Unravelling the relationships among Madrepora Linnaeus, 1758, Oculina Lamark, 1816 and Cladocora Ehrenberg, 1834 (Cnidaria: Anthozoa: Scleractinia)
Anna M. Addamo A B C F * , Melinda S. Modrell
A B C F * , Melinda S. Modrell  A , Marco Taviani
A , Marco Taviani  D E and Annie Machordom
D E and Annie Machordom  A
A
A
B
C
D
E
F
Abstract
Despite the widespread use of integrative taxonomic approaches, many scleractinian coral genera and species remain grouped in polyphyletic families, classified as incertae sedis or simply understudied. Oculinidae Gray, 1847 represents a family for which many taxonomic questions remain unresolved, particularly those related to some of the current genera, such as Oculina Lamark, 1816 or recently removed genera, including Cladocora Ehrenberg, 1834 and Madrepora Linnaeus, 1758. Cladocora is currently assigned to the family Cladocoridae Milne Edwards & Haime, 1857 and a new family, Bathyporidae Kitahara, Capel, Zilberberg & Cairns, 2024, was recently raised to accommodate Madrepora. However, the name Bathyporidae is not valid because this was not formed on the basis of a type genus name. To resolve taxonomic questions related to these three genera, the evolutionary relationships are explored through phylogenetic analyses of 18 molecular markers. The results of these analyses support a close relationship between the species Oculina patagonica and Cladocora caespitosa, indicating that these may belong to the same family (and possibly genus), and highlighting the need for detailed revisions of Oculina and Cladocora. By contrast, a distant relationship is found between these two species and Madrepora oculata, with the overall evidence supporting the placement of Madrepora in the resurrected family Madreporidae Ehrenberg, 1834. This study advances our knowledge of coral systematics and highlights the need for a comprehensive review of the genera Oculina, Cladocora and Madrepora.
Keywords: cold-water corals, incertae sedis, Mediterranean Sea, molecular markers, phylogeny reconstruction, polyphyly, systematics, taxonomy.
Introduction
The taxonomic classification of scleractinian corals has traditionally been based on skeletal morphology that can be problematic due to a lack of distinct diagnostic characters (e.g. Budd et al. 2010; Zlatarski and Stake 2012; Kitahara et al. 2016). In recent decades, advances in molecular genetic approaches have led to new phylogenetic hypotheses, providing insight into the evolutionary history of different lineages of the two major clades of Scleractinia: ‘complex’ and ‘robust’ (Romano and Palumbi 1996), respectively corresponding to the suborders Refertina and Vacatina (Okubo 2016) (e.g. Budd et al. 2010; Zlatarski and Stake 2012; Kitahara et al. 2016: Campoy et al. 2020). Recent integrative taxonomic studies analysing both molecular and morphological characters (described from morphometric, microstructural and anatomical analyses of coralla and polyps) have also contributed to major advancements in coral systematics (see e.g. Addamo et al. 2016; Benzoni et al. 2018; Terraneo et al. 2019; Arrigoni et al. 2020; Campoy et al. 2020; Capel et al. 2020; Kitahara et al. 2020; Oku et al. 2020; Juszkiewicz et al. 2022; Ramírez-Portilla et al. 2022; Seiblitz et al. 2022). In the order Scleractinia, systematics have been improved at all levels, with proposed changes in nomenclature and classification following new knowledge on phylogenetic relationships of taxa.
Despite these advances, five subfamilies (Cyathophorinae Vaughan & Wells, 1943; Desmophyllinae Vaughan & Wells, 1943; Dichocoeniinae Vaughan & Wells, 1943; Montastreinae Vaughan & Wells, 1943, and Stylophorinae Milne Edwards & Haime, 1849), four extant genera (Bachytrochus Duncan, 1876, Coenastrea Etallon, 1862, Dendrocora Duncan, 1876 and Solenastrea Milne Edwards & Haime, 1848) and the seven associated extant species (Bachytrochus simplex Duncan, 1876, Cladorbicella taiwanensis Yabe & Ehara, 1936, Dendrocora fissipara Duncan, 1876, Solenastrea bournoni Milne Edwards & Haime, 1849, Solenastrea hyades Dana, 1846, Solenastrea ecuadoriana Durham and Barnard, 1952 and Solenastrea spongiformis Duncan, 1864) remain classified as Scleractinia incertae sedis (Hoeksema and Cairns 2023a). Moreover, although many recent phylogenetic studies of corals have shown high support for certain relationships, resolving some taxonomic questions, these have also rendered inadequate some taxonomic diagnostic characters and dichotomous keys used for identification and classification (e.g. Cairns and Kitahara 2012). For example, Gittenberger et al. (2011) and Benzoni et al. (2012) demonstrated that several life history traits of mushroom corals (Fungiidae), such as monostomatism v. polystomatism and attached v. free-living corallum, do not have the diagnostic value once ascribed. Similarly, following an in-depth genetic and morphological study of Desmophyllum Ehrenberg, 1834, the new classification and nomenclature proposed by Addamo et al. (2016) make the first character of the dichotomous key for azooxanthellate corals (colonial v. solitary) no longer reliable for diagnosis.
Reconciling taxonomy with systematics knowledge is a challenge in the field and inconsistent classifications based on historically used morphological characters or molecular markers have driven the search for new characters. For example, mitochondrial cytochrome c oxidase subunit I (COI) has generally been regarded as an only moderately useful marker for universal barcoding of corals (Meyer and Paulay 2005; Huang et al. 2008; Shearer and Coffroth 2008; Bucklin et al. 2011; Krishna Krishnamurthy and Francis 2012). As a result, other molecular markers have been used to both clarify phylogenetic relationships and serve as barcodes for species identifications across the Tree of Life (e.g. Nosenko et al. 2013). Commonly used markers for describing coral species and making phylogenetic inferences include mitochondrial small subunit ribosomal RNA (12S), large subunit ribosomal RNA (16S), COI and cytochrome b (CYTB), and also nuclear small ribosomal RNA subunit (18S), large ribosomal RNA subunit (28S) and the internal transcribed spacer regions (internal transcribed spacer 1–5.8S ribosomal DNA–internal transcribed spacer 2, hereafter denoted as ITS) (see e.g. Stolarski et al. 2011; Addamo et al. 2012). At the same time, genome-based approaches and studies that assess the suitability of individual molecular markers for phylogenetic and taxonomic analyses (Quattrini et al. 2018; Cowman et al. 2020; Quek et al. 2020; Untiedt et al. 2021; Seiblitz et al. 2022) have increased the availability of other types of markers. Indeed, the use of a wide range of molecular-based approaches is key to achieving the shared goal of a comprehensive phylogeny that is well supported at all taxonomic levels. For this reason, Sanger sequencing-based studies of a subset of loci still allow for a broader (and more accessible) approach that can be integrated with genome-wide and direct sequencing studies (Quek and Huang 2022).
In our study, the main aim is to infer the phylogenetic relationships 3 geographically widespread genera, Madrepora Linnaeus, 1758, Oculina Lamarck, 1816 and Cladocora Ehrenberg, 1834, using 4 commonly used markers and 14 newly developed ones. Oculina, with the extant genera Bathelia Moseley, 1880, Cyathelia Milne Edwards & Haime, 1849, Petrophyllia Conrad, 1855 and Schizoculina Wells, 1937 belong to the family Oculinidae Gray, 1847 (Hoeksema and Cairns 2023b). Madrepora was also a genus of Oculinidae until very recently when the monogeneric family Bathyporidae fam. nov. Kitahara, Capel, Zilberberg & Cairns, 2024 was proposed to accommodate the genus following a molecular phylogenomic study (Capel et al. 2024). However, when the analyses presented here were conducted, this was still within Oculinidae. Species within Oculinidae are described as being colonial and hermatypic or ahermatypic (including extant and fossil species) with thickened corallites that are linked by a smooth coenostum (Hoeksema and Cairns 2023b). Phylogenetic studies have shown the non-monophyly of Oculinidae, with some genera being more closely related to members of other families than to those of Oculinidae (Fukami et al. 2004; Kitahara et al. 2010; Campoy et al. 2020; Kitahara and Cairns 2021). For example, in a study based on 12 commonly used markers, Oculina resolved as closely related to Astrangia Milne Edwards & Haime, 1849 within Astrangiidae Milne Edwards & Haime, 1857 or to Cladocora (then considered as Scleractinia incertae sedis), whereas Madrepora resolved as distant from all other Oculinidae genera analysed (Kitahara et al. 2016; Capel et al. 2024). By contrast, the taxonomic status of Cladocora has changed several times. This was initially considered a genus of the family Faviidae Milne Edwards & Haime, 1857 (Veron 1995) (also see Peirano et al. 1999; Cuif et al. 2003; Rodolfo-Metalpa et al. 2006; Kružić and Benković 2008) but was transferred to Caryophylliidae Dana, 1890 on the basis of molecular and morphological markers (Romano and Cairns 2000; Cairns et al. 2001) (see also Kersting and Linares 2012; Caroselli and Goffredo 2014; El Kateb et al. 2016; Mačić et al. 2019). Following an exhaustive molecular phylogenetic reconstruction of Scleractinia, Fukami et al. (2008) proposed Cladocora as a genus of Oculinidae (see also Casado-Amezua et al. 2011; Kersting et al. 2013a, 2013b; Caroselli and Goffredo 2016) but Benzoni et al. (2011) classified the genus as Scleractinia incertae sedis pending further review. Cladocora remained incertae sedis until assigned to the family Cladocoridae Milne Edwards & Haime, 1857 in 2023 (Hoeksema and Cairns 2023c).
