Tick-borne encephalitis and its global importance
Gerhard DoblerA Bundeswehr Institute of Microbiology, Neuherbergstrasse 11
B Tel: +49 89 992692 3974
C Fax: +49 89 992692 3983
D Email: gerharddobler@bundeswehr.org
Microbiology Australia 39(4) 191-194 https://doi.org/10.1071/MA18061
Published: 24 October 2018
Tick-borne encephalitis (TBE) is the most important tick-transmitted human viral disease in Europe and Asia with up to 10 000 human cases annually. The etiologic agents of TBE are the three subtypes of tick-borne encephalitis virus (TBEV), a member of the genus Flavivirus in the family Flaviviridae. The Far-Eastern subtype and the Siberian subtype are both mainly transmitted by Ixodes persulcatus; the European subtype is mainly transmitted by Ixodes ricinus. Besides tick bite, TBEV can be transmitted by unpasteurised milk from goat, sheep and cattle during the viremic phase of infection by the oral route of infection (alimentary form of TBE). There is no treatment for TBE available, but there are effective and well tolerated vaccines against TBE, which are recommended for people living or travelling to endemic countries with a risk of infection.
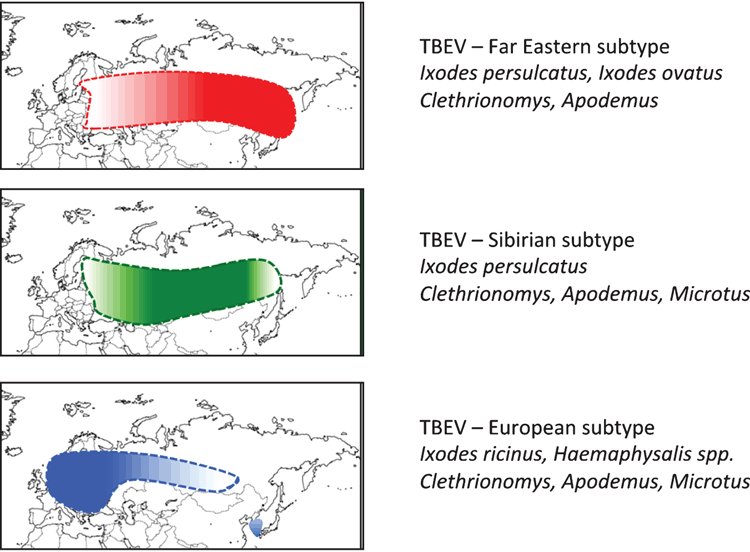
Tick-borne encephalitis is the most important tick-borne viral disease in Europe and Asia, but regarding numbers of patients it is the most important tick-borne viral disease in the world1. The disease is endemic exclusively in Europe and Asia. It is caused by a group of viruses of the genus Flavivirus in the family Flaviviridae. Three subtypes, the European, the Siberian and the Far-Eastern subtype can be distinguished by molecular methods and they are transmitted by different vectors and cause a different clinical picture of disease2 (Figure 1). An additional two other subtypes, the Baikalian (TBEV-Bkl) and the Himalayan subtype (TBEV-Him) have been described recently3,4.
|
The five subtypes of TBEV have a different, but partially overlapping geographical distribution and biological transmission cycles involving different tick species and rodents5. The European subtype (TBEV-EU) is geographically distributed mainly in Europe6. However, some TBEV-EU strains have been isolated and characterised in Siberia (Lake Baikal region) and also in South Korea. In Europe the most important vector of TBEV is I. ricinus. Goats, sheep and cattle shed TBEV into the milk during the viremic phase of infection without showing signs of disease. Infection with TBEV by the alimentary route from drinking unpasteurised virus containing milk or dairy products is a common way of infection in some European countries and occasionally also occurs in countries with a highly industrialised agriculture, as recently reported in Germany and Austria7,8.
