Exploiting the struggle for haem: a novel therapeutic approach against Haemophilus influenzae
Brianna Atto A C , David Gell B and Stephen Tristram AA School of Health Sciences, University of Tasmania, Newnham Drive, Launceston, Tas. 7248, Australia
B School of Medicine, University of Tasmania, 17 Liverpool Street, Hobart, Tas. 7000, Australia
C Email: brianna.atto@utas.edu.au
Microbiology Australia 42(3) 116-119 https://doi.org/10.1071/MA21032
Submitted: 1 July 2021 Accepted: 5 August 2021 Published: 6 September 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Over the past decade, nontypeable Haemophilus influenzae (NTHi) has gained recognition as a major opportunistic pathogen of the respiratory tract that imposes a substantial global burden of disease, owing to a high rate of morbidity and ensuing complications. Further amplifying the global impact of NTHi infections is the increasing spectrum and prevalence of antibiotic resistance, leading to higher rates of treatment failure with first- and second-line antibiotics regimes. The threat of antibiotic resistance was recognised by the World Health Organization in 2017, listing NTHi as a priority pathogen for which new therapies are urgently needed. Despite significant efforts, there are currently no effective vaccine strategies available that can slow the growing burden of NTHi disease. Consequently, alternative preventative or therapeutic approaches that do not rely on antibiotic susceptibility or stable vaccine targets are becoming more attractive. The nutritional dependency for haem at all stages of NTHi pathogenesis exposes a vulnerability that may be exploited for the development of such therapies. This article will discuss the therapeutic potential of strategies that limit NTHi access to this vital nutrient, with particular focus on a novel bacteriotherapeutic approach under development.
NTHi is a major respiratory pathogen for which new therapies are needed
Nontypeable Haemophilus influenzae (NTHi) is a common coloniser of the upper respiratory tract in healthy children (20–80%) and adults (20–30%), the prevalence of which varies considerably across geographical regions1–4. However, in susceptible individuals, NTHi represents a major cause of opportunistic infections in the respiratory tract, namely acute otitis media and sinusitis in children, and lower respiratory tract infections in elderly individuals or those with chronic obstructive pulmonary disease5. Collectively, these infections and subsequent long-term health complications, such as hearing loss or decline in lung function, impart a significant global disease burden5,6. Further amplifying the global impact of NTHi infections is the rapidly expanding spectrum and prevalence of antibiotic resistance, leading to treatment failure with first- and second-line antibiotics5,7. The high morbidity and long-term antibiotic prescription associated with NTHi infections, collectively expose a substantial proportion of the population to antimicrobial agents, driving resistance to a broad-spectrum of antibiotics in the community8,9. The threat of antibiotic resistance was recognised by the World Health Organization in 2017, listing NTHi as a priority pathogen for which new therapies are urgently needed10. Owing to the high genetic heterogeneity and phase-variable expression of conserved antigen targets, there are currently no effective vaccine strategies available that can slow the growing burden of NTHi disease11. Consequently, novel preventative or therapeutic approaches that do not rely on antibiotic susceptibility or stable vaccine targets are becoming more attractive.
Haem-iron acquisition is a major determinant of NTHi pathogenesis
The pathogenesis of NTHi is largely dictated by interactions with host airway epithelia. Although the exact mechanisms are poorly understood, NTHi adhesion and colonisation of the host pharyngeal epithelium, followed by migration to privileged anatomical sites, is required to elicit an infection12. Survival and persistence at the site of infection is mediated by host-cell internalisation, formation of biofilms, or modulation of the immune response that protects bacterial populations from immune or antibiotic clearance13–15. In addition to being an essential growth requirement, access to iron-containing haem plays an important role in the ability of NTHi to perform these interactions and as such, the ability to sequester host-derived sources of haem is a key determinant of pathogenesis16,17. The consequence of NTHi haem starvation, either by disruption of acquisition mechanisms or by environmental restriction, has been demonstrated to attenuate virulence in animal models of invasive disease and otitis media18–21. Strategies that interrupt NTHi acquisition or utilisation of host-derived sources of haem may therefore have a significant impact on the ability of NTHi to cause disease.
