Biodiscovery and the Queensland Plant Pathology Herbarium
Roger G Shivas A C , Dean R Beasley A , Kaylene Bransgrove A , Yu Pei Tan A and Geoff Bulow BA Queensland Plant Pathology Herbarium (BRIP), Department of Agriculture and Fisheries, Ecosciences Precinct, Dutton Park, Qld 4102, Australia
B Technology Commercialisation Fund Project (TCF), Department of Agriculture and Fisheries, 41 George Street, Brisbane, Qld 4000, Australia
C Email: roger.shivas@daf.qld.gov.au
Microbiology Australia 40(3) 134-137 https://doi.org/10.1071/MA19036
Published: 16 September 2019
The Queensland Plant Pathology Herbarium (BRIP) and its associated collection of fungal and bacterial cultures have obtained Australian and international recognition as critical resources for agricultural research and plant biosecurity. For decades, many key agricultural and mycological studies published in international journals have examined Australian reference specimens obtained from BRIP. The Queensland Plant Pathology Herbarium is now seeking to reposition itself as a significant provider of unique Australian cultures. This ambitious journey could unlock the potential of Australian specimens to provide novel bioactive natural products that may benefit society.
The Queensland Plant Pathology Herbarium was established in 1901. In 1966, the herbarium was registered under the acronym BRIP by its first curator, Dr John Alcorn (b. 1937), who held the position for almost 40 years (1956–1997). BRIP has a unique collection of microfungi that dates back to the 1850s. The earliest plant pathogens found in Queensland date back to the 1800s, when the botanist Frederick Manson Bailey (1827–1915) collected and sent specimens to European mycologists for study. Bailey conducted numerous expeditions across Queensland and collected more than 1000 specimens of fungi including plant pathogens. Bailey’s collection was housed in the Queensland Herbarium, Mount Coot-tha, Brisbane until 1968 when it was transferred to BRIP.
John Alcorn was a taxonomic mycologist who worked in an era when fungal taxonomy was determined by morphological differences. John Alcorn discovered and described many new species and genera of plant pathogenic fungi in Queensland. Importantly, the type specimens were deposited in BRIP with ex-type cultures preserved in the associated culture collection. John’s contribution to Australian taxonomic mycology has been recognised by the generic name Johnalcornia1, as well as the species Avettaea alcornii2, Colletotrichum alcornii3, Curvularia alcornii4, Muyocopron alcornii5, Teratosphaeria alcornii6 and Ustilago alcornii7. John Alcorn also introduced a computerised database that catalogued about 50 000 specimens at the time of his retirement in 1997.
Digitisation of plant disease records began in the early 1990s using the software Titan 3.2. The Plant Pathology Herbarium then migrated to KE Texpress in 1997 with an upgrade to KE EMu in 2002. In the late 1990s, John Alcorn and the curators of the plant pathology herbarium in New South Wales and Victoria formed the National Collection of Fungi (NCOF) and agreed to use the same database and standardised fields to enable data interchange8.
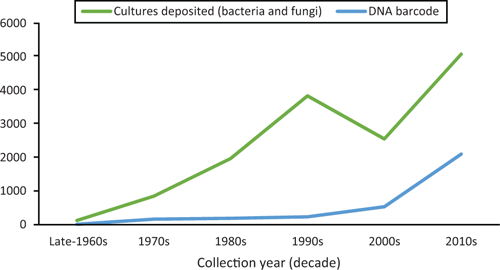
Since John Alcorn’s retirement, BRIP has grown to about 90 000 herbarium specimens and 23 000 living cultures preserved in a metabolically inactive state (Figure 1). Many of the herbarium specimens represent groups of obligate plant pathogens, e.g. rusts, smuts, downy mildews and powdery mildews, which cannot be cultured. Since the late 1990s the taxonomy of fungi and bacteria has been based on molecular phylogenetic analysis. Significantly, in 2012 the nuclear internal transcribed spacer (ITS) region, was recognised as the universal DNA barcode marker for most fungi9. Over the past two decades, staff at BRIP have taken a prominent role in using molecular methods to unravel cryptic diversity in plant pathogenic fungi. This work has led to revisions and taxonomic contributions to our knowledge of the diversity of Australian downy mildews10–12, smuts13–15, rusts16–19 and some important plant pathogenic ascomycetes (Bipolaris20, Botryosphaeriaceae21,22, Colletotrichum23,24, Curvularia1,25, Diaporthe26,27).
|
The Queensland Plant Pathology Herbarium holds authoritatively identified specimens of most of the known plant pathogenic fungi in Queensland. The Office of the Chief Scientist has recognised BRIP as a State Significant Collection. The collection records for almost 60 000 specimens are available through the website DAF Biological Collections (https://collections.daf.qld.gov.au/).
