The dynamic landscape of bat borne zoonotic viruses in Australia
Kim Halpin A and David N Durrheim BA Australian Animal Health Laboratory
5 Portarlington Road
East Geelong, Vic. 3219, Australia
Email: kim.halpin@csiro.au
B University of Newcastle
School of Medicine and Public
Health, Callaghan Campus
Newcastle, NSW 2287, Australia
Microbiology Australia 41(1) 6-9 https://doi.org/10.1071/MA20003
Published: 28 February 2020
This review discusses the history, epidemiology, diagnostics, clinical presentation in humans, as well as control and prevention measures, of the high-profile viruses Hendra virus (HeV) and Australian bat lyssavirus (ABLV). Since the discovery of HeV and ABLV in the 1990s, these viruses have only caused disease in areas where spill-over hosts, including humans, encounter the reservoir host.
Bats
Australia is home to over 90 species of bats, covering many different habitats. All but eight species belong to the suborder Microchiroptera (microbats). See Table 1 for a list of the eight species from the suborder Megachiropteran (megabats) found on mainland Australia, four of which belong to the genus Pteropus (commonly called flying foxes or fruit bats). Figure 1 provides a link to an interactive map showing flying fox camps in Australia.

|
The distribution of bats in Australia has changed over time. As their habitats are destroyed, many have been forced to adapt to life on the urban fringe. There are many successful flying fox camps in the heart of large and smaller cities across Australia – Brisbane, Sydney, Melbourne, Geelong and Cairns to name a few. In the past 10 years, we have seen the southern limit of the black flying fox (Pteropus alecto) distribution extend further south, and the south-western limit of the grey headed flying fox (Pteropus poliocephalus) distribution extend across into South Australia as well. By contrast, the very small footprint of the spectacled flying fox (Pteropus conspicillatus) in far north Queensland, is predicted to get even smaller over time1. The black flying fox will most likely fill this void. The ecological drivers behind these changes are complex but are highly likely to include loss of natural habitat, changes to food availability and warming climates.
Hendra virus
Since it was first described in Australia in 1994, HeV has caused horse and human illness and deaths. A high prevalence of neutralizing antibodies to HeV in bats of the genus Pteropus, and the isolation of Hendra virus from the same genus, confirmed flying foxes as reservoir hosts for this virus2. All four species of pteropus bats can be infected (Table 1). From recent work it appears that the risk of a spill-over event is greatest when either the black flying fox or the spectacled flying-fox is present3. The reservoir host appears to co-exist with this virus in complete harmony. The virus spreads easily amongst flying-foxes with the HeV seroprevalence in flying-fox colonies fluctuating over time and geography. The theory of viral co-evolution with chiropteran hosts has been previously suggested, and all field observations and experimental evidence to date supports this hypothesis4. Figure 1 provides a link to the results of Hendra virus research conducted in Australia, as well as information for horse owners.
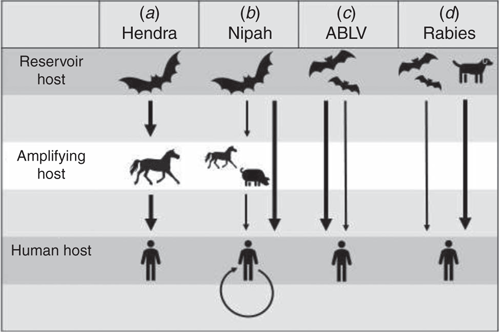
Figure 2 compares the routes of transmission for HeV and ABLV and other closely related bat viruses which result in human infection. For HeV, horses are the main spill-over host and serve as amplifying hosts, capable of infecting humans. The disease in horses exhibits seasonality with more spill-over events occurring in winter. Since it was discovered in 1994, only 95 horses have died to date. Horses in paddocks where flying foxes either roost or come to feed, are at risk of exposure to infection. Infection in horses most likely occurs after close contact with bat urine and birthing material which contain sufficiently high titres of virus to infect a horse15.
|
Extreme care must be taken in the handling of samples collected for HeV diagnostic testing. HeV is a Biosafety level four (BSL4) agent, in recognition of its status as one of the most dangerous zoonotic agents. Safety precautions during field investigation and in the laboratory are of paramount importance. Blood collected in an EDTA tube, as well as tissue samples from lung, spleen and kidney can be tested by PCR, which is specific for Hendra virus. For the detection of antibodies to HeV in serum either a virus neutralisation test (VNT) or an ELISA can be conducted.
There have been seven known human cases, four of which were fatal. Clinical presentations ranged from self-limiting influenza-like illness, to severe pneumonia and encephalitis16. The typical incubation period in humans was 5–21 days, although one person experienced an initial aseptic meningitis, appeared to fully recover, but succumbed to severe encephalitis 13 months later. All human cases had high level exposure to infected horse secretions or tissues16. Human to human HeV transmission has not been described to date, unlike the closely related Nipah virus where human to human spread has been reported overseas10.
