (at photosensitiser’s λmax). Photosensitisers become excited on illumination and generate cytotoxic ROS through subsequent de-excitation and collision with the surrounding oxygen molecules4 (Fig. 1). The produced ROS exhibit a multi-target attack towards different intracellular components of microorganisms present in the food, such as their proteins, lipids, and nucleic acids, resulting in cellular death. Therefore, because of direct and non-selective oxidative damage to essential biomolecules required for cell integrity and function, there is also a low probability of the emergence of microbial resistance using photodynamic treatment.5 Another advantage of this treatment is being chemical free that is in line with the growing demand for ‘clean label’ food products.
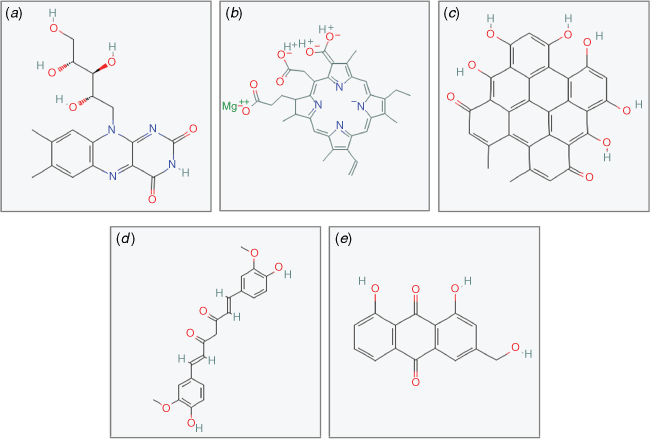
Generally, photosensitisers can be endogenous such as porphyrins, which already exist within some fungal and bacterial cells, or exogenous such as curcumin, chlorophyll, and riboflavin isolated from plant material (Fig. 2). However, the poor water solubility of some potent photosensitisers such as curcumin can limit the wide application of the treatment on different food products. This can be overcome with the aid of encapsulation technology using hydrocolloids isolated from natural resources. This results in better bonding of the photosensitiser with microbial cells and better accumulation in the vicinity of target cells and therefore an enhanced photodynamic antimicrobial effect. Furthermore, another feature of this photodynamic treatment is its low energy requirement and no toxic by-products or residue generation,5 thus making it a safe and eco-compatible technology.
|
Photodynamic application for food decontamination
In 2004, Lukšien and colleagues explored the application of innovative and promising photodynamic inactivation for microbial food safety. The authors reported a complete in vitro photoinactivation of common food crop spoilage fungi, namely Rhizopus oryzae, Aspergillus flavus, Trichothecium roseum, and Fusarium avenaceum, using hematoporphyrin dimethyl ether as a photosensitiser.8 Further studies have since shown the efficiency of photodynamic treatment in inactivating a wide variety of food spoilage and pathogenic microorganisms (i.e. vegetative cells, spores, biofilms) such as Listeria monocytogenes, Salmonella enterica,9 Vibrio parahaemolyticus,10 Enterococcus faecalis,11 Pseudomonas fluorescens, Shigella flexneri,12 Staphylococcus aureus,13 Bacillus cereus,14 Candida albicans, Aspergillus niger, Penicillium griseofulvum, Fusarium oxysporum, Zygosaccharomyces bailii,15 Aspergillus flavus,16 and Botrytis cinerea.17
Several studies have shown that photodynamic treatment has the potential to be applied on a variety of food products including fruits and vegetables, seeds and grains, meat and aquatic products, and juices. This also has the advantage of minimal influence on nutritional and sensorial properties compared to conventional thermal processing technologies. A few examples of decontamination efficiency of photodynamic treatment of various food matrices include reducing the population of V. parahaemolyticus on cooked oysters,18 of L. monocytogenes on smoked salmon19 and on fresh-cut pears,20 of E. coli on fresh-cut pineapple,21 of Staph. saprophyticus on fresh dough sheet,22 of P. fluorescens on Minas Frescal cheese,23 of A. flavus on maize kernel and flour24 and on peanuts,25 and of Botrytis cinerea on apples.26 The use of photodynamic treatment in disinfecting the water in fish farming was also suggested by Wohllebe and colleagues. The authors successfully decontaminated the water containing larvae (of human pathogenic parasites) using low concentrations of chlorophyll acid27 before introducing the fish. They further suggested chlorophyll-mediated photodynamic treatment as a practical and inexpensive treatment for controlling ectoparasites such as Ichthyophthirius mulftifiliis in fish.28
