The global eradication of smallpox and the work of Frank Fenner
CR Robert George A and William Rawlinson AVirology Division, SEALS Microbiology
Prince of Wales Hospital
Randwick, NSW 2031, Australia
Email: w.rawlinson@unsw.edu.au
Microbiology Australia 35(3) 165-168 https://doi.org/10.1071/MA14043
Published: 26 August 2014
The 1950s and 1960s represented a golden era in scientific discovery when many believed science would solve the world’s greatest problems. It was the era when colour television was introduced and the role of DNA described, space programmes, the introduction of vaccines for polio, measles and mumps, and the structures of proteins began to be described. Many discoveries were controversial, but there was a strong belief science was taking the world forward and reducing medical problems rapidly. The Intensified Smallpox Eradication Program (ISEP) won united support from both the Union of Soviet Socialist Republics (USSR) and the United States of America (USA). The initiative was passed by only a small margin (two votes) and came on the back of several failed disease eradication programmes1.
As documented in Professor Yenen’s accompanying paper, smallpox was a scourge with a long history of different interventions that had been only been partially successful. Contrary to this long history of partially successful interventions using different methods in different countries, the ISEP was unequivocally successful globally. The programme resulted in eradication of smallpox in every country where case-notification was undertaken, marking the first infectious disease of humans to be eradicated via human intervention. It demonstrated that coordinated vaccination programmes could succeed on a global scale despite political, economic and scientific barriers. Instrumental to documenting the ISEP’s success was Professor Frank Fenner, an Australian scientist and clinician who was elected Chairman of the committee certifying the programme’s success, the Global Commission for the Certification of Smallpox Eradication (GCCSE). His work had put Australia at the forefront of poxvirus research, and he was closely associated with international poxvirus research. Multiple obituaries document his life, achievements, and his death in November 20102–10.
A DNA virus called variola (Orthopoxvirus, Poxviridae) causes smallpox. Case fatality rates varied depending on the infection type, reaching up to 30% in the historically most common form variola major11. Smallpox has influenced the course of history and decimated populations. For example, historians have debated whether up to one-third of the Aztec population was decimated by smallpox in 1520 after Hernando Cortes accidently introduced the virus thus facilitating the European conquest of South America12. Elsewhere, native Virginian inhabitants were depopulated to one-third of their former number in 1689, and numerous North American tribes were affected13. Similarly, Australian Aborigines suffered widespread variola following European colonisation of Australia14. In remedy, various methods were attempted to control the disease with varying success. There were numerous methods tried, and this included establishing dedicated hospitals (in 1374 a Japanese Emperor established a smallpox hospital after repeated epidemics)15, and many different vaccination methods, as documented elsewhere in this volume of Microbiology Australia. Variolation, a method of exposing skin to material from smallpox pustules and thus inducing immunity had been employed by several cultures, and was eventually introduced to Great Britain (see Professor Yenen’s accompanying paper). Utilising this technique, in 1793, Haygarth proposed an eradication programme for Great Britain16. In 1796 Edward Jenner famously demonstrated immunity in an individual inoculated with a related poxvirus from a cow’s udder17. It may have seemed that resolution was within grasp. However, another 184 years would pass before Fenner announced to the World Health Assembly that smallpox had been eradicated. In the intervening period, incremental steps were made, and slowly the burden of disease was reduced. Cowpox was gradually replaced with vaccinia as the vaccine. Freeze dried vaccine was introduced, and national vaccination programmes begun by many nations. By 1950, although in decline, the disease remained endemic in areas of Central and South America, Africa, and Asia1. In 1958, Professor Victor Zhdanov, the chief of Soviet delegation to the World Health Assembly proposed a global eradication programme that resulted in resolution WHA11.54 planning eradication within 10 years1. While some success was achieved (for example, China), eradication was not achieved within the ambitious timeframe, in part due to resource constraints. In response, in 1967 the World Health Organization (WHO) launched the IESP with the renewed objective of global eradication, with the Smallpox Eradication unit headed by Donald Henderson from 1966 to 1977.
