Biocorrosion of materials and sick building syndrome
Olga Ilinskaya A C , Alina Bayazitova A B and Galina Yakovleva AA Microbiology Department, Institute for Fundamental Medicine and Biology, Kazan Federal (Volga-Region) University
B Kazan Research Institute of Epidemiology and Microbiology, Tatarstan, Russia
C Tel: +7 843 233 78 55, Email: Ilinskaya_kfu@mail.ru
Microbiology Australia 39(3) 129-132 https://doi.org/10.1071/MA18040
Published: 7 August 2018
The problem of biological damage of mineral building materials and structures based on them is multifaceted and covers all types of industry. The most destructive biocorrosion impacts are on building materials in cities with a large water area. Various types of microorganisms, including pathogens, and especially the filamentous fungi of the genera Aspergillus, Penicillium, Trichoderma, etc., occupy the surfaces of mineral building materials, cause their destruction, disturb the ecological balance of cities and endanger the human health. The term ‘sick building syndrome’ (SBS) is used to describe a situation when the residents of a building experience acute health- or comfort-related effects that seem to be linked directly to the time spent in the building wherein no specific illness or cause can be identified. Biological contaminants, in particular micromycetes, can present one of the possible causes of SBS. Here, we assessed the biodeterioration level of structural materials on the basis of fine-grained concrete widely used in construction practice and architecture. First, we determined the strength characteristics of the material that has been biologically damaged; second, we identified the damaging micromycetes and analysed their metabolic activity related both to the induction of biocorrosion and to the impacts of fungi on human health. Applying a new integrated approach, which combines methods of molecular microbiology and genetic toxicology with standard methods for determining the strength of building structures, we confirmed the relation between biodestructive and pathogenic properties of micromycete isolates.
At present, the world’s losses from biodeterioration are estimated at billions of dollars and are more than 7% of the total cost of industrial products. Physical, chemical and biological factors of mortar and concrete corrosion are in close interrelation. Because concrete is a capillary-porous body, microorganisms, settling on the surface, then spread into the interior causing corrosive processes by the secreted metabolites. Biocorrosion of construction materials and structures has now become an important environmental problem, as according to statistics, the urban residents spend about 95% of their time indoors. Since the prospects for the overwhelming majority of the population are in city housing, the relevance of studying the biocorrosion of building materials and its consequences for the environment and human health is constantly increasing. One of the consequences is sick building syndrome (SBS), which is considered to be a multifactorial health problem, being a medical, psychological and social phenomenon1–3 at the same time. The spaces that contain sufficient levels of chemicals, allergens and microorganisms to make those who live or work in the space sick are known as ‘sick buildings’. These are mainly incommodious old buildings contaminated by microorganisms, especially filamentous fungi inducing biocorrosion of construction materials. At least 600 species of fungi are in contact with humans, and less than 50 are frequently identified and described in epidemiologic studies of indoor environments2. In this study we found the connection between destructive and health-damaging properties of filamentous micromycetes.
According to the Federal State Statistics Service (2016), the population of the Republic of Tatarstan is 3 868 730 people, of which the urban population is 76.41%. Biological damage to building structures is a common problem, especially in cities. Filamentous fungi dramatically worsen the characteristics of materials on which they grow causing partial or complete biodegradation. We have isolated micromycetes from the inner walls of some old buildings in Kazan city and found that fungal communities represented the class Eurocytomycetes and family Trichocomaceae. Pan-fungal primers specific for the conserved sequences of 18S and 28S rRNA genes common to all fungi have been used. The predominant genera (97%) were filamentous fungi Penicillium and Aspergillus: A. niger and A. fumigatus were the most common species. In some cases, micromycetes of the genera Alternaria and Trichoderma were found. Simultaneously, our studies of fungal infection in patients who visited the Laboratory of fungal diseases at the Kazan Research Institute of Epidemiology and Microbiology revealed the prevalence of aspergillosis. This is partly due to the contamination of many buildings with micromycetes of the genus Aspergillus, which can cause mycogenic allergies, atopic dermatitis and mycotoxicoses4, the probability of which is significantly increased in environments with high numbers of these organisms, which contribute to SBS1.
