Avian influenza. Why the concern?
Ian G Barr A B D and Frank YK Wong CA WHO Collaborating Centre for Reference and Research on Influenza, VIDRL, Doherty Institute 792 Elizabeth Street߬Melbourne, Vic. 3000, Australia
B The University of Melbourne Melbourne, Vic. 3010, Australia
C CSIRO Australian Animal Health Laboratory߬East Geelong, Vic. 3220, Australia
D Email: Ian.Barr@influenzacentre.org
Microbiology Australia 37(4) 162-166 https://doi.org/10.1071/MA16055
Published: 22 September 2016
Avian influenza normally has little impact on poultry and wild birds but since 1996, highly virulent viruses have emerged and continue to circulate in many countries. The results of these viruses have been devastating in domestic poultry and they have also spilled over into humans, infecting and killing hundreds and raising the opportunities for the virus to further adapt and possibly cause a future influenza pandemic. This article briefly details these events and discusses the consequences of these viruses continuing to circulate and cause disease.
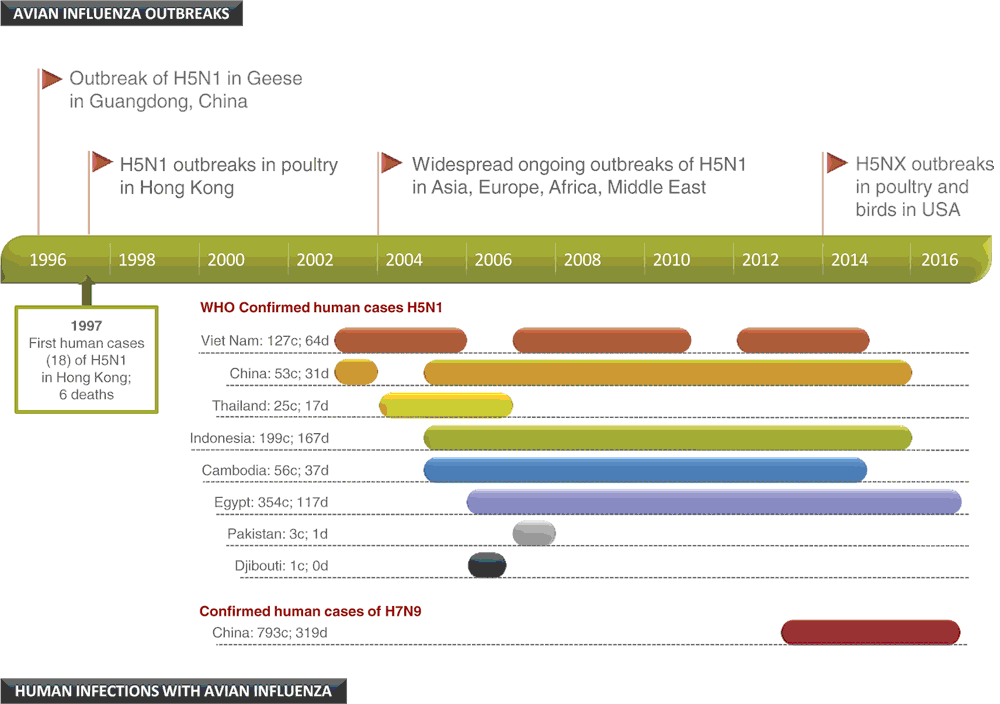
The year 2016 marks a milestone for avian influenza, often referred to as ‘bird flu’. It is now 20 years since the outbreaks of highly pathogenic avian influenza (HPAI) in farmed geese in the Guangdong Province of southern China1. These viruses are now recognised as the progenitors of the zoonotic H5N1 avian viruses that caused global concern in Hong Kong in 1997 with 18 human cases and 6 deaths and led to extensive poultry culling and changes to live bird markets (Figure 1). These viruses continued to evolve and rapidly spread from China throughout Asia resulting in a HPAI panzootic (a global disease epidemic in animals) event which continues to this day. The ‘H’ in H5N1 refers to the serotype of the haemagglutinin protein and the ‘N’ is the serotype of the neuraminidase protein. H and the N proteins are both abundant on the surface of the influenza virus and play key roles in the attachment (H) to cells and the release (N) from infected cells. The H protein has a proteolytic cleavage site (PCS) where host proteases cleave the protein into two subunits HA1 and HA2, an essential step in producing infectious virus. Only strong proteases present in the respiratory tract of mammals and birds, and the gastrointestinal tract of birds, cleave the H of low pathogenicity influenza viruses (LPAI). However, in HPAI the H protein acquires an insert of additional basic amino acids at the PCS allowing proteases in many organs to cleave the protein and virus to spread widely in the body. These HPAI viruses can be extremely deadly in some poultry species especially chickens and can lead to high mortality in flocks within a day or two of infection. HPAI viruses with the multi-basic PCS have been restricted to the avian influenza H5 or H7 types to date. The majority of influenza viruses circulating in avian species lack this insert and therefore do not cause significant disease in birds (including most H5 and H7 viruses).