Financial support was provided through a special budget of US$2.4 million per annum to the IESP, a figure overshadowed by the estimated US$315 million spent during the life of the programme18. Meanwhile smallpox remained endemic in 31 countries, with 10–15 million cases of annually19. Decisions were made to reduce the risk of early failure1. Reference centres were established for vaccine, and a quality programme established to ensure all vaccines used (including those produced in endemic countries) met strict standards regarding potency, stability and purity. The approach involved systematic vaccination, with rigorous surveillance and containment, whereby cases were reported weekly and special containment teams targeted outbreaks20. Rewards were paid to those who identified new cases21. This approach uniquely engaged and empowered local staff, whilst also minimising depletion of vaccine.
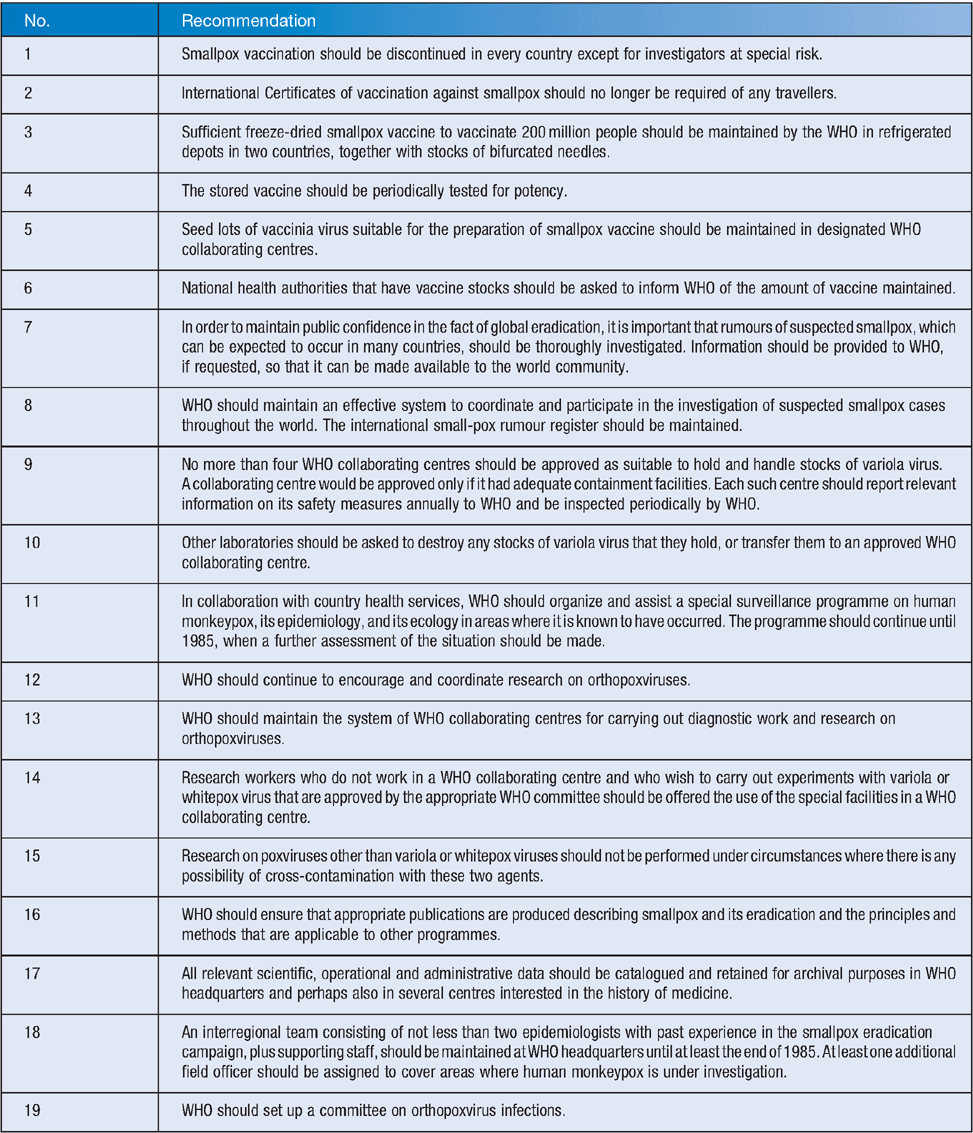
Between 1975 and 1977, smallpox had been contained to Bangladesh, Ethiopia, Kenya, and Somalia. The last case from Ethiopia was on 9 August 1976, from Kenya on 5 February 1977, and from Somalia on 26 October 1977 (the last wild case)22,23. At this time, the GCCSE was established to certify the eradication of smallpox, with Frank Fenner elected Chair. Eradication was defined as no cases from a country for 2 years21. Between inception of the GCCSE and 8 May 1980 when the final report was presented to the World Health Assembly, ongoing field studies, many of which Frank Fenner directly participated in, were performed to ensure no further cases occurred24. It was only after this work had been completed that the GCCSE was able to report: ‘1. Smallpox eradication has been achieved throughout the world. 2. There is no evidence that smallpox will return as an endemic disease’. The GCCSE made 19 recommendations to the World Health Assembly (Table 1), which have informed modern conduct in relation to smallpox.