Three main processes cause biodeterioration: mechanical, assimilative (because building materials are a source of nutrition and energy for microorganisms) and dissimilative (the interaction of building materials with aggressive metabolites of microorganisms)5,6. Since the ability of fungi to produce secondary metabolites like single-, double- and tribasic organic acids and enzymes plays an important role in the process of dissimilative biodeterioration7,8 as well as in pathogenesis2,9,10, we focused our experimental work on measurements of secreted proteases and lipases of Aspergillus strains isolated from old buildings and from patients diagnosed with onychomycosis and otomycosis.
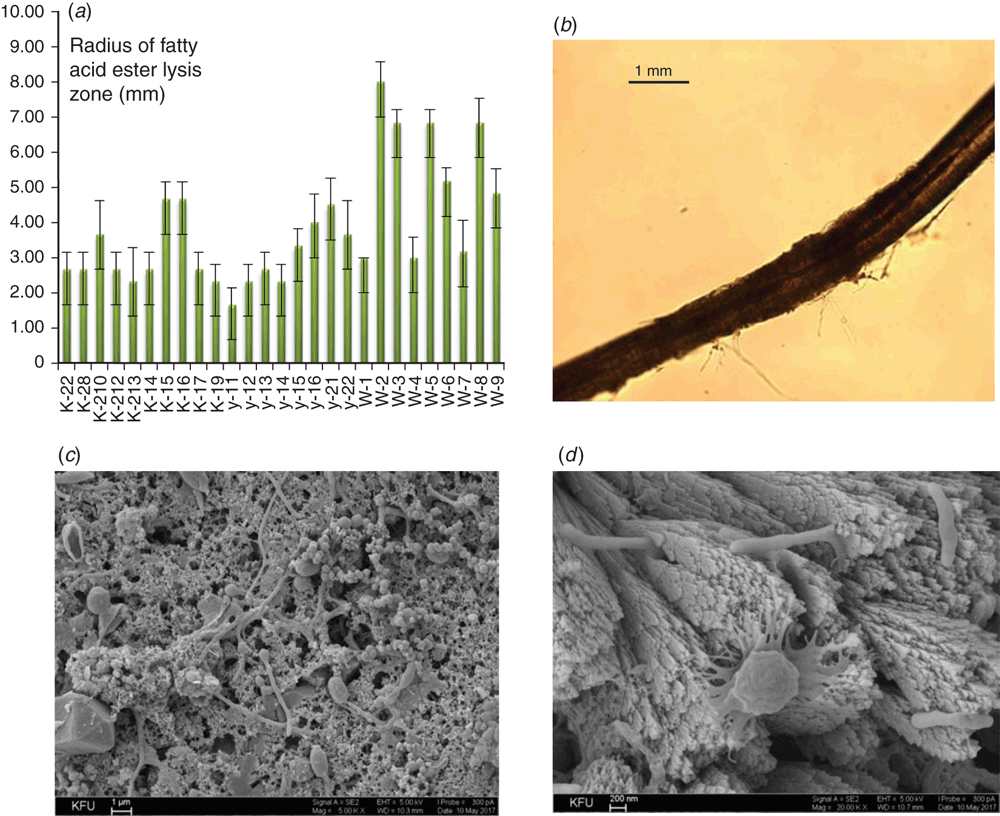
Pathogenic fungi secrete hydrolytic enzymes that enable them to breach and invade host tissues. The most highly recognised extracellular hydrolytic enzymes include proteases and lipolytic enzymes. Proteases from fungi induce inflammatory responses by altering the permeability of the epithelial barrier and by inducing the proinflammatory cytokines through protease-activated receptors10. Lipolytic enzymes have been shown to influence growth, morphology, adherence, and dissemination of fungal cells across the host9. We found that isolates from buildings showed a higher level of lipolytic activity compared to clinical strains (Figure 1a). The level of proteases was low in both groups, with slight prevalence in the activity of clinical isolates. Growing on keratin-containing substrate, Aspergillus strains produced a coat around the hair, but did not destroy the hair itself (Figure 1b). All the isolates studied are able to synthesise oxalic acid. The malic acid was found in culture fluids of 93% micromycetes, acetic acid was synthesised by 67%, citric acid by 60%, lactic acid by 33% and acetic acid by 13% of isolates. Acids promote the wash-out of calcium from concrete thus increasing the biodeterioration. On the other hand, pathogenic fungi acidify the environment as a strategy to damage host tissues, and acidification of the host tissues promotes the expression and activity of fungal proteases11.
