International postal, quarantine and safety regulations
David Smith A B and Matthew Ryan AA CAB International
Bakeham LaneEgham
Surrey TW20 9TY, UK
B Tel.: +44 1491829114
Email: d.smith@cabi.org
Microbiology Australia 40(3) 117-120 https://doi.org/10.1071/MA19032
Published: 16 September 2019
There are numerous legislative regulations that impact on microbiology, microbial Biological Resource Centres (mBRCs) and culture collections, with which all microbiologists must comply. These affect access for collection, handling, distribution/shipping and utilisation of microbial resources. Areas where regulations are triggered are international post, quarantine and safety. The legislation and supporting documents are often difficult to find and understand, therefore the World Federation for Culture Collections (WFCC) has a long history in providing advice and guidance to help compliance with such legislation. A visit to the WFCC library (http://www.wfcc.info/wfcc_library/) will provide information on postal requirements shipping dangerous goods and on control measures in place for biosecurity to control access to dangerous pathogens. This paper will update such communications and provide relevant information on: Health and Safety (H&S); Quarantine regulations; and Postal Regulations and Safety. Other papers in this special issue will address elements that impact on distribution and use of microorganisms for example in packaging, legislation on the proliferation, distribution and misuse of dangerous pathogens, export licensing measures, the Convention on Biological Diversity and the Nagoya Protocol, ownership of Intellectual Property Rights (IPR) and the provision of safety information to the recipient of microorganisms. The advice is generic and users are advised to refer to their own National guidance and implementation acts to ensure they are compliant. The work was compiled from authors’ efforts in their management of an mBRC and most recently contributions to the EMBRIC project (http://www.embric.eu/) in particular Deliverable 6.1 ‘Microbial pipeline from environment to active compounds’ (http://www.embric.eu/deliverables).
Overview
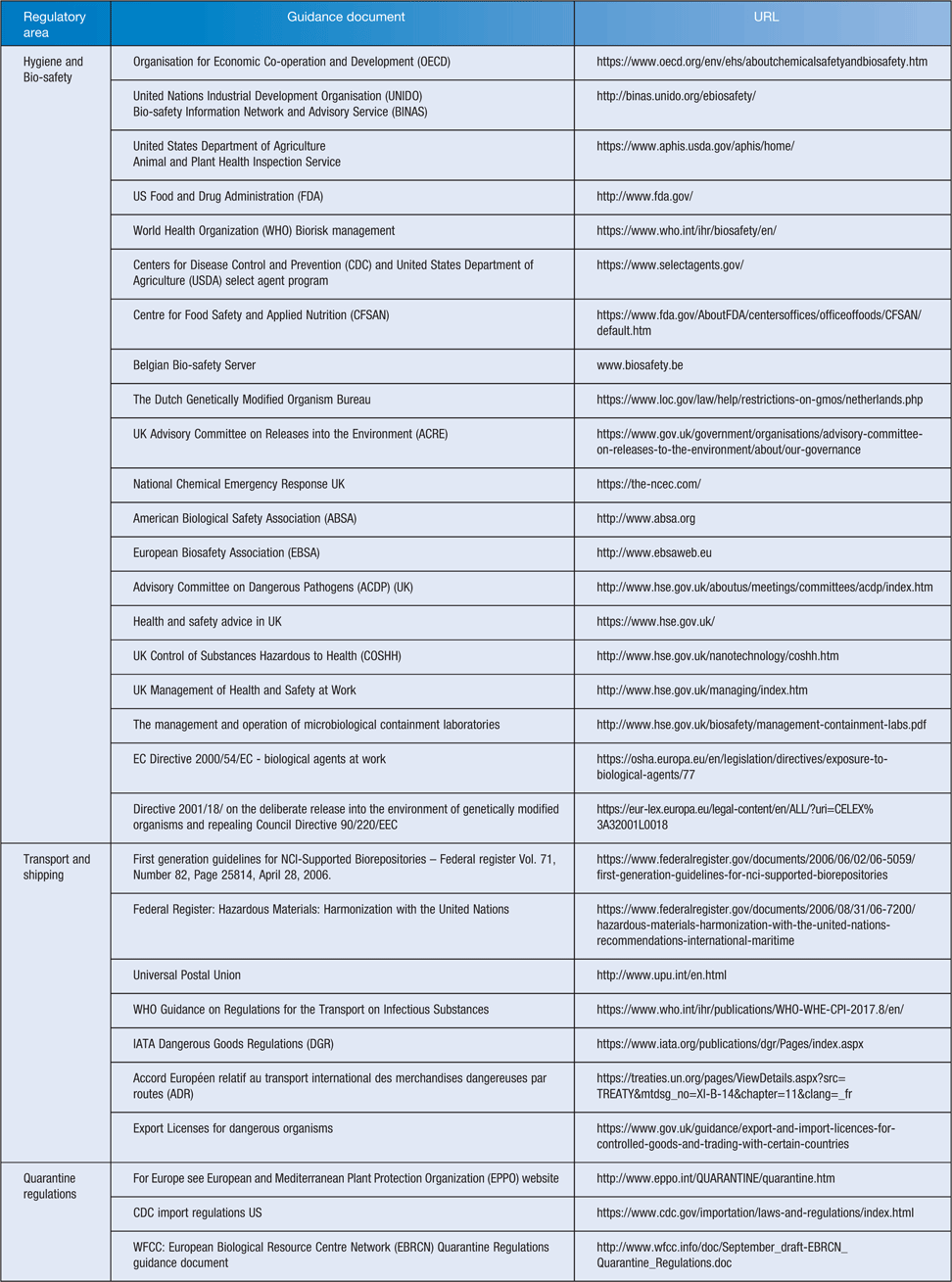
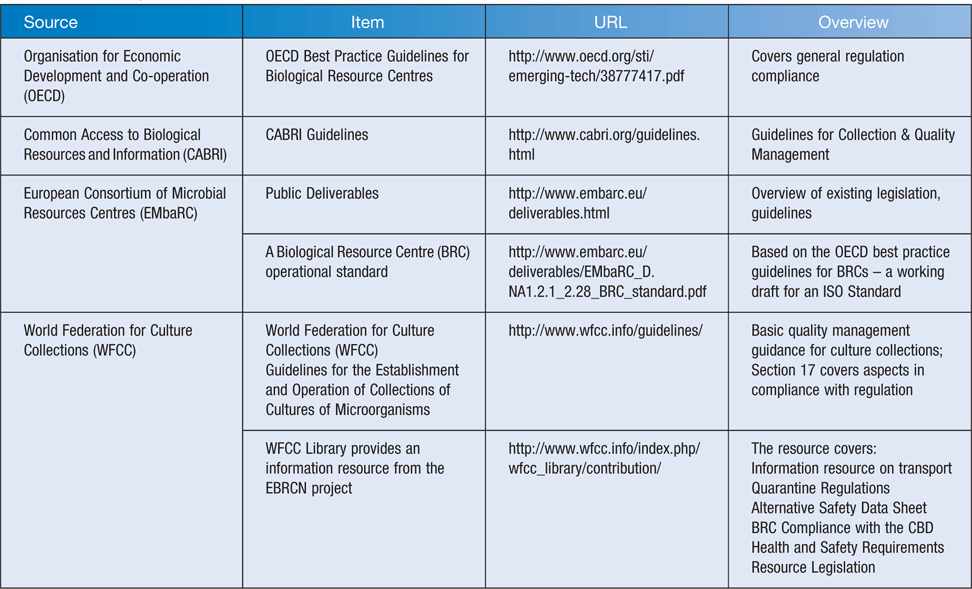
Work with microorganisms involves sampling, isolation, characterisation and may extend to generation of extracts, purification and assaying of nucleic acids, proteins and potential bioactive compounds, with the goal to place products on the market. Table 1 lists some of the relevant regulations and guidance on international postal, quarantine and safety regulations and provides links to the source; community best practice is listed in Table 2.
|
|
