The importance of a One Health approach to public health and food security in Australia – a perspective from the Chief Medical Officer
Christopher BaggoleyAustralian Government
Chief Medical Officer
Department of Health and Ageing
Tel (02) 6272 4644
Email Chris.Baggoley@health.gov.au
Microbiology Australia 33(4) 143-147 https://doi.org/10.1071/MA12143
Published: 1 November 2012
I have had the privilege of being Australia’s Chief Medical Officer for the past eighteen months which has given me a unique perspective on a range of health related matters. My role is to provide advice to the Minister and the Department of Health and Ageing (DoHA) including input to the development and administration of major health reforms for all Australians and ensuring the development of evidence based public health policy. I am responsible for the DoHA’s Office of Health Protection and Chair the Australian Health Protection Principal Committee which advises and makes recommendation to the Australian Health Ministers’ Advisory Council on national approaches to public health emergencies, communicable disease threats and environmental threats to public health.
Before and while studying human medicine I spent the early part of my working life as a practising veterinarian. This career path sparked an early and ongoing interest in zoonotic diseases and their impact on society. I am aware of the complex interactions between human and animal health and the environment and the value of the One Health concept. In my current role I regularly participate in animal disease related fora where there are potential human health implications. Examples include the Department of Agriculture, Fisheries and Forestry’s (DAFF) Consultative Committee on Emergency Animal Diseases and the Australian Animal Health Laboratory’s Strategic Policy Committee.
What is One Health?
A German scholar from the mid 1800s, Rudolf Virchow was an early proponent of one health. He said, “Between animal and human medicine there is no dividing line nor should there be”1. Described in the same publication from the US Department of Agriculture that cited Virchow, the concept of One Health is stated: “the health of animals, the health of people, and the viability of ecosystems are inextricable linked”. Over time One Health has evolved into a more holistic approach to disease preparedness, prevention and control at the human-animal-ecosystem interface. One Health now touches many different inter-sectoral problems such as emerging infectious diseases, food safety and security, international trade and international economic growth. However, there remains a need to clearly articulate the One Health concept so that we can progress activities under the One Health banner.
One Health Internationally
The concept of One Health is now recognised by several countries and organisations and continues to evolve. A range of initiatives on One Health have led to a number of high level international meetings which have raised political awareness. There has also been increased collaboration between international organisations such as the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO), the World Organisation for Animal Health (OIE), and the World Bank. In addition a number of international frameworks and action plans have been developed on One Health including: Contributing to One World, One Health: a strategic framework for reducing risks of infectious diseases at the animal-human-ecosystems interface (WHO, FAO, OIE, UNICEF, UNSIC and the World Bank), the APEC One Health Action Plan, and Zoonotic Diseases: A guide to establishing collaboration between animal and human health sectors at the country level (WHO, FAO and OIE).
Some countries have established formal mechanisms to address One Health concerns. This includes the joint Human Animal Infections and Risk Surveillance (HAIRS) group in the United Kingdom; the One Health Office in the Centers for Disease Control and Prevention and the One Health Initiative in the United States2-4. The European Centre for Disease Prevention and Control on Food- and Waterborne Diseases and Zoonoses and Emerging and Vector-borne diseases programmes are linked by veterinary and environmental health professionals. These programmes aim to strengthen these links into the future. In the Public Health Agency of Canada, the Centre for Food-borne, Environmental and Zoonotic Infectious Disease convened an expert consultation in 2009, One World One Health: from ideas to action.
Public Health in Australia
Public health in Australia is broadly defined as the organised response by society to the protection and promotion of health and the prevention of illness, injury and disability. Defining measurable health outcomes allows us to assess improvements in the overall health of the community. In order to achieve health improvements we need to strengthen our community capacity to manage and reduce health risks through the investigation of diseases and their associated risk factors; through regulation to ensure a healthy and safe environment; through organised population wide prevention and early detection programs; and by optimal planning of health services delivery.
