Acute treatment with monoclonal antibodies: their design and their use
Anthony D KelleherThe Kirby Institute, UNSW Sydney, NSW, Australia. Email: akelleher@kirby.unsw.edu.au
Microbiology Australia 42(1) 39-43 https://doi.org/10.1071/MA21011
Submitted: 1 February 2021 Accepted: 18 February 2021 Published: 9 April 2021
Journal Compilation © The Authors 2021 Open Access CC BY, published (by CSIRO Publishing) on behalf of the ASM
Abstract
Passive anti-viral immunotherapy, including monoclonal antibodies (mAb), was identified early as a promising therapeutic avenue for COVID-19 with a rapid development pathway. This has been driven by the lack of existing effective direct acting antivirals for coronaviruses, the marginal clinical impact of remdesivir and the relative lack of efficacy of antivirals against other respiratory pathogens, combined with the failure of repurposed drugs. This review explores the potential utility of mAb targeting SARS-CoV-2, to prevent or treat COVID-19 infection. The use of mAb against host factors (e.g. tocilizumab targeting IL-6 receptor and canakinumab targeting IL1-β) to mitigate the inflammatory response seen in progressive disease will not be considered. This review will primarily consider mAb that have direct neutralising activity via their targeting of the SARS-CoV-2 Spike (S) protein focussing on: the targets of mAb; how they mediate viral neutralisation; their propensity to generate escape mutants; their clinical use so far, and their likely place in the therapeutic play book.
The role of mAb in treatment and prevention of infectious diseases
While monoclonal antibodies (mAb) have a rapidly expanding role in the treatment of cancers and autoimmune conditions, their role in treating or preventing infections has been more constrained, primarily focused on viral infections.
The earliest example of clinical deployment of anti-viral mAbs is the humanised mAb, palivizumab, against Respiratory syncytial virus (RSV) infection in children1. Currently, second generation RSV mAb, engineered to have longer half-lives are being explored as seasonal prophylaxis for RSV in paediatric populations. However, the cost benefit of this approach, even in susceptible populations, has been questioned2. More recently, mAb used as either monotherapy or in cocktails have demonstrated efficacy in treatment of Ebola3, prevention of CMV recrudescence post kidney transplantation4, and prevention of recurrent C. difficle infection5. A range of mAb against Envelope proteins of HIV is being assessed in clinical trials as adjuvant therapy and prophylaxis6,7.
SARS-CoV-2 Spike protein
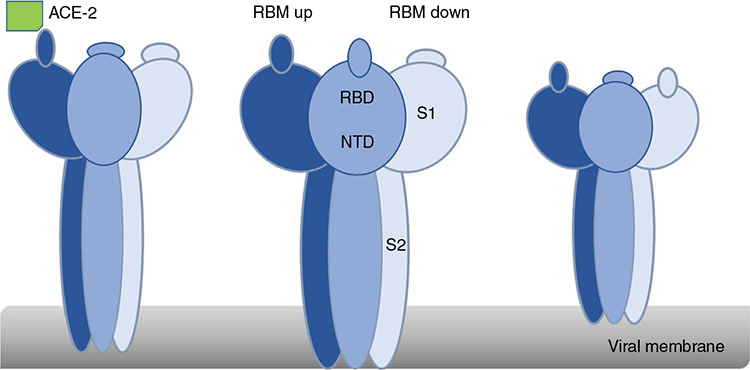
The Spike (S) protein of SARS-CoV-2 is responsible for determining the tropism of the virus, mediating both receptor binding and fusion of viral and target cell membranes (Figure 1). Spike trimeric class I fusion protein contains three identical protomers8. Each protomer has 2 subunits: S1 and S2. S1 contains the receptor binding domain (RBD) that binds to the host ligand, angiotensin-converting enzyme 2 (ACE-2), and an N terminal domain (NTD)9,10. S2 is membrane proximal, containing the viral fusion machinery. Fusion is activated after the binding of RBD to ACE-2 and is dependent on a host encoded serine protease (TMPRSS2)11. Neuropilin may be a cofactor for infection acting after S1/S2 cleavage12.