Interest in bacteria and fungi as an untapped resource for novel bioactive natural products (enzymes, proteins, primary and secondary metabolites) has gained momentum in recent years. Over 50% of all pharmaceutical drugs currently on the market are directly derived from or inspired by natural products28. This resurgence was considered due to novel approaches towards bioprospecting, which included: (1) the targeting of species not known to produce bioactive natural products; (2) exploring non-traditional environmental niches and methods for the isolation of species; and (3) genome mining29. Based on these criteria, many specimens in BRIP are potential candidates for bioprospecting.
The culture collection associated with BRIP holds about 22 000 fungal cultures and 1500 bacterial cultures. Some of the significant taxonomic groups represented amongst BRIP cultures are (1) entomopathogenic fungi from insects and spiders found in tropical Australian rainforests; (2) diverse yeasts found on the leaves of Australian native plants, including the spectacular red and orange ballistosporic species that discharge their spores into the environment with an acceleration of 25 000 times the force of gravity; (3) ascomycetes (helminthosporioid and cercosporoid fungi) that cause specific diseases on native plants, and (4) endophytic fungi that live symbiotically inside the plant tissues of rainforest plants without causing disease.
The Queensland Plant Pathology Herbarium has many unique specimens. The collection houses 493 holotypes (the specimen on which the description and name of the species is based), of which 129 are available as ex-holotype cultures. The genera with most ex-holotype cultures in BRIP are Bipolaris (16 species), Curvularia (24), Diaporthe (18) and Pseudocercospora (7). Each of these genera contain many well-known plant pathogenic ascomycetes. Recent taxonomic studies of the helminthosporioid genera (Bipolaris, Curvularia, Drechslera, Exserohilum)1,20,25,30,31 that mostly cause leaf spots on grasses, were built upon the work started by Alcorn 60 years ago.
During 2018, as part of a Queensland Government initiative called the Technology Commercialisation Fund (TCF), the provision of cultures from BRIP to organisations or companies for commercial purposes rather than solely for traditional research purposes was investigated. The TCF aims to find opportunities and pathways for the commercialisation of research outputs from the Department of Agriculture and Fisheries (DAF). The aim of the culture collection project is to make BRIP less financially dependent on government funding and use funds generated to increase staff levels for the effective long-term maintenance of the collection. Through an open Expression of Interest (EOI) process in late 2018, three companies made submissions to express their interest in accessing the collection. DAF and representatives from these companies are currently in discussion regarding terms and access arrangements. All parties interested in accessing the cultures in BRIP have to comply with the Queensland Government legislation, the Biodiscovery Act 2004. A Biodiscovery Plan approved by the Department of the Environment and Science and a Benefit Sharing Agreement (or Commercialisation Agreement) approved by the Minister for Science are required.
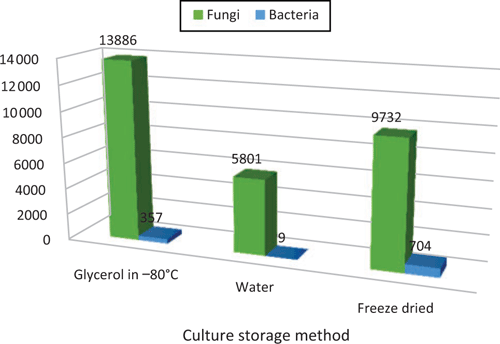
BRIP is currently reviewing the collection and testing of specimens to estimate their viability and biological diversity. Cultures in BRIP have been stored by several methods, including in freeze-dried ampoules; under water in vials; and under glycerol at –80°C (Figure 2). Initial results show a high level of viability and diversity, although there are some notable failures, such as the long-term storage of culturable of the oomycetes Phytophthora and Pythium.
|
Conclusion
BRIP is undergoing a transformation to make the collection more open and accessible for companies and researchers to undertake biodiscovery research. If successful this may lead to the development of commercial products that benefit society in diverse ways, including crop protection, drug development, and the production of fine chemicals. With the collection being primarily sourced from subtropical and tropical environments it offers a range of potentially unique cultures for research and development. The Department is willing to enter in to more arrangements should other companies want to access the collection.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Dr Sharon Bishop-Hurley for her contribution to the TCF project.
References
[1] Tan, Y.P. et al. (2014) Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas. Plant Pathol. 43, 589–603.| Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar |
[2] Sivanesan, A. and Sutton, B.C. (1985) Microfungi on Xanthorrhoea. Trans. Br. Mycol. Soc. 85, 239–255.
| Microfungi on Xanthorrhoea.Crossref | GoogleScholarGoogle Scholar |
[3] Crouch, J.A. (2014) Colletotrichum caudatum s.l. is a species complex. IMA Fungus 5, 17–30.
| Colletotrichum caudatum s.l. is a species complex.Crossref | GoogleScholarGoogle Scholar | 25083403PubMed |
[4] Manamgoda, D.S. et al. (2012) Two new species of Curvularia from nothern Thailand. Sydowia 64, 255–266.