|
The H5N1 HPAI problem
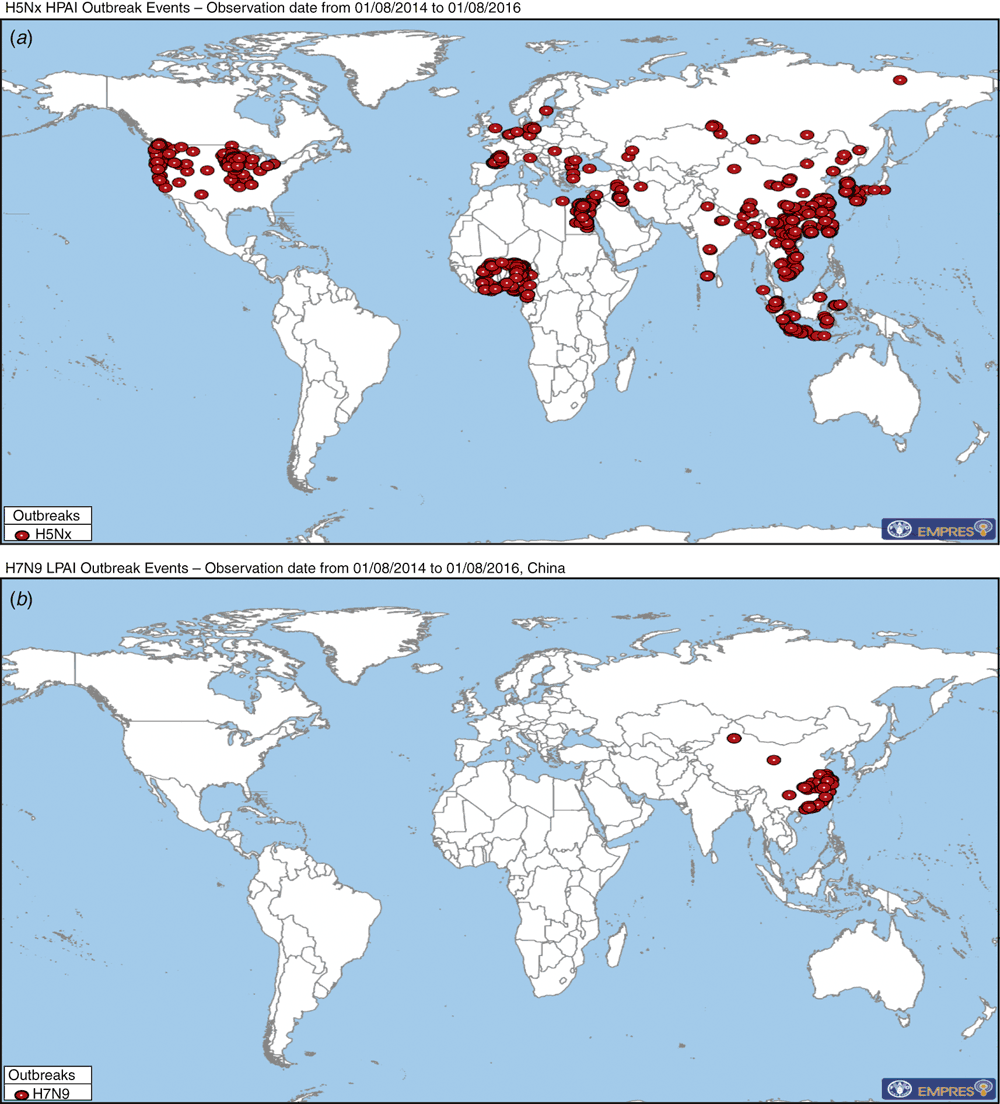
So what’s the problem 20 years on? Essentially it is the ongoing persistence of HPAI and its wide geographical spread (Figure 2) that have resulted in millions of birds being infected along with some humans, mostly through contact with infected poultry. The consequences of this panzootic have been dramatic, with millions of birds culled or dying from infection with H5N1 viruses. While human infections have been much fewer to date (854 human infections reported to WHO as of 19 July, 2016), the outcomes have been severe, with 450 deaths, fortunately without sustained human to human transmission2. The lost food supply and the costs and effort in vaccinating or culling birds, monitoring outbreaks and treating infections during this period have been enormous. Concomitantly has been the ongoing threat that these viruses, either by mutation, genetic reassortment or a combination of the two, might generate a virus that is more transmissible between humans and could cause a major human pandemic. Reassortment, where two or more influenza viruses infect the same cell resulting in a mixture of virus genes in the progeny can produce the most dramatic changes resulting in a unique virus. This has happened scores of times since 1996, most recently in North America and China where the H5 viruses have expanded their N type repertoire with HPAI H5N2 and H5N8 outbreaks in chickens and turkeys in North America and both avian and human infections in China with H5N6 from 2014–16, leading to these viruses being now referred to as H5Nx viruses. To date these viruses have retained their avian characteristics and have only occasionally infected humans: for example, there have been 14 cases of human infection with H5N6 reported in China since 2014.
|
A new problem, H7N9 LPAI
Adding to this mix of H5 viruses have been other avian influenza viruses that have also caused significant human infections. The most serious of these recently have been H7N9 LPAI infections, first detected in March 2013 in Southern China that have since recurred annually, with at least 793 human cases and 319 deaths, mostly associated with exposure to H7N9 infected birds especially with elderly men at places like live bird markets (Figure 3). As with H5 HPAI, these sporadic human H7N9 infections from birds and the continued endemic circulation in live bird markets and farms in China, mean that the public health risk from exposure to these and potentially novel reassortant viruses