Furthermore, encouraged by promising antimicrobial efficacy of this treatment, photodynamic-mediated food packaging films have been recently investigated. The photosensitiser is embedded in the film forming matrix to add the antimicrobial activity to the food packaging material. Examples of photodynamically active food packaging are riboflavin-incorporated chitosan-based film as a salmon packaging material, with the ability to reduce L. monocytogenes, V. parahaemolyticus and Shewanella baltica populations,29 carbon nitride-incorporated konjac glucomannan-based film as a cherry tomato packaging material with the ability to inhibit E. coli and Staph. aureus growth,30 curcumin-incorporated cellulose laurate-based film as a pork packaging material with the ability to reduce Staph. aureus population,31 β-cyclodextrin/curcumin complex-incorporated 2,3-dialdehyde cellulose-based film as a salmon packaging material with the ability to inactivate L. monocytogenes, V. parahaemolyticus, and Shew. putrefaciens,32 and aloe emodin-incorporated poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based film as a packaging material for fresh-cut papaya, pork belly and pork bologna with the ability to inactivate E. coli.33 It is also possible that the photosensitiser-incorporated food packaging material may improve the shelf life of the products by being exposed to artificial lighting in the retail stores. However, this needs further investigation.
Summary
In general, various studies have shown the efficiency of photodynamic treatment as a naturally based, cost effective, clean label and eco-friendly decontamination technique. The key benefit of this technology is that it is a non-thermal process and retains the nutritional and organoleptic qualities of food. It is easily scalable and can be implemented in the food industry as it can operate using an existing conveyor system. It does not require expensive equipment and the materials used such as curcumin and visible light are already approved and being used in the food industry. It is effective against a wide range of food related microorganisms including viruses, bacteria, yeasts, and moulds.
Besides the diverse applications of photodynamic treatment in post-harvest and processed foods from plant or animal sources it has applications in decontamination of fruit and vegetable washing water, making it attractive for adoption by industry. It can also be investigated as an antibacterial, antifungal, and possibly pesticide treatment for the photosensitiser to be applied directly on fresh produce and be photoactivated with the aid of artificial lighting for example in greenhouses, where the intensity of light and time of exposure can be controlled. However, the efficiency of the treatment is dependent on the processing conditions such as photosensitiser concentration, light dose, and wavelength, as well as the food matrix properties. Optimising and validating the treatment conditions, including photosensitiser formulation and illumination dosage, are the future challenges to effectively implementing the treatment in the food industry to replace the conventional decontamination techniques, while maintaining similar or higher decontamination efficiency.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] World Health Organization (2020) Food safety fact sheet. who.int[2] Huang, M et al.. (2019) Recent development in the application of alternative sterilization technologies to prepared dishes: a review. Crit Rev Food Sci Nutr 59, 1188–1196.
| Recent development in the application of alternative sterilization technologies to prepared dishes: a review.Crossref | GoogleScholarGoogle Scholar | 29359947PubMed |
[3] Pexara, A and Govaris, A (2020) Foodborne viruses and innovative non-thermal food-processing technologies. Foods 9, 1520.
| Foodborne viruses and innovative non-thermal food-processing technologies.Crossref | GoogleScholarGoogle Scholar |
[4] Penha, CB et al.. (2017) Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT 76, 198–202.
| Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin.Crossref | GoogleScholarGoogle Scholar |
[5] Costa, L et al.. (2011) Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of a PDT. Antivir Res 91, 278–282.
| Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of a PDT.Crossref | GoogleScholarGoogle Scholar | 21722673PubMed |
[6] Seidi Damyeh, M et al.. (2020) An insight into curcumin-based photosensitization as a promising and green food preservation technology. Compr Rev Food Sci 19, 1727–1759.
| An insight into curcumin-based photosensitization as a promising and green food preservation technology.Crossref | GoogleScholarGoogle Scholar |
[7] PubChem Identifier, CID, 493570 (riboflavin), 123798 (chlorophyllin A), 3663 (hypericin), 969516 (curcumin), 10207 (aloe-emodin). PubChem (nih.gov).
