Interaction of Candida albicans with human gut epithelium in the presence of Live Biotherapeutic Products (LBPs)
Bronwyn Smit A D , Anna Kuballa B , Samantha Coulson C and Mohammad Katouli AA School of Science, Technology and Education, University of the Sunshine Coast, Maroochydore DC, Qld 4556, Australia
B School of Health and Behavioural Sciences, University of the Sunshine Coast, Maroochydore DC, Qld 4556, Australia
C Servatus Biopharmaceuticals, Coolum Beach, Qld 4573, Australia
D Email: bronwyn.smit@research.usc.edu.au
Microbiology Australia 42(3) 120-124 https://doi.org/10.1071/MA21035
Submitted: 21 July 2021 Accepted: 30 August 2021 Published: 13 September 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Candida albicans is a semi-ubiquitous pathobiont that is known to significantly impact human health and wellbeing, causing a significant financial strain on the medical system. Due to increasing antifungal resistance, there is a growing need for novel fungal therapeutics to treat diseases caused by this fungus. The development and use of Live Biotherapeutic Products (LBPs) is an innovative and novel approach to potentially treating Candidiasis and other comorbidities associated with C. albicans infection. To evaluate their anti-pathogenic efficacy, it is necessary to understand the underlying mechanisms involved, via the use of biomimetic cell models. In this study, six LBPs were chosen to investigate their competitive inhibitory effect against C. albicans using a co-culture of Caco-2 cells and mucous-secreting HT29-MTX cells to mimic human gut epithelium. The LBP strains were supplied by Servatus Biopharmaceuticals and identified as SVT 01D1, SVT 04P1, SVT 05P2, SVT 06B1, SVT 07R1 and SVT 08Z1. Five out of the six LBPs showed a significant reduction in the adhesion of C. albicans and all six LBPs reduced C. albicans invasion in the co-culture cells to varying degrees. There was no significant difference between co-inoculation of C. albicans with the LBPs or pre-inoculation of LBPs before the addition of C. albicans. The potential of these LBPs as novel anti-fungal therapeutics for the treatment of C. albicans diseases can be further documented in clinical trials.
Introduction
The gut microbiome is arguably one of the most mysterious ‘organs’ of the human body and is vital to all aspects of human health and our sense of wellbeing. Over the past few decades significant research has been conducted to understand the dynamics involved in microbe-to-microbe and microbe-to-gut interactions and how these impact on overall human health. One microbe that has been the subject of ongoing investigation is Candida albicans, an opportunistic pathogenic yeast found in about 70% of people1. As a polymorphic fungus, it is generally considered to inhabit the body as a commensal, kept under control by the host’s beneficial microbiota. However, in certain circumstances, such as in immunocompromised individuals2, and during prolonged antibiotic therapy3, C. albicans is able to overgrow within its local environment, that is, within the gastrointestinal (GI) tract or translocate across the gut epithelium leading to systemic Candidiasis. This disease has a high morbidity and mortality ranging from 20% to 49%4–6. Studies suggest that C. albicans adversely affects inflammatory bowel disease (IBD) exacerbating inflammation in the gut and delaying healing of ulcerative colitis, for example in humans and mice7–9. An increased abundance of C. albicans has been observed in patients with IBD compared with healthy subjects suggesting that fungi may play a role in its pathogenesis10,11.
Current gaps and future direction
Scientific investigations have identified various fungal genes known to play a role in C. albicans pathogenicity. However, there are still gaps in current knowledge of atypical virulence mechanisms, particularly in understanding the ability of C. albicans to invade gut epithelial cells. The majority of studies have focused on the interaction of C. albicans with oral/vaginal epithelial cells as opposed to gastrointestinal (GI) epithelial cells12. While antibiotics are still the drug of choice to treat bacterial and fungal infections in clinic, Live Biotherapeutic Products (LBPs) have been suggested as an alternative for treating infections. LBPs are defined by the Food and Drug Administration (FDA) Centre for Biologic Evaluation and Research (CBER) as ‘a live biological product that: (1) contains live organisms, such as bacteria; (2) is applicable to the prevention, treatment or cure of a disease or condition of human beings; and (3) is not a vaccine’13. They are further described as ‘medicinal products containing live micro-organisms (bacteria or yeasts) for human use’ by the European Pharmacopoeia (Ph. Eur.) (which excludes faecal microbiota transplants and gene therapy agents from this category)14. The investigation of LBPs in treating inflammatory, autoimmune and even malignant conditions is accelerating at an astonishing rate, being recognised as novel drug candidates that aim to change the medical paradigm in treating human illness15. In this study we investigated the competitive inhibitory effects of six LBPs on adhesion and invasion of C. albicans using a co-culture of Caco-2:HT29-MTX cells as a model of human gut epithelium to provide insight into the potential of these LBPs for managing Candidiasis.
