Microbial cooperation improves bioleaching recovery rates
Melissa K Corbett A and Elizabeth LJ Watkin A BA School of Pharmacy and Biomedical Sciences, CHIRI Biosciences, Curtin University, Perth, WA, Australia
B Tel: +61 2 9266 2955, Email: e.watkin@curtin.edu.au
Microbiology Australia 39(1) 50-52 https://doi.org/10.1071/MA18013
Published: 16 February 2018
Whilst bioleaching is primarily used to recover minerals from low-grade ores, the increasing demand for Rare Earth elements combined with supply chain concerns is opening up new avenues of extraction from mine tailings, waste products and recyclable materials. Exploration of new, novel and economically viable techniques are required to manage the coming shortage and volatility of global markets with more environmentally sound alternatives to traditional mining operations holding the key.
The exploitation of microbes in the industrial application of bioleaching has been underway since the 1950s1 due to their ability to mobilise minerals from ore bodies, with either heap leaching implemented for the recovery of Cu, Zn, Ni2 or stirred tank reactors for U or Au3. With fewer discoveries of large high grade mineral deposits occurring4 it is anticipated that demand for raw minerals will outstrip reserves for not only these elements, but also for Rare Earth Elements (REEs). REEs are fundamental components of mobile phones, lasers, electric batteries and superconductors5. With dwindling supplies of high grade REE stocks, ever increasing demand for new technologies and a push for the mining industry to ‘go green’, the processing of lower grade ores, recycling of electronic waste and treatment of discarded mining by-products using bioleaching applications is proving attractive. Due to this the use and application of bioleaching techniques is expanding as they are more cost efficient, less energy intensive and employ more eco-friendly techniques2.
REEs (15 elements with atomic numbers ranging from 57 to 71)6 are located amid carbonates, placer deposits, pegmatites and marine phosphates7. However, current bioleaching applications utilise the autotrophic oxidation of ferrous and reduced sulphur compounds for mineral release and subsequent recovery, which are found in low amounts in REE ore bodies. Nevertheless, studies of REE mineral extraction from phosphate ores by bioleaching are in their infancy8–10. These bioleaching activities utilise acidophilic and heterotrophic phosphate solubilising microorganisms (PSMs), those often employed to increase soluble phosphate levels in agricultural settings. Species currently identified with the potential to recovery REEs from phosphate laden ores include Pseudomonas, Acinetobacter, Bacillus, Microbacterium, Aspergillus, Penicillium and Cladosporium10. Primarily, the focus of REE bioleaching investigations have utilised pure cultures on sterile ore and have resulted in varied rates of recovery depending on both the microbial species employed and mineralogical characteristics of the ore used.
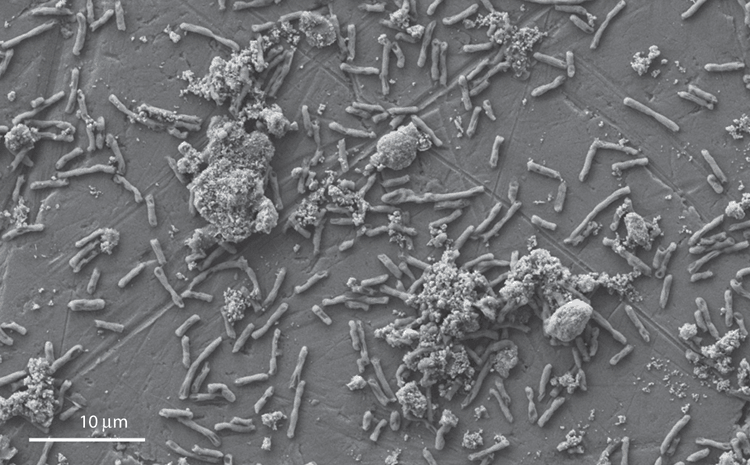
For example, the fungal species Penicillium tricolor was shown to leach 30–70% of available REEs from red mud9 compared to Bacillus megaterium, which leached less than 1% from monazite11. Industrial operations with ferrous and sulphide ores often involve two or more species due to the leaching chemistry requirements, size of the processes and the inability to maintain sterility. It has been shown that mixed acidophilic populations increase recovery rates of copper12 compared to pure cultures. Our research initially conducted with pure cultures13 (Figure 1) demonstrated low recovery rates of REEs from a concentrated Western Australian monazite, whereas when bioleaching was performed using non-sterile ore complete with the native population and an introduced PSM (Figure 2), REE leaching rates increased tenfold with some species14. These leaching rates were much greater than those recorded with either pure cultures or the native consortia alone. Cultures with a fungal starter organism such as Penicillium sp. had higher leaching rates than those commenced with a bacterial isolate such as Enterobacter or Pseudomonas sp. As the indigenous microbial population present on the ore is expected to be limited in number2, the addition of a heterotrophic PSM aids in initiating leaching processes during the early operating phases.
Unlike the chemolithoautrophic pathways employed by the microbial consortia for growth during ferrous and sulphide leaching operations, heterotrophic leaching of REE phosphates requires the addition of a carbon source, usually in the form of glucose, which can be cost prohibitive on a large scale. The provision of molasses, a waste product generated from sugarcane refining is a financially more viable possibility that will meet microbial growth needs for optimum ongoing leaching. Fermentation of glucose by an introduced heterotroph to the non-sterile leaching environment results in the manufacture of numerous ligands, predominately organic acids including acetic, citric, formic, oxalic and pyruvic depending on the PSM employed, which drives a significant portion of the REE leaching process. This initial consumption of glucose by the introduced species and the resultant availability of secondary metabolites can enable the growth of heterotrophic, mixotrophic and acidophilic microorganisms already existing on the ore, with the presence of native Firmicutes notably increasing leaching rates14. In this symbiotic association, with the generation of secondary carbon compounds, a lowered pH environment is established and as the system stabilises with increased numbers of indigenous species, the need to add further glucose is reduced. As it has been demonstrated that the native consortia alone are capable of REE leaching albeit at very low levels, initiation of indigenous activity appears to require one or more unknown metabolites that arise as a result of the inoculant species fermenting glucose.