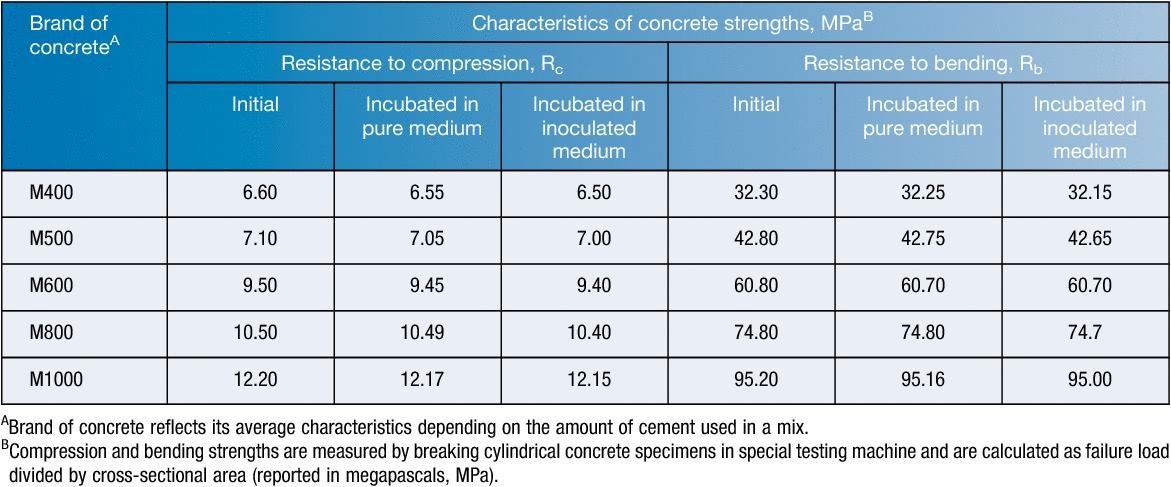
To date, there are more than 200 different methods to test biostability of building materials. Along with international standards, there are national standards for particular countries (STD 141C/6271/2-86 for USA; BS 1133 for UK, DIN 53739-84 for Germany, NF X 41-514-81 for France; JIS Z 2911-87 for Japan, GOST 9.048-89 for Russia). According to our data, the most aggressive strain was A. niger W-2 isolated from an old hospital building in Kazan city. This isolate possessed the highest levels of lipolytic activity and acid production. After 28 days of contact with A. niger W-2 in the growth medium, the values of concrete sample stability were determined as described earlier12. As seen from Table 1, the resistance coefficients to bending and compression (Rb and Rc) after contact with the growing strain decreased by no more than 1%, indicating that there are no significant changes in the concrete specimens. However, these minimal changes determined by the standard test develop over time and gradually contribute to the destruction of concrete. Penetrating into the thickness and germinating in cracks, A. niger W-2 destroys the concrete (Figure 1c, d). Moreover, colonisation of the building material by toxigenic fungi raises the question of a subsequent exposure of residents to aerosolised mycotoxins. The greatest part of the aerosolised toxic load is found in particles whose size corresponds to spores or mycelium fragments. However, some toxins were also found on particles smaller than spores that are easily inhaled and can deeply penetrate into the respiratory tract13.
|
A list of major strains in hospital environments (Centre F. Baclesse, Normandy, France) compiled according to their frequency, concentration level, and/or capacity to produce mycotoxins in vitro, is as follows: A. fumigatus, A. melleus, A. niger, A. versicolor, Cladosporium herbarum, Purpureocillium lilacinum, and Penicillium brevicompactum14. No mutagenic activity was found in bioaerosols. The results of genotoxicity testing by the reversion of auxotrophic Salmonella typhymurium stains to prototrophy under exposure to the culture fluid of A. niger W-2 (Ames assay) were also negative. Nevertheless, some Aspergillus strains assigned to section Nigri are well known as ochratoxin and/or fumonisin producers and showed toxigenic potential in vitro15. It could be concluded that active biodegraders from the genus Aspergillus contribute to the onset of the SBS.
Acknowledgements
The authors thank Professor Viktor Stroganov and Professor Eugene Sagadeev from Kazan State University of Architecture and Engineering for measurements of concrete sample stability and fruitful discussion.
