DSMZ: the European Union’s first Registered Collection under the Nagoya Protocol
Andrey Yurkov A , Hilke Marie Püschner A and Amber Hartman Scholz A BA Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany
B Email: amber.h.scholz@dsmz.de
Microbiology Australia 40(3) 108-113 https://doi.org/10.1071/MA19030
Published: 17 September 2019
The Convention on Biological Diversity and the Nagoya Protocol have created new challenges for international microbiological research. With the implementation of the Nagoya Protocol in 2014, the European Union created a new voluntary legal mechanism, the Register of Collections, to help users of collections, including culture collections, have an easier path to Nagoya Protocol compliance by using a so-called ‘registered collection’. The Leibniz Institute DSMZ is the first, and so far only, collection to successfully be entered into the Register. The challenges and lessons learned during this process can be informative for culture collections and users of microbial resources beyond the EU and indeed around the world.
The 1992 UN Convention on Biological Diversity (CBD) and its supplementary agreement, the 2010 Nagoya Protocol (NP), aim to promote biodiversity conservation, ensure sustainable use of biodiversity, and enable fair and equitable benefit sharing from use of biodiversity. The NP entered into force on 12 October 2014 and has changed biological research by creating a new and complex set of administrative and legal hurdles for researchers studying preserved ex situ as well as isolating new living organisms from their natural habitats, known in CBD/NP-language as ‘genetic resources’ or GR, from a country that claims sovereign rights over its GR. The CBD and NP do not distinguish between basic and applied research or commercial or non-commercial and, as such, nearly all microbiological research dependent on environmentally-sourced organisms or samples, including identification, taxonomic classification, deposition, distribution and further utilisation, can be covered by both CBD and NP depending on the national legislation where and when access (sampling) took place.
In the European Union (EU), user compliance with the NP is regulated by Regulation No. 511/2014 of the European Parliament and establishes due diligence measures for users of GR in the EU, which basically means a user must prove they followed the laws in place when conducting research and sampling abroad. This regulation applies to GR collected in a country that is a Party to the NP after the entry into force of the NP for the EU (12 October 2014) or after the country implements national legislation1. The European law is not retroactive and does not apply to ‘old’ GR although the legal definition of access is important. In practice it makes a difference whether GR is newly isolated from an environment or acquired from an ex-situ collection and different countries handle this in different ways (see the discussion on the definition of Access to GR below). The law does not require collections to comply with non-Nagoya CBD obligations (i.e. GR that is out of NP) although the DSMZ is diligent about ensuring access and benefit sharing (ABS) obligations are fulfilled and requests documentation from depositors accordingly.
The role of culture collections as major suppliers of GR was recognised in the EU regulation with the establishment of a ‘Register of Collections’ in which a collection voluntarily fulfils part of the due diligence obligations of the user by seeking NP-related ‘permits’ before distributing GR. In order to do this systematically, a collection must demonstrate that their management practices enable them to comply with national NP regimes for all incoming material. Only collections that fall within EU jurisdiction can apply for a ‘registered’ status, although their products continue to be available for users around the world and the NP/CBD information they provide is undoubtedly still helpful in fulfilling non-EU due diligence obligations.
In March 2018, the Leibniz Institute German Collection of Microorganisms and Cell Cultures GmbH (DSMZ) became the first collection in the EU register. The DSMZ submitted a 14-page application (along with 11 supporting documents) to the German Agency for Nature Conservation (BfN) in November 2017, and was officially approved by BfN on 18 March 2018. The application took around four months to prepare by a two-person science-legal team (AHS and HMP) and was a high priority for the DSMZ Scientific Director, Professor Jörg Overmann. There were significant personnel investments from quality management, scientific, and administrative staff with costs estimated around €200 000. The application was also supported in a close partnership with the BfN. Below we share our experiences of obtaining the registered collection status, and challenges and opportunities that arose during this process.