The RBD can be further divided into two structural regions: a relatively conserved core region and a more variable receptor binding motif (RBM)9. The RBM binds ACE-2 with high affinity. Interestingly, the SARS-CoV Spike protein also binds to ACE-2, while MERS Spike binds dipeptidyl peptidase 413,14, implying potential cross reactivity of neutralising domains among these coronaviruses.
The Spike protein exists in different conformations (Figure 1). The RBM can exist in an ‘up’ or open state, capable of binding ACE2 or in a ‘down’ or closed state which cannot bind the ligand10. Protomers with a single trimer may be in different up or down states simultaneously15 (Figure 1). Binding to ACE2 induces specific conformational changes allowing proteolytic cleavage at the S1/S2 boundary and activating the fusion machinery10.
SARS-CoV-2 neutralising antibodies target the Spike protein
The Spike is highly immunogenic. In natural infection neutralising antibodies are detectable in vitro in >90% of individuals within several weeks of mild infection, though titres vary enormously from person to person16. Memory B cells producing these antibodies are relatively easily isolated from the peripheral blood of convalescent patients17. These antibodies and the B cells that encode them have provided the source of the majority of mAb with potent neutralising activity. Other sources include immunisation of transgenic mice, llamas and alpacas, phage display, production of nanobodies and the re-engineering of cross reactive mAb produced against SARS-CoV (for review see Finkelstein et al.18)
The propensity of humans to efficiently produce neutralising antibodies to SARS-CoV-2 has been recapitulated in vaccine trials with each of the lead constructs, whether based on RNA, protein or recombinant viruses, inducing relatively efficient production of neutralising antibodies in the majority of those immunised19–22.
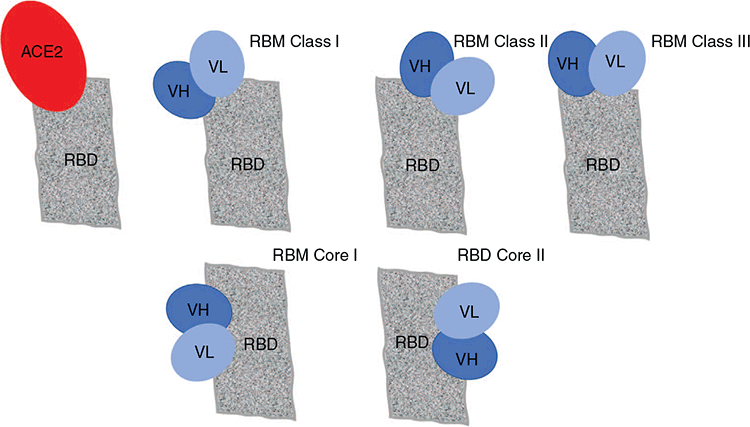
The neutralising domains or epitopes so far identified all impact on the interaction of Spike with ACE-2. Most are associated with the RBD or immediately adjacent regions of the Spike (S) protein. Using the crystal structures of neutralising mAb bound to Spike, initially 3 neutralising domains associated with the RBD and one outside the RBD were described23. With further study of the increasing numbers of monoclonals this classification has been further refined to include six separate functional targets within Spike18 (Figure 2). Understanding these structures will be likely to provide guidance with regards to mechanisms of immune escape or evasion and inform the rational design of cocktails of mAb.
The six neutralising domains and the mechanisms of mAb induced viral neutralisation are summarised below. There are two main subclasses of epitopes, those within the RBM and those outside the RBM.
(a) mAb targeting the RBM: all interfere with ACE-2 binding either directly or indirectly.
(1) RBM class I: This epitope overlaps the area of RBM that directly binds ACE-2 when it is in the up state (Figure 2). Almost all of the mAb targeting this epitope (with the notable exception of REGN1093324) use one of two almost identical VH chains. These antibodies are close to the germline and have short CDRH3 regions18. This lack of somatic hypermutation and affinity maturation likely explains why neutralising activity appears relatively easy to induce following both natural infection and immunisation.
(2) RBM class II: This epitope overlaps with the first epitope, but is available for mAb binding in both the up and down state. MAb targeting this epitope have been derived from convalescent patients, synthetic libraries and vaccinated llamas or alpacas (reviewed in Finkelstein et al.18).