The uptake of bioleaching as a viable alternative to traditional methods for the recovery of REEs has been slow due to the inherent unknowns in a biological based system and the uncertainty in value for money returns. In Australia there are currently no commercial REE bioleaching projects using heterotrophic or mixotrophic microorganisms despite Australia having one of the largest REE deposits in the world15. To encourage more mining corporations to opt for a more environmentally friendly approach to REE recovery, extensive research needs be undertaken to determine not only the best PSM to ‘prime’ the system, but also to examine the complex interactions occurring between the introduced PSM and native consortia. Armed with this evidence, optimisation of REE bioleaching operations (Figure 3) is an obtainable goal with improved leaching rates likely to allow the construction of long term reactor systems with decreased operating costs and lower environmental impacts.

|
References
[1] Agate, A.D. (1996) Recent advances in microbial mining. World J. Microbiol. Biotechnol. 12, 487–495.| Recent advances in microbial mining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmsFyit7w%3D&md5=efbd71f2b423308ac729687218ebe66dCAS |
[2] Rawlings, D.E. and Johnson, D.B. (2006) Biomining. Springer: Berlin, Heidelberg.
[3] Rawlings, D.E. (1998) Industrial practice and biology of leaching metals from ores. J. Ind. Microbiol. Biotechnol. 20, 268–274.
| Industrial practice and biology of leaching metals from ores.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXltlersLw%3D&md5=ab828ac2cc6dbf5323f40940bede41ecCAS |
[4] Watling, H.R. (2015) Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals (Basel) 5, 1–60.
| Review of biohydrometallurgical metals extraction from polymetallic mineral resources.Crossref | GoogleScholarGoogle Scholar |
[5] Humphries, M. (2010) Rare Earth Elements: The Global Supply Chain. DIANE Publishing Company.
[6] Long, K.R. et al. (2010) The principal rare earth elements deposits of the United States—A summary of domestic deposits and a global perspective. U.S. Geological Survey. Sci. Investig. Rep. 96.
[7] Massari, S. and Ruberti, M. (2013) Rare earth elements as critical raw materials: focus on international markets and future strategies. Resour. Policy 38, 36–43.
| Rare earth elements as critical raw materials: focus on international markets and future strategies.Crossref | GoogleScholarGoogle Scholar |
[8] Groudeva, V.I. et al. (2007) Bioleaching of a rich-in-carbonates copper ore at alkaline pH. Adv. Mat. Res. 20–21, 103–106.
| Bioleaching of a rich-in-carbonates copper ore at alkaline pH.Crossref | GoogleScholarGoogle Scholar |
[9] Qu, Y. and Lian, B. (2013) Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 136, 16–23.
| Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvV2ks7w%3D&md5=c9c49d7567384f4577a17c8c96da1cb7CAS |
[10] Barmettler, F. et al. (2016) Microbial mobilization of rare earth elements (REE) from mineral solids—a mini review. AIMS Microbiology 2, 190–204.
| Microbial mobilization of rare earth elements (REE) from mineral solids—a mini review.Crossref | GoogleScholarGoogle Scholar |
[11] Shin, D. et al. (2015) Use of phosphate solubilizing bacteria to leach rare earth elements from monazite bearing ore. Minerals (Basel) 5, 189–202.
| Use of phosphate solubilizing bacteria to leach rare earth elements from monazite bearing ore.Crossref | GoogleScholarGoogle Scholar |
[12] Akcil, A. et al. (2007) Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner. Eng. 20, 310–318.
| Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsFKlu7w%3D&md5=33fcbedbf84929a7318ccfe311cc4f0dCAS |
[13] Corbett, M.K. et al. (2017) Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: a study utilizing Western Australian monazite. Bioprocess Biosyst. Eng. 40, 929–942.
| Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: a study utilizing Western Australian monazite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXkvVWhtbg%3D&md5=547cdb4b4587c421dfe165b87bf1eb0bCAS |
[14] Corbett, M.K. et al. (2017) Incorporation of indigenous microorganisms increases leaching rates of rare earth elements from Western Australian monazite. Diffus. Defect Data Solid State Data Pt. B Solid State Phenom. 262, 294–298.
| Incorporation of indigenous microorganisms increases leaching rates of rare earth elements from Western Australian monazite.Crossref | GoogleScholarGoogle Scholar |
[15] BREE G.A. (2014) Australian Energey Resource Assessment, 2nd edn. (Economics, B.o.R.a.E., ed.), Geosciences Australia.
Biographies
Dr Melissa Corbett is a lecturer in microbiology at Curtin University within the School of Pharmacy and Biomedical Sciences. Dr Corbett’s post-doctoral research has focused on the use of microorganisms for bioleaching in saline conditions as well as investigating how to recover rare earth elements from radioactive sources using microbial action. For the past 5 years she has also lectured undergraduate students in the field of Microbiology.
Elizabeth Watkin is Professor of Microbiology at Curtin University. The overarching theme of her research is the microbial ecology of environmental systems and covers the fields of mining biotechnology and mineral resource recovery, microbial induced corrosion, and microbial fouling of water (particularly within mining systems).