Influenza B viruses: underestimated and overlooked
Marios Koutsakos A D and Stephen J Kent A B CA Department of Microbiology and Immunology, University of Melbourne, at the Peter Doherty Institute for Infection and Immunity, Melbourne, Vic. 3000, Australia
B Melbourne Sexual Health Centre and Department of Infectious Diseases, Alfred Hospital and Central Clinical School, Monash University, Melbourne, Vic. 3004, Australia
C ARC Centre for Excellence in Convergent Bio-Nano Science and Technology, University of Melbourne, Parkville, Vic. 3010, Australia
D Tel.: +61 3 9035 4179; Email: marios.koutsakos@unimelb.edu.au
Microbiology Australia 42(3) 110-115 https://doi.org/10.1071/MA21033
Submitted: 16 July 2021 Accepted: 19 August 2021 Published: 6 September 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Influenza B viruses circulate globally every year causing respiratory disease with significant clinical and socio-economic impacts. IBV are considered exclusive human pathogens with no established animal reservoirs, which suggests with concerted effort it may be possible to eradicate this virus from human circulation. However, this requires a deeper understanding of IBV virology and immunology and the design of vaccines that induce universal immunity to antigenic variants of IBV.
Introduction
Influenza A and B viruses (IAV and IBV) circulate annually causing seasonal epidemics around the world. Influenza viruses are single-stranded negative sense RNA viruses with segmented genomes belonging to the family of Orthomyxoviridae1. They replicate is the respiratory tract and cause influenza disease which can vary from asymptomatic and mild upper respiratory tract disease to severe lower respiratory tract disease and in some cases fatal disease1. Although IAV exists in a wide range of animal hosts, IBV does not have an established animal reservoir2. The potential of antigenically novel IAV viruses to ‘jump’ from animals into humans and cause severe disease, and is some instances global pandemics, has placed IAV in the spotlight. The lack of an established animal reservoir, and therefore lack of pandemic potential, for IBV has left this type of influenza virus considerably underestimated and overlooked. However, IBV has substantial health and socio-economic impacts annually. Additionally, the lack of an animal reservoir means that it may be possible to eradicate this virus from human circulation with highly effective, broadly protective vaccines and broad population coverage. To achieve that, a thorough understanding of IBV virology and immunology is needed.
The underestimated impact of IBV infections
Seasonal epidemics caused by IAV and IBV result in 3–5 million cases of severe disease and 290 000–650 000 deaths annually1. IBV accounts for on average 23% of the annual influenza burden3 but can comprise up to ~80% of infections in some countries in selected years4. It is estimated that IBV infections result in 7.9 million lower respiratory tract infections and 1.4 million hospitalisations annually5. Although the clinical severity of IBV was initially thought to be lower than that of IAV, recent studies have contested this notion, with hospitalisation and mortality rates in adults being similar for IAV and IBV6,7. Importantly, IBV incidence is higher in children, in which IBV can cause severe systemic complications and frequent hospitalisation and death, with up to 52% of influenza-related paediatric deaths being attributed to IBV8–10. Additionally, in children under the age of 16 years, IBV can have higher mortality rates than IAV and a significant rate of ICU admission11. Fatal infections of IBV in children are associated with secondary bacterial pneumonia as well as cardiac injury12. Lastly, IBV infections account on average for 37% of influenza-associated healthcare costs, with projected costs in the US of US$0.96–2.6 billion annually13. Overall, IBV has significant clinical and socioeconomic impacts. This impact could be minimised with highly effective vaccines and intervention strategies.
Known and unknowns of the IBV life cycle
IBV replicates in epithelial cells of the respiratory tract. The virus uses its surface glycoprotein haemagglutinin (HA) for attachment to sialic acid receptors on the cell surface and subsequent membrane fusion in endosomes. This results in the release of eight viral ribonucleoprotein (vRNP) complexes, which replicate in the nucleus of the cell. Viral RNA replication combined with protein expression are followed by assembly and budding of newly formed virions from the cell surface. The viral surface glycoprotein neuraminidase (NA) releases virions from attached sialic acid receptors on the cell surface14. During IBV infection, the viral non-structural 1 (NS1) protein of IBV has a critical role in counteracting immune recognition by innate receptors such as RIG-I as well as interferon-stimulated genes such as protein kinase R (PKR) and ISG-15, which are potent inhibitors of IBV14,15. Interestingly, the NS gene of IBV exhibits the highest rate of selection pressure among the genes of IBV16. Given its critical role in counteracting innate immune responses, understanding the evolution of the IBV NS1 protein in humans would be of great interest.