Compliance with the Nagoya Protocol
Many microbial collections collect up-front scientific and administrative information through accession forms, but the CBD and NP extend the information required and can complicate the deposit process. Here are the steps we took towards NP compliance in our collection:
(1) Geographical scope
The first step was to review our collection catalog and confirm that all strains collected in 2014 and beyond (NP entry into force) had a country of origin associated with them. For strains that pre-dated the CBD we accepted, if necessary, ‘country of origin unknown’ accompanied by evidence from our records or the literature that the collection date was pre-1992.
We then edited and standardised records with ‘imprecise’ countries of origin: states which no longer exist (e.g. Czechoslovakia), large geographic areas (e.g. Europe), islands (e.g. Hawaii), and dependent territories (e.g. Puerto Rico), duplicates (e.g. Burma and Myanmar), and spelling errors. We applied the ISO 3166 code for the representation of names of countries and their subdivisions and corrected our records accordingly to only include recognised sovereign states. With this list in hand, for all future deposits, we instituted the use of a drop down menu in our online accession form and mandatory geographical coordinates to avoid uncertainty. We added to the ISO list, areas not regulated by the Nagoya Protocol: Antarctica and the high seas (international waters), both regulated by separate UN treaties.
(2) Temporal scope
Whether a new strain is in or out of CBD/NP scope is dependent on geography and time. As such, sampling date is essential to determine whether access to GR is regulated. We reviewed all strains in the collection that had no sampling date information and an accession date after 1992. We used information from the deposit form, literature, and internal records to determine whether a sampling date could be determined. When no collection date was available, we substituted the accession date (when a strain was deposited) which is a more conservative value and added ‘sampled before + accession date’.
(3) Legal scope (PIC and MAT)
The CBD and NP invented a new vocabulary for granting access to GR – ‘prior informed consent’ (PIC) and ‘mutually agreed terms’ (MAT). These can be none, one, or two or more documents depending on how the national legislation works and they can carry different names such as ‘research permit’ or ‘material transfer agreement’ although their content must reflect PIC/MAT and be issued by the appropriate CBD/NP authority (see ABS-CH discussion below). At the beginning of our compliance check, very few strains in our collection had PIC/MAT documents, meaning they were ‘in scope’. But, recognising that the ‘in scope’ strains and, thus, their associated documents would grow over time, we established both a new standard operating procedure and IT system to enable transparent review of PIC/MAT.
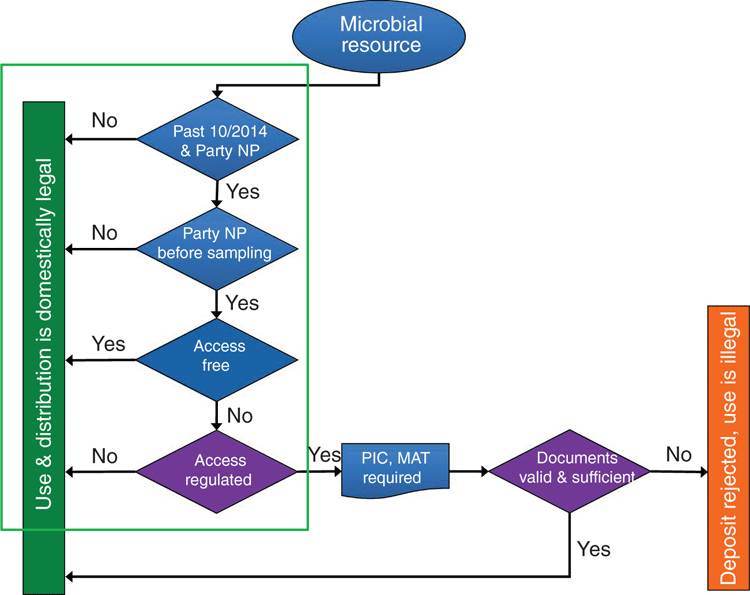
This decision tree for determining legal compliance is outlined in Figure 1 and is partially automated by receiving legal scope information from the ABS-Clearinghouse (ABS-CH) API (application programming interface) – specifically NP/CBD status (is the country a Party or not) and the date of entry into force. Based on this information from the ABS-CH, it can be determined whether PIC/MAT is required. Then the manual ‘legal check’ begins and the legal team verifies with the provider country (by directly contacting the national focal point, NFP) that the documentation received is adequate and sufficient. If necessary, an unofficial translation of the documents is also requested. If so, the strain is accepted; otherwise it is rejected. Besides the list of required documents, the legal team checks with the NFP which national authority is allowed to issue the documents. This procedure ensures that our holdings are legally compliant and we can pass this certainty on to our customers.