A new therapeutic approach: exploitive competition for haem-iron
Recently, we discovered strains of the closely related commensal Haemophilus haemolyticus (Hh) that also inhabit the pharyngeal niche and secrete a novel haemophore (since named haemophilin; Hpl) that elicits potent inhibitory activity against NTHi22,23. Functional and proteomic investigation demonstrated that Hpl is a previously unrecognised haem uptake mechanism of Hh, which inhibits NTHi growth through exploitative competition for haem. We have since conducted several investigations in vitro and in vivo to test the NTHi-inhibitory capacity of Hpl-producing strains of Hh (Hh-Hpl+) and propose their therapeutic utility as a respiratory probiotic.
In vitro investigations
In a broth co-culture system, NTHi strains were outcompeted by Hh-Hpl+ and suffered a complete loss of fitness over subsequent generations24. Similarly, in tissue culture models of nasopharyngeal (D562) and lung epithelia (A549), Hh strains with high levels of hpl expression protected cell monolayers against adhesion and invasion by NTHi25 (Figure 1). Significant inhibition of NTHi adherence and invasion was maintained when Hh-Hpl+ treatment doses were 10–100-fold lower than the NTHi challenge. In both in vitro models, NTHi-inhibitory activity correlated with levels of hpl expression and Hpl protein quantified from competition media. The absence of NTHi-inhibitory activity in a hpl knockout or native non-producing strains confirmed that the inhibitory phenotype was mediated by the ability to produce Hpl.
In vivo investigations
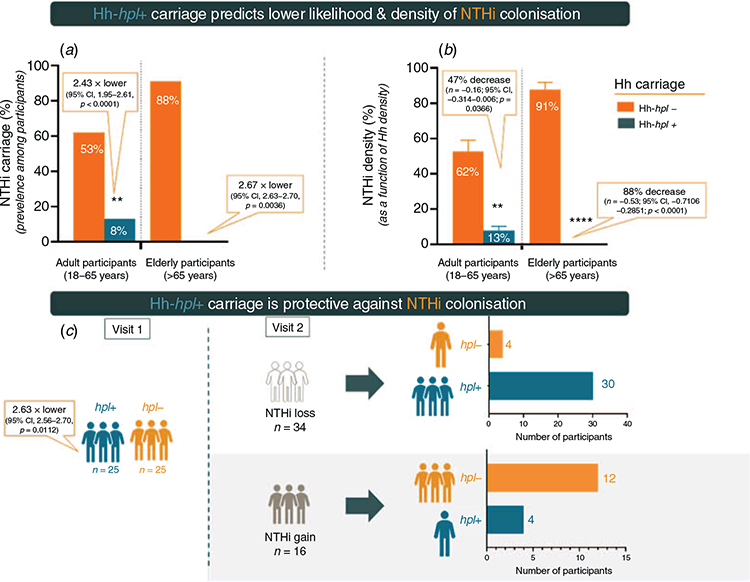
Considering the NTHi-inhibitory activity in vitro we hypothesised that natural pharyngeal carriage of Hh strains with the hpl open reading frame would be associated with a lower prevalence and/or density of NTHi colonisation in healthy individuals. Real-time PCR was used to quantitatively compare the oropharyngeal carriage load of NTHi and Hh populations with the Hh-hpl+ or Hh-hpl– genotype from 257 healthy adults in Australia. Compared to carriage of Hh-hpl– strains, adult (18–65 years) and elderly (>65 years) participants that were colonised with Hh-hpl+ were 2.43 (95% CI, 1.95–2.61; P < 0.0001), or 2.67 times (95% CI, 2.63–2.70; P = 0.0036) less likely to carry NTHi, respectively. Colonisation with high densities of Hh-hpl+ correlated with low NTHi carriage load and a 2.63-times (95% CI, 2.56–2.70, P = 0.0112) lower likelihood of acquiring/maintaining NTHi colonisation status between visits26 (Figure 2).
