The malaria war
Aya C Taki A and Peter M Smooker ABiotechnology and Biological Sciences Discipline
School of Applied Sciences
RMIT University
PO Box 71
Bundoora, Vic. 3083, Australia
Tel: +61 3 9925 7129
Fax: +61 3 9925 7110
Email: aya.taki@rmit.edu.au
Email: peter.smooker@rmit.edu.au
Microbiology Australia 35(3) 153-155 https://doi.org/10.1071/MA14046
Published: 26 August 2014
The 25th of April is a national day to honour the members of the Australian and New Zealand Army Corps (ANZAC), who gave their lives at Gallipoli during the First World War (WWI). The 25th of April has also been designated World Malaria Day by the World Health Organization (WHO), and is commemorated every year to bring awareness of deaths caused by malaria infection and global efforts to control infection. There is no coincidence that these two commemorative events are on the same day, as military campaigns suffered great burdens caused by malaria infection during WWI. Malaria infection is yet to be eradicated from human history; fundamental discoveries of malaria and its control were developed during WWI and the fight against malaria continues to this date. This article focuses on the discovery of malaria prior to WWI, the impact that malaria had on military in the war, and the development of control measures taken to minimize these effects and to subsequently eradicate the disease in many countries.
Malaria is one of the most prevalent parasitic diseases, and is caused by a eukaryotic protist of the genus Plasmodium, a member of the phylum Apicomplexa. The species of Plasmodium that are pathogenic for humans are: P. falciparum, responsible for approximately 80% of malaria cases, P. vivax, P. ovale, and P. malariae. These four were originally considered to be the only species to cause infection in humans, until in 2004 a zoonotic malaria species P. knowlesi identified in Malaysian Borneo was recognized as a fifth human malaria species1. The malaria parasite exhibits a complex life cycle, ultimately transmitted via a bite of a female Anopheles mosquito. Therefore, infection mainly occurs in the sub-Saharan African and South-East Asian regions, where the tropical climate provides a sustainable environment for the mosquito vector to flourish. In locations where the theatres of war and malaria transmission coincided there were significant impacts on military performance.
The mortality rate from malaria remains high and in 2012 was responsible for more than 200 million cases of infection worldwide and 627,000 deaths, predominantly in children under the age of 52. Infection begins with the inoculation of sporozoites, which travels from the injection site into the bloodstream and infects liver hepatocytes to proliferate to tens of thousands of haploid forms. It then re-enters the bloodstream where the intracellular parasite undergoes asexual replication and a cycle of erythrocyte infection proceeds. This rapid intra-erythrocyte multiplication stage of parasites is mainly responsible for the severe morbidity and mortality of malaria, in addition to the incidence of ‘cerebral malaria’ caused by the blockage of blood vessels in the brain in P. falciparum infections3.
WWI: malaria infection and the monumental discoveries in the 1880s to 1910s
In the 19th and early 20th Century, the Greek region was a major malaria endemic site and approximately 40% of British and French troops (120,000 soldiers out of 300,000) contracted malaria and became incapacitated in Macedonia during 1916 and 1917. There were 1.3 million hospital cases for the total of 1 million Allied forces in the theatre between 1916 and 19184. In the Middle Eastern region where the Tigris and Euphrates run, with an environment for the Anopheles mosquito to prosper, the Gallipoli campaign including ANZACs suffered as 90,000 soldiers were evacuated due to malaria.
Malaria caused a significant burden on European members during the time leading to, and also during WWI. Therefore, it is no surprise that the cause of infection and the mechanism of transmission were discovered by military surgeons during this time. In 1880, Charles Louis Alphonse Laveran, a French military physician posted in Algeria, discovered that the protozoa-bearing black pigment found in malaria patient’s blood was the cause of disease5. Laveran also showed that malaria parasites can be found in a patient’s brain, spleen and liver6: some of his drawings are shown in Figure 1. In 1897 Ronald Ross, of the British Indian Medical Service in India, elucidated that the transmission of malaria parasites was due to the Anopheles mosquito, by discovering the malaria protozoa in the stomach wall and salivary glands of the insect7. Both men were later awarded the Nobel Prize in Physiology or Medicine (in 1907 and 1902, respectively), for their invaluable contributions to the understanding of malaria infection.