(4) Depositor due diligence
In order to help our depositors understand the CBD/NP legal complexity, we published several websites2–4 including ‘Deposit of biological material at the DSMZ: Compliance with the Nagoya Protocol’2 and explained important terms and restrictions and provided links to resources which help to determine territorial waters, Exclusive Economic Zones (EEZ) in the Sea, and locate national focal points. We prepared an infographic to explain the minimal requirements on information applied to a deposited strain3.
(5) User due diligence
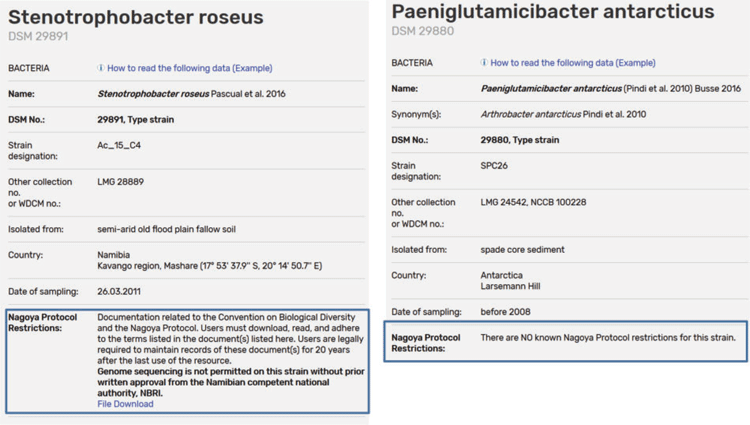
The DSMZ online catalog offers customers an overview of each bioresource’s relevant scientific information and, since Registration, the country of origin, sampling date, and any associated documentation (PIC/MAT or any other MTA). The documents can be downloaded and saved by the users ensuring the chain of custody required by the NP. If there are no known Nagoya restrictions this is also explicitly posted in the catalog (Figure 2). Strains accompanied with confidential documents cannot be accepted for deposit in the open collection and the depositor is informed during the deposit process that the documents will be published on our website and agrees to this as a condition of acceptance.
DSMZ also began using a Material Transfer Agreement (and accompanying Terms & Conditions) that explicitly require users to: (1) use bioresources for non-commercial research purposes only; (2) not distribute strains to third parties; and (3) to adhere to the terms listed in the Nagoya Restrictions section of the catalogue. With their signature the customer commits to the observance of these regulations. The first two restrictions have been in place in DSMZ before the admittance to the Register of collections. If we become aware of infringements, we report them to the responsible authorities, e.g. the regulatory authorities (like BfN) or other competent national authorities (CNA) which are responsible for ensuring that those users are compliant with EU Regulation 511/2014 or other national legislation.
The advantage of purchasing from a Registered Collection is that a European user can simply report the DSMZ and the strain number in their due diligence reports along with other required information creating a simpler path to compliance than gathering the information and PIC/MAT from the original depositor.
(6) Nagoya and the Bacteriological Code of Nomenclature
Strains deposited for the purpose of describing a new species of prokaryote must be deposited in the open collection. Furthermore, according to the International Code of Nomenclature of Prokaryotes (ICNP), type strains and the associated information must be available from at least two culture collections without restrictions. Strains from a country with strong NP/CBD restrictions can be disqualified from becoming a type strain of a new species under the ICNP because it is not possible to distribute these strains without restrictions. The Certificate of Availability for prospective type strains issued by the DSMZ follows the principle of unrestricted access to proposed type strains and the DSMZ does not accept type strains from countries with restrictions on distribution3.