(3) RBM Class III also overlaps with the Class II epitope but involves RBDs of the adjacent protomers in the trimer. MAb targeting this epitope disrupt ACE2 interaction by binding near the trimer apex cross linking the RBM of adjacent protomers, locking them in the closed state (reviewed in Finkelstein et al.18). Convalescent patients are the source of most of these antibodies.
These mAb represent the majority so far described and most are strain specific18.
(b) outside the RBM, within the more conserved core region of S1.
(4) RBD core I epitope is exposed when RBM is in the down position and the spike is in its closed conformation.
(5) RBD core 2 is buried on the opposite face of the core domain and is available for binding only when at least 2 of 3 RBM are in the up conformation.
MAb to both these sites block ACE2 binding by locking Spike into conformations that are unreceptive to ACE-225–27. This group of antibodies includes REGN 1098724, one of the antibodies currently in late-stage clinical trials. These regions are similar in SARS-CoV and SARS-CoV-2 representing cross reactive targets27.
(6) NTD epitope is less well described, but a similar region has been described in MERS24,28,29. MAb interfere with transition of prefusion to post fusion state after ACE-2 binding26.
Immune escape from mAb
SARS-CoV-2 is an RNA virus and like other coronaviruses has the capacity to escape the immune response through a variety of mechanisms including mutational escape. This may be through changes to the:
-
amino acid sequence contributing to an epitope;
-
conformational landscape of an epitope; or
-
capacity for glycosylation at sites that impact on the accessibility of an epitope18.
Several mutations in S change the capacity of the virus to be neutralised by polyclonal sera or mAb16. Direct pressure by mAb on an epitope has driven escape variants in other coronaviruses. Immune escape has been described with the clinical use of convalescent plasma in COVID-19. However, in the latter case escape viruses appear to be less fit and reverted to wild type in vivo in the absence of antibody pressure30. However, immune escape is a risk, especially with strain specific mAb. MAb targeting conserved, cross-reactive viral epitopes may be relatively resistant to escape, while cocktails of mAb that increase the genetic barrier or act synergistically (e.g. asirivimab or REGN10933 (RBM-specific Class I) and imdevimab or REGN10987 (RBD core specific)) may have advantages as the basis of therapeutic or prophylactic interventions.
MAb in clinical trials
Neutralising antibodies have been rapidly identified for development via clinical trial programs using compressed approaches incorporating rapid development through novel design of combined phase 1 to phase 2b/3 trials. Given the burden of severe disease and the lack of directly acting antivirals, the target population has included those with progressive disease, with end-points that have included mortality, time in ICU and time to discharge. Almost in parallel, trials have also targeted those with mild disease, identified early, including those treated as outpatients, with end-points that include measures of disease progression31–33.
Thus far, a single dose of 7.0 g of the mAb Ly-CoC555/bamlanivimab, which binds with high affinity to RBM or placebo has been tested in hospitalised patients with less than 12 days of symptoms, an average age of 61 years and a typical range of comorbidities. The primary end-point was based on a 7-point ordinal scale of disease severity at day 5 post infusion. The trial was stopped early due to lack of efficacy. Further, there was no impact on any secondary end-point, including time to sustained recovery or to time to hospital discharge31.
Two other trials in non-hospitalised patients early in the disease process, one testing a single dose of either LY-CoV555 (0.7 g, 2.8 g or 7.0 g) or placebo and the other testing single doses of a cocktail of 2 antibodies: casirivimab and imdevimab (2.4 g or 8 g) or placebo have reported interim results on completion of their phase 1/2 components. The two trial populations were similar with a median age of mid-40s. MAb or placebo was administered within 4–7 days of onset of symptoms. Both trials demonstrated modest reductions in viral loads of 0.25–0.5 log10 copies/mL, relative to the placebo arms at days 3–11 post infusion. This is on the background of viral loads of 3–5 log10 copies/mL. These early phase trials were not powered to detect differences in clinical end-points; however, both demonstrated potentially encouraging effects, reducing hospitalisations by approximately 50%. However, these effects were based on less than 10 cases in each trial (6% v 1.6% for Ly-CoV55, and 6% v 3% for the cocktail) and seem out of proportion to the viral load reductions. Both trials continue into their phase 3 components, which will provide more definitive insights into their potential to prevent disease progression32,33.
