Protozoa PCR: boon or bane
Colin Pham A and Harsha Sheorey BA St Vincent’s Hospital
Melbourne, Vic., Australia
Email: colin.pham@svha.org.au
B St Vincent’s Hospital
Melbourne, Vic., Australia
Email: harsha.sheorey@svha.org.au
Microbiology Australia 37(1) 46-49 https://doi.org/10.1071/MA16007
Published: 12 February 2016
Parasite detection in faeces has traditionally been performed by microscopy, a procedure that is labour-intensive and highly specialised. In addition, identification by microscopy based on morphological features alone is subjective and prone to wide variability. Although enzyme immunoassays (EIA) of high sensitivity have been developed1 they can detect only a limited range of pathogens. Given these factors the introduction of Polymerase Chain Reaction (PCR) into the routine diagnostic laboratory has improved parasite detection rates2. The ability to multiplex has enabled the detection of multiple targets from a single sample and provides an objective alternative to identification by morphology.
Results
In February 2014, St Vincent’s Pathology, Melbourne, introduced a new testing algorithm making use of a commercial multiplex PCR (LightMix®Gastroenteritis Parasite Kit, Roche Molecular Systems, Pleasanton, California, USA) for parasite detection. This assay is designed to detect Giardia intestinalis, Cryptosporidium species, Dientamoeba fragilis and Entamoeba histolytica, four human gastrointestinal protozoan pathogens3. Blastocystis was not included as a target in this multiplex assay, as the primers do not differentiate the 17 different subtypes now known4, many being of animal origin and most of doubtful clinical significance.
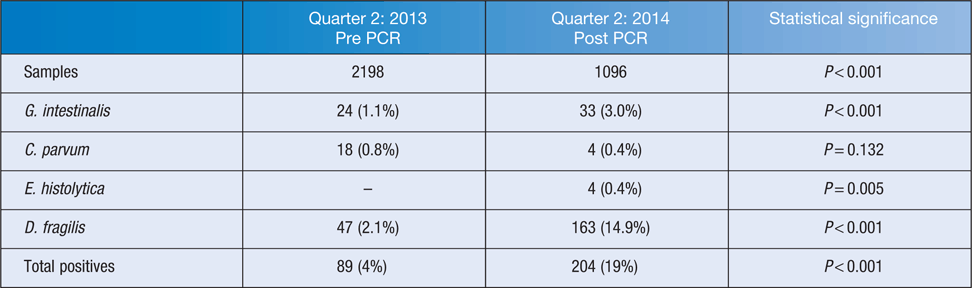
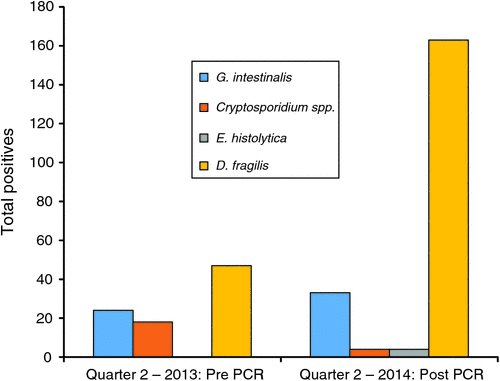
Since the commencement of testing by PCR, the rates of detection have improved when compared with results obtained based on the previous protocol that included microscopy and Giardia/Cryptosporidium antigen testing (Table 1). Comparison over a three month period showed that the range of common parasites detected in the population remains similar with D. fragilis accounting for the majority of the additional parasites detected (Figure 1). The reduction of Cryptosporidium cases from 18 (0.8%) to 4 (0.4%) was not statistically significant and is thought to relate to seasonal variation in prevalence. The additional E. histolytica cases were detected from microscopy-positive specimens referred from other laboratories. In addition, the assay was positive for E. histolytica in liver aspirates from four cases of suspected amoebic hepatitis.
|
|
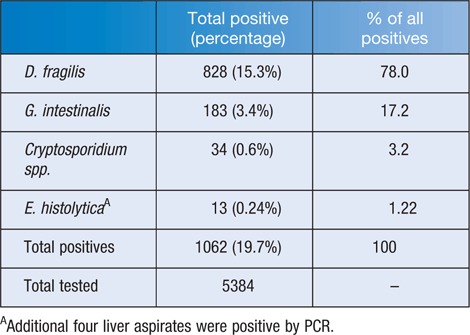
The detection of protozoa by molecular technology at St Vincent’s Pathology, Melbourne, over the 15 month period since implementation of the multiplex PCR assay is shown in Table 2. The rate of D. fragilis detection increased from 2.1% by microscopy to 15.3% with PCR; this represents the most significant finding, accounting for 78% of all parasites detected by this test.