The Siberian subtype is geographically distributed mainly in Russia east of the Ural Mountains, but it is also found in the Baltic countries and in localised places in Finland9. I. persulcatus, the Taiga tick, mainly transmits this subtype. The Far-Eastern subtype of TBEV is geographically distributed mainly in the far-eastern part of Russia and the northern parts of China. TBEV-FE is also found on the northern Japanese island of Hokkaido, where I. ovatus was identified as its vector10. The Baikalian subtype was detected at the Lake Baikal region in different Myodes spp. and in I. persulcatus3. The Himalayan subtype was identified two times from respiratory fluid of Marmota himalayana from the Qinghai-Tibet Plateau in China4.
Earlier serological data suggest that only about 30% of the TBEV infections present with clinical symptoms, ranging from febrile (‘flu-like’) disease to meningitis, encephalitis and encephalomyelitis1. In milk-borne and diary product-borne infections the manifestation index seems to be much higher ranging in many outbreaks to up to 100% of exposed individuals8.
The incubation period of TBE ranges from 5 to 14 days. In many of the human cases caused by the TBEV-EU a biphasic course is reported. In the first phase of the disease, symptoms of a general infection (‘flu-like’) are seen. Many patients report elevated temperatures, headache, muscle ache, fatigue and also gastrointestinal symptoms or symptoms of the respiratory tract. During this phase of disease, the TBE virus can be detected and isolated from the blood of the patients. The symptoms reflect the virus replication in the different organs of the body. The first phase lasts from four to seven days11.
After a symptomless phase of 4–7 days, symptoms of general infection of the central nervous system (CNS) may follow in ~30% of patients. The CNS symptoms may range from headache and mild meningitis to severe encephalomyelitis with a fatal outcome. Generally three clinical forms of the CNS disease of TBE infection can be distinguished11:
-
Meningitis: fever, headache, nuchal rigidity
-
Encephalitis: change of consciousness, stupor, coma, epileptic attacks, disorientation, dysarthria
-
Myelitis: flaccid paralysis of different muscles; mainly muscles of the upper musculo-skeletal system
The Siberian and the Far-Eastern forms of TBE infections follow a more severe clinical course. These infections mostly show a monophasic form with more severe encephalitic and myelitic features. The fatality rates range from 3% to 20%. In patients with the Siberian subtype virus infection a chronic form of TBE has been described. However, it is unclear, whether these more severe clinical courses in the Russian forms of TBE are due to differences in case-reporting, due to differences in medical interpretation or to genuine different pathogenicity of this TBE virus subtype12.
Despite the manifestation of central nervous system disease, the isolation or detection of TBE virus in the cerebrospinal fluid (CSF) of the patient is difficult and only rarely successful. Within the brain the virus seems to spread from one cell to the next without being shed into the CSF. Therefore the diagnostic method of choice is the detection of specific antibodies. The detection of IgM and IgG antibodies against TBE virus together with typical clinical CNS symptoms and tick exposure is strong evidence for a diagnosis of acute TBE infection11. However, there are many serological cross-reactions with other flaviviruses. The detection of antibodies in a single serum, without further serological follow up and diagnostic information, may make the diagnosis difficult. Also IgM might be very low or even missing in the case of pre-existing antibodies against other flaviviruses (e.g. dengue virus, Zika virus) or after vaccination against other flaviviruses (yellow fever virus, Japanese encephalitis virus)13. Therefore, the diagnosis of TBE should be made after excluding other flavivirus antibodies, e.g. against yellow fever virus, dengue fever viruses, or Japanese encephalitis virus.
So far, there is no effective treatment for TBE11. Treatment may include symptomatic therapy to lower temperature, to relieve pain and especially in case of encephalitis to avoid complications from inflammatory brain damage. In severe clinical forms patients are put into an artificial coma. Rehabilitation medicine plays an important part in the post-acute treatment to control the neurological and psychiatric sequelae. The fatality rate of the European form of TBE ranges from 0.5% to 2%10.