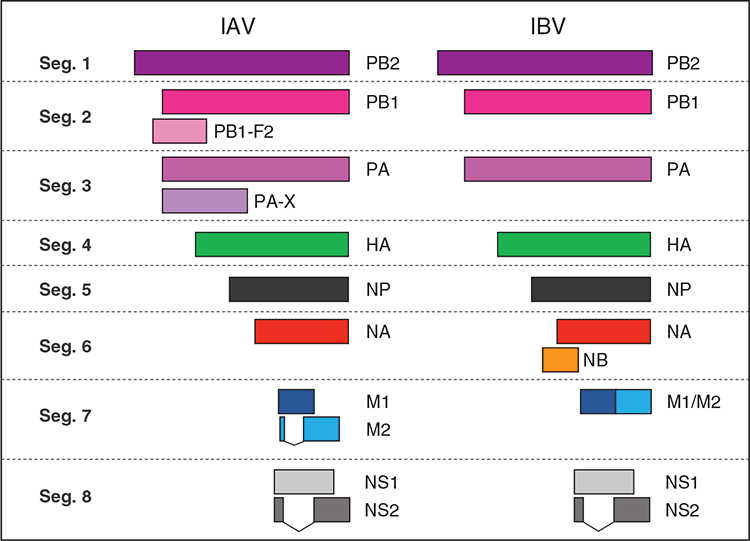
Although the life cycle of IAV and IBV is in many ways similar, it is pertinent to note that the two types of influenza viruses encode different sets of accessory proteins (Figure 1). Specifically, IBV lacks expression of immunomodulatory virulence factors PB1-F2 and PA-X found in IAV. Conversely, IBV encodes a unique open reading frame (ORF) called NB, that overlaps with the NA ORF15. NB is a small transmembrane protein that is heavily glycosylated and is incorporated in the IBV virion. Despite the high conservation of NB in IBV, NB expression is dispensable for virus viability and replication in vitro17,18 and its role in viral replication is unclear18. Dissecting the role and function of NB in the life cycle of IBV would assist the understanding of the IBV life cycle.
Host species tropism of IBV
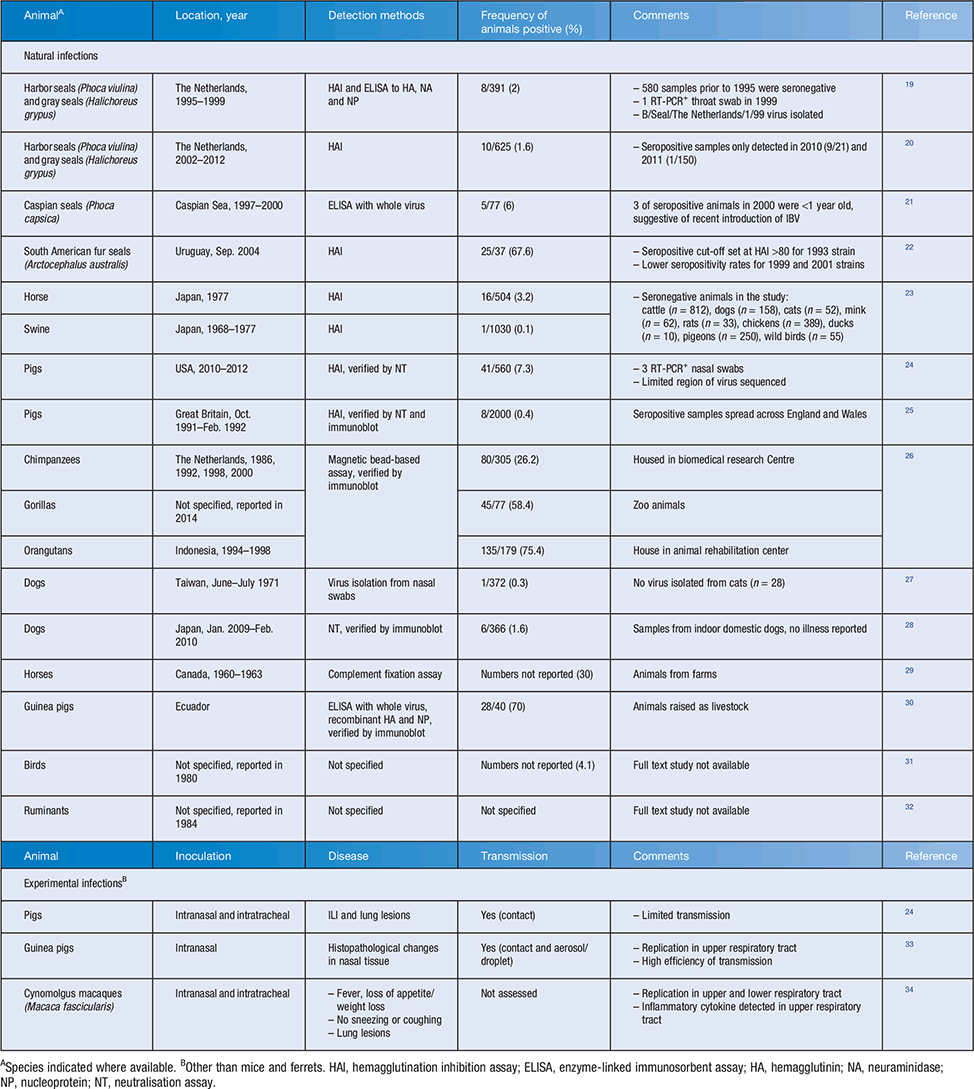
Another important difference between IAV and IBV is host species tropism. Although IAV can be found in many animal species, IBV is considered exclusively a human pathogen. However, it is important to note that natural infections of animals with IBV have been reported for a variety of species (Table 1) and some have been recapitulated experimentally. However, most of these infections have occurred in animals in proximity with humans (domestic and farm animals or animals in zoos/research centres) and likely represent isolated reverse zoonosis events. A notable exception is the presence of IBV in seals that has been detected across species of seals and geographical sites between 1995 and 201219–22. The single virus isolated from a seal in 1999 had high homology to a human IBV isolate. However, it is not known whether the presence of IBV in seals represents a single introduction from humans and subsequent spread amongst seals between 1995 and 2012 or multiple distinct reverse zoonosis events in that period. Overall, while a variety of mammals are susceptible to natural IBV infection, there is no evidence of established animal reservoirs in any species.
|