|
Discussion
The advent of PCR has definitely become a ‘boon’ for our laboratory in providing a rapid, less labour-intensive method for detecting the three pathogenic protozoa G. intestinalis, Cryptosporidium species and E. histolytica. PCR can detect low numbers easily missed by microscopy and also can differentiate pathogenic E. histolytica from morphologically similar, non-pathogenic Entamoeba dispar/moshkovskii5. During the initial validation period, 10 Entamoeba species identified by microscopy were tested by PCR and only two of these samples were positive for E. histolytica. The ability to differentiate between pathogenic E. histolytica and non-pathogenic Entamoeba species is clinically significant. Results concur with a prevalence study of Entamoeba in immigrants in Spain that showed that the majority of cyst-positive samples detected by microscopy were E. dispar and do not require treatment5.
On the other hand, PCR seemed to become a ‘bane’ by detecting a large number of D. fragilis in asymptomatic people, especially children6,7. Numerous parents of ‘well’ children get anxious about this so called ‘bad bug that does not go away’. Many of these ‘worried well’ get treated with various antiparasitic drugs and combinations (some of which are known to imbalance the normal gut flora). They ‘doctor shop’ and are being referred to specialists such as paediatricians, gastroenterologists and infectious diseases physicians (communications with General Practitioners and Australia and New Zealand Paediatric Infectious Diseases Group).
The reliance on morphological diagnosis by microscopy of fixed stained-smears and the recognition that parasite shedding may be intermittent, suggests that prior to PCR diagnosis, the true prevalence of this protozoon has been underestimated. A review of D. fragilis carriage has shown that prevalence ranges from 0.2% to 82% in numerous geographical settings, utilising a variety of detection methods8. In this review, the authors conclude that in ‘symptomatic patients who harbor Dientamoeba and no other pathogen, it should be considered as the etiological agent and treated’. However, they also mention that evidence is only circumstantial. There has been debate about the clinical significance of D. fragilis given such wide variation in prevalence estimates coupled with the observation that the protozoa may be found in symptomatic and asymptomatic individuals and variable responses to antiparasitic drugs. A recent case-controlled study by Krogsgaard et al.6 demonstrated that D. fragilis and Blastocystis were detected in a greater proportion of faecal samples from the asymptomatic background population in Denmark than from subjects with irritable bowel syndrome. Röser et al.7 presented data from four years of routine PCR testing and found that from a total of 22,484 samples, 43% were positive for D. fragilis. With the introduction of PCR into routine testing within our laboratory, our data suggest that the prevalence of asymptomatic D. fragilis carriage is higher than previously reported.
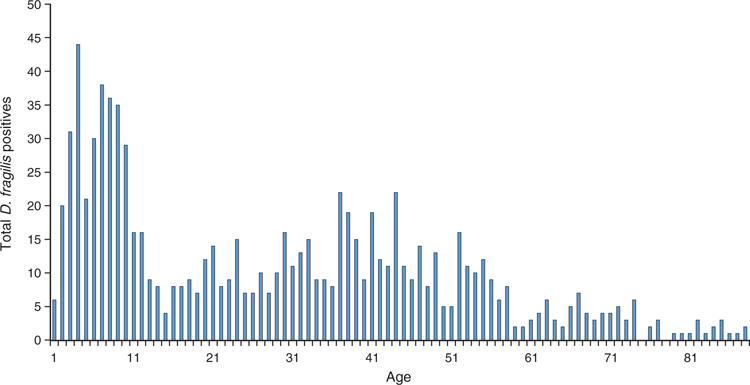
Of all D. fragilis detected in our study period, 40% of faeces were formed, 58% were semi-formed or unformed and only 2% were loose/liquid as observed in the laboratory. The clinical notes provided on pathology request forms ranged from diarrhoea to vague IBS like symptoms such as abdominal pain or discomfort, altered bowel habit and others unrelated to gastrointestinal tract. Samples that were positive for D. fragilis by PCR were predominantly in children below 10 years with a second peak in young adults in age group 30–40 years (Figure 2), a similar distribution to that described by Röser et al.7.
With the more widespread adoption of faecal parasite multiplex PCR assays, the higher positive rate of D. fragilis by PCR reported by laboratories (especially when diagnosed in those with non-specific symptoms), is confusing and of concern to clinicians. Many patients get unnecessary courses of antiparasitic drugs with more than half not clearing the parasite. A double-blinded, placebo-controlled metronidazole study by Röser et al.9 suggests that treatment is not associated with better clinical outcomes. Apart from optimal therapy not being known and a highly variable response to treatment, the pathogenicity of D. fragilis has not been reliably demonstrated.
It must be noted that the use of molecular technology is limited to the targeted screening of only a relatively small number of parasites in a low prevalence population. Microscopy still plays a key role in less common parasite identification, especially in specific patient groups where parasites other than those targeted by this multiplex are suspected. For parasite examination in populations from regions where there is high parasite endemicity, microscopy remains the ‘gold standard’ with the capacity to screen for the majority of pathogens.