Importantly these trials have not revealed significant safety issues. In particular, there has been no clear safety signals indicating antibody induced enhancement of infection, which has been seen in the treatment of animal models of coronavirus infection with mAb34.
Where to from here?
Multiple SARS-CoV-2 mAb have been identified, many with high affinity. Synergistic combinations are being identified. In general, passive immunotherapies for infections have been more effective as prophylaxis or immediate post exposure prophylaxis. Early indications are that mAb may be most effective if used early and may have a place in protecting those most vulnerable, who may have compromised responses to active vaccination: the frail elderly, or those who are immunocompromised or immunosuppressed. The data from clinical trials in variety of populations will define the therapeutic niche for mAb over the next several months.
Conflicts of interest
The author has no conflicts of interest apart from the receipt of the peer-reviewed funding sources stated in the acknowledgements.
Acknowledgements
ADK is in receipt of four MRFF grants related to; the immunology of COVID-19; the production of passive immunotherapies, both hyperimmune globulin and mAb; and for the development of a COVID-19 vaccine, either as a Chief or Associate Investigator.
References
[1] Man, W.H. et al. (2020) Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: a subanalysis of the MAKI randomised controlled trial. Lancet Respir. Med. 8, 1022–1031.| Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: a subanalysis of the MAKI randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 32203712PubMed |
[2] Blanken, M.O. et al. (2018) Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur. J. Pediatr. 177, 133–144.
| Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants.Crossref | GoogleScholarGoogle Scholar | 29168012PubMed |
[3] Mulangu, S. et al. (2019) A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 381, 2293–2303.
| A randomized, controlled trial of Ebola virus disease therapeutics.Crossref | GoogleScholarGoogle Scholar | 31774950PubMed |
[4] Ishida, J.H. et al. (2017) Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob. Agents Chemother. 61, e01794-16.
| Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients.Crossref | GoogleScholarGoogle Scholar | 27872061PubMed |
[5] Wilcox, M.H. et al. (2017) Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N. Engl. J. Med. 376, 305–317.
| Bezlotoxumab for prevention of recurrent Clostridium difficile infection.Crossref | GoogleScholarGoogle Scholar | 28121498PubMed |
[6] Cohen, Y.Z. et al. (2019) Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: a randomized, phase 1 study. PLoS One 14, e0219142.
| Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: a randomized, phase 1 study.Crossref | GoogleScholarGoogle Scholar | 31393868PubMed |
[7] Crowell, T.A. et al. (2019) Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV 6, e297–e306.
| Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial.Crossref | GoogleScholarGoogle Scholar | 31000477PubMed |
[8] Walls, A.C. et al. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 183, 1735.
| Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein.Crossref | GoogleScholarGoogle Scholar | 33306958PubMed |
[9] Wrapp, D. et al. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263.
| Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation.Crossref | GoogleScholarGoogle Scholar | 32075877PubMed |
[10] Cai, Y. et al. (2020) Distinct conformational states of SARS-CoV-2 spike protein. Science 369, 1586–1592.
| 32694201PubMed |
[11] Hoffmann, M. et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280 e8.
| SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor.Crossref | GoogleScholarGoogle Scholar | 32142651PubMed |
[12] Cantuti-Castelvetri, L. et al. (2020) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860.
| Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity.Crossref | GoogleScholarGoogle Scholar | 33082293PubMed |
[13] Li, W. et al. (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454.
| Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus.Crossref | GoogleScholarGoogle Scholar | 14647384PubMed |
[14] Wang, N. et al. (2013) Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 23, 986–993.
| Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4.Crossref | GoogleScholarGoogle Scholar | 23835475PubMed |
[15] Wang, Q. et al. (2020) Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181, 894–904.e9.
| Structural and functional basis of SARS-CoV-2 entry by using human ACE2.Crossref | GoogleScholarGoogle Scholar | 32275855PubMed |
[16] Tea, F. et al. (2021) SARS-CoV-2 neutralizing antibodies; longevity, breadth, and evasion by emerging viral variants. medRxiv. , .