Scope of this project
Current methods to investigate pathogenic interactions of microbes on gut epithelium rely on using cell lines that resemble biomimetic synthetic intestines, mainly Caco-2 or HT-29 cell lines16–18. Caco-2 cells are differentiated in culture medium to form a polarized cell monolayer with tight junctions and microvilli to resemble important characteristics of human intestinal mature enterocytes. The main drawback of this cell line is that it does not produce a sufficient mucus layer. HT29, with methotrexate (MTX) adaptation, differentiates in culture media to secret mucin19, an essential component of the gut epithelium. We used a co-culture of Caco-2 and HT29-MTX cells to investigate the interaction of C. albicans ATCC 10231 with the gut epithelium. Six LBP candidates were selected and provided by Servatus Biopharmaceuticals: SVT 01D1, SVT 04P1, SVT 05P2, SVT 06B1, SVT 07R1 and SVT 08Z1.
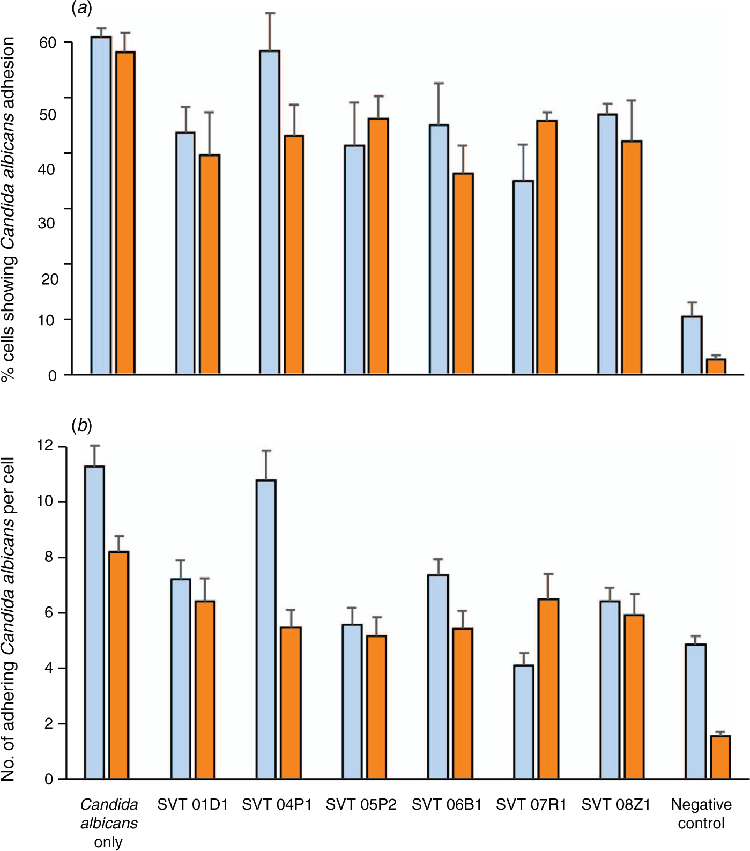
Interaction of C. albicans ATCC 10231 at a final concentration of 106 CFU/mL with the co-culture Caco-2:HT29-MTX (9:1) alone and in the presence of each of the LBPs (106 CFU/mL) was assessed by measuring reduction in C. albicans colonisation when co-inoculated with LBP strains, and when pre-inoculated for 60 min with LBP strains prior to inoculation of C. albicans. The number of adhering C. albicans per cell was recorded to identify the competitive ability of LBP strains to inhibit adherence of C. albicans per cell. The results indicated that the LBP strains (except SVT 04P1) reduced the colonisation of C. albicans on the co-culture cells by 21–43% (Figure 1a) and adhesion per cell by 21–64% (Figure 1b) both in co-inoculation and pre-inoculation assays.