|
Frank Fenner’s role in certifying smallpox eradication (as described in interviews in 1992–1993 with Dr Max Blythe) was to meet over three successive years, organising 21 international commissions that visited all countries where smallpox had been endemic since 1967. The goal was to obtain signatures from every country verifying that no cases of smallpox had occurred, thereby certifying disease elimination. In retrospect, it is interesting to see the degree of cooperation between the USA and USSR, with meetings of the monkeypox committee in Moscow in 1969 in the midst of the cold war. It is also interesting that, similar to recent early, mistaken diagnoses for other viruses, Russian investigators mistakenly believed that white-pock variants of monkeypox were identical to vaccinia virus. They were not, and were subsequently shown to be laboratory contaminants, again as has occurred with some recent emerging viruses.
Although it is not the intention here to revisit all of the considerable achievements of Frank Fenner, it is important to note that his training, interests and skills made him an ideal candidate for the position as Chair. He was predominantly a scientist of viruses including poxviruses of humans and animals, but was trained originally in medicine, with a research degree early in his career (1942). During World War II, he served as an officer in the Australian Army Medical Corps in Australia, Palestine, Egypt, PNG and Borneo. This included clinical work, as well as specific studies of malaria, and as pathologist in a general hospital. He had interests and training in tropical medicine, and during smallpox certification, he visited many of the tropical countries to review eradication first hand. Further, he undertook research in different institutes – the Walter and Eliza Hall Institute in Melbourne (studying mousepox), the Rockefeller in New York (studying tuberculosis), and the John Curtin School of Medical Research in Canberra (studying myxoma, then as Director gaining management experience). He was also the President of the International Committee on Taxonomy of Viruses (1970–1975), and his attention to detail combined with writing skills contributed to this work, and the subsequent publication 7 years following eradication of the definitive text on smallpox history and eradication – Smallpox and its eradication. This unique combination of basic science, research, medical, managerial, and specific knowledge of poxviruses made him an ideal Chair for the GCCSE, that culminated in his announcement to the World Health Assembly that smallpox was eradicated.
It is possibly less well known, but equally relevant, that Professor Fenner was a key member (and subsequently Chair) of the committee examining whether animal viruses, specifically monkeypox virus, might constitute an animal reservoir of smallpox. Fenner’s experience with animal poxviruses was key in this role, and in many ways presaged the more recent one-health initiatives for human and animal health programmes His other key was final certification, and authorship of the WHO document Smallpox and its eradication in 1988, along with Donald Henderson, Isao Arita (Chief of the Smallpox Eradication unit 1977–1984), Zdeněk Ježek (Chief of the Smallpox Eradication unit from 1985) and Ivan Ladnyi (Assistant Director-General of the WHO 1976–1983)1. Together, Fenner, Henderson and Arita were nominated for the Nobel Prize in Physiology or Medicine in 1985, 1986 and 1987, and shared the Japan prize in 1988.
Although the eradication of smallpox overseen by Frank Fenner and the GCSSE was widely praised, other issues remain controversial. While the virus is widely regarded as a potential biological weapon, it has been suggested that destruction of remaining stocks could set back scientific discovery and prevent the design of new antiviral agents in the event of a future outbreak25. In 1990 the WHO requested smallpox strain mapping, with subsequent stock destruction scheduled for 31 December 1993. Destruction has been repeatedly deferred and debate continues26. Meanwhile, the virus continues to play into international events. In 2002–2003 during a period of claims that the Iraq regime had amassed weapons of mass destruction, the White House released 28 news releases mentioning smallpox27. Vaccinia derived illness has occurred, particularly in relationship to vaccination28,29. Most recently, in 2014, vials reportedly from the 1950s and labelled ‘variola’ were discovered in a storage room in a US Food and Drug Administration laboratory in Maryland30,31, continuing the importance of variola in our lives.