Potential translation as a respiratory probiotic to prevent NTHi infections
The presence of healthy carriers of NTHi indicates that a complete eradication of NTHi is not necessary to prevent infection. Furthermore, higher NTHi pharyngeal carriage loads are correlated with an increased susceptibility to otitis media in vivo27–30 and an increased severity of airway inflammation, exacerbations, and daily symptoms in chronic obstructive pulmonary disease31,32. Thus, even small reductions in NTHi carriage might have beneficial clinical outcomes. Using a model designed to predict the risk of otitis media in children based on NTHi pharyngeal carriage load30, we could predict a ≈40% decrease in the risk of infection, provided the level of protection conferred by Hpl-producing Hh to model cell lines was preserved in the context of the respiratory tract. Hh also possesses favourable characteristics suited to probiotic applications; it has not been implicated as a causative agent of respiratory tract infection33,34 and as a normal pharyngeal inhabitant, is able to thrive in the niche amongst other microbial inhabitants35. Additionally, probiotic-based therapies have a narrow spectrum of activity that do not damage host tissue, provoke collateral damage to the healthy microbiome or promote enrichment of resistant clones36; properties which make them an asset against the emergence of antibiotic resistance.
In conclusion, Hpl-producing Hh may be a promising respiratory probiotic candidate for the prevention of NTHi infections by inhibiting requisite pharyngeal colonisation.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
The studies informing this work were funded by the Clifford Craig Medical Research Trust (CCF170, CCF192).
References
[1] Chi, D.H. et al. (2003) Nhasopharyngeal reservoir of bacterial otitis media and sinusitis pathogens in adults during wellness and viral respiratory illness. Am. J. Rhinol. 17, 209–214.| Nhasopharyngeal reservoir of bacterial otitis media and sinusitis pathogens in adults during wellness and viral respiratory illness.Crossref | GoogleScholarGoogle Scholar | 12962190PubMed |
[2] Greenberg, D. et al. (2004) Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J. Clin. Microbiol. 42, 4604–4609.
| Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age.Crossref | GoogleScholarGoogle Scholar | 15472316PubMed |
[3] Mackenzie, G.A. et al. (2010) Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect. Dis. 10, 304.
| Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease.Crossref | GoogleScholarGoogle Scholar | 20969800PubMed |
[4] Rawlings, B.A. et al. (2013) Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection. Am. J. Rhinol. Allergy 27, 39–42.
| Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection.Crossref | GoogleScholarGoogle Scholar | 23406599PubMed |
[5] Van Eldere, J. et al. (2014) Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 14, 1281–1292.
| Non-typeable Haemophilus influenzae, an under-recognised pathogen.Crossref | GoogleScholarGoogle Scholar | 25012226PubMed |
[6] Cerquetti, M. and Giufrè, M. (2016) Why we need a vaccine for non-typeable Haemophilus influenzae. Hum. Vaccin. Immunother. 12, 2357–2361.
| Why we need a vaccine for non-typeable Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 27171854PubMed |
[7] Tristram, S. et al. (2007) Antimicrobial resistance in Haemophilus influenzae. Clin. Microbiol. Rev. 20, 368–389.
| Antimicrobial resistance in Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 17428889PubMed |
[8] Venekamp, R.P. et al. (2015) Antibiotics for acute otitis media in children. Cochrane Database Syst. Rev. , .
| Antibiotics for acute otitis media in children.Crossref | GoogleScholarGoogle Scholar | 26465274PubMed |
[9] Ito, M. et al. (2010) Clonal spread of β-lactamase-producing amoxicillin–clavulanate-resistant (BLPACR) strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan. Int. J. Pediatr. Otorhinolaryngol. 74, 901–906.
| Clonal spread of β-lactamase-producing amoxicillin–clavulanate-resistant (BLPACR) strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan.Crossref | GoogleScholarGoogle Scholar | 20846501PubMed |
[10] World Health Organization (2017) Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis.