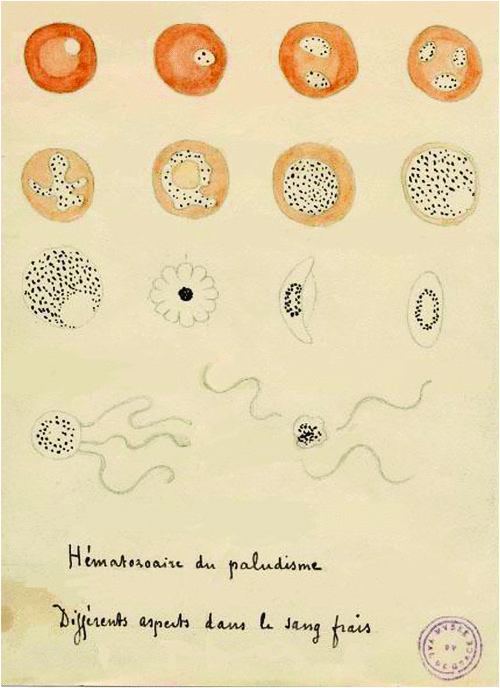
|
Malaria control in WWI and post war periods
After the discovery by Ross, the prevention of malaria included control of mosquito breeding sites and use of mosquito bed nets8, often supplemented with the use of quinine, a naturally occurring antimalarial compound from the bark of the Cinchona tree, native to South America. Quinine was the primary treatment for malaria throughout WWI; however, its strong adverse effects and the short action period of the drug made quinine a suboptimal treatment9. The Cinchona tree was cultivated into the world’s largest supply source in Java, monopolised by the Dutch. However, the plantation later fell into the hands of the Japanese who, as Britain’s ally in WWI, occupied South Pacific islands. Therefore, the Germans lost their supply of quinine, forcing them to develop alternative antimalarial drugs.
One of the first synthetic antimalarial drugs was introduced by German scientist, Paul Ehrlich, in 1891. He was the first to report the antimalarial property of methylene blue, which is particularly effective in staining and killing intracellular malaria parasites, and was shown to cure two patients10. This later led the German dye company Bayer to become a pharmaceutical company for the production of a methylene blue-based synthetic drugs for malaria treatment. A more defined compound from another dye, 9-amino acridine, related to methylene blue, was described in 1932 by Bayer and was later named as the new and more effective antimalarial drug Atabrine9. Atabrine production was greatly expanded in the United States and Britain when Java was captured once again by the Japanese Imperial army and also when Germany seized the quinine stored in Amsterdam during WWII. In 1945, Bayer released another synthetic antimalarial drug named chloroquine, which contributed to saving countless lives from the infection for the next 20 years11.
Towards the end of WWII, malaria control began in many European countries such as Spain, Italy and especially Greece, where a quarter of the total population was infected in the 1930s12. The eradication of malaria was achieved by the introduction of the insecticide DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane) by the Allies, which was initially used by the US Army to control lice vectors of typhus13. Several field studies conducted in these southern European countries demonstrated the powerful efficacy of DDT on Anopheles mosquitos, resulting in malaria almost being eradicated from some areas where it was previously endemic late 1940s13,14. Coating the surface of interior walls with residual DDT and spraying homes to control the Anopheles mosquito had a significant impact on the reduction of global malaria cases, and also helped eliminate the disease in the US by 195115,16. The Global Malaria Eradication Programme was launched by the WHO in 1955 with the use of chloroquine and DDT against malaria (the eradication programme was however discontinued in 1972 due to the financial burden on the WHO)17. Although malaria was permanently eliminated from some regions, the emergence of drug resistant parasites and insecticide resistant mosquitoes became apparent18,19.
Lessons from the past and challenges today
Sanitation and mosquito bite prevention are key to reducing malaria infection. Mosquito control has been shown to be one of the most effective control measures, as was clearly evident from the malaria eradication campaign. Development and administration of antimalarial drugs also greatly reduced the morbidity and mortality of malaria infection since the early 20th Century. While these intensive measures marked significant and lasting benefits in malaria history, they have not resulted in the complete eradication of disease. The threat of multi-drug resistance in the malaria parasite, as well as pesticide resistance in mosquito vectors coupled with the failure to continue the global eradication campaign due to unsustainable financial resources, left less developed African and South-Eastern Asian countries vulnerable. Moreover, the shortage or complete failure of the antimalarials can possibly lead to a catastrophic situation in countries where disease transmission is possible.
The prevention of malaria infection, especially in developing countries, has become one of the most challenging goals in the field of modern science. The development of an effective vaccine is required to achieve complete eradication and prevention from disease. Numerous malaria vaccine studies have been attempted, including the development of a liposome-based vaccine RTS,R as a most promising vaccine candidate20. However, there is insufficient efficacy to provide an adequate level of protection in all populations21. Nevertheless, research into malaria control has advanced greatly since the discovery of the parasite under the microscope prior to the WWI era by Laveran and Ross, and the eradication of disease was achieved in some parts of the world. However, infants and children are still dying every minute, and until the day that no casualties due to the parasite occur, the war against the disease continues.
References
[1] Singh, B. et al. (2004) A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363, 1017–1024.| A large focus of naturally acquired Plasmodium knowlesi infections in human beings.Crossref | GoogleScholarGoogle Scholar | 15051281PubMed |
[2] World Health Organization (2014) WHO | Malaria. WHO Fact Sheet .
[3] Idro, R. et al. (2010) Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 68, 267–274.
| Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome.Crossref | GoogleScholarGoogle Scholar | 20606600PubMed |
[4] Payne, D. (2008) Malaria in the Great War. The Western Front Association.