The reach of a laboratory’s H&S procedures extend beyond the laboratory where the work is carried out to cover all those who may come in contact with substances and products from that laboratory. A microorganism in transit will put postal staff, freight operators and recipients at risk. Some organisms are relatively low hazard whilst others are quite dangerous. It is essential that safety and shipping regulations are followed to ensure safe transit and that H&S requirements, quarantine regulations and postal regulations are met. It is critical that biological resource centres operate to high standards1,2 and currently there are guidelines available for adoption and use (Table 2).
Health and safety (H&S)
There are numerous national requirements for H&S; e.g. in the EU, H&S is covered by Directive 89/391/EEC, which implements measures to improve H&S at work. It is designed to encourage improvement in occupational H&S in all sectors, both public and private; promote workers’ rights to make proposals relating to H&S, to appeal to the competent authority and to stop work in the event of serious danger, seeking to adequately protect workers and ensure that they return home in good health at the end of the working day (http://ec.europa.eu/social/main.jsp?catId=148). The EU legal framework in the area of occupational safety and health (OSH) is outlined in the OSH Strategic Framework 2014–2020 (http://ec.europa.eu/social/main.jsp?catId=151&langId=en).
A risk assessment of handling and supply of organisms is required and should include an assessment of all hazards involved, not just infection, but others such as the production of toxic metabolites and the ability to cause allergic reactions. Organisms that produce volatile toxins or aerosols of spores or cells present a greater risk. It is the responsibility of the microbiologist to provide such assessment data to the recipient of a culture, to ensure its safe handling and containment. Adequate assessment of risks includes:
-
Provision of adequate control measures
-
Provision of H&S information
-
Provision of appropriate training
-
Establishment of record systems to allow safety audits to be undertaken
-
Implementation of good working procedures
Good working practice requires assurance that correct procedures are actually being followed and this requires a sound and accountable safety policy (http://www.hse.gov.uk/pubns/hsc13.htm). This demands suitable and sufficient assessment of the risks to H&S to which any person, whether employed or not may be exposed through their work (http://www.hse.gov.uk/risk/). These assessments must be regularly reviewed, especially when changes in procedures or regulations dictate, and must be recorded. The distribution of microorganisms to others outside the workplace extends these duties to protect others. In Europe the protection of workers from risks related to exposure to biological agents at work is addressed by Directive 2000/54/EC – biological agents at work of the European Parliament and of the Council of 18 September 2000 (https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77). This Directive lays down minimum requirements for the H&S of workers exposed to biological agents at work.