There are six inactivated and adjuvanted vaccines available against TBEV infection14,15. Two vaccines, Encepur (GSK) and FSME-Immun (Pfizer) are produced in Europe and contain European type TBEV. Three vaccines, TBE vaccine Moscow and EnceVir and Tick-E-Vac/Klesh-E-Vac are produced in Russia and contain Far Eastern TBEV strains. The name of the Chinese TBE vaccine is Sen Tai Bao; however, no further information on this vaccine is available. The two European vaccines need three doses for a basic immunisation. Two are given 1–3 months apart. Two weeks after the second dose a vaccine efficacy of more than 95% can be assumed. For both vaccines a third dose is recommended after 6–12 months after the start of immunisation. A fourth vaccine dose is recommended after three years. Depending on the patient’s age, subsequent boosters are recommended after 3 or 5 years. For both vaccines, rapid immunisations schemes are available, which might induce immunity as early as three weeks. Both European vaccines can be used in special formulations in children >12 months of age. The Russian Tick-E-Vac/Klesh-E-Vac can also be used for children >12 months of age. Data indicate that the European vaccines also provide protection against infections with the Siberian and the Far Eastern subtype of TBEV. Due to the close genetic relatedness a similar assumption may be made also for the three Russian vaccines in relation to the European subtypes.
The two Russian TBE vaccines, TBE vaccine Moscow and EnceVir, are not licensed for children <3 years of age. They are administered in two doses 1–2 and 1–7 months apart. A third dose is recommended after 12 months. Further booster doses should be given after 3 years. TBE-E-Vac/Klesh-E-Vac is given in two doses 1–7 months apart. A third dose is recommended one year after the second dose.
In the northern hemisphere tick-borne encephalitis is the most important tick-borne virus infection. An estimated 10 000 human cases occur every year in Europe and Asia16. Incidence rates of TBE infection in endemic areas of Europe range from 0.1 to 20/100 000 inhabitants. The European countries with the highest incidence rates are the Baltic countries and Slovenia, followed by the Czech Republic, Slovakia, Poland and Austria (non-vaccinated population)17. However, the incidence rates may vary on a local district level. For example, in some German districts incidence rates of >10/100 000 per annum are recorded, while in the whole country the incidence rate is below 0.5/100 000 per annum (G. Dobler, personal observation).
Besides the imminent risk of infection in residents of endemic areas, TBE is becoming an important travel-related disease. According to estimates, the risk of exposure for acquiring a TBE infection was calculated at 1 case per 77 000 to 200 000 visitors18. Travel-related cases have been reported from Israel, The Netherlands, Australia, United States and England19–22. The Australian patient travelled by car from Moscow to Novosibirsk with ample contact in nature although it was unclear whether he was infected by a tick bite or by the alimentary route. He developed a generalised infection with drowsiness, fatigue and lower limb myalgia. After acute symptoms subsided the patient noticed a severe depressiveness and changes in his handwriting, which was explained as a cerebellar dysfunction of the right upper limb. However, the clinical course was complete recovery after one month22.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] Bogovic, P. et al. (2015) Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 3, 430–441.| Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management.Crossref | GoogleScholarGoogle Scholar |
[2] Ecker, M. et al. (1999) Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J. Gen. Virol. 80, 179–185.
| Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia.Crossref | GoogleScholarGoogle Scholar |
[3] Kovalev, S.Y. et al. (2017) Reconsidering the classification of tick-borne encephalitis virus within the Siberian subtype gives new insights into its evolutionary history. Infect. Genet. Evol. 55, 159–165.
| Reconsidering the classification of tick-borne encephalitis virus within the Siberian subtype gives new insights into its evolutionary history.Crossref | GoogleScholarGoogle Scholar |
[4] Dai, X. et al. (2018) A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 7, 74.
| A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China.Crossref | GoogleScholarGoogle Scholar |
[5] Heinze, D.M. et al. (2012) Revisiting the clinal concept of evolution and dispersal for the tick-borne flaviviruses by using phylogenetic and biogeographic analyses. J. Virol. 86, 8663–8671.
| Revisiting the clinal concept of evolution and dispersal for the tick-borne flaviviruses by using phylogenetic and biogeographic analyses.Crossref | GoogleScholarGoogle Scholar |
[6] Süss, J. (2008) Tick-borne encephalitis in Europe and beyond—the epidemiological situation as of 2007. Euro Surveill. 13, 18916.
| Tick-borne encephalitis in Europe and beyond—the epidemiological situation as of 2007.Crossref | GoogleScholarGoogle Scholar |
[7] Holzmann, H. et al. (2009) Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Emerg. Infect. Dis. 15, 1671–1673.