[5] Cox, F.E. (2010) History of the discovery of the malaria parasites and their vectors. Parasit Vectors
[6] Haas, L.F. (1999) Charles Louis Alphonse Laveran (1845–1922). J. Neurol. Neurosurg. Psychiatry 67, 520.
| Charles Louis Alphonse Laveran (1845–1922).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MvhslOmtA%3D%3D&md5=007da2b374439b13ad32b717e348e3c0CAS | 10486402PubMed |
[7] Ross, R. (1897) On some peculiar pigmented cells found in two mosquitos fed on malarial blood. BMJ 2, 1786–1788.
| On some peculiar pigmented cells found in two mosquitos fed on malarial blood.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MzhvVOlsg%3D%3D&md5=640d7c924058e1a0627c3dca2e7954e5CAS | 20757493PubMed |
[8] Agyepong, I.A. (1992) Malaria: Ethnomedical perceptions and practice in an Adangbe farming community and implications for control. Soc. Sci. Med. 35, 131–137.
| Malaria: Ethnomedical perceptions and practice in an Adangbe farming community and implications for control.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zns1KjtQ%3D%3D&md5=673fd842dd8763474bc75710328a049dCAS | 1509302PubMed |
[9] Kitchen, L.W. et al. (2006) Reviews of anti‐infective agents: Role of US military research programs in the development of US food and drug administration–approved antimalarial drugs. Clin. Infect. Dis. 43, 67–71.
| Reviews of anti‐infective agents: Role of US military research programs in the development of US food and drug administration–approved antimalarial drugs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntV2ks70%3D&md5=73e8bdbc60732af7aa2925cb58da7efcCAS | 16758420PubMed |
[10] Guttmann, P. and Ehrlich, P. (1891) Über die Wirkung des Methylenblau bei Malaria (On the effect of methylene blue on malaria) Berliner Klinische Wochenschrift 28, 953–956.
[11] Krafts, K. et al. (2012) From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy. Parasitol. Res. 111, 1–6.
| From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy.Crossref | GoogleScholarGoogle Scholar | 22411634PubMed |
[12] Kamarck, A.M. (1976) Tropics and economic development: a provocative view into the poverty of nations, Johns Hopkins University.
[13] Majori, G. (2012) Short history of malaria and its eradication in Italy with short notes on the fight against the infection in the Mediterranean basin. Mediterr. J. Hematol. Infec. Dis. 4, 1.
| Short history of malaria and its eradication in Italy with short notes on the fight against the infection in the Mediterranean basin.Crossref | GoogleScholarGoogle Scholar |
[14] Breman, J.G. (2001) The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64, 1–11.
| 1:STN:280:DC%2BD3MzmsVKquw%3D%3D&md5=ee32661be8e90593ef2f5de8da89aa45CAS | 11425172PubMed |
[15] Bruce-Chwatt, L.J. and de Zulueta, J. (1980) The rise and fall of malaria in Europe, Oxford University Press on behalf of the Regional Office for Europe of the World Health Organization.
[16] Greenwood, B.M. et al. (2008) Malaria: progress, perils, and prospects for eradication. J. Clin. Invest. 118, 1266–1276.
| Malaria: progress, perils, and prospects for eradication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkt1CntLg%3D&md5=9b1c58c583f44c71bdeb64c05d1f6e38CAS | 18382739PubMed |
[17] Nájera, J.A. et al. (2011) Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med. 8, e1000412.
| Some lessons for the future from the Global Malaria Eradication Programme (1955–1969).Crossref | GoogleScholarGoogle Scholar | 21311585PubMed |
[18] Sidhu, A.B.S. (2002) Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298, 210–213.
| Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsFSgs70%3D&md5=a13ecc1a8c75b3b7090b48d3aa198b0bCAS |
[19] Coetzee, M. and Koekemoer, L.L. (2013) Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus. Annu. Rev. Entomol. 58, 393–412.
| Molecular systematics and insecticide resistance in the major African malaria vector Anopheles funestus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXivVWjtL8%3D&md5=79682b0b94dba73693cf733c45c16890CAS | 23317045PubMed |
[20] Regules, J.A. et al. (2011) The RTS,S vaccine candidate for malaria. Expert Rev. Vaccines 10, 589–599.
| The RTS,S vaccine candidate for malaria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsFymu7o%3D&md5=99b82a02d24b9fcec1578f7f5dedb2b0CAS | 21604980PubMed |
[21] Bejon, P. et al. (2013) Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect. Dis. 13, 319–327.
| Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjslWqs7Y%3D&md5=9b61c0c3960d65eed58ca4ee831284cbCAS | 23454164PubMed |
Biographies
Aya Taki is a PhD candidate in Biotechnology who has recently submitted her thesis at RMIT University, Australia in June 2014. Her research interest is in vaccine development against infectious diseases using nanoparticles as an antigen delivery system. A particular interest is in the field of malarial protein engineering and vaccine design.
Peter Smooker is a Professor of Biotechnology and head of the Biotechnology laboratory at RMIT University. The laboratory’s main activities are in vaccine design, including antigen identification and engineering, and vector development.