In this study, the type species Madrepora oculata Linnaeus, 1758 and Cladocora caespitosa (Linnaeus, 1767) were chosen to represent the respective genera. As neither samples nor sequencing data were available for Oculina virginea (Linnaeus, 1758), the type species of Oculina, Oculina patagonica de Angelis D’Ossat, 1908 was used to represent the genus. Madrepora oculata is a framework building, deep-water azooxanthellate coral that is distributed worldwide, except in polar regions (Roberts et al. 2009). Cladocora caespitosa is an endangered endemic shallow-water zooxanthellate species that is currently restricted to the Mediterranean Basin (Zibrowius 1980) that has been highly affected by climatic and more recent anthropogenic changes extending back to the Pliocene (Peirano et al. 1998). Oculina patagonica is a shallow-water zooxanthellate species (Zibrowius 1974) that has long been and continues to be considered a species that was likely introduced from the Atlantic Ocean to the Mediterranean Sea during the most recent period, the Holocene (i.e. unofficial unit of geological times known as Anthropocene) (Zibrowius 1974, 1980; Zibrowius and Ramos 1983; Fine and Loya 1995; Bitar and Zibrowius 1997; Ballesteros et al. 1998; Çinar et al. 2006; Izquierdo et al. 2007; Sartoretto et al. 2008; Cvitković et al. 2013; Salomidi et al. 2013; Serrano et al. 2013; Rubio-Portillo et al. 2014a, 2014b; Schwindt et al. 2014; Terrón-Sigler et al. 2015; López et al. 2019; Cutajar et al. 2020; Martinez et al. 2021). However, molecular evidence suggests that O. patagonica is a native species that has become invasive within the range owing to environmental changes (Leydet and Hellberg 2015). In contrast to C. caespitosa that has a substantial fossil record, O. patagonica is unknown in the paleontological record of the Mediterranean basin (Vertino et al. 2014), although the species was described from fossil remains from South America (Zibrowius 1974). Regardless, this species seems to compete with C. caespitosa (Boudouresque 1994; Streftaris et al. 2005; Katsanevakis et al. 2012) that may have a negative effect on the long-term survival and conservation management of the latter species in the Mediterranean Sea.
Employing commonly used and newly developed molecular markers, this study aims to test the hypothesis that O. patagonica and C. caespitosa are closely related species that are distantly related to M. oculata that could indicate the need for taxonomic reassignments at multiple levels.
Methods
Sample collection and preservation
Some of the coral specimens analysed were freshly collected in the field and immediately stored in absolute ethanol for the molecular study. Most of the other analysed specimens, currently preserved in ethanol, were from specimens previously collected and stored in museum or personal collections. The specimens analysed represent 17 families, 54 genera and 103 species of scleractinians. Specimens included in this study are listed in Supplementary Table S1.
DNA extraction, amplification and sequencing
For all specimens, total genomic DNA (gDNA) was extracted either from entire polyps or mesenteric tissue using the Qiagen BioSprint 15 DNA Blood Kit, following the manufacturer’s protocol with slight modifications, including the optional RNase treatment and an overnight incubation in proteinase K lysis buffer at 55°C. DNA concentration was quantified using a Qubit 2.0 Fluorometer (ThermoFisher Scientific). Polymerase chain reactions (PCRs) were performed in a total volume of 50 μL containing 1× PCR Biotools Standard Reaction Buffer including 2 mM of MgCl2, 0.5 μM of forward and reverse primers, 0.2 mM of each dNTP, 1.5 U of DNA polymerase (Biotools) and 2 ng of template DNA. All reactions were run in a Veriti Thermal Cycler (Applied Biosystems). For the markers 16S, COI, 28S and ITS, thermocycling conditions described by Addamo et al. (2012) were used. For remaining markers, the ramped cycling profile described by Palumbi et al. (1991) was used, with some modifications: initial denaturation at 94°C for 5 min, followed by 40 cycles at 94°C for 30 s, annealing at 48°C for 10 s and an up-ramp from 48 to 72°C for 2 min, and a final extension at 72°C for 10 min. When this profile did not amplify a specific PCR product, other starting annealing temperatures were tested (e.g. TA/R 45, 50, 52 or 56°C). Amplified products were visualised on 1.5% agarose gels. Sequencher (ver. 4.10.1, Gene Codes Corporation) was used to verify sequence chromatograms and trim primer sequences from alignments.
Phylogeny reconstructions
Phylogenetic analyses were based mainly on two concatenated data sets consisting of sequences of the following markers: (1) the widely used mitochondrial genes 16S and COI, and nuclear 28S and ITS; and (2) 7 of the 14 markers developed from a genome screen (Actin, AMPt1, AMPt2, ß-Actin, NAD3, NAD5 and SIAH1) (Table 1, see the ‘Description of the 14 markers developed from the genome screen’ section in the Supplementary material and Supplementary Tables S2–S3). These seven markers were selected because due to providing the most complete data set without including missing data (i.e. species without sequences for all included markers), thereby avoiding spurious results due to a lack of data. In addition, each gene was analysed individually. All new sequences were deposited in NCBI GenBank (https://www.ncbi.nlm.nih.gov/) under the following accession numbers (gene): MW139432–MW139480 (Actin); MW110483–MW110531 (AMPt1); MW139384–MW139431 (AMPt2); MW139592–MW139607 (ATP6/NAD4); MW139481–MW139511 (ß-Actin); MW110608–MW110632 (Creatine kinase); MW139577–MW139591 (Heat shock-like); MW139558–MW139576 (Helicase); MW139364–MW139383 (NAD3); MW139608–MW139642 (NAD5); MW110532–MW110556 (NCAH-like); MW139512–MW139557 (SIAH1) and MW110557–MW110566 (UBB). The new sequences of 16S rDNA (covering a different part of the 16S gene) and ITS were deposited under accession numbers MW110567–MW110604 and OP466710–OP466715 respectively. Sample data and voucher codes are provided in Table S1.
| Gene | Forward Primer 5′–3′ (source) | Reverse Primer 5′–3′ (source) | |
|---|---|---|---|
| Actin | TCAACTGYCCAGCYATGTAYG | CAGGNAGCTCRTAGCTCTTCTC | |
| AMPt1 | TRCCYTCRACRGCATTRGAATGR | CTTAAACTNGCTYTGGANATG | |
| AMPt2 | GTTGAAACAAGNATGGCNGT | GTCCATTGGCRTCAGTRAAT | |
| ATP6-NAD4 | AAGCRCGAACYTTTTCTTCYC | GTNTNTTTGAATGTGYTGGG | |
| ß-Actin | CAGATYATGTTCGAGACYTTCCA | RAANAGTGCTTCNGGRCATC | |
| CK | GTGCAGAARCGCTCGAANAC | CGYTRGACTCNCTNGATGGCG | |
| COI | COIcoralF (Addamo et al. 2012) | HCO2 (Folmer et al. 1994) | |
| Heat shock-like | ATCCACTTCYTCAATAGTAGGTCCA | GTCCAAGGANGACATAGA | |
| Helicase | CCAATGTTACCRGCTTGTTTRGTTT | TGCTGAGAAAAARGCTGATGTNGAT | |
| ITS | ITS2.1 (Hugall et al. 1999) | ITS2.2 (Hugall et al. 1999) | |
| NAD3 | CAYTCTARNCCCYCCTYTTARTC | CAATNGCAGCRGCNGAGTCTTC | |
| NAD5 | TTTYCTTCARTTRTTTATTGGNTG | CCCTAAAACYTTTCGTTCTGC | |
| NCAH-like | TGGGNAARCAAAACAGTAARCTCAA | GAACTTGACGCATCRCAYTG | |
| SIAH1 | TCNGCTTGTTTACGTGTTCCAAT | ATGAATCGCCAANCAAGYTC | |
| UBB | CTAAAAATAGCNCATTATGAATTG | GRGTRGACTCYTTCTGGAT | |
| 16S | LP16SF (Le Goff-Vitry et al. 2004) | LP16SR (Le Goff-Vitry et al. 2004) | |
| 16S rDNA | GTACTGTGAAGGAAAGTTGAAAGAG | TGAYACCATTCATACCGGYCAA | |
| 28S | C1′ (Cuif et al. 2003) | D2MAD (Cuif et al. 2003) |
Bold gene markers are those used in one of the two concatenated data sets.