References
[1] Fenner, F. et al. (1988) Smallpox and its eradication. Geneva: World Health Organization.[2] Wilks, C.R. and Studdert, M.J. (2011) Frank Fenner: 1914–2010. Aust. Vet. J. 89, 81.
| Frank Fenner: 1914–2010.Crossref | GoogleScholarGoogle Scholar |
[3] Murphy, F.A. (2011) In memoriam: Frank John Fenner (1914–2010). Emerg. Infect. Dis. 17, 759–762.
| In memoriam: Frank John Fenner (1914–2010).Crossref | GoogleScholarGoogle Scholar | 21470486PubMed |
[4] Murphy, F.A. (2011) In memoriam: Frank John Fenner (1914–2010). Arch. Virol. 156, 363–367.
| In memoriam: Frank John Fenner (1914–2010).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtleisLk%3D&md5=50acc0bdf500b991ac748924b7572d7cCAS | 21221675PubMed |
[5] Lessi, A. (2011) Obituary: Professor Frank Fenner (1914–2010). N S W Public Health Bull. 22, 33.
| Obituary: Professor Frank Fenner (1914–2010).Crossref | GoogleScholarGoogle Scholar |
[6] Hodgkin, P.D. (2011) Remembering Frank Fenner. Immunol. Cell Biol. 89, 497–498.
| Remembering Frank Fenner.Crossref | GoogleScholarGoogle Scholar |
[7] Henderson, D.A. (2011) Frank Fenner (1914–2010). Nature 469, 35.
| Frank Fenner (1914–2010).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitl2rsw%3D%3D&md5=c33b4274bd6f83944ccbf73e7ecdb63dCAS | 21209651PubMed |
[8] Sweet, M. (2010) Frank Fenner: helped eradicate smallpox. BMJ 341, 1218.
[9] Pincock, S. (2011) Frank John Fenner. Lancet 377, 24.
| Frank John Fenner.Crossref | GoogleScholarGoogle Scholar |
[10] Brett-Crowther, M. (2011) Frank Fenner, AC, CMG, MBE, FAA, FRS. Int. J. Environ. Stud. 68, 141–143.
| Frank Fenner, AC, CMG, MBE, FAA, FRS.Crossref | GoogleScholarGoogle Scholar |
[11] Breman, J.G. and Henderson, D.A. (2002) Diagnosis and management of smallpox. N. Engl. J. Med. 346, 1300–1308.
| Diagnosis and management of smallpox.Crossref | GoogleScholarGoogle Scholar | 11923491PubMed |
[12] Brooks, F.J. (1993) Revising the Conquest of Mexico: smallpox, sources, and populations. J. Interdiscip. Hist. 24, 1–29.
| Revising the Conquest of Mexico: smallpox, sources, and populations.Crossref | GoogleScholarGoogle Scholar |
[13] King, R. (1850) Address to the Ethnological Society of London delivered at the anniversary, 25th May 1844. Journal of the Ethnological Society of London (1848–1856) 2, 9–42.
[14] Bennett, M.J. (2009) Smallpox and cowpox under the Southern Cross: the smallpox epidemic of 1789 and the advent of vaccination in colonial Australia. Bull. Hist. Med. 83, 37–62.
| Smallpox and cowpox under the Southern Cross: the smallpox epidemic of 1789 and the advent of vaccination in colonial Australia.Crossref | GoogleScholarGoogle Scholar | 19329841PubMed |
[15] Irwin, F. (1910) Smallpox in Japan. Public Health Reports (1896–1970) 25, 1205–1208.
[16] Haygarth, J. (1793) A sketch of a plan to exterminate the casual small-pox: from Great Britain; and to introduce general inoculation: to which is added, a correspondence on the nature of variolous contagion... London: J. Johnson.
[17] Jenner, E. (1801) On the origin of the vaccine inoculation. London: D. N. Shury.