The role of DoHA is to achieve the Australian Government’s priorities (outcomes) for health and ageing. This is done through the development of evidence-based policies and management programs and via research and regulatory activities. DoHA also works closely with other agencies to achieve results for the Australian Government and community, and engages in open and constructive consultation with professionals, providers, industry and community groups.
Australia’s constitutional arrangements give primary responsibility for public health action to the states and territories. At the Commonwealth level therefore, surveillance, response and preparedness efforts are based on cooperation. These arrangements have been shown to work well during health emergencies such as pandemic H1N1(2009). Any new approaches to public health must complement and draw on existing expertise rather than overriding current arrangements. There are many opportunities to enhance linkages between public health, veterinary and other sectors. This is particularly important for emerging infectious diseases and some foodborne disease.
The One Health concept provides a useful framework for building on linkages between human and animal health, where there is a clear inter-relationship. Whilst this represents only a small subset of overall public health issues, the collaborative and consultative nature of the One Health concept is consistent with the overall public health approach.
One Health in Australia and the region
Internationally, Australia works closely with a number of organisations including the World Health Organization and the Food and Agriculture Organisation to assist neighbouring countries improve their surveillance, preparedness and responsiveness to infectious diseases. We also participate in many international forums to develop a better understanding of the emergence of disease threats at the animal-human-environment interface and to develop appropriate and sustainable means to reduce such threats.
The Australian Government’s international development assistance for pandemics and emerging infectious disease is government by the Pandemics and Emerging Infectious Diseases Framework 2010 – 2015. While the Australian Agency for International Development (AusAID) is primarily responsible for this framework, DoHA, DAFF and the Australian Centre for International Agriculture Research are consulted and involved.
The One Health approach requires multi-sectoral and multi-agency cooperation. Domestically, DoHA has a history of inter-sectoral partnerships especially with other government departments. In particular, the Office of Health Protection was established by the Department to protect the health of the Australian community in partnership with key stakeholders. There are already a number of collaborations occurring at the national level that fall within the One Health concept. These interactions are depicted in Figure 1. While acknowledging these existing collaborations, it important to emphasise that there is always room to build on these. For example there could be better collaboration on laboratory testing, multidisciplinary risk assessments, and research. Also there would be benefits in integrating relevant areas of animal and human communicable disease surveillance systems including better analysis, co-ordination and use of the data collected.
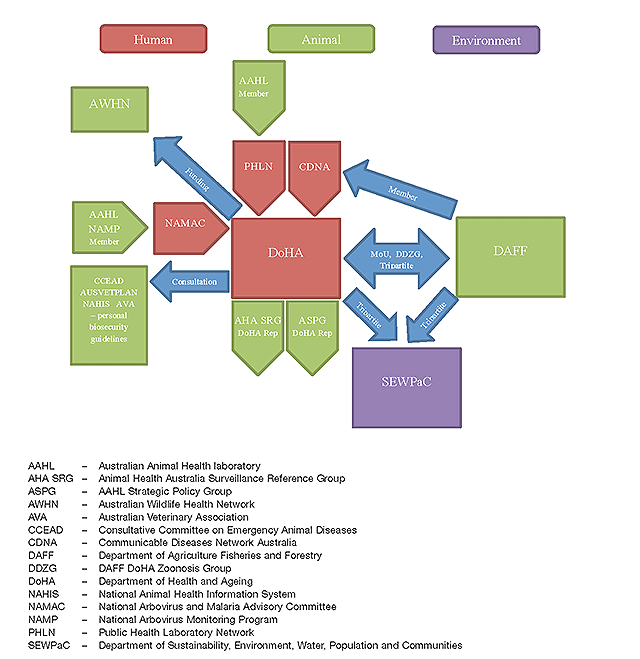
|
Emerging infectious diseases
The driver for the One Health initiative comes from our knowledge that viruses and other agents of disease can move between species. Most emerging infectious diseases are of animal origin5, therefore surveillance response and preparedness efforts need to incorporate One Health. The emergence of henipaviruses (Nipah virus and Hendra Virus) provides a classic illustration. Large fruit bats (Pteropus sp.) are commonly acknowledged to be the reservoir of henipavirus6-9. Hendra virus emerged in Australia in 1994 with two separate outbreaks10,11. Nipah virus was first recognised following outbreaks on the Malaysian peninsular and Singapore in 1998-199912,13, and recurrent outbreaks have been described in Bangladesh since then14,15. Multiple modes of transmission of henipaviruses have been described during spillover events; from bats to humans via an amplifying host (pigs in Malaysia, horses in Australia), ingestion of contaminated fruit or fruit products (Bangladesh, India) and person-to-person (Bangladesh) 10-15. In Australia, animal health authorities make recommendations for horse management that reduce the likely exposure of horses to bat “spats” and excrement, but regular spillover events continue to occur. The prevention of horse-to-horse and horse-to-human transmission and the development of a vaccine for horses requires the involvement of public health, animal health and scientific researchers. Preventing spillover events of henipaviruses may require the involvement of more than the veterinary and public health sectors. Changes to bat habitat and nutritional stress are hypothesised to have contributed to the emergence of Hendra virus and the risk of Hendra spillover events which tend to be seasonal16,17. Preventing human infections therefore requires the involvement of environmental scientists and town planners. Nipah virus prevention in Bangladesh has required the development of novel solutions to prevent bats from having access to date palm sap which was implicated in at least one outbreak15.
Collaboration
In Australia, the Government recognises that to address any inter-sectoral problem, the analysis and policy response needs to incorporate whole of government, whole of society and whole of science. The One Health concept firmly sits within this mantra. The emergence of three diseases of concern in the past 20 years in Australia (Australian Bat Lyssavirus, Hendra Virus and Menangle virus) reminds us that it is essential to be able to detect new diseases early so that we are able to respond as they arise. We must be able to conduct timely research on these diseases, develop diagnostic tests and develop vaccines and treatments. Public health agencies may contribute funding and expertise to the early work on emerging infections which may occur in the animal health sector.
A One Health approach is vitally important in relation to early warning and surveillance of the vectorborne disease Murray valley Encephalitis (MVE) in Australia. The ecology of MVE virus is a complex relationship between humans, vertebrate hosts, mosquito vectors and the environment. The primary vector during epidemics is the fresh water breeding mosquito Culex annulirostris, the common banded mosquito, though other mosquito species may be involved in other aspects of MVE virus ecology. The primary vertebrate hosts of the virus are thought to be water birds such as herons and egrets, which act as reservoirs or amplifiers for infection. The principal virus cycle exists between these birds and the mosquito vectors. MVE virus also infects a wide range of native and exotic animals. Surveillance mechanisms for MVE include mosquito monitoring, virus isolation from mosquitoes and sentinel chicken surveillance. Data on Southern Oscillation Index, rainfall and temperature obtained from Bureau of Meteorology are used by members of the New South Wales Arbovirus Surveillance and Vector Monitoring Program to predict mosquito-breeding capabilities; climatic data are used to predict MVE outbreaks18. The National Arbovirus and Malaria Advisory Committee is developing a framework for the control of Murray Valley Encephalitis, emphasising a One Health approach. The framework aims to provide an overarching approach for routine public health activities and response to MVEV at the local, state and national level, and consider future policy and research options.
Food security and food safety
Communicable diseases can threaten the food supply in a number of ways. With increasing globalisation, the persistence of transboundary animal diseases (such as foot and mouth disease, classical swine fever and Rift Valley Fever) can pose risks to food security and jeopardise international trade19. While the majority of the issues surrounding food security are dealt with by DAFF (particularly about ensuring people experiencing social or geographical isolation or socio-economic disadvantage have access to food), I can discuss the food safety aspect.
Foodborne illness remains a significant public health problem, and is estimated to cost $1.2 billion per year20. Australia has comparatively high rates of some foodborne diseases such as campylobacteriosis when compared to other similar countries. Three-quarters of Campylobacter infections are foodborne21, and research has demonstrated that a significant proportion of these infections are associated with the consumption of poultry meat22,23. In New Zealand, in 2007, the New Zealand Food Safety Authority (NZFSA) released a Campylobacter Risk Management Strategy which aimed to reduce the incidence of human campylobacteriosis24. The strategy included performance targets for Campylobacter prevalence in broiler chicken, auditing of primary and secondary processors of poultry meat and microbiological monitoring of food products. In the second phase of the strategy (2010–2013) the NZFSA aims to achieve a 50% reduction in the annual incidence of campylobacteriosis within 5 years25. The risk management strategy has had a demonstrable effect on rates of infection; in 2008, the annual campylobacteriosis notification rate in New Zealand was 161.5/100,000 population, representing a 54% decline compared with the average annual rate of 353.8 per 100,000 for 2002–2006, with a 74% reduction in the number of cases attributed to poultry26. There was also a 13% decline in hospitalisations for Gullian-Barré Syndrome, an infrequent severe outcome of Campylobacter infection27.
If Australia is to make similar inroads into rates of foodborne disease, we must improve our integration of surveillance from on farm through to the point of sale, including compiling laboratory data from human and animal surveillance. DoHA funds OzFoodNet to enhance surveillance of foodborne disease nationally. OzFoodNet works with food regulators and food safety agencies to compare data on pathogens from humans with other sources where possible. However there are barriers to this such as a lack of access to results of tests conducted in private laboratories, and lack of a consistent typing scheme between jurisdictions. OzFoodNet and DoHA work together closely along with Food Standards Australia New Zealand (FSANZ). OzFoodNet data on outbreaks of foodborne illness is integral to the development of food standards including the Primary Production and Processing Standards. FSANZ recently developed the Primary Production and Processing Standard for Poultry Meat which has been in place since May 2012, and requires that poultry producers and processors must control food safety hazards and must be able to trace their products28.
Conclusion
While One Health has only recently come to the forefront, it is an important concept that encourages collaboration between many different areas. However, when looking to implement actions under the One Health banner it is important that we consider how we can build on our current systems and relationships, identify practical and cost effective actions to be taken and most importantly how we set priorities relevant to each discipline involved.
Acknowledgements
I would like to acknowledge the contributions of Jennie Hood and Katrina Knope in the Department of Health and Ageing for assisting with the preparation of this article.
References
[1] US. Department of Agriculture, Animal and Plant Health Inspection Service Veterinary Services 2015 One Health. “What is One Health?”.[2] The Health Protection Agency. Human Animal Infections and Risk Surveillance group http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/EmergingInfections/HAIRS/ (accessed 12 July 2012).
[3] Centers for Disease Control and Prevention One Health. http://www.cdc.gov/onehealth/ (accessed 12 July 2012).
[4] The One Health Initiative http://www.onehealthinitiative.com/ (accessed 12 July 2012).
[5] Taylor, L. H. et al.. (2001) Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 983–989.
| Risk factors for human disease emergence.Crossref | GoogleScholarGoogle Scholar |
[6] Yob, J. M. et al.. (2001) Nipah virus infection in bats (order Chiroptera) in peninsular Maylaysia. Emerg. Infect. Dis. 7, 439–441.
[7] Chua, K. et al.. (2002) Isolation of Nipah virus from Malaysian Island flying foxes. Microbes Infect. 4, 145–151.
| Isolation of Nipah virus from Malaysian Island flying foxes.Crossref | GoogleScholarGoogle Scholar |
[8] Olson, J. et al.. (2002) Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg. Infect. Dis. 8, 987–988.
| Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia.Crossref | GoogleScholarGoogle Scholar |
[9] Reynes, J. M. et al.. (2005) Nipah virus in Lyle’s flying foxes, Cambodia. Emerg. Infect. Dis. 11, 1042–1047.
| Nipah virus in Lyle’s flying foxes, Cambodia.Crossref | GoogleScholarGoogle Scholar |
[10] Selvey, L. A. et al.. (1995) Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 162, 642–645.
[11] O’Sullivan, J. D. et al.. (1997) Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 349, 93–95.
| Fatal encephalitis due to novel paramyxovirus transmitted from horses.Crossref | GoogleScholarGoogle Scholar |
[12] Chua, K. B. (2003) Nipah virus outbreak in Malaysia. J. Clin. Virol. 26, 265–75.
| Nipah virus outbreak in Malaysia.Crossref | GoogleScholarGoogle Scholar |
[13] Centers for Disease Control and Prevention (1999) Update: outbreak of Nipah virus—Malaysia and Singapore. MMWR Morb. Mortal. Wkly. Rep. 48, 335–337.
[14] Luby, S. P. et al.. (2009) Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007. Emerg. Infect. Dis. 15, 1229–1235.
| Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001-2007.Crossref | GoogleScholarGoogle Scholar |
[15] Luby, S. P. et al.. (2006) Foodborne transmission of Nipah virus, Bangladesh. Emerg. Infect. Dis. 12, 1888–1894.
| Foodborne transmission of Nipah virus, Bangladesh.Crossref | GoogleScholarGoogle Scholar |
[16] Field, H. et al.. (2001) The natural history of Hendra and Nipah viruses. Microbes Infect. 3, 307–314.
| The natural history of Hendra and Nipah viruses.Crossref | GoogleScholarGoogle Scholar |
[17] Plowright, R. K. et al.. (2008) Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapularus). Proc. Biol. Sci. 275, 861–869.
| Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapularus).Crossref | GoogleScholarGoogle Scholar |
[18] Spencer, J. D. et al.. (2001) Murray Valley encephalitis virus surveillance and control initiatives in Australia. Commun. Dis. Intell. 25, 33–47.
[19] Domenech, J. et al.. (2006) Regional and international approaches on prevention and control of animal transboundary and emerging diseases. Ann. N. Y. Acad. Sci. 1081, 90–107.
| Regional and international approaches on prevention and control of animal transboundary and emerging diseases.Crossref | GoogleScholarGoogle Scholar |
[20] Hall, G. et al.. (2005) Estimating foodborne gastroenteritis, Australia. Emerg. Infect. Dis. 1, 1257–1264.
| Estimating foodborne gastroenteritis, Australia.Crossref | GoogleScholarGoogle Scholar |
[21] Stafford, R. et al.. (2007) A multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in Australia. Epidemiol. Infect. 135, 978–988.
| A multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in Australia.Crossref | GoogleScholarGoogle Scholar |
[22] Stafford, R. J. et al.. (2008) Population-attributable risk estimates for risk factors associated with Campylobacter infection, Australia. Emerg. Infect. Dis. 14, 895–901.
| Population-attributable risk estimates for risk factors associated with Campylobacter infection, Australia.Crossref | GoogleScholarGoogle Scholar |
[23] Unicomb, L. E. et al.. (2008) Age-specific risk factors for sporadic Campylobacter infection in regional Australia. Foodborne Pathog. Dis. 5, 79–85.
| Age-specific risk factors for sporadic Campylobacter infection in regional Australia.Crossref | GoogleScholarGoogle Scholar |
[24] New Zealand Food Safety Authority. Campylobacter Risk Management Strategy 2008 - 2011. http://www.foodsafety.govt.nz/elibrary/industry/Campylobacter_Risk-Aims_Acheive.pdf.
[25] N. Z. F. S. Authority, Campylobacter Risk Management Strategy 2010 - 2013. http://www.foodsafety.govt.nz/elibrary/industry/Campylobacter_Risk-Comprehensive_Aimed.pdf5.Accessed 24 August 2012.
[26] Sears, A. et al.. (2011) Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand. Emerg. Infect. Dis. 17, 1007–1015.
| Marked campylobacteriosis decline after interventions aimed at poultry, New Zealand.Crossref | GoogleScholarGoogle Scholar |
[27] Baker, M. G. et al.. (2012) Declining Guillain-Barré syndrome after campylobacteriosis control, New Zealand, 1988–2010. Emerg. Infect. Dis. 18, 226–233.
[28] Food Standards Australia New Zealand. Primary Production and Processing Standard for poutry meat. http://www.foodstandards.gov.au/foodstandards/primaryproductionprocessingstandardsaustraliaonly/poultrystandards.cfm Accessed 23 August 2012.