[8] Luksiene, Z et al.. (2004) Inactivation of fungi in vitro by photosensitization: preliminary results. Ann Agric Environ Med 11, 215–220.
| 15627327PubMed |
[9] Kairyte, K et al.. (2012) Effective inactivation of food pathogens Listeria monocytogenes and Salmonella enterica by combined treatment of hypericin‐based photosensitization and high power pulsed light. J Appl Microbiol 112, 1144–1151.
| Effective inactivation of food pathogens Listeria monocytogenes and Salmonella enterica by combined treatment of hypericin‐based photosensitization and high power pulsed light.Crossref | GoogleScholarGoogle Scholar | 22469030PubMed |
[10] Chen, B et al.. (2020) Eradication of planktonic Vibrio parahaemolyticus and its sessile biofilm by curcumin-mediated photodynamic inactivation. Food Control 113, 107181.
| Eradication of planktonic Vibrio parahaemolyticus and its sessile biofilm by curcumin-mediated photodynamic inactivation.Crossref | GoogleScholarGoogle Scholar |
[11] Chiniforush, N et al.. (2020) The effect of antimicrobial photodynamic therapy using chlorophyllin–phycocyanin mixture on Enterococcus faecalis: the influence of different light sources. Appl Sci 10, 4290.
| The effect of antimicrobial photodynamic therapy using chlorophyllin–phycocyanin mixture on Enterococcus faecalis: the influence of different light sources.Crossref | GoogleScholarGoogle Scholar |
[12] Liang, Z et al.. (2022) Photodynamic inactivation of Shigella flexneri by curcumin. LWT 153, 112491.
| Photodynamic inactivation of Shigella flexneri by curcumin.Crossref | GoogleScholarGoogle Scholar |
[13] Shi, Y-g et al.. (2022) Ultra-efficient antimicrobial photodynamic inactivation system based on blue light and octyl gallate for ablation of planktonic bacteria and biofilms of Pseudomonas fluorescens. Food Chem 374, 131585.
| Ultra-efficient antimicrobial photodynamic inactivation system based on blue light and octyl gallate for ablation of planktonic bacteria and biofilms of Pseudomonas fluorescens.Crossref | GoogleScholarGoogle Scholar | 34802804PubMed |
[14] Oliveira, A et al.. (2009) Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J Appl Microbiol 106, 1986–1995.
| Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores.Crossref | GoogleScholarGoogle Scholar | 19228253PubMed |
[15] Al-Asmari, F et al.. (2017) A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J Photochem Photobiol B: Biol 173, 301–306.
| A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin.Crossref | GoogleScholarGoogle Scholar |
[16] Temba, BA et al.. (2016) Inactivation of Aspergillus flavus spores by curcumin-mediated photosensitization. Food Control 59, 708–713.
| Inactivation of Aspergillus flavus spores by curcumin-mediated photosensitization.Crossref | GoogleScholarGoogle Scholar |
[17] Huang, L et al.. (2021) The inactivation by curcumin-mediated photosensitization of Botrytis cinerea spores isolated from strawberry fruits. Toxins 13, 196.
| The inactivation by curcumin-mediated photosensitization of Botrytis cinerea spores isolated from strawberry fruits.Crossref | GoogleScholarGoogle Scholar | 33803254PubMed |
[18] Chen, B et al.. (2021) Effects of the curcumin-mediated photodynamic inactivation on the quality of cooked oysters with Vibrio parahaemolyticus during storage at different temperature. Int J Food Microbiol 345, 109152.
| Effects of the curcumin-mediated photodynamic inactivation on the quality of cooked oysters with Vibrio parahaemolyticus during storage at different temperature.Crossref | GoogleScholarGoogle Scholar | 33725529PubMed |
[19] Josewin, SW et al.. (2018) Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon. Food Control 84, 354–361.
| Antibacterial effect of 460 nm light-emitting diode in combination with riboflavin against Listeria monocytogenes on smoked salmon.Crossref | GoogleScholarGoogle Scholar |
[20] Chai, Z et al.. (2021) Antibacterial mechanism and preservation effect of curcumin-based photodynamic extends the shelf life of fresh-cut pears. LWT 142, 110941.
| Antibacterial mechanism and preservation effect of curcumin-based photodynamic extends the shelf life of fresh-cut pears.Crossref | GoogleScholarGoogle Scholar |
[21] Zou, Y et al.. (2021) Effects of curcumin-based photodynamic treatment on quality attributes of fresh-cut pineapple. LWT 141, 110902.