[5] Hernández-Restrepo, M. et al. (2019) Re-evaluation of Mycoleptodiscus species and morphologically similar fungi. Persoonia 42, 205–227.
| Re-evaluation of Mycoleptodiscus species and morphologically similar fungi.Crossref | GoogleScholarGoogle Scholar |
[6] Vánky, K. (2000) Taxonomical studies on Ustilaginales. XX. Mycotaxon 74, 161–215.
[7] Crous, P.W. et al. (2009) Unravelling Mycosphaerella: do you believe in genera? Persoonia 23, 99–118.
| Unravelling Mycosphaerella: do you believe in genera?Crossref | GoogleScholarGoogle Scholar | 20198164PubMed |
[8] Shivas, R.G. et al. (2006) Specimen-based databases of Australian plant pathogens: past, present and future. Australas. Plant Pathol. 35, 195–198.
| Specimen-based databases of Australian plant pathogens: past, present and future.Crossref | GoogleScholarGoogle Scholar |
[9] Schoch, C.L. et al. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 109, 6241–6246.
| Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi.Crossref | GoogleScholarGoogle Scholar | 22454494PubMed |
[10] McTaggart, A.R. et al. (2015) Plasmopara sphagneticolae sp. nov. (Peronosporales) on Sphagneticola (Asteraceae) in Australia. Australas. Plant Pathol. 44, 81–85.
| Plasmopara sphagneticolae sp. nov. (Peronosporales) on Sphagneticola (Asteraceae) in Australia.Crossref | GoogleScholarGoogle Scholar |
[11] Shivas, R.G. et al. (2012) Peronosclerospora australiensis sp. nov. and Peronosclerospora sargae sp. nov., two newly recognised downy mildews in northern Australia, and their biosecurity implications. Australas. Plant Pathol. 41, 125–130.
| Peronosclerospora australiensis sp. nov. and Peronosclerospora sargae sp. nov., two newly recognised downy mildews in northern Australia, and their biosecurity implications.Crossref | GoogleScholarGoogle Scholar |
[12] Thines, M. et al. (2015) Baobabopsis, a new genus of graminicolous downy mildews from tropical Australia, with an updated key to the genera of downy mildews. IMA Fungus 6, 483–491.
| Baobabopsis, a new genus of graminicolous downy mildews from tropical Australia, with an updated key to the genera of downy mildews.Crossref | GoogleScholarGoogle Scholar | 26734551PubMed |
[13] Vánky, K. and Shivas, R.G. (2008) Fungi of Australia: The Smut Fungi. Australian Biological Resources Study/CSIRO Publishing.
[14] Li, Y.-M. et al. (2017) Ten new species of Macalpinomyces on Eriachne in northern Australia. Mycologia 109, 408–421.
| Ten new species of Macalpinomyces on Eriachne in northern Australia.Crossref | GoogleScholarGoogle Scholar | 28636469PubMed |
[15] Li, Y.-M. et al. (2019) Diversity of Moesziomyces (Ustilaginales, Ustilaginomycotina) on Echinochloa and Leersia (Poaceae). MycoKeys 52, 1–16.
| Diversity of Moesziomyces (Ustilaginales, Ustilaginomycotina) on Echinochloa and Leersia (Poaceae).Crossref | GoogleScholarGoogle Scholar | 31139007PubMed |
[16] McTaggart, A.R. et al. (2015) A co-evolutionary relationship exists between Endoraecium (Pucciniales) and its Acacia hosts in Australia. Persoonia 35, 50–62.
| A co-evolutionary relationship exists between Endoraecium (Pucciniales) and its Acacia hosts in Australia.Crossref | GoogleScholarGoogle Scholar | 26823628PubMed |
[17] Shivas, R.G. et al. (2014) Online identification guides for Australian smut fungi (Ustilaginomycotina) and rust fungi (Pucciniales). IMA Fungus 5, 195–202.
| Online identification guides for Australian smut fungi (Ustilaginomycotina) and rust fungi (Pucciniales).Crossref | GoogleScholarGoogle Scholar | 25734028PubMed |
[18] Doungsa-ard, C. et al. (2018) Diversity of gall-forming rusts (Uromycladium, Pucciniales) on Acacia in Australia. Persoonia 40, 221–238.
| Diversity of gall-forming rusts (Uromycladium, Pucciniales) on Acacia in Australia.Crossref | GoogleScholarGoogle Scholar | 30505002PubMed |
[19] McTaggart, A.R. et al. (2016) Host jumps shaped the diversity of extant rust fungi (Pucciniales). New Phytol. 209, 1149–1158.