All LBP strains (except SVT 04P1) demonstrated a significant reduction in colonisation and adhesion per cell of C. albicans (P < 0.01 in both co- and pre-inoculation). Overall, reduction in number of adhering C. albicans ATCC 10231 was seen for both co-inoculation and pre-inoculation, with SVT 07R1 showing the highest reduction (P = 0.0005).
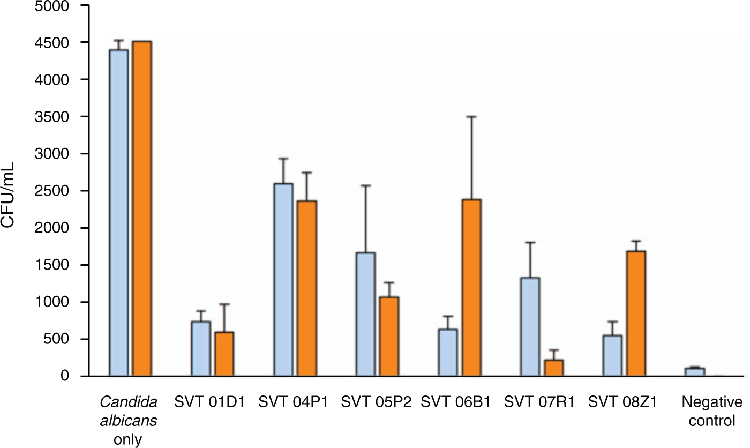
In scanning electron microscopy (Figure 2) of the co-culture assay, C. albicans ATCC 10231 was shown to be highly invasive in that the C. albicans hypha was seen penetrating the epithelial cell monolayer. A similar procedure to the adhesion assay was used for co-inoculation and pre-inoculation in the invasion assay. A suspension of C. albicans was inoculated into 96-well plates at a final concentration of 107 CFU/well. After 90 min the wells were inoculated with nystatin (24 μg/mL) for 60 min to kill any extracellular C. albicans, followed by incubation with 0.1% Triton-X-10 (Sigma-Aldrich) for 15 min to lyse the monolayer releasing invading pathogens and enumerating them. The results showed a reduction in invasion of C. albicans in the presence of LBP strains that demonstrated variable efficacy (Figure 3) with SVT 01D1 showing the highest reduction overall.

|
Discussion
The escalating need to develop alternative approaches for managing C. albicans infection has highlighted the potential for the use of LBPs as anti-fungal therapeutics. We showed that most LBPs used in this study showed a significant reduction in the adhesion and invasion of C. albicans in our human gut epithelium cell culture model, although these effects varied among the LBPs. The potential use of these LBPs as a therapeutic or as a prophylactic measure was also tested using co-inoculation and pre-inoculation models of the LBPs against the C. albicans. While there was a significant reduction in colonisation and invasion of the cells by C. albicans in the presence of LBPs, we did not observe a significant difference between co-inoculation and pre-inoculation of LBPs one hour before the addition of C. albicans. Poupet et al. studied the curative effect of LBP L. rhamnosus Lcr35® on Caenorhabditis elegans survival after C. albicans exposure, and found that the 2-h and the 4-h pre-inoculation periods were most protective against C. albicans infection20. Future studies to investigate the effect of longer incubation periods of LBPs used in our study can provide a better understanding of the impact of pre-inoculation time in clinical studies aiming to assess the prophylactic effect of LBPs against C. albicans. Furthermore, it could prove insightful to explore the efficacy of various other LBP strains against C. albicans. Similarly, the use of further Candida strains in future studies for comparison would strengthen our findings.