remains a great concern. Other influenza A subtypes such as H9N2, H10N8 and H6N1 have also been implicated in human infections in China, some of which have been fatal, while others such as H7N7 cases in the Netherlands, have been associated with much milder human infections. As endemic LPAI viruses such as the Chinese H7N9 and H9N2 viruses have little pathogenicity in poultry, there is little warning of their presence, resulting in increased risk of human exposures.
The big questions that still remain today
Can these viruses be eliminated or controlled in poultry and if not, will any adapt and increase their tropism for humans, leading to widespread outbreaks or a potential human pandemic of unknown severity? Thankfully the H5N1/H5Nx HPAI viruses have so far failed to become more transmissible in humans, with only a few possible clusters of H5N1 human-to-human transmission and there has been little increase in the number of human cases even with ongoing poultry outbreaks and human exposure. This is supported by testing in ferret models of influenza where H5Nx viruses did not transmit from infected to naïve animals even when co-housed, nor could they be transmitted to ferrets via virus infected aerosols. The situation for recent Chinese H7N9 viruses is less clear cut. Similar to H5Nx, there have been few human secondary infections or infection clusters recorded to date, but ferret studies demonstrated that these H7N9 viruses were easily transmitted by close contact and even by aerosols, although still not as efficiently as human seasonal influenza A viruses. The possible emergence of a virus variant of these or other subtypes that is able to replicate and transmit by the aerosol route more efficiently in man, would be an immediate pandemic concern since modern air travel means that infected persons can easily and quickly spread their infections at a global level before transboundary infectious disease mitigation strategies can be effectively implemented. This was highlighted by the rapid worldwide spread of the 2009 pandemic H1N1 virus that emerged from swine. The establishment of the Asian lineage H5N1 HPAI in the poultry of many other countries in Asia and Africa (Figure 2), and the recent emergence of related H5Nx viruses affecting birds in several Southeast and East Asian countries and further afield in Europe and North America, also demonstrate the potential for their widespread distribution by either cross-border poultry trade or carriage by migratory wild waterbirds.
Some possible answers?