Understanding the factors that contribute to the exclusivity of IBV in humans is of great importance. The host species restriction of avian IAV in birds, and the requirement for significant adaption for efficient replication and transmission in humans, occurs at many stages of the life cycle, including HA mediated attachment and entry as well the activity of the influenza virus replication machinery35. Interestingly, it was recently reported that the IBV HA exhibits optimal activity in the pH and temperature conditions of the human upper respiratory tract, more so than IAV strains tested in that study, indicating significant host adaptation in the human host environment36. Additionally, IBV can interact with mammalian (human and murine) but not avian homologues of host proteins required to support viral replication37,38, providing a potential mechanistic basis for the lack of IBV in avian species. The IBV NS1 protein can counteract the effects of the antiviral protein ISG-15 in a species-specific manner, by interacting with human and non-human primate ISG-15 but not with canine or murine homologues39. Overall, these studies demonstrate considerable adaptation of IBV to mammalian and often specifically human hosts, which may restrict the ability of IBV to efficiently replicate in other species. It is important to note the recent discovery of IBV-like viruses in lower vertebrates40. These viruses show similar genome architecture to human IBVs40 and encode functional homologues of HA and NA but are not recognised by human serum samples41. Understanding the virology and host-restriction of these viruses could provide novel insights into IBV evolution and host species tropism.
Antigenic diversity and immune responses to IBV
Two antigenically and genetically distinct lineages of IBV co-circulate globally. These lineages, named B/Yamagata/16/1988-like (or B/Yamagata) and B/Victoria/2/1987-like (or B/Victoria), are estimated to have diverged in the 1970s42. While B/Victoria viruses were dominant in the late 1980s in most countries, B/Yamagata viruses dominated in the 1990s, during which B/Victoria viruses were virtually absent globally, except for a 1996/1997 outbreak in Asia42. B/Victoria viruses re-emerged in 2001 and the two lineages have co-circulated since42,43.
Both IBV lineages undergo gradual antigenic drift by accumulating escape mutations in the head domain of the HA protein – the major antigenic target of protective antibodies14. Mutations are primarily focused on sites surrounding the receptor binding site of the HA and overlap with sites of antibody recognition. Interestingly, since 2015 the B/Yamagata HA has not acquired any mutations in those sites. Instead, it has acquired 7 mutations on the NA protein16, although the effects of these mutations in antigenic evolution and immune escape are unclear. In contrast, since 2015 the B/Victoria viruses have undergone significant diversification of their HA gene, including the recurrent but independent emergence of viruses with 2–3 amino acid deletions in one of the antigenic sites16. These deletions significantly alter the antigenicity of those domains and have necessitated the inclusion of these strains in the influenza vaccine44. Intriguingly, similar amino acid deletions have been previously detected in IBV strain from 1940–198816, an observation that warrants further investigation as it indicates this might be a common escape mechanism of IBV.
Although such mutations can escape antibody recognition, conserved domains of the HA protein can be recognised by broadly cross-reactive antibodies45,46. These can target highly conserved sites of the HA head as well as the HA stem domain and cross-react with both IBV lineages46. Cross-recognition of the two lineages can also occur by cytotoxic T cells, which can recognise and kill virally infected cells, providing an additional level of immune protection47. The repeated isolation of multiple broadly cross-reactive antibodies in different studies indicates that such antibody responses may not be uncommon, although their prevalence and abundance in serum samples is unknown. Nonetheless, their discovery indicates that universal immunity across both lineages of IBV is feasible. Antibodies to the IBV NA also show broad cross-reactivity across both lineages and can mediate protection from challenge48. Understanding how such broadly cross-reactive immune responses to HA and NA are generated through infections and vaccination during the human lifespan will assist in the design of broadly cross-protective vaccines.
Vaccination strategies against IBV
Influenza vaccines primarily comprise unadjuvanted inactivated split virions or recombinant proteins that induce antibodies towards the HA and vaccine composition needs to be updated annually to accommodate for the emergence of escape mutants. A live attenuated influenza vaccine (LAIV) is also approved in some countries. Traditionally, a trivalent influenza vaccine (TIV) has been used that includes two IAV strains along with one IBV strain from the lineage predicted to dominate the upcoming influenza season. However, due to the frequent mismatch of the predicted and the circulating IBV lineage8, in 2012 the WHO recommended where possible the use of a quadrivalent vaccine (QIV) that includes one IBV strain from each lineage. Despite this, the average vaccine effectiveness for IBV is only 54%49 and the advantages of the QIV formulation remain contested50. An alternative to annual administration of a strain-specific vaccine would be the design of a universal vaccine that induces broadly cross-reactive immunity and does not require annual reformulation. This can be achieved by rationally designing vaccines that focus the immune response to highly conserved sites of the IBV HA and NA proteins, although such vaccines are only in pre-clinical development. Overall, despite the introduction of QIV, current vaccination strategies against IBV only provide modest and partial protection and further research is needed to improve vaccine effectiveness. The development of more effective IBV vaccines will assist efforts to eliminate IBV from human circulation.
Future directions
Despite the consistent seasonal circulation globally and the significant health and socio-economic impacts of IBV, initial misconceptions of relatively lower clinical severity have left IBV underestimated and overlooked. As a result, there is only limited focus on the control of IBV infections. Significant advances in the last decade have demonstrated the potential for universal immunity across both lineages of IBV. The lack of animal reservoir and subsequently pandemic potential, once a reason for neglecting IBV, is now considered its Achilles’ heel and could allow for the high-level suppression or even elimination of this virus. However, this can only be achieved by global concerted efforts to understand the antigenic evolution of IBV, the generation of broadly cross-reactive immunity and the rational design of universal vaccines.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive specific funding.
References
[1] Krammer, F. et al. (2018) Influenza. Nat. Rev. Dis. Primers 4, 3.| Influenza.Crossref | GoogleScholarGoogle Scholar | 29955068PubMed |
[2] van de Sandt, C.E. et al. (2015) Influenza B viruses: not to be discounted. Future Microbiol. 10, 1447–1465.
| Influenza B viruses: not to be discounted.Crossref | GoogleScholarGoogle Scholar | 26357957PubMed |
[3] Caini, S. et al. (2019) The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS One 14, e0222381.
| The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century.Crossref | GoogleScholarGoogle Scholar | 31869381PubMed |
[4] Tafalla, M. et al. (2016) A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum. Vaccin. Immunother. 12, 993–1002.