In 2012 a vaccine was released for use in horses, to prevent infection with Hendra virus. This subunit vaccine based on the G glycoprotein of Hendra virus is very immunogenic and affords protection against HeV challenge in experimental infections17. Since the vaccine was released, no vaccinated horse has been diagnosed with Hendra virus infection. Vaccination of horses provides a public health and workplace health and safety benefit by reducing the risk of HeV transmission from horses to humans and other susceptible animals. Whenever HeV infection is suspected, even in vaccinated horses, appropriate biosecurity precautions, including personal protective equipment (PPE), should be used by all people in contact with sick horses.
ABLV
In 1996 a five-month-old female black flying fox was found under a fig tree in Wollongbar, NSW, unable to fly. From this bat, a virus with close serologic and genetic relationships to members of the Lyssavirus genus of the family Rhabdoviridae was isolated18. ABLV has since been found in all four flying fox species and in one species of microbat, the yellow-bellied sheath tailed bat13. It is assumed that all Australian bat species have the potential to carry and transmit ABLV. ABLV is transmitted to humans by bites or scratches from an infected bat.
No laboratory tests are currently available to diagnose ABLV in humans before the onset of clinical disease. In the early stages of disease, saliva and cerebrospinal fluid (CSF) can be tested by PCR. Antibody testing can also be performed on CSF. A positive serum antibody test is diagnostic of lyssavirus infection provided the person has never been immunised against rabies and may assist in the diagnosis of lyssavirus clinical disease. Any negative test on a symptomatic person is not definitive, as viral shedding in body secretions is intermittent and early tests may be negative for antibody. Therefore, repeat testing is often indicated.
For post mortem testing in humans and animals including bats, the standard diagnostic techniques include positive fluorescent antibody test (FAT) and PCR on fresh brain smears, and PCR from tissues.
ABLV infection has resulted in three human deaths, two adults and an eight-year-old child, in Queensland, Australia; 1996, 1998 and 201319. Transmission from flying foxes and an insectivorous microbat were implicated, with all three cases displaying features of encephalitic (furious) rabies before their demise. The incubation period is thought to mirror rabies (usually 3–8 weeks, but potentially as short as a few days or as long as several years). Exposure through wounds close to the central nervous system on the head and neck or richly innervated areas like the fingers, carry an increased infection risk and may result in a shorter incubation period. In furious rabies, prodromal symptoms may precede sensorineural dysfunction, with progression to hyperactivity, aerophobia and/or hydrophobia, followed by convulsions20. The clinical course following symptom onset is usually rapid, almost invariably progressing to death within a few days.
Regarding prevention, the key strategy is for untrained and unvaccinated people to avoid handling bats. Public health authorities promote this message particularly during periods of high bat activity, including fruiting periods, and heat stress events when bats and especially pups drop to the ground. Prompt post-exposure vigorous wound cleaning, submission of the bat’s brain for ABLV testing (where possible), rabies vaccination and administration of rabies immunoglobulin, are recommended following bat bites or scratches. Figure 1 provides a link to statistics on ABLV surveillance in Australia, as well as answers to questions about flying foxes and possible impacts on human health from NSW Department of Health.
Conclusions
ABLV and HeV can both cause an encephalitis syndrome in humans, sometimes with significant delay or recrudescence. Bats are the reservoirs of these viruses and may well be implicated in transmission of yet to be identified zoonotic pathogens. As the distribution of these reservoir hosts changes, so too does the risk of spill-over events that may involve humans.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Martin, G. et al. (2018) Climate change could increase the geographic extent of Hendra virus spillover risk. EcoHealth 15, 509–525.| Climate change could increase the geographic extent of Hendra virus spillover risk.Crossref | GoogleScholarGoogle Scholar | 29556762PubMed |
[2] Halpin, K. et al. (2000) Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Gen. Virol. 81, 1927–1932.
| Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus.Crossref | GoogleScholarGoogle Scholar | 10900029PubMed |
[3] Edson, D. et al. (2015) Routes of Hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS One 10, e0140670.
| Routes of Hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk.Crossref | GoogleScholarGoogle Scholar | 26625128PubMed |
[4] Halpin, K. et al. (2007) Emerging viruses: coming in on a wrinkled wing and a prayer. Clin. Infect. Dis. 44, 711–717.
| Emerging viruses: coming in on a wrinkled wing and a prayer.Crossref | GoogleScholarGoogle Scholar | 17278066PubMed |
[5] Kirkland, P.D. et al. (2015) Hendra virus infection in dog, Australia, 2013. Emerg. Infect. Dis. 21, 2182–2185.