Although the most reliable model to establish the impact of LBPs against C. albicans and other enteric pathogens is clinical trials in humans, in vitro studies utilising a co-culture of Caco-2 and HT29-MTX cells lines as used in this study, provide a suitable model to mimic the human gut epithelium. Caco-2 cells can be differentiated in the culture medium to form a polarized cell monolayer with tight junctions and microvilli that resemble important characteristics of human intestinal mature enterocytes. The other cell line, HT29, with methotrexate (MTX) adaptation, differentiates in culture media to secret mucin. In this study we used this co-culture model to investigate the efficacy of the LBPs against C. albicans, however, to achieve a far more reliable and robust gut epithelium model which resembles biomimetic molecular mechanisms in the intestinal niche, further improvements of this model such as the use of secretory IgAs and/or various other crucial antibodies/cytokines necessary for managing gut microbiome homeostasis are necessary21.
This fascinating field of research has significant potential for determining the link in a chain of events involving interactions between C. albicans and the gut epithelium where LBPs are used to treat the invading pathogens.
Future studies
We are currently investigating the cellular response of the gut epithelial cells to C. albicans colonisation by comparing global gene expression (using RNA sequencing) with and without co-inoculation of LBPs. The RNAs will represent a snapshot of interaction/non-interaction of C. albicans with gut epithelium cells following the competitive adhesion of C. albicans with and without LBPs and identify genes that play a major role during these interactions. This will further our understanding of the mechanisms associated with using LBPs to treat invading pathogens.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by a Research Training Program (RTP) scholarship from University of the Sunshine Coast and a top-up scholarship from Servatus Biopharmaceuticals.
References
[1] Schulze, J. and Sonnenborn, U. (2009) Yeasts in the gut: from commensals to infectious agents. Dtsch. Arztebl. Int. 106, 837–842.| 20062581PubMed |
[2] Myerowitz, R.L. et al. (1977) Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am. J. Clin. Pathol. 68, 29.
| Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology.Crossref | GoogleScholarGoogle Scholar | 868803PubMed |
[3] Verghese, A. et al. (1988) Synchronous bacterial and fungal septicemia. A marker for the critically ill surgical patient. Am. Surg. 54, 276–283.
| 3364864PubMed |
[4] Kumamoto, C.A. (2011) Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 14, 386–391.
| Inflammation and gastrointestinal Candida colonization.Crossref | GoogleScholarGoogle Scholar | 21802979PubMed |
[5] Gudlaugsson, O. et al. (2003) Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37, 1172–1177.
| Attributable mortality of nosocomial candidemia, revisited.Crossref | GoogleScholarGoogle Scholar | 14557960PubMed |
[6] Bassetti, M. et al. (2011) Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One 6, e24198.
| Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy.Crossref | GoogleScholarGoogle Scholar | 21935385PubMed |
[7] Jawhara, S. et al. (2008) Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 197, 972–980.
| Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3.Crossref | GoogleScholarGoogle Scholar | 18419533PubMed |
[8] Sonoyama, K. et al. (2011) Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice. Med. Mycol. 49, 237–247.
| Gut colonization by Candida albicans aggravates inflammation in the gut and extra-gut tissues in mice.Crossref | GoogleScholarGoogle Scholar | 20807027PubMed |
[9] Yan, L. et al. (2016) Effect of Candida albicans on intestinal ischemia-reperfusion injury in rats. Chin. Med. J. (Engl.) 129, 1711.
| Effect of Candida albicans on intestinal ischemia-reperfusion injury in rats.Crossref | GoogleScholarGoogle Scholar | 27411459PubMed |
[10] Sokol, H. et al. (2017) Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048.
| Fungal microbiota dysbiosis in IBD.Crossref | GoogleScholarGoogle Scholar | 26843508PubMed |
[11] Standaert-Vitse, A. et al. (2009) Candida albicans colonization and ASCA in familial Crohn’s disease. Am. J. Gastroenterol. 104, 1745–1753.
| Candida albicans colonization and ASCA in familial Crohn’s disease.Crossref | GoogleScholarGoogle Scholar | 19471251PubMed |
[12] Erdogan, A. and Rao, S.S. (2015) Small intestinal fungal overgrowth. Curr. Gastroenterol. Rep. 17, 16.
| Small intestinal fungal overgrowth.Crossref | GoogleScholarGoogle Scholar | 25786900PubMed |
[13] U.S. Department of Health and Human Services (2016) Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information; guidance for industry. https://www.fda.gov/media/82945/download
[14] Rouanet, A. et al. (2020) Live biotherapeutic products, a road map for safety assessment. Front. Med. 7, 237.
| Live biotherapeutic products, a road map for safety assessment.Crossref | GoogleScholarGoogle Scholar |
[15] Charbonneau, M.R. et al. (2020) Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738.
| Developing a new class of engineered live bacterial therapeutics to treat human diseases.Crossref | GoogleScholarGoogle Scholar | 32269218PubMed |
[16] Chen, X.M. et al. (2010) Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J. Pharmacol. Toxicol. Methods 61, 334–342.
| Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design.Crossref | GoogleScholarGoogle Scholar | 20159047PubMed |
[17] Béduneau, A. et al. (2014) A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 87, 290–298.
| A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure.Crossref | GoogleScholarGoogle Scholar | 24704198PubMed |
[18] Hilgendorf, C. et al. (2000) Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 89, 63–75.
| Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport.Crossref | GoogleScholarGoogle Scholar | 10664539PubMed |
[19] Lesuffleur, T. et al. (1990) Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 50, 6334.
| 2205381PubMed |
[20] Poupet, C. et al. (2019) Curative treatment of candidiasis by the live biotherapeutic microorganism Lactobacillus rhamnosus Lcr35® in the invertebrate model Caenorhabditis elegans: first mechanistic insights. Microorganisms 8, 34.
| Curative treatment of candidiasis by the live biotherapeutic microorganism Lactobacillus rhamnosus Lcr35® in the invertebrate model Caenorhabditis elegans: first mechanistic insights.Crossref | GoogleScholarGoogle Scholar |
[21] Weis, A.M. and Round, J.L. (2021) Microbiota-antibody interactions that regulate gut homeostasis. Cell Host Microbe 29, 334–346.
| Microbiota-antibody interactions that regulate gut homeostasis.Crossref | GoogleScholarGoogle Scholar | 33705705PubMed |
Biographies
Born in South Africa, Bronwyn Smit is a naturalised New Zealand citizen, currently residing in Queensland. She completed her undergraduate degree in Biomedical Science at the University of Auckland, followed by a master’s degree in forensic science. Aside from briefly working in environmental science, Bronwyn explored career opportunities in the arts and hospitality industries before developing a fascination with the gut microbiome and its overwhelming impact on human health and disease. This led to her current PhD project in Microbiology at University of the Sunshine Coast, investigating the competitive inhibition of Candida albicans by live biotherapeutic products in the human gut.
Dr Anna Kuballa obtained a Bachelor of Biomedical Science degree (Hons) majoring in microbiology from the James Cook University. She graduated with a Doctor of Philosophy in the field of molecular biology from the University of Queensland, Brisbane in 2007. She continued her research as a post-doctoral research fellow at the University of the Sunshine Coast where she currently holds an academic research and teaching position. Dr Kuballa’s published contributions centre around the molecular pathways involved in inflammation and microbial infection, with a special interest in the microbiome of inflammatory bowel disease and the breast milk microbiome.
Samantha Coulson joined Servatus in 2018 as Head of the Clinical Research Department and holds a PhD in Medicine from the University of Queensland. With over 15 years’ experience, Samantha has a diverse background in both academia and industry with extensive knowledge of the human microbiome. She is adept in designing, initiating, leading and completing multidisciplinary research projects and also in managing product research, innovation and development programs. As Head of Clinical Research Samantha manages all aspects of Servatus’ human clinical trial projects and preclinical studies, together with a small but highly experienced research team and global collaborators.
Associate Professor Mohammad Katouli obtained his PhD in 1980 from University of Ulster in UK. He then joined the Research and Development Department of DP Pharmaceuticals. In 1985, he took the position of the Head of Microbiology Department at the Pasteur Institute in Tehran. Between 1988 and 1998, he worked as a senior research fellow at the Microbiology and Tumor Biology Centre of the Karolinska Institute, Stockholm, Sweden. In 1998, he took a teaching and research position at University of the Sunshine Coast. His current interest is gut microbiota and the role of E. coli in pathogenesis of IBD.