| A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries.Crossref | GoogleScholarGoogle Scholar | 26890005PubMed |
[5] Lafond, K.E. et al. (2021) Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: a systematic review and meta-analysis. PLoS Med. 18, e1003550.
| Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 33914729PubMed |
[6] Su, S. et al. (2014) Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin. Infect. Dis. 59, 252–255.
| Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection.Crossref | GoogleScholarGoogle Scholar | 24748521PubMed |
[7] Sharma, Y. et al. (2020) Clinical characteristics and outcomes of influenza A and B virus infection in adult Australian hospitalised patients. BMC Infect. Dis. 20, 913.
| Clinical characteristics and outcomes of influenza A and B virus infection in adult Australian hospitalised patients.Crossref | GoogleScholarGoogle Scholar | 33261559PubMed |
[8] Paul Glezen, W. et al. (2013) The burden of influenza B: a structured literature review. Am. J. Public Health 103, e43–e51.
| The burden of influenza B: a structured literature review.Crossref | GoogleScholarGoogle Scholar | 23327249PubMed |
[9] Shang, M. et al. (2018) Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics 141, e20172918.
| Influenza-associated pediatric deaths in the United States, 2010-2016.Crossref | GoogleScholarGoogle Scholar | 29440502PubMed |
[10] Zaraket, H. et al. (2021) Burden of influenza B virus infection and considerations for clinical management. Antiviral Res. 185, 104970.
| Burden of influenza B virus infection and considerations for clinical management.Crossref | GoogleScholarGoogle Scholar | 33159999PubMed |
[11] Tran, D. et al. (2016) Hospitalization for influenza A versus B. Pediatrics 138, e20154643.
| Hospitalization for influenza A versus B.Crossref | GoogleScholarGoogle Scholar | 27535144PubMed |
[12] Paddock, C.D. et al. (2012) Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J. Infect. Dis. 205, 895–905.
| Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection.Crossref | GoogleScholarGoogle Scholar | 22291193PubMed |
[13] Yan, S. et al. (2017) US healthcare costs attributable to type A and type B influenza. Hum. Vaccin. Immunother. 13, 2041–2047.
| US healthcare costs attributable to type A and type B influenza.Crossref | GoogleScholarGoogle Scholar | 28700268PubMed |
[14] Hensen, L. et al. (2020) Innate and adaptive immunity toward influenza B viruses. Future Microbiol. 15, 1045–1058.
| Innate and adaptive immunity toward influenza B viruses.Crossref | GoogleScholarGoogle Scholar | 32811172PubMed |
[15] Koutsakos, M. et al. (2016) Knowns and unknowns of influenza B viruses. Future Microbiol. 11, 119–135.
| Knowns and unknowns of influenza B viruses.Crossref | GoogleScholarGoogle Scholar | 26684590PubMed |
[16] Virk, R.K. et al. (2020) Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 117, 619–628.
| Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity.Crossref | GoogleScholarGoogle Scholar | 31843889PubMed |
[17] Hatta, M. and Kawaoka, Y. (2003) The NB protein of influenza B virus is not necessary for virus replication in vitro. J. Virol. 77, 6050–6054.
| The NB protein of influenza B virus is not necessary for virus replication in vitro.Crossref | GoogleScholarGoogle Scholar | 12719596PubMed |
[18] Elderfield, R.A. et al. (2016) NB protein does not affect influenza B virus replication in vitro and is not required for replication in or transmission between ferrets. J. Gen. Virol. 97, 593–601.
| NB protein does not affect influenza B virus replication in vitro and is not required for replication in or transmission between ferrets.Crossref | GoogleScholarGoogle Scholar | 26703440PubMed |
[19] Osterhaus, A.D. et al. (2000) Influenza B virus in seals. Science 288, 1051–1053.
| Influenza B virus in seals.Crossref | GoogleScholarGoogle Scholar | 10807575PubMed |
[20] Bodewes, R. et al. (2013) Recurring influenza B virus infections in seals. Emerg. Infect. Dis. 19, 511–512.
| Recurring influenza B virus infections in seals.Crossref | GoogleScholarGoogle Scholar | 23750359PubMed |
[21] Ohishi, K. et al. (2002) Serological evidence of transmission of human influenza A and B viruses to Caspian seals (Phoca caspica). Microbiol. Immunol. 46, 639–644.