Matrices were aligned using ClustalX (ver. 2.1, see http://www.clustal.org/clustal2/; Thompson et al. 1997) with the default settings and resulting alignments were manually checked with Se-Al (ver. 2.0a11, A. Rambaut, see http://tree.bio.ed.ac.uk/software/seal/). Maximum likelihood (ML) and Bayesian inference (BI) approaches were used to construct phylogenetic trees using PhyML (ver. 3.0, see http://www.atgc-montpellier.fr/phyml/; Guindon and Gascuel 2003) and MrBayes (ver. 3.2.7a, see https://github.com/NBISweden/MrBayes/; Ronquist and Huelsenbeck 2003) for the ML and BI analyses respectively. The model that best fitted the data was selected according to the Smart Model Selection (SMS) option (Lefort et al. 2017) with the Bayesian information criterion for ML and the number of substitution types (nst) = mixed (which explores multiple combinations of change rates) for BI. To assess the relative robustness of tree branches in the ML analyses, bootstrapping (Felsenstein 1985) was used (1000 pseudoreplicates). For the BI analyses, double parallel runs were performed for 100 million generations with one cold and three heated Markov Chains Monte Carlo (MCMC) for each run, sampling trees every 10,000 generations (5000 trees were saved during the MCMC of each run), until the average standard deviation of split frequencies between runs was less than 0.01, indicating convergence of the runs. Nodal support was evaluated by posterior probabilities. Sequences of non-scleractinian cnidarians available from GenBank were used as outgroups (e.g. Nematostella vectensis Stephenson, 1935; Hydra Linnaeus, 1758 and Ricordea florida Duchassaing & Michelotti, 1860).
Results
The phylogenetic relationships among Madrepora, Oculina and Cladocora representatives inferred by the two sets of molecular markers (i.e. commonly used and new ones) were generally congruent (Fig. 1 and 2). In addition, the gene trees resulting from the individual analysis of the 14 new markers generally support the relationship observed among the three genera with the two concatenated matrices (Supplementary Fig. S1).
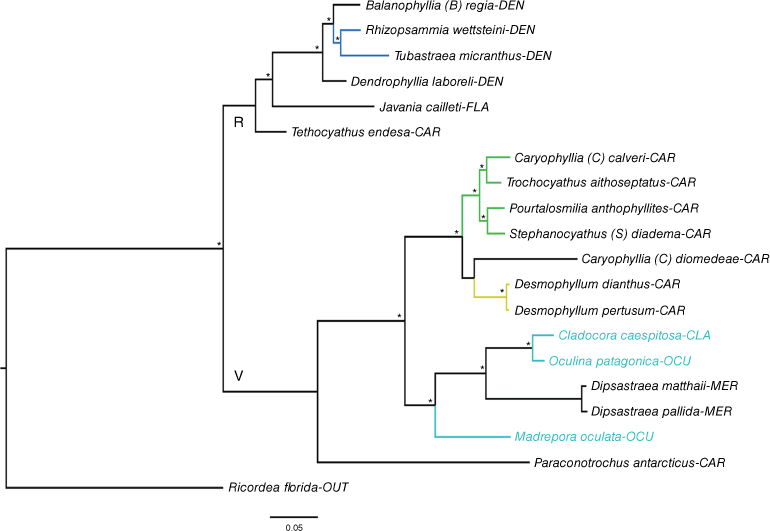
Phylogeny reconstruction based on BI and ML approaches, and a concatenated data set consisting of 16S, COI, 28S and ITS sequences. An asterisk (*) indicates a well-supported node (pp ≥ 95; bootstrap > 70). Cladorora caespitosa, Madrepora oculata and Oculina patagonica are highlighted in light blue. Clades showing other relationships worthy of further investigation are indicated in blue, green and yellow. Three-letter codes after the taxon names are the family codes – see Table 1 and Table S3 for marker information, Table S1 for species information, and ‘Supplementary results’ section in the Supplementary material for additional results. OUT, outgroup; R, Refertina suborder; V, Vacatina suborder.
Phylogeny reconstruction based on BI and ML approaches and a concatenated data set consisting of 7 of the 14 newly developed markers: Actin, ß-Actin, AMPt1, AMPt2, NAD3, NAD5 and SIAH1. An asterisk (*) indicates a well-supported node (pp ≥ 95; bootstrap > 70). Cladorora caespitosa, Madrepora oculata and Oculina patagonica are highlighted in light blue. Clades showing other relationships worthy of further investigation are indicated in blue, pink, red, yellow and green. Three-letter codes after the taxon names are the family codes – see Table 1 and Supplementary Tables S1 and S3 for marker information, Supplementary Table S1 for species information, and ‘Supplementary results’ section in the Supplementary material for additional results. OUT, outgroup; R, Refertina suborder; V, Vacatina suborder.
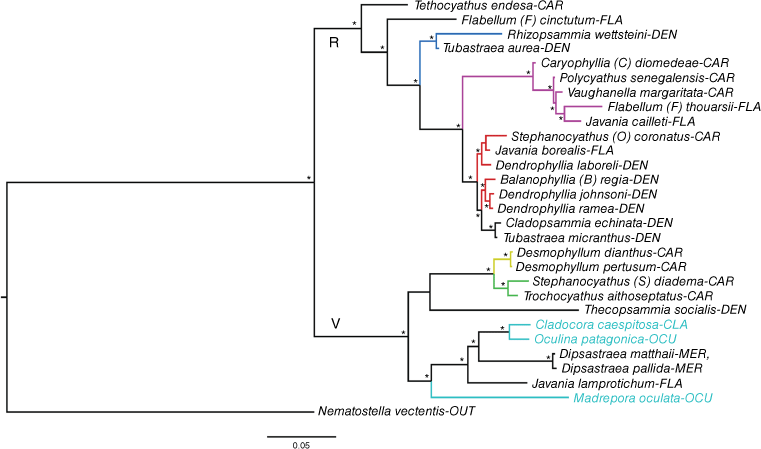
In the two main phylogenies, M. oculata (Bathyporidae, see Capel et al. 2024), O. patagonica (Oculinidae) and C. caespitosa (Cladocoridae) grouped within the robust clade (Vacatina suborder). Madrepora oculata and O. patagonica resolved as distantly related species. In contrast, a close relationship was observed between O. patagonica and C. caespitosa (Fig. 1, 2, S1b–h, j, l, m, o). Only NAD5 and COI support a close relationship between M. oculata, O. patagonica and C. caespitosa (Fig. S1e, g) of the eight single-locus phylogenies that included all three species. In the main phylogenies inferred, the clade O. patagonica + C. caespitosa resolved as the sister group of the clade comprising species belonging to the family Merulinidae Milne Edwards & Haime, 1857. In the first phylogeny, the sister taxon of this larger clade was M. oculata (Fig. 1), whereas in the second, this was Javania lamprotichum (Moseley, 1880), a species of the family Flabellidae Bourne, 1905 (Fig. 2; for additional results, see the ‘Supplementary results’ section in the Supplementary material). Further insights on other scleractinian genera and families are provided in the ‘Supplementary results’.
Capel et al. (2024) proposed the new monogeneric family Bathyporidae Kitahara, Capel, Zilberberg & Cairns, 2024 to accommodate Madrepora Linnaeus, 1758. This proposal aimed to provide a more fitting classification for the genus Madrepora and alleviate confusion related with the name Madreporidae. Capel et al.’s (2024) rationale for erecting Bathyporidae stems from the argument that the family names Madreporidae Ehrenberg 1834, Madreporidae Dana, 1846 and Madreporidae Gray, 1847 should all be considered invalid because these are based on the junior homonymy. In this context, Madreporidae is argued to be unavailable under Article 39 of the International Code of Zoological Nomenclature (ICZN) that pertains to ‘invalidity due to homonymy or suppression of the name of the type genus’.
Without doubt, the taxonomic history behind the nomenclature of Madreporidae (and Madrepora) is complicated owing to certain author restrictions and questionable decisions made by earlier taxonomists (e.g. Dana 1846; Gray 1847; Verrill 1901). However, Capel et al.’s (2024) rationale for adopting the nomenclature of the new proposed family Bathyporidae is disputable. Despite Capel et al.’s (2024) assertion to the contrary, Ehrenberg (1834) did not designate Madrepora Ehrenberg, 1834 as the type genus of the new family Madreporidae (see Ehrenberg 1834, pp. 299–344). Therefore, the family name Madreporidae remains available according to Article 10.6 of the ICZN:
Effect of invalidity upon availability: A name once available remains so irrespective of its invalidity as a junior synonym, a junior homonym, an unjustified emendation, an unnecessary substitute name, or a suppressed name, unless the Commission has ruled otherwise [Arts. 78.1, 78.2]. Even if the taxon concerned is no longer classified as animal its name remains available [Art. 2.2]
Furthermore, the establishment of the new proposed monogeneric family Bathyporidae was not based on an available type genus name (e.g. Bathyporus, Bathypora or Bathyporum) infringing on ICZN Article 11.7.1.1, regarding criteria for family-group names when first published and Article 29.1:
Formation of family-group names: A family-group name is formed by adding to the stem of the name [Art. 29.3] of the type genus, or to the entire name of the type genus [see Article 29.6], a suffix as specified in Article 29.2
Consequently, the new family Bathyporidae should not be accepted with respect to accommodating Madrepora.
In addition to adhering to ICZN rules, we assert that resurrecting Madreporidae would more effectively mitigate taxonomic confusion associated with the name Madreporidae compared with introducing the new family Bathyporidae. Consequently, we advocate for the application of Madreporidae as the new family name accommodating the genus Madrepora Linnaeus, 1758.
Family MADREPORIDAE Ehrenberg, 1834
Colonial, extratentacular sympodial budding forming dendroid colonies. Coenosteum dense. Costae absent or faint. Theca porcellanous or finely granulated. Usually three cycles of septa. Paliform lobes usually absent but if present, rudimentary and positioned before the first and second septal cycles. Columella usually absent but if present, papillose or rudimentary.
Madrepora oculata Linnaeus, 1758, by subsequent designation (Verrill 1901).
Madrepora arbuscula (Moseley, 1880); Madrepora astroites Forskål, 1775; Madrepora carolina (Pourtalès, 1871); Madrepora minutiseptum Cairns & Zibrowius, 1997; Madrepora oculata Linnaeus, 1758; Madrepora piresae Kitahara, Capel & Zilberberg, 2024; Madrepora porcellana (Moseley, 1880).
The genus has been widely described as cosmopolitan deep-sea corals belonging to the family Oculinidae. The molecular analysis presented in this study, however, supports the reassignment of the genus Madrepora from Oculinidae to a different family, namely Madreporidae. The diagnosis reported includes only the skeletal characters; other characters can be found in the original species descriptions.
Discussion
In agreement with previous studies (e.g. Kitahara et al. 2010; Addamo et al. 2012; Capel et al. 2024), our results demonstrate not only the consistent grouping of O. patagonica (Oculinidae) and C. caespitosa (Cladocoridae) in a well-supported clade but also the lack of a close relationship with M. oculata.
The close evolutionary relationship observed between the specimens of C. caespitosa and O. patagonica analysed in this study provide support for the potential reassignment of O. patagonica to the genus Cladocora. However, before such a taxonomic act is formally proposed, the taxonomic status of the species should be resolved as two species may seemingly be attributed to O. patagonica: an extinct species on which the original description of the species is based (on fossil remains from Argentina) (De Angelis D’Ossat 1908) and an extant species that inhabits the Mediterranean but originated from the north-western Atlantic (Leydet and Hellberg 2015). Given that the extant species has likely been misattributed to O. patagonica, the name should be preserved for the extinct species. We propose that the Mediterranean specimens attributed to O. patagonica be renamed following a formal, detailed morphological description. Whether this should be an Oculina sp. or a Cladocora sp. remains to be determined and would require in-depth morphological and molecular analyses with other species of both genera.
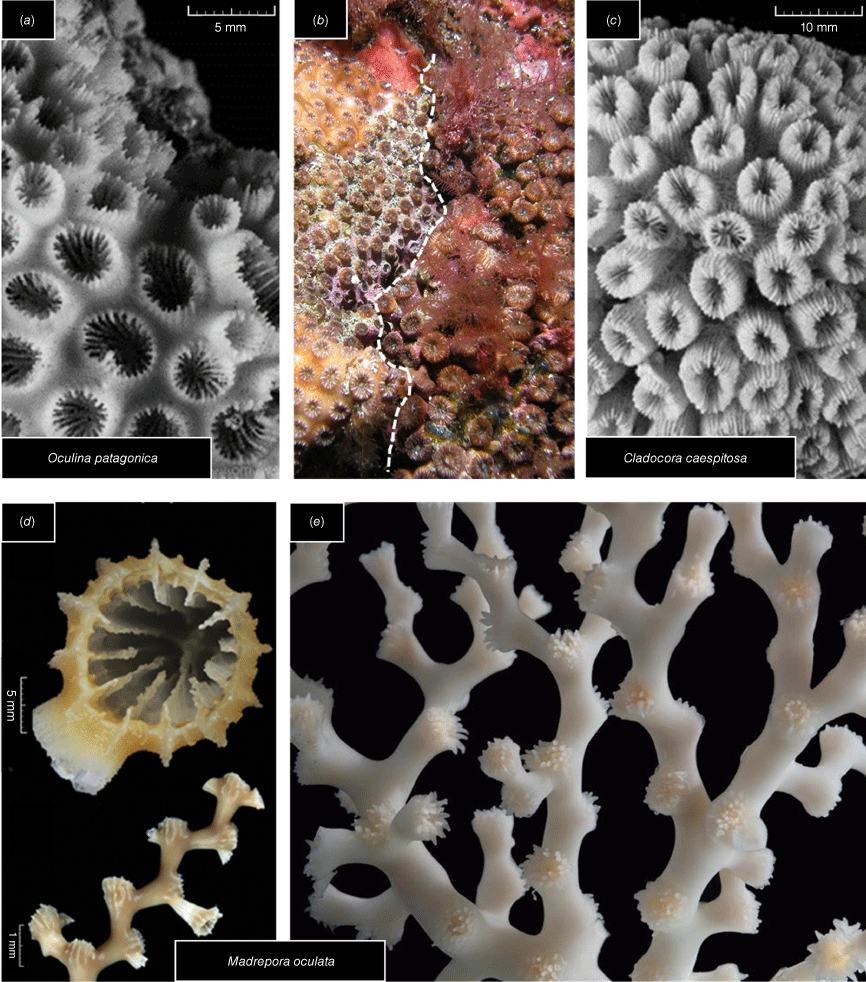
The taxonomic assignment of Oculina and Cladocora to different families stems from differences in the skeletons: a coenosteum composed of costate is absent in Oculina but present in Cladocora (Cairns and Kitahara 2012). These also differ in the encrusting colonial morphology with O. patagonica forming mainly plocoid corallites (Fine et al. 2001) and C. caespitosa, pillow-shaped colonies with a phaceloid growth form (Hoeksema and Ocaña Vicente 2014) (see Table 2, Fig. 3a–c). However, O. patagonica displays phenotypic plasticity in response to environmental factors and, for example, the colonial morphology changes from plocoid to phaceloid-like under bleaching conditions (Zaquin et al. 2019), suggesting that this character may not be useful for defining the species.
Colony and corallites of Cladocora caespitosa, Madrepora oculata and Oculina patagonica. (a) Corallites of O. patagonica from the Mediterranean Sea. Photograph: © Emre Turak and Charlie Veron (adapted from Veron et al. 2016a). (b) Coral colonies of O. patagonica (left side of the dashed line) and C. caespitosa (right side of the dash line) found near the Balearic Islands at a depth of 8 m. Photography: © Diego K. Kersting, used with permission. (c) Corallites of C. caespitosa from the Mediterranean Sea. Photograph: © Emre Turak and Charlie Veron (adapted from Veron et al. 2016b). (d) Calicular (top) and colony (bottom) views of M. oculata (adapted from Cairns and Kitahara 2012, licence CC BY 3.0). (e) Colony of M. oculata. Photograph: © Dugornay Olivier (adapted from Dugornay 2019, license CC BY 4.0).
| Madrepora oculata | Oculina patagonica | Cladocora caespitose | |
|---|---|---|---|
| Colonial, dendroid colonies with delicate distal branches | Colonial, encrusting colonies with plocoid or phaceloid-like growth form | Colonial, pillow-shaped colonies with a phaceloid growth form | |
| Coenosteum finely granular, coenoscarc present | Coenosteum dense, coenoscarc present | Coenosteum costate, coenosarc absent in most colonies | |
| Costae and pali absent | Costae and pali absent | Costae and pali present | |
| Columella papillose | Columella papillose | Columella papillose |
Regardless of whether the Mediterranean specimens attributed to O. patagonica belong to Oculina or Cladocora, the fact that these species inhabit and compete in the Mediterranean may have important implications for conservation of the endangered C. caespitosa. Genetic information can be used to help establish or change conservation priorities for endangered corals and the community of species that rely on these (Beger et al. 2014). Though numerous ecological and biological studies have been published on C. caespitosa (e.g. Peirano et al. 2004; Kersting et al. 2013a, 2013b; Rubio-Portillo et al. 2018; Pons-Fita et al. 2020), less is known about the genetic diversity and population structure. Recent work has shown that, despite the supposed low dispersal ability and high self-recruitment of C. caespitosa, the populations are characterised by low levels of genetic differentiation, indicating a relatively high degree of connectivity (Repullés et al. 2022), though regional and local differentiation was observed among different basin populations (López-Márquez et al. 2019, 2021; Kersting et al. 2022). The regional differentiation is associated with differences in reproductive strategies (sexual v. clonal) and the response to thermal and climate-related stressors. This finding highlights the importance of knowing the status of local populations throughout the Mediterranean and the potential effect on the diversity and conservation of the species. Given that the expansion of O. patagonica might have consequences for the distribution of C. caespitosa, future genetic studies of co-occurring populations of the two species will be necessary to uncover any association between a change in the abundance of C. caespitosa and the presence of O. patagonica.
The third genus under study, Madrepora, was originally included within the family Oculinidae owing to similarities in the morphological diagnoses – colonial, extratentacular sympodial budding forming dendroid colonies, coenosteum dense, costae and pali absent, columella papillose or absent (Vaughan and Wells 1943) (see Table 2, Fig. 3d, e). However, molecular phylogenies (Le Goff-Vitry et al. 2004; Kitahara et al. 2010, 2016; Campoy et al. 2020; this study) and mitochondrial gene rearrangements (Lin et al. 2012) suggested that Madrepora does not belong to Oculinidae. Kitahara and Cairns (2021), instead of transferring Madrepora to a new family, proposed a temporary clade (‘C’) within Oculinidae comprising species of Madrepora until the morphological synapomorphy (or synapomorphies) characterising oculinids could be identified. Capel et al. (2024) recently proposed the new monogeneric family Bathyporidae to accommodate the genus Madrepora following an analysis based on microsatellite markers, and ultraconserved elements (UCE) and exons. However, as mentioned, the name chosen by Capel et al. (2024), though intended to reduce confusion associated with the name Madreporidae, violates Art. 11.7.1.1 and Art 29.1 of the ICZN. Regardless, our study provides additional molecular evidence that supports the movement of Madrepora to a different family and we contend that Madreporidae is still an available name and should be resurrected to accommodate the genus as this appears to be a more appropriate (and less confusing) family name.
Undoubtedly, the use of genome-scale approaches among other technological advances has transformed the scope of coral studies (see e.g. Bhattacharya et al. 2016; Mao et al. 2018; Quattrini et al. 2018; Cowman et al. 2020; Quek et al. 2020; McFadden et al. 2021; Quek and Huang 2021; Terraneo et al. 2021; Untiedt et al. 2021). Although these approaches can be cost-effective relative to the expense of Sanger sequencing and often successful with DNA samples of insufficient quality (e.g. Untiedt et al. 2021), these are not yet widely used in the field. Moreover, new species or phylogenetic reconstructions are still mainly described using few genes (e.g. Arrigoni et al. 2021; Cairns et al. 2021; Núñez-Flores et al. 2021). Indeed, Ramírez-Portilla et al. (2022) suggest that single gene sequencing may still be the most cost-effective method for DNA barcoding of large samples. For these reasons, multi-loci studies such as our study are still highly relevant as these provide a greater breadth of molecular markers for taxonomic and phylogenetic study at a lower scale.
In summary, the overall congruence among the phylogenies (concatenated matrices and gene trees) supports not only the utility of the analysed markers but also the close relationship between extant Oculina and Cladocora species. This also supports the distant relationship between these genera and Madrepora and highlights the long-standing need for the revision of the taxonomy of O. patagonica and C. caespitosa.
Data availability
Data supporting this study are available in the article and accompanying online supplementary material, and in NCBI GenBank (https://www.ncbi.nlm.nih.gov/) under the following accession numbers (gene): MW110483–MW110531 (AMPt1); MW110532–MW110556 (NCAH-like); MW110557–MW110566 (UBB); MW110567–MW110604 (16S); MW110608–MW110632 (Creatine kinase); MW139364–MW139383 (NAD3); MW139384–MW139431 (AMPt2); MW139432–MW139480 (Actin); MW139481–MW139511 (ß-Actin); MW139512–MW139557 (SIAH1); MW139558–MW139576 (Helicase); MW139577–MW139591 (Heat shock-like); MW139592–MW139607 (ATP6/NAD4); MW139608–MW139642 (NAD5); and OP466710–OP466715 (ITS).
Declaration of funding
This research was supported by the Spanish Ministry of Science Innovation and Universities grants (CGL2011-23306, CTM2014-57949-R and PDI2019-108644GB-I00); project CORALIEN (Fundación Biodiversidad, Ministerio para la Transición Ecológica y el Reto Demográfico, BM_2019); and the Seventh Framework Programme European Commission (FP7-KBBE – Specific Programme ‘Cooperation’: Food, Agriculture and Biotechnology) project CoCoNET – ‘Towards Coast to Coast Networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential’ (contract number 287844).
Acknowledgements
We thank the captains, crew and scientific parties on board the RRVV Meteor, Urania, Belgica, Universitatis, Marion Dufresne and Celtic Explorer for collaboration at sea during the following oceanographic cruises: M70-1, CORSARO, MEDCOR 2009 and Belgica 2012/16 (‘CWC Moira Mounds’, FP7/2007-2013 EuroFLEETS under grant agreement number 228344). Marco Taviani further acknowledges the programmes EU FP6 Hermes (GOCE-CT-2005-511234-1) and EU FP7 Hermione (grant agreement number 226354). We are particularly grateful to Stephen Cairns (NMNH) and Aude Andouche (MNHN) for loaning coral samples from the Smithsonian National Museum of Natural History and the Museum national d’Histoire naturelle respectively, and Virginia Polonio (Control Union UK Ltd) and Javier Cristobo (Instituto Español de Oceanografía) for providing samples from the South Pacific Ocean (Expeditions SS02/2007, TAN 0803) and South Atlantic Ocean (Patagonia 0209). We acknowledge Francesca Benzoni (KAUST), Roberto Arrigoni (SZN), Eric Dutrieux (CREOCEAN), Claude-Henri Chaineau (Total SA), Robert Hirst and M. Abdul Aziz (YLNG) for tissue from and identification of samples collected in Yemen. We are grateful to Ana Navarro Campoy for assistance with amplifying the ITS sequences of the coral species Stephanocyathus diadema (Moseley, 1876), Caryophyllia diomedeae Marenzeller, 1904, Trochocayhtus aithospetatum Cairns, 1984, Dipsastraea matthaii (Vaughan, 1918) and D. pallida (Dana, 1846). We are grateful to Miguel Ángel Alonso-Zarazaga and Agostina Vertino for insightful discussions on coral taxonomy and the rules of the ICZN, and the two anonymous reviewers for helpful comments and suggestions that improved the quality of the manuscript. This is the Ismar-CNR, Bologna scientific contribution number 1887.
References
Addamo AM, Reimer JD, Taviani M, Freiwald A, Machordom A (2012) Desmophyllum dianthus (Esper, 1794) in the scleractinian phylogeny and its intraspecific diversity. PLoS ONE 7, e50215.
| Crossref | Google Scholar | PubMed |
Addamo AM, Vertino A, Stolarski J, García-Jiménez R, Taviani M, Machordom A (2016) Merging scleractinian genera: the overwhelming genetic similarity between solitary Desmophyllum and colonial Lophelia. BMC Evolutionary Biology 16, 108.
| Crossref | Google Scholar | PubMed |
Arrigoni R, Berumen ML, Mariappan KG, Beck PSA, Hulver AM, Montano S, Pichon M, Strona G, Terraneo TI, Benzoni F (2020) Towards a rigorous species delimitation framework for scleractinian corals based on RAD sequencing: the case study of Leptastrea from the Indo-Pacific. Coral Reefs 39, 1001-1025.
| Crossref | Google Scholar |
Arrigoni R, Huang D, Berumen ML, Budd AF, Montano S, Richards ZT, Terraneo TI, Benzoni F (2021) Integrative systematics of the scleractinian coral genera Caulastraea, Erythrastrea and Oulophyllia. Zoologica Scripta 50, 509-527.
| Crossref | Google Scholar |
Ballesteros E (1998) Addicions a la fauna d’invertebrats bentonics marins de l’Arxipelag de Cabrera (Illes Balears, Mediterrania Occidental). Bolletí de la Societat Història Natural de les Balears 41, 41-8 [In French].
| Google Scholar |
Beger M, Selkoe KA, Treml E, Barber PH, von der Heyden S, Crandall ED, Toonen RJ, Riginos C (2014) Evolving coral reef conservation with genetic information. Bulletin of Marine Science 90, 159-185.
| Crossref | Google Scholar |
Benzoni F, Arrigoni R, Stefani F, Pichon M (2011) Phylogeny of the coral genus Plesiastrea (Cnidaria, Scleractinia). Contributions to Zoology 80, 231-249.
| Crossref | Google Scholar |
Benzoni F, Arrigoni R, Stefani F, Reijnen BT, Montano S, Hoeksema BW (2012) Phylogenetic position and taxonomy of Cycloseris explanulata and C. wellsi (Scleractinia: Fungiidae): lost mushroom corals find their way home. Contributions to Zoology 81, 125-146.
| Crossref | Google Scholar |
Benzoni F, Arrigoni R, Berumen ML, Taviani M, Bongaerts P, Frade PR (2018) Morphological and genetic divergence between Mediterranean and Caribbean populations of Madracis pharensis (Heller 1868) (Scleractinia, Pocilloporidae): too much for one species? Zootaxa 4471, 473-492.
| Crossref | Google Scholar | PubMed |
Bhattacharya D, Agrawal S, Aranda M, Baumgarten S, Belcaid M, Drake JL, Erwin D, Foret S, Gates RD, Gruber DF, Kamel B, Lesser MP, Levy O, Liew YJ, MacManes M, Mass T, Medina M, Mehr S, Meyer E, Price DC, Putnam HM, Qiu H, Shinzato C, Shoguchi E, Stokes AJ, Tambutté S, Tchernov D, Voolstra CR, Wagner N, Walker CW, Weber APM, Weis V, Zelzion E, Zoccola D, Falkowski PG (2016) Comparative genomics explains the evolutionary success of reef-forming corals. eLife 5, e13288.
| Crossref | Google Scholar | PubMed |
Bitar G, Zibrowius H (1997) Scleractinian corals from Lebanon, eastern Mediterranean, including a non-lessepsian invading species (Cnidaria: Scleractinia). Scientia Marina 61, 227-31.
| Google Scholar |
Boudouresque C-F (1994) Les espèces introduites dans les eaux côtières d’Europe et de Méditerranée: État de la question et conséquences. In ‘Introduced Species in European Coastal Waters’. (Eds C-F Boudouresque, F Briand, C Nolan) Ecosystems Research Report 8, pp. 8–27. (European Commission/CIESM: Luxembourg) [In French]
Bucklin A, Steinke D, Blanco-Bercial L (2011) DNA barcoding of marine Metazoa. Annual Review of Marine Science 3, 471-508.
| Crossref | Google Scholar | PubMed |
Budd AF, Fukami H, Smith ND, Knowlton N (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia) (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society 166(3), 465-529.
| Crossref | Google Scholar |
Cairns S, Kitahara M (2012) An illustrated key to the genera and subgenera of the recent azooxanthellate Scleractinia (Cnidaria, Anthozoa), with an attached glossary. Zookeys 227, 1-47.
| Crossref | Google Scholar |
Cairns S, Hoeksema BW, van der Land J (2001) Scleractinia. In ‘European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification’. (Eds MJ Costello, C Emblow, R White) pp. 109–110. (Publications Scientifiques du Muséum National d’Histoire Naturelle: Paris, France)
Cairns S, Cordeiro RTS, Xu Y, Zhan Z, Alderslade P (2021) A new family and two new genera of calcaxonian octocoral, including a redescription of Pleurogorgia militaris (Cnidaria: Octocorallia: Chrysogorgiidae) and its placement in a new genus. Invertebrate Systematics 35, 282-297.
| Crossref | Google Scholar |
Campoy AN, Addamo AM, Machordom A, Meade A, Rivadeneira MM, Hernández CE, Venditti C (2020) The origin and correlated evolution of symbiosis and coloniality in scleractinian corals. Frontiers in Marine Science 7, 461.
| Crossref | Google Scholar |
Capel KCC, López C, Moltó-Martín I, Zilberberg C, Creed JC, Knapp ISS, Hernández M, Forsman ZH, Toonen RJ, Kitahara MV (2020) Atlantia, a new genus of Dendrophylliidae (Cnidaria, Anthozoa, Scleractinia) from the eastern Atlantic. PeerJ 8, e8633.
| Crossref | Google Scholar | PubMed |
Capel KCC, Zilberberg C, Carpes RM, Morrison CL, Vaga CF, Quattrini AM, Queck RZB, Huang D, Carins SD, Kitahara MV (2024) How long have we been mistaken? Multi-tools shedding light into the systematics of the widespread deep-water genus Madrepora Linnaeus, 1758 (Scleractinia). Molecular Phylogentics and Evoluation 191, 107994.
| Crossref | Google Scholar |
Casado-Amezúa P, García-Jiménez R, Kersting DK, Templado J, Coffroth MA, Merino P, Acevedo I, Machordom A (2011) Development of microsatellite markers as a molecular tool for conservation studies of the Mediterranean reef builder coral Cladocora caespitosa (Anthozoa, Scleractinia). Journal of Heredity 102(5), 622-626.
| Crossref | Google Scholar | PubMed |
Çinar ME, Bilecenoglu M, Öztürk B, Can A (2006) New records of alien species on the Levantine coast of Turkey. Aquatic Invasions 1, 84-90.
| Crossref | Google Scholar |
Cowman PF, Quattrini AM, Bridge TCL, Watkins-Colwell GJ, Fadli N, Grinblat M, Roberts TE, McFadden CS, Miller DJ, Baird AH (2020) An enhanced target-enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Molecular Phylogenetics and Evolution 153, 106944.
| Crossref | Google Scholar | PubMed |
Cuif JP, Lecointre G, Perrin C, Tillier A, Tillier S (2003) Patterns of septal biomineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework. Zoologica Scripta 32(5), 459-473.
| Crossref | Google Scholar |
Cutajar M, Evans J, Borg JA, Abela H, Schembri PJ (2020) Distribution, abundance and colony size of the invasive coral Oculina patagonica de Angelis, 1908 (Cnidaria, Scleractinia) in Malta. BioInvasions Records 9, 737-744.
| Crossref | Google Scholar |
Cvitković I, Despalatović M, Nikolić V, Žuljević A (2013) The first record of Oculina patagonica (Cnidaria, Scleractinia) in the Adriatic Sea. Acta Adriatica 54, 87-92.
| Google Scholar |
De Angelis D’Ossat G (1908) Altri Zoantari del Terziario della Patagonia. Anales del Museo Nacional de Buenos Aires, Serie 3 9, 93-102 [In Spanish].
| Google Scholar |
Dugornay O (2019) Colonie de corail blanc (Madrepora oculata). (Ifremer) Available at https://image.ifremer.fr/data/00615/72696/ [In French]
Ehrenberg CG (1834) Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen, und besonders des rothen Meeres, nebst einem Versuche zur physiologischen Systematik derselben. Abhandlungen der Königlichen Akademie der Wissenschaften, Berlin 1, 225-380 [In German].
| Google Scholar |
El Kateb A, Stalder C, Neururer C, Pisapia C, Spezzaferri S (2016) Correlation between pollution and decline of Scleractinian Cladocora caespitosa (Linnaeus, 1758) in the Gulf of Gabes. Heliyon 2(11), e00195.
| Crossref | Google Scholar | PubMed |
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783-791.
| Crossref | Google Scholar | PubMed |
Fine M, Loya Y (1995) The coral Oculina patagonica: a new immigrant to the Mediterranean coast of Israel. Israel Journal of Zoology 41, 81.
| Google Scholar |
Fine M, Zibrowius H, Loya Y (2001) Oculina patagonica: a non-lessepsian scleractininan coral invading the Mediterranean Sea. Marine Biology 138, 1195-1203.
| Crossref | Google Scholar |
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology Biotechnology 3(5), 294-299.
| Google Scholar | PubMed |
Fukami H, Budd AF, Paulay G, Solé-Cava A, Chen CA, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427, 832-835.
| Crossref | Google Scholar | PubMed |
Fukami H, Chen C, Budd A, Collins A, Wallace C, Chuang Y, Chen C, Dai C, Iwao K, Sheppard C, Knowlton N (2008) Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS ONE 3, e3222.
| Crossref | Google Scholar | PubMed |
Gittenberger A, Reijnen BT, Hoekseama BW (2011) A molecularly based phylogeny reconstruction of mushroom corals (Scleractinia: Fungiidae) with taxonomic consequences and evolutionary implications for life history traits. Contributions to Zoology 80, 107-132.
| Crossref | Google Scholar |
Gray JE (1847) XV.—An outline of an arrangement of stony corals. Annals and Magazine of Natural History 19, 120-128.
| Crossref | Google Scholar |
Guindon S, Gascuel O (2003) A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52, 696-704.
| Crossref | Google Scholar | PubMed |
Hoeksema BW, Cairns S (2023a) Scleractinia incertae sedis. In ‘World Register of Marine Species: World List of Scleractinia’. Available at https://marinespecies.org/aphia.php?p=taxdetails&id=266986
Hoeksema BW, Cairns S (2023b) Oculinidae Gray, 1847. In ‘World Register of Marine Species: World List of Scleractinia’. Available at https://www.marinespecies.org/scleractinia/aphia.php?p=taxdetails&id=135078
Hoeksema BW, Cairns S (2023c) Cladocoridae Milne Edwards & Haime, 1857. In ‘World Register of Marine Species: World List of Scleractinia’. Available at https://www.marinespecies.org/aphia.php?p=taxdetails&id=1579839
Hoeksema BW, Ocaña Vicente O (2014) First record of the central Indo-Pacific reef coral Oulastrea crispata in the Mediterranean Sea. Mediterranean Marine Science 15, 429-436.
| Crossref | Google Scholar |
Huang D, Meier R, Todd P, Chou L (2008) Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. Journal of Molecular Evolution 66, 167-174.
| Crossref | Google Scholar | PubMed |
Hugall A, Stanton J, Moritz C (1999) Reticulate evolution and origins of ribosomal internal transcribed spacer diversity in apomictic. Molecular Biology and Evolution 14(1), 40-48.
| Crossref | Google Scholar |
Izquierdo A, Loya A, Diaz-Valdez M, Ramos-Espla AA (2007) Non-indigenous species at the Alicante Harbour (SE-Spain): Oculina patagonica de Angelis, 1908 and Botrycapulus aculeatus (Gmelin, 1791). Rapports Commission Internationale Pour l’Exploration Scientifique de la Mer Méditerranée 38, 506.
| Google Scholar |
Juszkiewicz DJ, White NE, Stolarski J, Benzoni F, Arrigoni R, Hoeksema BW, Wilson NG, Bunce M, Richards ZT (2022) Phylogeography of recent Plesiastrea (Scleractinia: Plesiastreidae) based on an integrated taxonomic approach. Molecular Phylogenetics and Evolution 172, 107469.
| Crossref | Google Scholar | PubMed |
Katsanevakis S, Bogucarskis K, Gatto F, Vandekerkhove J, Deriu I, Cardoso AC (2012) Building the European Alien Species Information Network (EASIN): a novel approach for the exploration of distributed alien species data. BioInvasions Records 1, 235-245.
| Crossref | Google Scholar |
Kersting DK, Linares C (2012) Cladocora caespitosa bioconstructions in the Columbretes Islands Marine Reserve (Spain, NW Mediterranean): distribution, size structure and growth. Marine Ecology 33(4), 427-436.
| Crossref | Google Scholar |
Kersting DK, Bensoussan N, Linares C (2013a) Long-term responses of the endemic reef-builder Cladocora caespitosa to Mediterranean warming. PLoS ONE 8, e70820.
| Crossref | Google Scholar | PubMed |
Kersting DK, Casado C, López-Legentil S, Linares C (2013b) Unexpected patterns in the sexual reproduction of the Mediterranean scleractinian coral Cladocora caespitosa. Marine Ecology Progress Series 486, 165-171.
| Google Scholar |
Kersting D, Casado de Amezua P, Goffredo S (2022) Mediterranean pillow coral Cladocora caespitosa. In ‘The IUCN Red List of Threatened Species 2022’. e.T133142A75872554. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/133142/165739749
Kitahara MV, Cairns SD, Stolarski J, Blair D, Miller D (2010) A comprehensive phylogenetic analysis of the Scleractinia (Cnidaria, Anthozoa) based on mitochondrial CO1 sequence data. PLoS ONE 5, e11490.
| Crossref | Google Scholar | PubMed |
Kitahara MV, Capel KCC, Migotto AE (2020) Coenocyathus sebroecki sp. nov.: a new azooxanthellate coral (Scleractinia, Caryophylliidae) from southeastern Brazil. Marine Biodiversity 50, 59.
| Crossref | Google Scholar |
Krishna Krishnamurthy P, Francis R (2012) A critical review on the utility of DNA barcoding in biodiversity conservation. Biodiversity and Conservation 21, 1901-1919.
| Crossref | Google Scholar |
Kružić P, Benković L (2008) Bioconstructional features of the coral Cladocora caespitosa (Anthozoa, Scleractinia) in the Adriatic Sea (Croatia). Marine Ecology 29(1), 125-139.
| Crossref | Google Scholar |
Lefort V, Longueville J-E, Gascuel O (2017) SMS: smart model selection in PhyML. Molecular Biology and Evolution 34, 2422-2424.
| Crossref | Google Scholar | PubMed |
Le Goff-Vitry MC, Rogers AD, Baglow D (2004) A deep-sea slant on the molecular phylogeny of the Scleractinia. Molecular Phylogenetics and Evolution 30, 167-177.
| Crossref | Google Scholar | PubMed |
Leydet KP, Hellberg ME (2015) The invasive coral Oculina patagonica has not been recently introduced to the Mediterranean from the western Atlantic. BMC Evolutionary Biology 15, 79.
| Crossref | Google Scholar | PubMed |
Lin MF, Kitahara MV, Tachikawa H, Fukami H, Miller DJ, Chen CA (2012) Novel organization of the mitochondrial genome in the deep-sea coral, Madrepora oculata (Hexacorallia, Scleractinia, Oculinidae) and its taxonomic implications. Molecular Phylogenetics and Evolution 65, 323-328.
| Crossref | Google Scholar | PubMed |
López C, Clemente S, Moreno S, Ocaña O, Herrera R, Moro L, Monterroso O, Rodríguez A, Brito A (2019) Invasive Tubastraea spp. and Oculina patagonica and other introduced scleractinians corals in the Santa Cruz de Tenerife (Canary Islands) harbor: Ecology and potential risks. Regional Studies in Marine Science 29, 100713.
| Crossref | Google Scholar |
López-Márquez V, Cushman SA, Templado J, Wan HY, Bothwell HM, Kruschel C, Mačić V, Machordom A (2019) Seascape genetics and connectivity modelling for an endangered Mediterranean coral in the northern Ionian and Adriatic seas. Landscape Ecology 34, 2649-2668.
| Crossref | Google Scholar |
López-Márquez V, Lozano-Martín C, Hadjioannou L, Acevedo I, Templado J, Jimenez C, Taviani M, Machordom A (2021) Asexual reproduction in bad times? The case of Cladocora caespitosa in the eastern Mediterranean Sea. Coral Reefs 40, 663-677.
| Crossref | Google Scholar | PubMed |
Mačić V, Đorđević N, Petović S (2019) First monitoring of Cladocora caespitosa (Anthozoa, Scleractinia) in the Boka Kotorska Bay (Montenegro). Studia Marina 32(1), 26-32.
| Crossref | Google Scholar |
Mao Y, Economo EP, Satoh N (2018) The roles of introgression and climate change in the rise to dominance of Acropora corals. Current Biology 28, 3373-3382.
| Crossref | Google Scholar | PubMed |
Martinez S, Bellworthy J, Ferrier-Pagès C, Mass T (2021) Selection of mesophotic habitats by Oculina patagonica in the Eastern Mediterranean Sea following global warming. Scientific Reports 11, 18134.
| Crossref | Google Scholar | PubMed |
McFadden SC, Quattrini AM, Brugler MR, Cowman PF, Dueñas LF, Kitahara MV, Paz-García DA, Reimer JD, Rodríguez E (2021) Phylogenomics, origin, and diversification of Anthozoans (Phylum Cnidaria). Systematic Biology 70, 635-647.
| Crossref | Google Scholar | PubMed |
Meyer CP, Paulay G (2005) DNA barcoding: error rates based on comprehensive sampling. PLoS Biology 3, e422.
| Crossref | Google Scholar | PubMed |
Nosenko T, Schreiber F, Adamska M, Adamski M, Eitel M, Hammel J, Maldonado M, Müller WE, Nickel M, Schierwater B, Vacelet J, Wiens M, Wörheide G (2013) Deep metazoan phylogeny: when different genes tell different stories. Molecular Phylogenetics and Evolution 67, 223-233.
| Crossref | Google Scholar | PubMed |
Núñez-Flores M, Uchida-Gomez D, López-González PJ (2021) Molecular systematics of Thouarella (Octocorallia: Primnoidae) with the description of three new species from the Southern Ocean based on combined molecular and morphological evidence. Invertebrate Systematics 35, 655-674.
| Crossref | Google Scholar |
Oku Y, Iwao K, Hoeksema BW, Dewa N, Tachikawa H, Koido T, Fukami H (2020) Fungia fungites (Linnaeus, 1758) (Scleractinia, Fungiidae) is a species complex that conceals large phenotypic variation and a previously unrecognized genus. Contributions to Zoology 89, 188-209.
| Crossref | Google Scholar |
Okubo N (2016) Restructuring the traditional suborders in the order Scleractinia based on embryogenetic morphological characteristics. Zoological Science 33(1), 116-123.
| Crossref | Google Scholar | PubMed |
Peirano A, Morri C, Mastronuzzi G, Bianchi CN (1998) The coral Cladocora caespitosa (Anthozoa, Scleractinia) as a bioherm builder in the Mediterranean Sea. Memorie Descrittive della Carta Geologica d’Italia 52, 59-74.
| Google Scholar |
Peirano A, Morri C, Bianchi CN (1999) Skeleton growth and density pattern of the temperate, zooxanthellate scleractinian Cladocora caespitosa from the Ligurian Sea (NW Mediterranean). Marine Ecology Progress Series 185, 195-201.
| Crossref | Google Scholar |
Peirano A, Morri C, Bianchi CN, Aguirre J, Antonioli F, Calzetta G, Carobene L, Matronuzzi G, Orrù P (2004) The Mediterranean coral Cladocora caespitosa: a proxy for past climate fluctuations? Global Planetary Change 40, 195-200.
| Crossref | Google Scholar |
Pons-Fita A, Verdura J, Santamaría J, Kersting DK, Ballesteros E (2020) Coexistence of the reef-building coral Cladocora caespitosa and the canopy forming alga Treptacantha ballesterosii: description of a new Mediterranean habitat. Scientia Marina 84, 263-271.
| Crossref | Google Scholar |
Quattrini AM, Faircloth BC, Dueñas LF, Bridge TCL, Brugler MR, Calixto-Botía IF, DeLeo DM, Forêt S, Herrera S, Lee SMY, Miller DJ, Prada C, Rádis-Baptista G, Ramírez-Portilla C, Sánchez JA, Rodríguez E, McFadden CS (2018) Universal target-enrichment baits for anthozoan (Cnidaria) phylogenomics: new approaches to long-standing problems. Molecular Ecology Resources 18, 281-295.
| Crossref | Google Scholar | PubMed |
Quek RZB, Huang D (2022) Application of phylogenomic tools to unravel anthozoan evolution. Coral Reefs 41, 475-495.
| Crossref | Google Scholar |
Quek RZB, Jain SS, Neo ML, Rouse GW, Huang D (2020) Transcriptome-based target-enrichment baits for stony corals (Cnidaria: Anthozoa: Scleractinia). Molecular Ecology Resources 20(3), 807-818.
| Crossref | Google Scholar |
Ramírez-Portilla C, Baird AH, Cowman PF, Quattrini AM, Harii S, Sinniger F, Flot J-F (2022) Solving the coral species delimitation conundrum. Systematic Biology 71(2), 461-475.
| Crossref | Google Scholar | PubMed |
Repullés M, López-Márquez V, Templado J, Taviani M, Machordom A (2022) Genetic structure of the endangered coral Cladocora caespitosa matches the main bioregions of the Mediterranean Sea. Frontiers in Genetics 13, 889672.
| Crossref | Google Scholar | PubMed |
Rodolfo-Metalpa R, Richard C, Allemand D, Ferrier-Pagès C (2006) Growth and photosynthesis of two Mediterranean corals, Cladocora caespitosa and Oculina patagonica, under normal and elevated temperatures. Journal of Experimental Biology 209(22), 4546-4556.
| Crossref | Google Scholar | PubMed |
Romano S, Cairns S (2000) Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bulletin of Marine Science 67, 1043-1068.
| Google Scholar |
Romano S, Palumbi S (1996) Evolution of scleractinian corals inferred from molecular systematics. Science 271, 640-642.
| Crossref | Google Scholar |
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572-1574.
| Crossref | Google Scholar | PubMed |
Rubio-Portillo E, Souza-Egipsy V, Ascaso C, de los Rios Murillo A, Ramos-Esplá AA, Antón J (2014a) Eukarya associated with the stony coral Oculina patagonica from the Mediterranean Sea. Marine Genomics 17, 17-23.
| Crossref | Google Scholar |
Rubio-Portillo E, Vázquez-Luis M, Izquierdo Muñoz A, Ramos Esplá AA (2014b) Distribution patterns of alien coral Oculina patagonica De Angelis D’Ossat, 1908 in western Mediterranean Sea. Journal of Sea Research 85, 372-378.
| Crossref | Google Scholar |
Rubio-Portillo E, Kersting DK, Linares C, Ramos-Esplá AA, Antón J (2018) Biogeographic differences in the microbiome and pathobiome of the coral Cladocora caespitosa in the Western Mediterranean Sea. Frontiers in Microbiology 9, 22.
| Crossref | Google Scholar | PubMed |
Salomidi M, Katsanevakis S, Issaris Y, Tsiamis K, Katsiaras N (2013) Anthropogenic disturbance of coastal habitats promotes the spread of the introduced scleractinian coral Oculina patagonica in the Mediterranean Sea. Biological Invasions 15, 1961-1971.
| Crossref | Google Scholar |
Sartoretto S, Harmelin J-G, Bachet F, Bejaoui N, Lebrun O, Zibrowius H (2008) The alien coral Oculina patagonica De Angelis, 1908 (Cnidaria, Scleractinia) in Algeria and Tunisia. Aquatic Invasions 3, 173-180.
| Crossref | Google Scholar |
Schwindt E, López Gappa J, Raffo MP, Tatián M, Bortolus A, Orensanz JM, Alonso G, Diez ME, Doti B, Genzano G, Lagger C, Lovrich G, Priz ML, Mendez MM, Savoya V, Sueiro MC (2014) Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Marine Environmental Research 99, 60-68.
| Crossref | Google Scholar | PubMed |
Seiblitz IGL, Vaga CF, Capel KCC, Cairns SD, Stolarski J, Quattrini AM, Kitahara MV (2022) Caryophylliids (Anthozoa, Scleractinia) and mitochondrial gene order: insights from mitochondrial and nuclear phylogenomics. Molecular Phylogenetics and Evolution 175, 107565.
| Crossref | Google Scholar | PubMed |
Serrano E, Coma R, Ribes M, Weitzmann B, García M, Ballesteros E (2013) Rapid northward spread of a zooxanthellate coral enhanced by artificial structures and sea warming in the Western Mediterranean. PLoS ONE 8, e52739.
| Crossref | Google Scholar | PubMed |
Shearer TL, Coffroth MA (2008) DNA BARCODING. Barcoding corals: limited by interspecific divergence, not intraspecific variation. Molecular Ecology Resources 8, 247-255.
| Crossref | Google Scholar | PubMed |
Stolarski J, