Cytomegalovirus and ageing of the immune system: a controversial cause of ageing
Wendy van ZuijlenVirology Division
SEALS Microbiology
Prince of Wales Hospital
Randwick, NSW 2031, Australia
School of Medical Sciences
The University of New South Wales
Kensington, NSW 2033, Australia
Tel: +61 2 9382 9096
Fax: +61 2 9382 8533
Email: wendy.vanzuijlen@sesiahs.health.nsw.gov.au
Microbiology Australia 34(3) 157-159 https://doi.org/10.1071/MA13052
Published: 4 September 2013
Human cytomegalovirus (HCMV) is a herpesvirus that infects 30–90% of the population in developed countries, establishing lifelong latent infection. Infection during foetal development, in immunocompromised and immunosuppressed individuals might cause severe disease, whereas in the adult immunocompetent host HCMV infection is generally considered asymptomatic. However, recent studies suggest that HCMV affects ageing of the immune system in healthy individuals. These recent findings provide a novel area of research into ageing and the current controversy is discussed here.
Human cytomegalovirus
HCMV is a herpesvirus that spreads between individuals by close contact with infected body fluids such as saliva, milk, blood or urine. HCMV infection rates increase gradually with age with 70–80% of individuals older than 60 years being HCMV-seropositive in western countries1. After primary infection (first infection in life), HCMV is not cleared from the host but persists throughout life in a latent form. While primary infection in healthy adults rarely elicits symptoms, primary infection during pregnancy can lead to HCMV crossing the placental barrier and infecting the foetus, resulting in congenital HCMV. The prevalence of HCMV infection at birth, worldwide, is ~0.7% and is a leading cause of birth defects and developmental disabilities2,3. In addition, congenital HCMV infection has been associated with adverse pregnancy outcomes, including stillbirth4.
Reactivation of latent HCMV infection is a significant health issue in immunocompromised and immunosuppressed individuals as it is associated with increased risk of morbidity and mortality. Interestingly, recent clinical, epidemiological and immunological studies suggest that HCMV infection might have clinical importance in the immunocompetent, and might affect immune senescence.
Ageing and immune senescence
Immune senescence is described as the age-related alteration and dysfunction of the immune system, which leads to impaired protective immunity. This is likely to be a multifactorial process involving molecular, cellular, genetic and environmental factors. Interestingly, all components of the immune system undergo age-related alterations; however, the T-cell compartment seems to be affected the most. A shift in T-cell subset distributions with a decline in the naïve T-cell population plus an increase in end-differentiated T cells (CD45RA+CD57+CD28- T cells) have been described as biomarkers of human immune senescence5.
Long-term effects of HCMV on the T-cell compartment
An association of HCMV-seropositivity with an altered distribution of T-cell phenotypes was first reported by Looney and colleagues6. Interestingly, they and others observed that HCMV-seropositive individuals have a similar peripheral blood lymphocyte profile as seen in the ageing immune system. This profile consisted of a large population of HCMV-specific CD8+ and CD4+ T cells (with a late-differentiated phenotype), fewer naïve T cells, and a decreased CD4:CD8 T-cell ratio7 (Figure 1).
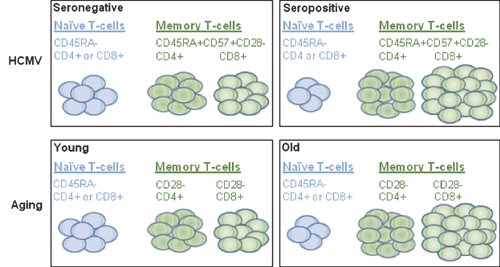
|
These biomarkers of human immune senescence, with the exception of reduced naïve T cells, were later identified as part of an immune risk profile (IRP) in the Swedish OCTO and NONA immune longitudinal studies8,9. This IRP was associated with persistent HCMV-infection and shown to be predictive of an increased mortality in Swedish individuals between 86 and 94 years of age. More recently, Strindhall and collegues10 identified an identical association with HCMV seropositivity in the HEXA longitudinal study of Swedish individuals at 66 years of age, suggesting that HCMV might also be connected to the generation of late-differentiated CD8+ T cells in this age group. Notably, data from the Swedish NONA study at 6-year follow up demonstrated that 80.6% (25 of 31) of individuals between 92 and 101 years of age were CMV-seropositive, although given the ubiquitous nature of CMV infection, this was not surprising11.
The overall findings from the Swedish OCTO and NONA studies have led to the assumption that ageing-related alterations to the cellular composition are detrimental to survival. However, data from the Leiden 85-plus Study suggests that this may not necessarily be true. In this study, Derhovanessian and colleagues12 observed that low naïve CD8+ T-cell frequencies and an accumulation of late-differentiated effector memory CD8+ T cells in CMV-seropositive individuals correlated significantly with longer survival on 8-year follow up. So the controversy continues.
Long-term effects of HCMV on the immune response
There is a clear evidence for an association of CMV-seropositivity with pronounced changes of cellular components of the immune system. However, the data suggesting a causative role for CMV in immune dysfunction or impaired immunity are highly controversial.
Khan and colleagues13 observed that HCMV-infection might affect the immune response to other viruses, since lower frequencies of Epstein–Barr virus (EBV)-specific CD8+ T cells were observed in HCMV-seropositive elderly donors. However, others demonstrated that absolute numbers and phenotype of pre-existing EBV-specific memory T cells were not affected by the appearance of HCMV-specific CD8+ T cells on primary HCMV infection14.
Interestingly, Cicin-Sain and colleagues15 found that latent/prior murine CMV (MCMV)-infection led to lower CD8+ T-cell responses to influenza virus, herpes simplex virus type I (HSV-1) and West-Nile Virus (WNV)15. However, in a rhesus CMV (RhCMV) macaque model specific CD8+ T-cell responses to a super-physiologic RhCMV-infection were equivalent in adult and old monkeys, even though latent RhCMV-infection demonstrated the features of immune senescence found in humans16.
Furthermore, the findings from Trzonkowski and colleagues17 suggest that HCMV might interfere with the development of an adequate immune response, since a serological response to influenza vaccine could not be observed in participants with high concentrations of anti-HCMV antibodies. However, these findings could not be confirmed by others18.
There is also some evidence suggesting that latent HCMV might induce the development of dysfunctional CD8+ T cells. Ouyang and colleagues19 found that only a small fraction of HCMV-specific CD8+ T cells from elderly individuals were able to mount an immune response on stimulation. However, Gillespie and colleagues20 showed that stimulated HCMV-specific CD8+ T cells were fully functional as these cells expressed both cytokines and chemokines and were capable of cytotoxicity.
Conclusion
There is mounting evidence indicating an association of HCMV infection and biomarkers of immune senescence; however, a causative role for HCMV is yet to be proven. This controversial area remains an important subject for research, and if CMV does affect immune senescence, then it provides a method for intervening in ageing.
Additional efforts are needed to establish whether, and how, latent HCMV infection might influence immunity. If future studies demonstrate that HCMV is involved in immune senescence, then eliminating or reducing HCMV viral load potentially will not only reduce the risk of morbidity and mortality in immunocompromised and immunosuppressed individuals, but might also aid in maintaining appropriate immunity.
References
[1] Chidrawar, S. et al. (2009) Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 155, 423–432.| Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M7lt1eltQ%3D%3D&md5=af8cd1099a67d24000d49c4226ca2a24CAS | 19220832PubMed |
[2] McMullan, B.J. et al. (2011) Congenital cytomegalovirus – time to diagnosis, management and clinical sequelae in Australia: opportunities for earlier identification. Med. J. Aust. 194, 625–629.
| 21692718PubMed |
[3] Dollard, S.C. et al. (2007) New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17, 355–363.
| New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 17542052PubMed |
[4] Iwasenko, J.M. et al. (2011) Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J. Infect. Dis. 203, 1526–1533.
| Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy.Crossref | GoogleScholarGoogle Scholar | 21592980PubMed |
[5] Pawelec, G. and Derhovanessian, E. (2011) Role of CMV in immune senescence. Virus Res. 157, 175–179.
| Role of CMV in immune senescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXltFaisrw%3D&md5=43e06c4e67b280a4ab3bbf02434d4cbeCAS | 20869407PubMed |
[6] Looney, R.J. et al. (1999) Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin. Immunol. 90, 213–219.
| Role of cytomegalovirus in the T cell changes seen in elderly individuals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M7otVOqsQ%3D%3D&md5=3be900b1ab4dc8f9afd02b82a947b8d5CAS | 10080833PubMed |
[7] Pawelec, G. et al. (2009) Cytomegalovirus and human immunosenescence. Rev. Med. Virol. 19, 47–56.
| Cytomegalovirus and human immunosenescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXitF2ht78%3D&md5=c7730ac543b469148a3698ccb8385933CAS | 19035529PubMed |
[8] Olsson, J. et al. (2000) Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121, 187–201.
| 1:CAS:528:DC%2BD3MXnsVWrsg%3D%3D&md5=64e2dd2054121f00cbdac07e806ba292CAS | 11164473PubMed |
[9] Wikby, A. et al. (2002) Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37, 445–453.
| Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitlKq&md5=0920b64776b4a848c8fb881862b56f0bCAS | 11772532PubMed |
[10] Strindhall, J. et al. (2013) The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study. Age (Dordr) 35, 985–991.
| The inverted CD4/CD8 ratio and associated parameters in 66-year-old individuals: the Swedish HEXA immune study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmvFWiu7Y%3D&md5=cd1b1e81e7bbf034ffd3dc4150127b7bCAS | 22415616PubMed |
[11] Strindhall, J. et al. (2007) No immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study. Exp. Gerontol. 42, 753–761.
| No immune risk profile among individuals who reach 100 years of age: findings from the Swedish NONA immune longitudinal study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotVWlsrw%3D&md5=2ca2657726cf1a2dfd3fbedd0f163644CAS | 17606347PubMed |
[12] Derhovanessian, E. et al. (2013) Lower proportion of naive peripheral CD8+ T cells and an unopposed pro-inflammatory response to human Cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age (Dordr.) 35, 1387–1399.
| Lower proportion of naive peripheral CD8+ T cells and an unopposed pro-inflammatory response to human Cytomegalovirus proteins in vitro are associated with longer survival in very elderly people.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtV2ku7jL&md5=f33c06313caf6816a3533b6501e927c5CAS | 22661297PubMed |
[13] Khan, N. et al. (2004) Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173, 7481–7489.
| 1:CAS:528:DC%2BD2cXhtVCqurrJ&md5=d8ec1f182de14c3273d65f88ae2487dbCAS | 15585874PubMed |
[14] van Leeuwen, E.M.M. et al. (2006) Differential usage of cellular niches by cytomegalovirus versus EBV- and influenza virus-specific CD8+ T cells. J. Immunol. 177, 4998–5005.
| 1:CAS:528:DC%2BD28XhtVansr3F&md5=5480cd1a2a66bcf82da84cead8ff025aCAS |
[15] Cicin-Sain, L. et al. (2012) Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 8, .
| Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Cns7bE&md5=f3590bc906e41f94313e0f7187cf1b90CAS | 23271968PubMed |
[16] Cicin-Sain, L. et al. (2011) Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J. Immunol. 187, 1722–1732.
| Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpslOrtLs%3D&md5=c4c01b1c2a61df61a18119526f385982CAS | 21765018PubMed |
[17] Trzonkowski, P. et al. (2003) Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination – an impact of immunosenescence. Vaccine 21, 3826–3836.
| Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination – an impact of immunosenescence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmt1Gkurw%3D&md5=419dd02c88d1fa3c77eca15ae626d5efCAS | 12922116PubMed |
[18] den Elzen, W.P.J. et al. (2011) Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine 29, 4869–4874.
| Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntlGju7w%3D&md5=69f03a703f52b8ec782baca49f5f1db3CAS |
[19] Ouyang, Q. et al. (2004) Dysfunctional CMV-specific CD8+ T cells accumulate in the elderly. Exp. Gerontol. 39, 607–613.
| Dysfunctional CMV-specific CD8+ T cells accumulate in the elderly.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXisF2nu70%3D&md5=0dce2146bc16b26759c2707d3ad3db6cCAS | 15050296PubMed |
[20] Gillespie, G.M.A. et al. (2000) Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74, 8140–8150.
| Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlsl2ns7k%3D&md5=212af682f9bcabd5f5cbc63ee64489abCAS |
Biography
Wendy van Zuijlen is a postdoctoral scientist in the School of Medical Sciences at the University of New South Wales. She recently joined the Virology Research Laboratory at the Prince of Wales Hospital to combine her interests in immunology and virology. Her current research aims to understand the pathogenesis of congenital cytomegalovirus infection.