| Host jumps shaped the diversity of extant rust fungi (Pucciniales).Crossref | GoogleScholarGoogle Scholar | 26459939PubMed |
[20] Tan, Y.P. et al. (2016) Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP). Mycol. Prog. 15, 1203–1214.
| Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP).Crossref | GoogleScholarGoogle Scholar |
[21] Tan, Y.P. et al. (2019) Australian cultures of Botryosphaeriaceae held in Queensland and Victoria plant pathology herbaria revisited. Australas. Plant Pathol. 48, 25–34.
| Australian cultures of Botryosphaeriaceae held in Queensland and Victoria plant pathology herbaria revisited.Crossref | GoogleScholarGoogle Scholar |
[22] Burgess, T.I. et al. (2019) Current status of the Botryosphaeriaceae in Australia. Australas. Plant Pathol. 48, 35–44.
| Current status of the Botryosphaeriaceae in Australia.Crossref | GoogleScholarGoogle Scholar |
[23] Shivas, R.G. and Tan, Y.P. (2009) A taxonomic re-assessment of Colletotrichum acutatum in Australia, introducing C. fioriniae comb. nov. and C. simmondsii sp. nov. Fungal Divers. 39, 111–122.
[24] Shivas, R.G. et al. (2016) Colletotrichum species in Australia. Australas. Plant Pathol. 45, 447–464.
| Colletotrichum species in Australia.Crossref | GoogleScholarGoogle Scholar |
[25] Tan, Y.P. et al. (2018) Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium. MycoKeys 35, 1–25.
| Cryptic species of Curvularia in the culture collection of the Queensland Plant Pathology Herbarium.Crossref | GoogleScholarGoogle Scholar |
[26] Tan, Y.P. et al. (2013) Molecular phylogenetic analysis reveals six new species of Diaporthe from Australia. Fungal Divers. 61, 251–260.
| Molecular phylogenetic analysis reveals six new species of Diaporthe from Australia.Crossref | GoogleScholarGoogle Scholar |
[27] Thompson, S.M. et al. (2015) Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia. Persoonia 35, 39–49.
| Green and brown bridges between weeds and crops reveal novel Diaporthe species in Australia.Crossref | GoogleScholarGoogle Scholar | 26823627PubMed |
[28] Newman, D.J. and Cragg, G.M. (2016) Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661.
| Natural products as sources of new drugs from 1981 to 2014.Crossref | GoogleScholarGoogle Scholar | 26852623PubMed |
[29] Sekurova, O.N. et al. (2019) Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb. Biotechnol. 12, 828–844.
| Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering.Crossref | GoogleScholarGoogle Scholar | 30834674PubMed |
[30] Manamgoda, D.S. et al. (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex. Fungal Divers. 56, 131–144.
| A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex.Crossref | GoogleScholarGoogle Scholar |
[31] Hernández-Restrepo, M. et al. (2018) Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 41, 71–108.
| Multi-locus phylogeny and taxonomy of Exserohilum.Crossref | GoogleScholarGoogle Scholar | 30728600PubMed |
Biographies
Professor Roger Shivas is a plant pathologist and co-curator of the Queensland Plant Pathology Herbarium, as well as a mycologist in the Centre for Crop Health, University of Southern Queensland. His research interests are the systematics of fungi, especially those that cause diseases of plants and insects. He has described and classified over 500 new species of Australian fungi.
Dr Dean Beasley is a plant pathologist who has developed and maintains the Queensland Plant Pathology Herbarium and Insect Collection database. He has developed computer based diagnostic tools for plant pathogenic fungi. He has extensive experience in south-east Asian countries, training plant pathologists in biosecurity and diagnostics.
Kaylene Bransgrove is a plant pathologist, mycologist, co-curator of the Queensland Plant Pathology Herbarium and a research scientist at the University of Queensland. She has worked in Australian and international herbaria in mycological and botanical taxonomic roles and has a keen interest in both micro- and macro-fungi. Current taxonomic groups of interest include the ascomycete plant pathogens Phyllosticta and the powdery mildews.
Dr Yu Pei Tan is a molecular biologist and mycologist who uses phylogenetic analyses to identify and classify fungal plant pathogens. She has made significant taxonomic contributions for a diverse range of plant pathogenic fungi, including Botryosphaeriaceae, Colletotrichum, Elsinoë, Fusarium, helminthosporioid fungi and downy mildews (Oomycetes).
Geoff Bulow is responsible for the commercialisation of technologies, and as a planner and organiser provides leadership in managing the requirements and processes for technology commercialisation within a government framework, overall project management and resource allocation.