To help manage the risk of these avian influenza viruses becoming a threat to mankind, various systems have been developed to assess avian and swine influenza viruses. One such system, IRAT (Influenza Risk Assessment Tool), was developed by the Influenza Division at CDC, Atlanta, USA. This assessment takes into account a number of factors that may be important in avian influenza viruses making the ‘jump’ from being an avian virus to becoming a human virus. These include both virus and host characteristics, such as virus’ H receptor specificity, pathogenicity in man and in animal models, background levels of immunity in the human population, transmissibility in man and in animal models, the number of infected birds and many other factors. These factors are combined and influenza virus types are ranked by plotting them according to their potential risk to achieve ‘sustained human-to-human transmission’ (emergence risk) and potential ‘for significant impact on public health’ (impact risk). In a recent publication3 from a small number of existing avian influenza viruses tested in the IRAT model, the Chinese H7N9 virus achieved the highest score (above H5N1 and a swine virus H3N2v) and this risk factor had increased slightly since a previous assessment in April 2013. This is not to say that an H7N9 outbreak is imminent but these rankings are meant to help guide public health measures such as early vaccine development and to also encourage virus control in the avian population, or to introduce measures to avoid human infection.
In addition to these risk assessments, researchers are exploring the factors that allow interspecies transmission but limit human transmission. A recent study with an H7N9 isolate suggests a ‘genetic bottleneck’ during infection of ferrets, and possibly humans, whereby the virus becomes less fit and therefore unlikely to be easily transmitted4. However, until we fully understand the mechanisms that allow ongoing human to human transmission it would be prudent to try to eliminate from poultry flocks those viruses with the highest risk to man (e.g. H7N9) and those of greatest risk to the domestic poultry population and to the global food supply (e.g. H5Nx HPAI) by targeted culling, effective poultry vaccines or in the future breeding poultry genetically resistant to HPAI. Meanwhile it remains important to maintain surveillance for novel influenza viruses in animals and humans and plan measures to combat any emerging virus in the human population, including appropriate vaccines and effective anti-viral drugs.
Acknowledgement
The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health.
References
[1] Wan, X.F. (2012) Lessons from emergence of A/goose/Guangdong/1996-like H5N1 highly pathogenic avian influenza viruses and recent influenza surveillance efforts in southern China. Zoonoses Public Health 59, 32–42.| Lessons from emergence of A/goose/Guangdong/1996-like H5N1 highly pathogenic avian influenza viruses and recent influenza surveillance efforts in southern China.Crossref | GoogleScholarGoogle Scholar | 22958248PubMed |
[2] Uyeki, T.M. (2008) Global epidemiology of human infections with highly pathogenic avian influenza A (H5N1) viruses. Respirology 13, S2–S9.
| Global epidemiology of human infections with highly pathogenic avian influenza A (H5N1) viruses.Crossref | GoogleScholarGoogle Scholar | 18366524PubMed |
[3] Trock, S.C. et al. (2015) Development of framework for assessing influenza virus pandemic risk. Emerg. Infect. Dis. 21, 1372–1378.
| Development of framework for assessing influenza virus pandemic risk.Crossref | GoogleScholarGoogle Scholar | 26196098PubMed |
[4] Zaraket, H. et al. (2015) Mammalian adaptation of influenza A(H7N9) virus is limited by a narrow genetic bottleneck. Nat. Commun. 6, 6553.
| Mammalian adaptation of influenza A(H7N9) virus is limited by a narrow genetic bottleneck.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtF2lu7jE&md5=d5542d39d023b17fa9295f5df183ecbeCAS | 25850788PubMed |
Biographies
Professor Ian Barr is the Acting Director of the WHO Collaborating Centre for Reference and Research on Influenza based at the Doherty Institute in Melbourne. He joined the Centre 16 years ago and has previously worked in commercial, research and academic scientific positions. The Centre is one of six in the world that supports a WHO-led global human influenza surveillance network.
Dr Frank Wong is a Research Team Leader with the CSIRO Australian Animal Health Laboratory. Frank is the current World Organisation for Animal Health (OIE) expert focal point on avian influenza for Australia. He is also a contributor to the Joint OIE/FAO Network of Expertise on Animal Influenza (OFFLU), and currently represents OFFLU at the World Health Organization (WHO) vaccine consultations on zoonotic influenza viruses for pandemic preparedness purposes. He also serves as a Steering Committee member of the Wildlife Health Australia National Avian Influenza Wild Bird Surveillance Program. Altogether, Frank has more than 15 years’ experience in the molecular characterisation of microbiological pathogens.