| Hendra virus infection in dog, Australia, 2013.Crossref | GoogleScholarGoogle Scholar | 26583697PubMed |
[6] Middleton, D.J. et al. (2017) Experimental Hendra virus infection of dogs: virus replication, shedding and potential for transmission. Aust. Vet. J. 95, 10–18.
| Experimental Hendra virus infection of dogs: virus replication, shedding and potential for transmission.Crossref | GoogleScholarGoogle Scholar | 28124415PubMed |
[7] Ching, P.K. et al. (2015) Outbreak of henipavirus infection, Philippines, 2014. Emerg. Infect. Dis. 21, 328–331.
| Outbreak of henipavirus infection, Philippines, 2014.Crossref | GoogleScholarGoogle Scholar | 25626011PubMed |
[8] Chua, K.B. et al. (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435.
| Nipah virus: a recently emergent deadly paramyxovirus.Crossref | GoogleScholarGoogle Scholar | 10827955PubMed |
[9] Rahman, M.A. et al. (2012) Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 12, 65–72.
| Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008.Crossref | GoogleScholarGoogle Scholar | 21923274PubMed |
[10] Luby, S.P. et al. (2009) Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007. Emerg. Infect. Dis. 15, 1229–1235.
| Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007.Crossref | GoogleScholarGoogle Scholar | 19751584PubMed |
[11] Yadav, P.D. et al. (2019) Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018. Emerg. Infect. Dis. 25, 1003–1006.
| Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018.Crossref | GoogleScholarGoogle Scholar | 31002049PubMed |
[12] Annand, E.J. and Reid, P.A. (2014) Clinical review of two fatal equine cases of infection with the insectivorous bat strain of Australian bat lyssavirus. Aust. Vet. J. 92, 324–332.
| Clinical review of two fatal equine cases of infection with the insectivorous bat strain of Australian bat lyssavirus.Crossref | GoogleScholarGoogle Scholar | 25156050PubMed |
[13] Si, D. et al. (2016) Potential exposures to Australian bat lyssavirus notified in Queensland, Australia, 2009–2014. PLoS Negl. Trop. Dis. 10, e0005227.
| Potential exposures to Australian bat lyssavirus notified in Queensland, Australia, 2009–2014.Crossref | GoogleScholarGoogle Scholar | 28033365PubMed |
[14] WHO (2013) Expert consultation on rabies second report. Geneva: WHO.
[15] Halpin, K. et al. (2011) Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 85, 946–951.
| Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission.Crossref | GoogleScholarGoogle Scholar | 22049055PubMed |
[16] Playford, E.G. et al. (2010) Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg. Infect. Dis. 16, 219–223.
| Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008.Crossref | GoogleScholarGoogle Scholar | 20113550PubMed |
[17] Middleton, D. et al. (2014) Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg. Infect. Dis. 20, 372–379.
| Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health.Crossref | GoogleScholarGoogle Scholar | 24572697PubMed |
[18] Fraser, G.C. et al. (1996) Encephalitis caused by a Lyssavirus in fruit bats in Australia. Emerg. Infect. Dis. 2, 327–331.
| Encephalitis caused by a Lyssavirus in fruit bats in Australia.Crossref | GoogleScholarGoogle Scholar | 8969249PubMed |
[19] Young, M.K. and McCall, B.J. (2010) Potential exposure to Australian bat lyssavirus in South East Queensland: what has changed in 12 years? Commun. Dis. Intell. 34, 334–338.
[20] Merritt, T. et al. (2018) Australian bat lyssavirus. Aust. J. Gen. Pract 47, 93–96.
| Australian bat lyssavirus.Crossref | GoogleScholarGoogle Scholar | 29621841PubMed |
Biographies
Kim Halpin leads the Pathology and Pathogenesis Group at the Australian Animal Health Laboratory (AAHL). She is a veterinary graduate from the University of Queensland and has worked in research, diagnostic and commercial settings. Her focus has been on emerging infectious diseases. After completing her PhD on Hendra virus, Kim did her postdoc at the Centres for Disease Control and Prevention in Atlanta, USA, working on a Nipah virus reverse genetics project. In 2003 she returned to Australia and conducted henipavirus experimental transmission studies at AAHL. Kim is the OIE Reference Expert for Hendra virus and Nipah virus.
David Durrheim is Conjoint Professor of Public Health Medicine, University of Newcastle, and Director - Health Protection, Hunter New England Health. He is a Public Health Physician with an established track record in conducting research that has an operational focus and is translational in nature. Professor Durrheim is an outspoken advocate for equitable global access to effective public health measures, particularly immunisation. He has been instrumental in developing novel surveillance systems to detect and facilitate response to emerging infectious disease risks.