[11] Jalalvand, F. and Riesbeck, K. (2018) Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev. Vaccines 17, 503–512.
| Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development.Crossref | GoogleScholarGoogle Scholar | 29863956PubMed |
[12] Duell, B.L. et al. (2016) Host–pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen. FEBS Lett. 590, 3840–3853.
| Host–pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen.Crossref | GoogleScholarGoogle Scholar | 27508518PubMed |
[13] Clementi, C.F. and Murphy, T.F. (2011) Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front. Cell. Infect. Microbiol. 1, 1.
| Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract.Crossref | GoogleScholarGoogle Scholar | 22919570PubMed |
[14] Goyal, M. et al. (2015) Cellular interaction of nontypeable Haemophilus influenzae triggers cytotoxicity of infected type II alveolar cells via apoptosis. Pathog. Dis. 73, 1.
| 25227327PubMed |
[15] Moriyama, S. et al. (2009) Formation of biofilm by Haemophilus influenzae isolated from pediatric intractable otitis media. Auris Nasus Larynx 36, 525–531.
| Formation of biofilm by Haemophilus influenzae isolated from pediatric intractable otitis media.Crossref | GoogleScholarGoogle Scholar | 19135325PubMed |
[16] Harrison, A. et al. (2013) Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae. Infect. Immun. 81, 1221–1233.
| Ferric uptake regulator and its role in the pathogenesis of nontypeable Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 23381990PubMed |
[17] Rodríguez-Arce, I. et al. (2019) Moonlighting of Haemophilus influenzae heme acquisition systems contributes to the host airway-pathogen interplay in a coordinated manner. Virulence 10, 315–333.
| Moonlighting of Haemophilus influenzae heme acquisition systems contributes to the host airway-pathogen interplay in a coordinated manner.Crossref | GoogleScholarGoogle Scholar | 30973092PubMed |
[18] Morton, D.J. et al. (2004) Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin–haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media. Microb. Pathog. 36, 25–33.
| Reduced severity of middle ear infection caused by nontypeable Haemophilus influenzae lacking the hemoglobin/hemoglobin–haptoglobin binding proteins (Hgp) in a chinchilla model of otitis media.Crossref | GoogleScholarGoogle Scholar | 14643637PubMed |
[19] Morton, D.J. et al. (2009) The heme-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant. Int. J. Med. Microbiol. 299, 479–488.
| The heme-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant.Crossref | GoogleScholarGoogle Scholar | 19451029PubMed |
[20] Seale, T.W. et al. (2006) Complex role of hemoglobin and hemoglobin-haptoglobin binding proteins in Haemophilus influenzae virulence in the infant rat model of invasive infection. Infect. Immun. 74, 6213–6225.
| Complex role of hemoglobin and hemoglobin-haptoglobin binding proteins in Haemophilus influenzae virulence in the infant rat model of invasive infection.Crossref | GoogleScholarGoogle Scholar | 16966415PubMed |
[21] Szelestey, B.R. et al. (2013) Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity. PLoS Pathog. 9, e1003709.
| Haemophilus responses to nutritional immunity: epigenetic and morphological contribution to biofilm architecture, invasion, persistence and disease severity.Crossref | GoogleScholarGoogle Scholar | 24130500PubMed |
[22] Latham, R.D. et al. (2017) An isolate of Haemophilus haemolyticus produces a bacteriocin-like substance that inhibits the growth of nontypeable Haemophilus influenzae. Int. J. Antimicrob. Agents 49, 503–506.
| An isolate of Haemophilus haemolyticus produces a bacteriocin-like substance that inhibits the growth of nontypeable Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 28242259PubMed |
[23] Latham, R.D. et al. (2020) A heme‐binding protein produced by Haemophilus haemolyticus inhibits non‐typeable Haemophilus influenzae. Mol. Microbiol. 113, 381–398.
| A heme‐binding protein produced by Haemophilus haemolyticus inhibits non‐typeable Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 31742788PubMed |
[24] Atto, B. et al. (2020) In vitro anti-NTHi activity of haemophilin-producing strains of Haemophilus haemolyticus. Pathogens 9, 243.