The complex NP legal ‘potpourri’
All Parties to the CBD are required under international law to provide information on their national legislation and contact information. However, in practice, the ABS-CH is not legally binding for users although the NP countries are technically required to use it, which leads to a great deal of uncertainty. Over the course of two weeks in September 2017, we (HMP) wrote to all 198 countries under the contact email address found in the ABS-CH (either NFP or CNA) asking what would need to be done in order to accept a microorganism from their country for deposit in our collection. The results were discouraging: 30 (16%) replied with information, 33 (18%) replied and promised to provide a detailed answer later, and the majority 120 (64%) did not reply. Over the next year, we continued to reach out to national focal points as new deposits came into the collection, which only slightly improved the response rate: informative replies were received from 63 (34%) countries and 28 (15%) are still expected to provide us the NP-relevant information. No answer was received from 83 (44%) countries. Astoundingly, despite the legal requirement in international law to use the ABS-CH, 14 (7%) countries either do not have contact information in the ABS-CH system or the emails bounced. A further complication is that national legislation and laws are not always translated into English and country representatives do not respond because of language barriers. These data taken together show that, in some cases, a legal deposit will be nearly impossible.
As our experience with the NP grows, we continue to be astonished by the intricacies and diversity of the national regulations implementing the NP as well the grand challenge of gathering information from provider countries. Even in the EU the situation is not easy as there are a wide variety of access regimes in place: some countries have granted free access (e.g. Germany), while others have restricted access (e.g. France and Spain), others do not have any legislation in place (e.g. Italy), or have proposed different rules for different regions within the country (e.g. Belgium). Legislation between mainland and overseas territories may differ as well. For example, Denmark’s ratification of the Nagoya Protocol does not apply to Greenland.
There is much room for clarification of definitions of ‘access’ and ‘utilisation’, as well as key dates from which a national legislation applies. The original EU regulation refers to date of access. A few countries have defined it as ‘date of sampling’ others as ‘whenever you have access for the first time to the sample from the country of origin’, so that old material sampled before the ratification of NP (and potentially CBD) and preserved in an ex-situ collection would fall under the EU regulation. Likewise, there is no common position regarding the difference between utilisation of GR, basic research on GR and quality control (QC) of a strain deposited in a culture collection. This problem is amplified in light of the ongoing debate on the regulation of Digital Sequence Information (DSI).
The NP provides a legal framework for access and benefit sharing among Parties and the EU Register of Collections is restricted to NP Parties. However, it is important to remember that a non-NP-Party may have national laws that govern access to their resources. Brazil is probably the most well-known example of a NP non-party country which has developed its own national legislation regulating access to GR5. Despite its non-Party status, the DSMZ is doing its best to follow Brazilian national laws.
Outlook
In a time of increasing legal overheads, it is important that microbial culture collections continuously exchange information and share their experience with competent authorities, national and foreign, and with other collections and colleagues through professional associations like the World Federation for Culture Collections, and regional networks such as the European Culture Collections’ Organization (ECCO), the Asian Consortium for the Conservation and Sustainable Use of Microbial Resources, and the Latin American Federation of Culture Collections.
But even with good exchange and networking, the process of strain deposition is simply becoming more complex. Several authors have pointed out the negative impact of the NP regulations on biodiversity assessments and conservation efforts6,7. The situation in microbiological research is not much different. Long and complicated applications for sampling permits discourage many scientists (e.g. 8–10) and additional problems can be expected when a strain needs to be sent out of the country of origin and deposited in an open collection. The number of strains deposited in the DSMZ for the purpose of taxonomic description has rapidly dropped down from nearly half of deposits (48% and 46% in 2014 and 2015, respectively) to 16% in 2018. We also have observed a trend towards growing numbers of strains originating from countries who declared free access to their GR or from the U.S., a country that is not a Party to the CBD. Another interesting trend is the growing interest on the deposition of ‘old’ material, strains isolated before the NP and CBD, as a part of safeguarding collections from retiring scientists.