Quarantine regulations
Clients who want to obtain cultures of non-indigenous plant pathogens must obtain a permit to import, handle and store from the appropriate Government Department. Under the terms of such a licence, the shipper is required to see a copy of the Ministry permit before such strains are supplied. The BRC must do its best to ensure that non-indigenous pathogens are not distributed unless the recipient has a current licence.
Quarantine legislation is in place in countries world-wide restricting the import of non-indigenous plant and animal pathogens. Those who want to import such organisms must hold the relevant import permit, which can be obtained from the relevant country authority, for example, Canada, UK and USA. Information on the transport of plant pathogens throughout Europe can be obtained from the European and Mediterranean Plant, Protection Organisation (EPPO; https://www.eppo.int/).
Postal regulations and safety
Countries have their own national regulations governing the packaging and transport of biological material in their domestic mail. International Postal Regulations covering postage of human and animal pathogens are very strict on account of the safety hazard they present. There are several organisations that set regulations controlling the international transfer of such material (Table 1). It is common practice to send microorganisms by post, as this is more convenient and less expensive than air freight. However, many countries prohibit the movement of biological substances through their postal services. The International Bureau of the UPU in Berne publishes all import and export restrictions for biological materials by national postal services. IATA Dangerous Goods Regulations (DGR) require that packaging used for the transport of hazard group 2, 3 or 4 must meet defined standards3. There are several other regulations that impose export restrictions on the distribution of microorganisms. These include control of distribution of agents that could be used in biological warfare; EU Council Regulation 3381/94/EEC on the control of export of dual-use goods (Official J. L 367, p1). There are several qualified courier services that can be engaged to ensure shipping regulations are followed. There is an obligation to have a certified shipper for class A organisms in the IATA guidelines and it is the responsibility of the microbiologist that organisms are labelled, packed and shipped correctly by such a certified shipper.
It is critical that microbiologists are aware of and follow legislation; see Guidance on regulations for the Transport of Infectious Substances 2007– 2008. WHO/CDS/EPR/2007.2 World Health Organization 2007 (https://www.who.int/csr/resources/publications/biosafety/WHO_CDS_EPR_2007_2cc.pdf). Article III of the Biological Weapons Convention (BWC) obliges the States Parties not to transfer to any recipient whatsoever, directly or indirectly, and not in any way to assist, encourage, or induce any States, group of States or international organizations to manufacture or otherwise acquire any of the agents, toxins, weapons, equipment or means of delivery specified in article I of the Convention (https://www.un.org/disarmament/wmd/bio/). This is a legally binding obligation. A number of countries have implemented national export licensing measures as an effective means of implementing these obligations and to avoid the possibility of the inadvertent supply of any item that could be used in a BW program.
A safety data sheet must be despatched with an organism indicating which hazard group it belongs to and what containment and disposal procedures are necessary. Article 10 of the EU Directive 90/379/EEC regulates that manufacturers, importers, distributors and suppliers must provide safety data sheets in a prescribed format (https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31990D0379).
Summary
All microbiologists must keep abreast of regulatory requirements. Although there are numerous publications on the operation of mBRCs4 the requirements extend to all microbiological laboratories. Participation in microbiological and culture collection communities such as the WFCC will help ensure you are aware of your compliance needs.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
CABI is an international intergovernmental organisation, and we gratefully acknowledge the core financial support from our member countries (and lead agencies) including the United Kingdom (Department for International Development), China (Chinese Ministry of Agriculture), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), Netherlands (Directorate-General for International Cooperation), and Switzerland (Swiss Agency for Development and Cooperation). See https://www.cabi.org/about-cabi/who-we-work-with/key-donors/ for full details.
References
[1] Smith, D. (2012) Culture collections. Adv. Appl. Microbiol. 79, 73–118.| Culture collections.Crossref | GoogleScholarGoogle Scholar | 22569518PubMed |
[2] Martin, D. et al. (2015) MIRRI promoting quality management systems for microbiology. EC Microbiol. 2.2: 278–287. https://www.ecronicon.com/ecmi/pdf/ECMI-02-000036.pdf
[3] International Air Transport Association (IATA) (2019) Dangerous goods regulations (DGR). 60th edition. https://www.iata.org/publications/dgr/Pages/index.aspx
[4] Smith, D. et al. E (2013) Public Service Collections and Biological Resource Centres of Microorganisms. In The Prokaryotes – Prokaryotic Biology and Symbiotic Associations (Rosenberg, E. et al., eds). pp. 267–304. Springer-Verlag Berlin, Heidelberg.
Biographies
David Smith is Director Biological Resources at CAB International. His research interests are in the conservation and sustainable use of microorganisms in agriculture and the environment.
Mathew J Ryan is a microbiologist at CAB International. His specific interests are in pure and applied mycology, biodeterioration, natural product research and the cryopreservation of fungi.