| In vitro anti-NTHi activity of haemophilin-producing strains of Haemophilus haemolyticus.Crossref | GoogleScholarGoogle Scholar |
[25] Avadhanula, V. et al. (2006) Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74, 830–838.
| Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression.Crossref | GoogleScholarGoogle Scholar | 16428725PubMed |
[26] Atto, B. et al. (2021) Oropharyngeal carriage of hpl-containing Haemophilus haemolyticus predicts lower prevalence and density of NTHi colonisation in healthy adults. Pathogens 10, 577.
| Oropharyngeal carriage of hpl-containing Haemophilus haemolyticus predicts lower prevalence and density of NTHi colonisation in healthy adults.Crossref | GoogleScholarGoogle Scholar | 34068621PubMed |
[27] de Gier, C. et al. (2019) PCV7-and PCV10-vaccinated otitis-prone children in New Zealand have similar pneumococcal and Haemophilus influenzae densities in their nasopharynx and middle ear. Vaccines (Basel) 7, 14.
| PCV7-and PCV10-vaccinated otitis-prone children in New Zealand have similar pneumococcal and Haemophilus influenzae densities in their nasopharynx and middle ear.Crossref | GoogleScholarGoogle Scholar |
[28] Hill, A.T. et al. (2000) Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 109, 288–295.
| Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis.Crossref | GoogleScholarGoogle Scholar | 10996579PubMed |
[29] Hood, D. et al. (2016) A new model for non-typeable Haemophilus influenzae middle ear infection in the Junbo mutant mouse. Dis. Model. Mech. 9, 69–79.
| 26611891PubMed |
[30] Smith-Vaughan, H. et al. (2006) Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 6, 10.
| Measuring nasal bacterial load and its association with otitis media.Crossref | GoogleScholarGoogle Scholar | 16686940PubMed |
[31] Desai, H. et al. (2014) Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 11, 303–309.
| Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease.Crossref | GoogleScholarGoogle Scholar | 24423399PubMed |
[32] Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Research 29, e45.
| A new mathematical model for relative quantification in real-time RT–PCR.Crossref | GoogleScholarGoogle Scholar | 11328886PubMed |
[33] Kirkham, L.-A.S. et al. (2010) Nasopharyngeal carriage of Haemophilus haemolyticus in otitis-prone and healthy children. J. Clin. Microbiol. 48, 2557–2559.
| Nasopharyngeal carriage of Haemophilus haemolyticus in otitis-prone and healthy children.Crossref | GoogleScholarGoogle Scholar |
[34] Murphy, T.F. et al. (2007) Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J. Infect. Dis. 195, 81–89.
| Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae.Crossref | GoogleScholarGoogle Scholar | 17152011PubMed |
[35] De Boeck, I. et al. (2021) Lactic acid bacteria as probiotics for the nose? Microb. Biotechnol. 14, 859–869.
| Lactic acid bacteria as probiotics for the nose?Crossref | GoogleScholarGoogle Scholar | 33507624PubMed |
[36] Hols, P. et al. (2019) Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol. 27, 690–702.
| Mobilization of microbiota commensals and their bacteriocins for therapeutics.Crossref | GoogleScholarGoogle Scholar | 30987817PubMed |
Biographies
Brianna Atto is a final year PhD candidate at the University of Tasmania. She is interested in the development of novel therapeutic strategies that overcome antibiotic resistance to treat and prevent infections. Her research is currently exploring the therapeutic value of upper respiratory commensals to tackle the growing disease burden of the major respiratory pathogen nontypeable Haemophilus influenzae.
David Gell is a protein chemist at the University of Tasmania with an interest in the role of haem-binding proteins at the interface of host-microbe interactions.
Stephen Tristram lectures in Medical Microbiology in the School of Health Sciences at the University of Tasmania and is an Associate Professor and Academic Lead of the Laboratory Medicine Programs. He has been researching antibiotic resistance and pathogenesis in Haemophilus influenzae for over 20 years.