In summary, molecular testing can offer accurate results for the detection of the four commonly seen parasites in low prevalence populations. It has been shown to be comparable to EIA for G. intestinalis and Cryptosporidium diagnosis and superior to microscopy for D. fragilis and E. histolytica detection. Our new testing algorithm for parasite detection utilising PCR has shown to improve sensitivity and specificity. It has significantly reduced the time taken to stain fixed smears and microscopy, allowing for a more efficient use of labour, whilst improving parasite detection rates. However, laboratories should consider the limitations of reporting parasites of doubtful clinical significance such as D. fragilis and Blastocystis species, and should alert physicians of the possibility of this being non-pathogenic in immunocompetent people.
| Summary of current faecal protozoa molecular testing: |
|
References
[1] Vidal, A.M. and Catapani, W.R. (2005) Enzyme-linked immunosorbent assay (ELISA) immunoassaying versus microscopy: advantages and drawbacks for diagnosing giardiasis. Sao Paulo Med. J. 123, 282–285.| Enzyme-linked immunosorbent assay (ELISA) immunoassaying versus microscopy: advantages and drawbacks for diagnosing giardiasis.Crossref | GoogleScholarGoogle Scholar | 16444388PubMed |
[2] Bruijnesteijn van Coppenraet, L.E. et al. (2009) Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin. Microbiol. Infect. 15, 869–874.
| Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1WhtLrN&md5=194b9e9c6d9eb424bd98c62fd7ebcbbbCAS | 19624500PubMed |
[3] Slack, A. (2012) Parasitic causes of prolonged diarrhoea in travellers – diagnosis and management. Aust. Fam. Physician 41, 782–786.
| 23210100PubMed |
[4] Stensvold, C.R. (2013) Blastocystis: genetic diversity and molecular methods for diagnosis and epidemiology. Trop. Parasitol. 3, 26–34.
| Blastocystis: genetic diversity and molecular methods for diagnosis and epidemiology.Crossref | GoogleScholarGoogle Scholar | 23961438PubMed |
[5] Gutiérrez-Cisneros, M.J. et al. (2010) Application of real-time PCR for the differentiation of Entamoeba histolytica and E. dispar in cyst-positive faecal samples from 130 immigrants living in Spain. Ann. Trop. Med. Parasitol. 104, 145–149.
| Application of real-time PCR for the differentiation of Entamoeba histolytica and E. dispar in cyst-positive faecal samples from 130 immigrants living in Spain.Crossref | GoogleScholarGoogle Scholar | 20406581PubMed |
[6] Krogsgaard, L.R. et al. (2015) The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: a population-based case-control study. Clin. Gastroenterol. Hepatol. 13, 507–513.
| 25229421PubMed |
[7] Röser, D. et al. (2013) Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1303–1310.
| Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR.Crossref | GoogleScholarGoogle Scholar | 23609513PubMed |
[8] Barratt, J.L. et al. (2011) A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2, 3–12.
| A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness.Crossref | GoogleScholarGoogle Scholar | 21637013PubMed |
[9] Röser, D. et al. (2014) Metronidazole therapy for treating dientamoebiasis in children is not associated with better clinical outcomes: a randomized, double-blinded and placebo-controlled clinical trial. Clin. Infect. Dis. 58, 1692–1699.
| Metronidazole therapy for treating dientamoebiasis in children is not associated with better clinical outcomes: a randomized, double-blinded and placebo-controlled clinical trial.Crossref | GoogleScholarGoogle Scholar | 24647023PubMed |
Biographies
Colin Pham, is a Medical Scientist with over 10 years’ experience in bacteriology, serology and molecular testing. He underwent his training at St Vincent’s Melbourne in 2003, and started his career in Microbiology at the Monash Medical Centre. Since re-joining St Vincent’s, he has specialised in molecular testing and emerging molecular technology. He completed a Masters of Applied Science (Medical Science) at RMIT in 2014, with his thesis titled ‘Impact of molecular testing on the laboratory diagnosis of Giardia intestinalis, Cryptosporidium parvum, Dientamoeba fragilis and Entamoeba histolytica from clinical stool samples’.
Dr Harsha Sheorey, is a Clinical Microbiologist at St Vincent’s Hospital in Melbourne. His special interest is in clinical parasitology and tropical medicine and is the author of Clinical Parasitology – a handbook for medical practitioners and microbiologists: a second edition has recently been published. He is an invited writer for the Nematodes chapter in ASM Manual of Clinical Microbiology. He coordinates the Victorian branch of Parasitology & Tropical Medicine SIG and is actively involved in organisation of the Australian Parasitology and Tropical Medicine Master Class.