[18] Nelson, A.M. (1999) The cost of disease eradication. Smallpox and bovine tuberculosis. Ann. N. Y. Acad. Sci. 894, 83–91.
| The cost of disease eradication. Smallpox and bovine tuberculosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7ks1Gmtw%3D%3D&md5=50e915d3982fd768d050d081c0777d33CAS | 10681974PubMed |
[19] Henderson, D.A. (1987) Principles and lessons from the smallpox eradication programme. Bull. World Health Organ. 65, 535–546.
| 1:STN:280:DyaL1c%2FotVOrsQ%3D%3D&md5=b2f362708125ac9c94165b3d00da5044CAS | 3319270PubMed |
[20] Henderson, D.A. and Klepac, P. (2013) Lessons from the eradication of smallpox: an interview with D. A. Henderson. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130113.
| Lessons from the eradication of smallpox: an interview with D. A. Henderson.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sjls1Cruw%3D%3D&md5=883d74ec793fa7346b67456649f00d06CAS | 23798700PubMed |
[21] Strassburg, M.A. (1982) The global eradication of smallpox. Am. J. Infect. Control 10, 53–59.
| The global eradication of smallpox.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL383gsVemtg%3D%3D&md5=33eab6b9fbddb98885870510777cd7ebCAS | 7044193PubMed |
[22] World Health Organization (1978) SME/78.3: Plan of action for the smallpox eradication programme in Somalia 1978/1979. World Health Organization.
[23] World Health Organization (1977) Smallpox surveillance. Wkly. Epidemiol. Rec. 52, 389–396.
[24] Breman, J.G. and Arita, I. (1980) The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303, 1263–1273.
| The confirmation and maintenance of smallpox eradication.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M%2FjtVOqsA%3D%3D&md5=3ffbd67204a2ce58f40c6605592842c3CAS | 6252467PubMed |
[25] Berche, P. (2001) The threat of smallpox and bioterrorism. Trends Microbiol. 9, 15–18.
| The threat of smallpox and bioterrorism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvFemsLk%3D&md5=526a86a0ee9d71601155f3af8211cf61CAS | 11166237PubMed |
[26] Tucker, J.B (2011) Breaking the deadlock over destruction of the smallpox virus stocks. Biosecurity and Bioterrorism : Biodefense Strategy, Practice, and Science 9, 55–67.
[27] Cohen, H.W. et al. (2004) The pitfalls of bioterrorism preparedness: the anthrax and smallpox experiences. Am. J. Public Health 94, 1667–1671.
| The pitfalls of bioterrorism preparedness: the anthrax and smallpox experiences.Crossref | GoogleScholarGoogle Scholar | 15451727PubMed |
[28] Arness, M.K. et al. (2004) Myopericarditis following smallpox vaccination. Am. J. Epidemiol. 160, 642–651.
| Myopericarditis following smallpox vaccination.Crossref | GoogleScholarGoogle Scholar | 15383408PubMed |
[29] Halsell, J.S. et al. (2003) Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 289, 3283–3289.
| Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel.Crossref | GoogleScholarGoogle Scholar | 12824210PubMed |
[30] Cohen, J. (2014) Lab safety. Alarm over biosafety blunders. Science 345, 247–248.
| Lab safety. Alarm over biosafety blunders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlagt77E&md5=421acbf627fc9361eabe145e1632b308CAS | 25035464PubMed |
[31] McCarthy, M. (2014) Smallpox samples are found in FDA storage room in Maryland. BMJ 349, g4545.
| Smallpox samples are found in FDA storage room in Maryland.Crossref | GoogleScholarGoogle Scholar | 25013209PubMed |
Biographies
Dr Robert George is the Microbiology Registrar at SEALS Randwick. He completed a PhD at the University of Queensland where he worked on spatial modelling of entomological outbreak systems. He has also completed a BA majoring in history.
Professor William Rawlinson is head of the Division of Virology, in the Department of Microbiology SEALS. He has conjoint positions in the Department of Infectious Diseases, Prince of Wales Hospital, and as Professor in the School of Medical Science and the School of Biotechnology and Biomolecular Sciences at The University of New South Wales. He supervises PhD, Masters and science Honours students in studies of viral pathogenesis, with his research funded by NHMRC, ARC, and others. He is Director of the TGA licensed laboratory testing all increased risk donors in NSW for blood borne viruses. This laboratory has established new algorithms for testing, and continues to publish findings in evaluation of donors for infection.