| Serological evidence of transmission of human influenza A and B viruses to Caspian seals (Phoca caspica).Crossref | GoogleScholarGoogle Scholar | 12437032PubMed |
[22] Blanc, A. et al. (2009) Serologic evidence of influenza A and B viruses in South American fur seals (Arctocephalus australis). J. Wildl. Dis. 45, 519–521.
| Serologic evidence of influenza A and B viruses in South American fur seals (Arctocephalus australis).Crossref | GoogleScholarGoogle Scholar | 19395764PubMed |
[23] Kawano, J. et al. (1978) Distribution of antibodies in animals against influenza B and C viruses. Jpn. J. Vet. Res. 26, 74–80.
| 739713PubMed |
[24] Ran, Z. et al. (2015) Domestic pigs are susceptible to infection with influenza B viruses. J. Virol. 89, 4818–4826.
| Domestic pigs are susceptible to infection with influenza B viruses.Crossref | GoogleScholarGoogle Scholar | 25673727PubMed |
[25] Brown, I.H. et al. (1995) Serological studies of influenza viruses in pigs in Great Britain 1991–2. Epidemiol. Infect. 114, 511–520.
| Serological studies of influenza viruses in pigs in Great Britain 1991–2.Crossref | GoogleScholarGoogle Scholar | 7781739PubMed |
[26] Buitendijk, H. et al. (2014) Retrospective serology study of respiratory virus infections in captive great apes. Viruses 6, 1442–1453.
| Retrospective serology study of respiratory virus infections in captive great apes.Crossref | GoogleScholarGoogle Scholar | 24662675PubMed |
[27] Chang, C.P. et al. (1976) Influenza virus isolations from dogs during a human epidemic in Taiwan. Int. J. Zoonoses 3, 61–64.
| 977232PubMed |
[28] Horimoto, T. et al. (2014) Serological evidence of infection of dogs with human influenza viruses in Japan. Vet. Rec. 174, 96.
| Serological evidence of infection of dogs with human influenza viruses in Japan.Crossref | GoogleScholarGoogle Scholar | 24336761PubMed |
[29] Ditchfield, J. and Macpherson, L.W. (1965) Zbitnew a: upper respiratory disease in thouroughbred horses: studies of its viral etiology in the Toronto area, 1960 to 1963. Can. J. Comp. Med. Vet. Sci. 29, 18–22.
| 14230908PubMed |
[30] Leyva-Grado, V.H. et al. (2012) Influenza virus infection in guinea pigs raised as livestock, Ecuador. Emerg. Infect. Dis. 18, 1135–1138.
| Influenza virus infection in guinea pigs raised as livestock, Ecuador.Crossref | GoogleScholarGoogle Scholar | 22710350PubMed |
[31] Romvary, J. et al. (1980) Susceptibility of birds to type-B influenza virus. Acta Microbiol. Acad. Sci. Hung. 27, 279–287.
| 6258401PubMed |
[32] Lopez, J.W. and Woods, G.T. (1984) Influenza virus in ruminants: a review. Res. Commun. Chem. Pathol. Pharmacol. 45, 445–462.
| 6390588PubMed |
[33] Pica, N. et al. (2012) Transmission of influenza B viruses in the guinea pig. J. Virol. 86, 4279–4287.
| Transmission of influenza B viruses in the guinea pig.Crossref | GoogleScholarGoogle Scholar | 22301149PubMed |
[34] Kitano, M. et al. (2010) Establishment of a cynomolgus macaque model of influenza B virus infection. Virology 407, 178–184.
| Establishment of a cynomolgus macaque model of influenza B virus infection.Crossref | GoogleScholarGoogle Scholar | 20822788PubMed |
[35] Long, J.S. et al. (2019) Host and viral determinants of influenza A virus species specificity. Nat. Rev. Microbiol. 17, 67–81.
| Host and viral determinants of influenza A virus species specificity.Crossref | GoogleScholarGoogle Scholar | 30487536PubMed |
[36] Laporte, M. et al. (2019) Hemagglutinin cleavability, acid stability, and temperature dependence optimize influenza B virus for replication in human airways. J. Virol. 94, e01430-19.
| Hemagglutinin cleavability, acid stability, and temperature dependence optimize influenza B virus for replication in human airways.Crossref | GoogleScholarGoogle Scholar | 31597759PubMed |
[37] Staller, E. et al. (2019) ANP32 proteins are essential for influenza virus replication in human cells. J. Virol. 93, e00217-19.