| Effects of curcumin-based photodynamic treatment on quality attributes of fresh-cut pineapple.Crossref | GoogleScholarGoogle Scholar |
[22] Wang, Z et al.. (2021) Antimicrobial photodynamic inactivation with curcumin against Staphylococcus saprophyticus, in vitro and on fresh dough sheet. LWT 147, 111567.
| Antimicrobial photodynamic inactivation with curcumin against Staphylococcus saprophyticus, in vitro and on fresh dough sheet.Crossref | GoogleScholarGoogle Scholar |
[23] Saraiva, BB et al.. (2021) Photodynamic inactivation of Pseudomonas fluorescens in Minas Frescal cheese using curcumin as a photosensitizer. LWT 151, 112143.
| Photodynamic inactivation of Pseudomonas fluorescens in Minas Frescal cheese using curcumin as a photosensitizer.Crossref | GoogleScholarGoogle Scholar |
[24] Nguenha, RJ et al.. (2022) Effect of solvents on curcumin as a photosensitizer and its ability to inactivate Aspergillus flavus and reduce aflatoxin B1 in maize kernels and flour. J Food Process Preserv 46, e16169.
| Effect of solvents on curcumin as a photosensitizer and its ability to inactivate Aspergillus flavus and reduce aflatoxin B1 in maize kernels and flour.Crossref | GoogleScholarGoogle Scholar |
[25] Mukubesa, N et al.. (2022) Curcumin-based photosensitization, a green treatment in inactivating Aspergillus flavus spores in peanuts. Foods 11, 354.
| Curcumin-based photosensitization, a green treatment in inactivating Aspergillus flavus spores in peanuts.Crossref | GoogleScholarGoogle Scholar | 35159505PubMed |
[26] Wei, C et al.. (2021) Photosensitization effect of curcumin for controlling plant pathogen Botrytis cinerea in postharvest apple. Food Control 123, 107683.
| Photosensitization effect of curcumin for controlling plant pathogen Botrytis cinerea in postharvest apple.Crossref | GoogleScholarGoogle Scholar |
[27] Wohllebe, S et al.. (2009) Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances. Parasitol Res 104, 593–600.
| Photodynamic control of human pathogenic parasites in aquatic ecosystems using chlorophyllin and pheophorbid as photodynamic substances.Crossref | GoogleScholarGoogle Scholar | 19009310PubMed |
[28] Wohllebe, S et al.. (2012) Chlorophyllin for the control of Ichthyophthirius multifiliis (Fouquet). Parasitol Res 111, 729–733.
| Chlorophyllin for the control of Ichthyophthirius multifiliis (Fouquet).Crossref | GoogleScholarGoogle Scholar | 22476598PubMed |
[29] Su, L et al.. (2021) Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int J Biol Macromol 172, 231–240.
| Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging.Crossref | GoogleScholarGoogle Scholar | 33453253PubMed |
[30] Ni, Y et al.. (2021) Enhanced antimicrobial activity of konjac glucomannan nanocomposite films for food packaging. Carbohydr Polym 267, 118215.
| Enhanced antimicrobial activity of konjac glucomannan nanocomposite films for food packaging.Crossref | GoogleScholarGoogle Scholar | 34119169PubMed |
[31] Ma, T et al.. (2021) Cellulose laurate films containing curcumin as photoinduced antibacterial agent for meat preservation. Int J Biol Macromol 193, 1986–1995.
| Cellulose laurate films containing curcumin as photoinduced antibacterial agent for meat preservation.Crossref | GoogleScholarGoogle Scholar | 34767881PubMed |
[32] Chen, L et al.. (2021) Novel 2, 3-dialdehyde cellulose-based films with photodynamic inactivation potency by incorporating the β-cyclodextrin/curcumin inclusion complex. Biomacromolecules 22, 2790–2801.
| Novel 2, 3-dialdehyde cellulose-based films with photodynamic inactivation potency by incorporating the β-cyclodextrin/curcumin inclusion complex.Crossref | GoogleScholarGoogle Scholar | 34077200PubMed |
[33] Le, TD et al.. (2021) Development of an antimicrobial photodynamic poly (3-hydroxybutyrate-co-3-hydroxyvalerate) packaging film for food preservation. Food Packag Shelf Life 30, 100749.
| Development of an antimicrobial photodynamic poly (3-hydroxybutyrate-co-3-hydroxyvalerate) packaging film for food preservation.Crossref | GoogleScholarGoogle Scholar |