References
[1] Joshi, S.M. (2008) The sick building syndrome. Indian J. Occup. Environ. Med. 12, 61–64.| The sick building syndrome.Crossref | GoogleScholarGoogle Scholar |
[2] Haleem Khan, A.A. and Mohan Karuppayil, S. (2012) Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 19, 405–426.
| Fungal pollution of indoor environments and its management.Crossref | GoogleScholarGoogle Scholar |
[3] Gomzi, M. and Bobić, J. (2009) Sick building syndrome. Do we live and work in unhealthy environment? Periodicum Biologorum 111, 79–84.
[4] van Burik, J.-A.H. and Magee, P.T. (2001) Aspects of fungal pathogenesis in humans. Annu. Rev. Microbiol. 55, 743–772.
| Aspects of fungal pathogenesis in humans.Crossref | GoogleScholarGoogle Scholar |
[5] Allsopp, D. et al. (2004) Introduction to biodeterioration. 2nd edn. Cambridge University Press.
[6] Jayakumar, S. (2012) Studies on the biodeterioration of concrete by marine algae. Shodhganga Repository of Indian Electronic Theses and Dissertations. Pondicherry University School of Engineering. http://shodhganga.inflibnet.ac.in/handle/10603/5590
[7] Wei, S. et al. (2013) Microbiologically induced deterioration of concrete. Braz. J. Microbiol. 44, 1001–1007.
| Microbiologically induced deterioration of concrete.Crossref | GoogleScholarGoogle Scholar |
[8] Bertron, A. et al. (2004) Cement paste alteration by liquid manure organic acids: chemical and mineralogical characterization. Cement Concr. Res. 34, 1823–1835.
| Cement paste alteration by liquid manure organic acids: chemical and mineralogical characterization.Crossref | GoogleScholarGoogle Scholar |
[9] Park, M. et al. (2013) Lipolytic enzymes involved in the virulence of human pathogenic fungi. Mycobiology 41, 67–72.
| Lipolytic enzymes involved in the virulence of human pathogenic fungi.Crossref | GoogleScholarGoogle Scholar |
[10] Yike, I. (2011) Fungal proteases and their pathophysiological effects. Mycopathologia 171, 299–323.
| Fungal proteases and their pathophysiological effects.Crossref | GoogleScholarGoogle Scholar |
[11] Vylkova, S. (2017) Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 13, e1006149.
| Environmental pH modulation by pathogenic fungi as a strategy to conquer the host.Crossref | GoogleScholarGoogle Scholar |
[12] Yakovleva, G. et al. (2016) Assessment of biodamage resistance of various concrete grades. Int. J. Pharm. Technol. 8, 24291–24299.
[13] Aleksic, B. et al. (2017) Aerosolization of mycotoxins after growth of toxinogenic fungi on wallpaper. Appl. Environ. Microbiol. , .
| Aerosolization of mycotoxins after growth of toxinogenic fungi on wallpaper.Crossref | GoogleScholarGoogle Scholar |
[14] Heutte, N. et al. (2017) Assessment of multi-contaminant exposure in a cancer treatment center: a 2-year monitoring of molds, mycotoxins, endotoxins, and glucans in bioaerosols. Environ. Monit. Assess. 189, 31.
| Assessment of multi-contaminant exposure in a cancer treatment center: a 2-year monitoring of molds, mycotoxins, endotoxins, and glucans in bioaerosols.Crossref | GoogleScholarGoogle Scholar |
[15] Zhang, J. et al. (2016) A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger. J. Agric. Food Chem. 64, 9680–9688.
| A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger.Crossref | GoogleScholarGoogle Scholar |
Biographies
Professor Olga Ilinskaya is the Head of Microbiology Department at Kazan Federal (Volga-Region) University (Tatarstan, Russia). She took her Doctor degree in Microbiology and Biochemistry from I.M. Sechenov First Moscow State Medical University. She is a Full Member of the Academy of Sciences, Laureate of the state award of Tatarstan Republic, and a member of the Russian Interregional Microbiological Society. Her research interests include molecular microbiology, genetic toxicology and biomedicine.
Dr Galina Yakovleva is an assistant professor at the above university. She took her PhD in Microbiology from Kazan Federal University and is a member of the Russian Interregional Microbiological Society. She is an expert in microbial biotechnology and biodegradation of xenobiotics.
Alina Bayazitova is a young researcher at the Laboratory of Fungal Diseases, Kazan Research Institute of Epidemiology and Microbiology, and a PhD student at Kazan Federal University. She majors in clinical mycology.