| Tick-borne encephalitis from eating goat cheese in a mountain region of Austria.Crossref | GoogleScholarGoogle Scholar |
[8] Brockmann, S.O. et al. (2018) A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurized goat milk and cheese in Germany, May 2016. Euro. Surveill. 23, pii=17-00336.
| A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurized goat milk and cheese in Germany, May 2016.Crossref | GoogleScholarGoogle Scholar |
[9] Kuivanen, S. et al. (2018) Fatal tick-borne encephalitis virus infections caused by Siberian and European subtypes, Finland, 2015. Emerg. Infect. Dis. 24, 946–948.
| Fatal tick-borne encephalitis virus infections caused by Siberian and European subtypes, Finland, 2015.Crossref | GoogleScholarGoogle Scholar |
[10] Yoshii, K. et al. (2017) Tick-borne encephalitis in Japan, Republic of Korea and China. Emerg. Microbes Infect. 6, e82.
| Tick-borne encephalitis in Japan, Republic of Korea and China.Crossref | GoogleScholarGoogle Scholar |
[11] Taba, P. et al. (2017) EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur. J. Neurol. 24, 1214-e61.
| EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis.Crossref | GoogleScholarGoogle Scholar |
[12] Varlacher, J.F. et al. (2015) Tick-borne encephalitis. Rev. Sci. Tech. 34, 453–466.
| Tick-borne encephalitis.Crossref | GoogleScholarGoogle Scholar |
[13] Dobler, G. et al. (1997) Cross reactions of patients with acute dengue fever to tick-borne encephalitis. Wien. Med. Wochenschr. 147, 463–464.
[14] Lehrer, A.T. and Holbrook, M.R. (2011) Tick-borne encephalitis vaccines. J. Bioterror. Biodef. 2011, .
| Tick-borne encephalitis vaccines.Crossref | GoogleScholarGoogle Scholar |
[15] WHO (2011) Vaccines against tick-borne encephalitis: WHO position paper – recommendations. Vaccine 29, 8769–8770.
| Vaccines against tick-borne encephalitis: WHO position paper – recommendations.Crossref | GoogleScholarGoogle Scholar |
[16] Süss, J. (2011) Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2, 2–15.
| Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview.Crossref | GoogleScholarGoogle Scholar |
[17] ECDC Technical Report (2012) Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries.
[18] Steffen, R. (2016) Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western Europe and conclusions on vaccination recommendations. J. Travel Med. 23, taw018.
| Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western Europe and conclusions on vaccination recommendations.Crossref | GoogleScholarGoogle Scholar |
[19] Meltzer, E. et al. (2017) Travel-related tick-borne encephalitis, Israel, 2006–2014. Emerg. Infect. Dis. 23, 119–121.
| Travel-related tick-borne encephalitis, Israel, 2006–2014.Crossref | GoogleScholarGoogle Scholar |
[20] Reusken, C. et al. (2011) Case report: tick-borne encephalitis in two Dutch travellers returning from Austria, Netherlands, July and August 2011. Euro Surveill. 16, 20003.
[21] CDC (2010) Tick-borne encephalitis among US travelers to Europe and Asia – 2000–2009. MMWR Morb. Mortal. Wkly. Rep. 59, 335–338.
[22] Chaudhuri, A. and Ruzek, D. (2013) First documented case of imported tick-borne encephalitis in Australia. Intern. Med. J. 43, 93–96.
| First documented case of imported tick-borne encephalitis in Australia.Crossref | GoogleScholarGoogle Scholar |
Biography
Gerhard Dobler is a medical doctor and specialist in medical microbiology. He is head of the Department of Virology and Rickettsiology at the Bundeswehr Institute of Microbiology in Munich and associate professor at the Unit of Parasitology of the Institute of Zoology at the University of Hohenheim. He is head of the German reference laboratory for tick-borne encephalitis (TBE). His main areas of research are the molecular phylogeny, the eco-pathogenesis of TBE virus and the eco-epidemiology of TBE and other tick-borne diseases.