| ANP32 proteins are essential for influenza virus replication in human cells.Crossref | GoogleScholarGoogle Scholar | 31217244PubMed |
[38] Zhang, Z. et al. (2020) Selective usage of ANP32 proteins by influenza B virus polymerase: implications in determination of host range. PLoS Pathog. 16, e1008989.
| Selective usage of ANP32 proteins by influenza B virus polymerase: implications in determination of host range.Crossref | GoogleScholarGoogle Scholar | 33045004PubMed |
[39] Sridharan, H. et al. (2010) Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins. J. Biol. Chem. 285, 7852–7856.
| Species specificity of the NS1 protein of influenza B virus: NS1 binds only human and non-human primate ubiquitin-like ISG15 proteins.Crossref | GoogleScholarGoogle Scholar | 20093371PubMed |
[40] Parry, R. et al. (2020) Divergent Influenza-like viruses of amphibians and fish support an ancient evolutionary association. Viruses 12, 1042.
| Divergent Influenza-like viruses of amphibians and fish support an ancient evolutionary association.Crossref | GoogleScholarGoogle Scholar |
[41] Arunkumar G.A.et al. (2021 ) Functionality of the putative surface glycoproteins of the Wuhan spiny eel influenza virus.bioRxiv.
[42] Chen, R. and Holmes, E.C. (2008) The evolutionary dynamics of human influenza B virus. J. Mol. Evol. 66, 655–663.
| The evolutionary dynamics of human influenza B virus.Crossref | GoogleScholarGoogle Scholar | 18504518PubMed |
[43] Vijaykrishna, D. et al. (2015) The contrasting phylodynamics of human influenza B viruses. eLife 4, e05055.
| The contrasting phylodynamics of human influenza B viruses.Crossref | GoogleScholarGoogle Scholar | 25594904PubMed |
[44] WHO (2013) Summary of status of development and availability of influenza B (Victoria and Yamagata lineages) candidate vaccine viruses and potency testing reagents.
[45] Liu, Y. et al. (2019) Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat. Commun. 10, 324.
| Cross-lineage protection by human antibodies binding the influenza B hemagglutinin.Crossref | GoogleScholarGoogle Scholar | 30659197PubMed |
[46] Tan, J. et al. (2018) Universal influenza virus vaccines and therapeutics: where do we stand with influenza B virus? Curr. Opin. Immunol. 53, 45–50.
| Universal influenza virus vaccines and therapeutics: where do we stand with influenza B virus?Crossref | GoogleScholarGoogle Scholar | 29677684PubMed |
[47] Koutsakos, M. et al. (2019) Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 20, 613–625.
| Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses.Crossref | GoogleScholarGoogle Scholar | 30778243PubMed |
[48] Madsen, A. et al. (2020) Human antibodies targeting influenza B virus neuraminidase active site are broadly protective. Immunity 53, 852–863.e7.
| Human antibodies targeting influenza B virus neuraminidase active site are broadly protective.Crossref | GoogleScholarGoogle Scholar | 32976769PubMed |
[49] Belongia, E.A. et al. (2016) Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 16, 942–951.
| Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies.Crossref | GoogleScholarGoogle Scholar | 27061888PubMed |
[50] Gaglani, M. et al. (2021) Effectiveness of trivalent and quadrivalent inactivated vaccines against influenza B in the United States, 2011–2012 to 2016–2017. Clin. Infect. Dis. 72, 1147–1157.
| Effectiveness of trivalent and quadrivalent inactivated vaccines against influenza B in the United States, 2011–2012 to 2016–2017.Crossref | GoogleScholarGoogle Scholar | 32006430PubMed |
Biographies
Dr Marios Koutsakos is an NHMRC Emerging Leadership Investigator and Research Fellow in Professor Kent’s group in the Department of Microbiology and Immunology, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity. His research focuses on understanding immune responses to IBV and the rational design of universal vaccines as well as IBV–host interactions.
Professor Stephen Kent is an infectious diseases physician and viral immunologist in the Department of Microbiology and Immunology, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity. He heads a lab studying immunity to HIV, influenza, COVID-19 and the development of vaccines against such pathogens, from preclinical development to human clinical trials. Stephen remains active in infectious diseases clinical medicine at the Alfred Hospital and Melbourne Sexual Health Centre.