| SARS-CoV-2 neutralizing antibodies; longevity, breadth, and evasion by emerging viral variants.Crossref | GoogleScholarGoogle Scholar |
[17] Rodda, L.B. et al. (2021) Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 184, 169–183.e17.
| Functional SARS-CoV-2-specific immune memory persists after mild COVID-19.Crossref | GoogleScholarGoogle Scholar | 33296701PubMed |
[18] Finkelstein, M.T. et al. (2021) Structural analysis of neutralizing epitopes of the SARS-CoV-2 spike to guide therapy and vaccine design strategies. Viruses 13, 134.
| Structural analysis of neutralizing epitopes of the SARS-CoV-2 spike to guide therapy and vaccine design strategies.Crossref | GoogleScholarGoogle Scholar | 33477902PubMed |
[19] Jackson, L.A. et al. (2020) An mRNA vaccine against SARS-CoV-2: preliminary report. N. Engl. J. Med. 383, 1920–1931.
| An mRNA vaccine against SARS-CoV-2: preliminary report.Crossref | GoogleScholarGoogle Scholar | 32663912PubMed |
[20] Folegatti, P.M. et al. (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478.
| Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.Crossref | GoogleScholarGoogle Scholar | 32702298PubMed |
[21] Zhu, F.C. et al. (2020) Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396, 479–488.
| Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial.Crossref | GoogleScholarGoogle Scholar | 32702299PubMed |
[22] Walsh, E.E. et al. (2020) Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 383, 2439–2450.
| Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates.Crossref | GoogleScholarGoogle Scholar | 33053279PubMed |
[23] Barnes, C.O. et al. (2020) SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588, 682–687.
| SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies.Crossref | GoogleScholarGoogle Scholar | 33045718PubMed |
[24] Hansen, J. et al. (2020) Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014.
| Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail.Crossref | GoogleScholarGoogle Scholar | 32540901PubMed |
[25] Huo, J. et al. (2020) Neutralization of SARS-CoV-2 by destruction of the prefusion spike. Cell Host Microbe 28, 497.
| Neutralization of SARS-CoV-2 by destruction of the prefusion spike.Crossref | GoogleScholarGoogle Scholar | 32910920PubMed |
[26] Liu, L. et al. (2020) Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584, 450–456.
| Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike.Crossref | GoogleScholarGoogle Scholar | 32698192PubMed |
[27] Yuan, M. et al. (2020) A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 368, 630–633.
| A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV.Crossref | GoogleScholarGoogle Scholar | 32245784PubMed |
[28] McCallum M.et al2021 N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2.bioRxiv.
[29] Wang, N. et al. (2019) Structural definition of a neutralization-sensitive epitope on the MERS-CoV S1-NTD. Cell Rep. 28, 3395–3405 e6.
| Structural definition of a neutralization-sensitive epitope on the MERS-CoV S1-NTD.Crossref | GoogleScholarGoogle Scholar | 31553909PubMed |
[30] Kemp S.A.et al. (2020 ) Neutralising antibodies in Spike mediated SARS-CoV-2 adaptation.medRxiv.
[31] ACTIV-3/TICO LY-CoV555 Study Group et al. (2020) A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N. Engl. J. Med. , .
| 33394576PubMed |
[32] Chen, P. et al. (2021) SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 384, 229–237.
| SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19.Crossref | GoogleScholarGoogle Scholar | 33113295PubMed |
[33] Weinreich, D.M. et al. (2021) REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 384, 238–251.
| REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19.Crossref | GoogleScholarGoogle Scholar | 33332778PubMed |
[34] Wang, S.F. et al. (2014) Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 451, 208–214.
| Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins.Crossref | GoogleScholarGoogle Scholar | 25073113PubMed |
Biography
Professor Anthony (Tony) Kelleher is a clinician scientist and Director of the Kirby Institute at UNSW Sydney. He is also head of the Immunovirology and Pathogenesis program at the Kirby Institute and Principal of the Infection Immunology and Inflammation Theme at UNSW Sydney. As a clinical academic at St Vincent’s Hospital Sydney, Professor Kelleher is responsible for clinical care of patients with HIV infection and autoimmune diseases as well as providing consultative input into the running of the NSW State HIV Reference laboratory.