Obtaining the Registered collection status is a long-term investment which is aimed to provide additional service and value for our customers, who would otherwise be responsible for obtaining and checking the NP documents themselves and to demonstrate good will and competence to provider countries. To date, the registered collection status has not led to an increase in sales, but we have heard from colleagues that they appreciate the additional quality control and legal clarity. Over the past year, there has been growing interest from our customers in the strain-related information regarding the NP, perhaps due to the year-old EU due diligence declaration4. Overall, we feel strongly that the Registered Collection was the right decision on balance and look forward to working with European and international colleagues in assisting microbiologists with the legal overhead that is now our daily reality.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge the leadership and commitment of Jörg Overmann in deciding to undertake the registered collection process. We also thank the ABS staff of the German Agency for Nature Conservation, especially Thomas Greiber and Ellen Frederichs, for their collegial exchange and support navigating a new legal mechanism for the first time together. We are deeply appreciative of the DSMZ curators that did the individual checks of hundreds of strains and remain on the daily front lines of Nagoya compliance. This research did not receive any specific funding.
References
[1] Boundy‐Mills, K.L. et al. (2016) Yeast culture collections in the twenty‐first century: New opportunities and challenges. Yeast 33, 243–260.| Yeast culture collections in the twenty‐first century: New opportunities and challenges.Crossref | GoogleScholarGoogle Scholar | 27144478PubMed |
[2] https://www.dsmz.de/about-us/nagoya-protocol.html
[3] https://www.dsmz.de/deposit/nagoya-protocol.html
[4] https://www.dsmz.de/about-us/nagoya-protocol/due-diligence.html
[5] https://absch.cbd.int/countries/BR
[6] Overmann, J. and Scholz, A.H. (2017) Microbiological research under the Nagoya Protocol: facts and fiction. Trends Microbiol. 25, 85–88.
| Microbiological research under the Nagoya Protocol: facts and fiction.Crossref | GoogleScholarGoogle Scholar | 27887771PubMed |
[7] Prathapan, K.D. et al. (2018) When the cure kills—CBD limits biodiversity research. Science 360, 1405–1406.
| When the cure kills—CBD limits biodiversity research.Crossref | GoogleScholarGoogle Scholar | 29954970PubMed |
[8] Bockmann, F.A. et al. (2018) Brazil’s government attacks biodiversity. Science 360, 865.
| 29798874PubMed |
[9] Neumann, D. et al. (2018) Global biodiversity research tied up by juridical interpretations of access and benefit sharing. Org. Divers. Evol. 18, 1–12.
| Global biodiversity research tied up by juridical interpretations of access and benefit sharing.Crossref | GoogleScholarGoogle Scholar |
[10] Wynberg, R. and Laird, S.A. (2018) Fast science and sluggish policy: the herculean task of regulating biodiscovery. Trends Biotechnol. 36, 1–3.
| Fast science and sluggish policy: the herculean task of regulating biodiscovery.Crossref | GoogleScholarGoogle Scholar | 28964595PubMed |
Biographies
Andrey Yurkov studied soil sciences and microbiology at the Lomonosov Moscow State University in 1998-2002 and completed his doctorate thesis in 2006. Since 2012, he is the curator for fungi and yeasts at the DSMZ. He is a member of executive boards of the World Federation for Culture Collections (WFCC) and the International Mycological Association (IMA).
Hilke Marie Püschner studied in Hannover and Göttingen and completed her doctorate in medical law. She finished her education in Braunschweig, among others at the Higher Regional Court Braunschweig. Since 2017 she is a lawyer at the DSMZ and among other things responsible for the observance of the Nagoya Protocol.
Dr Amber Hartman Scholz is the Deputy to the Director at the Leibniz Institute DSMZ, the German Collection for Microorganisms and Cell Cultures, in Braunschweig, Germany. She headed the team that led to the DSMZ becoming the first Registered Collection under the Nagoya Protocol in the European Union, demonstrating the collection’s voluntary and stringent compliance with EU Regulation 511/2014. Her broader work at the DSMZ focuses on internationalization, strategic development, and science policy. Dr Scholz has broad experience in science and policy through her work in the United States at the White House Office of Science and Technology Policy (OSTP) as Executive Director to the President’s Council of Advisors on Science and Technology from, the National Cancer Institute as a Policy Advisor, and as a Science Fellow to the California State Senate Environmental Quality Committee. She received her PhD in Biology with a focus on the human intestinal microbiome and bioinformatics methods development in 2009 from the Johns Hopkins University.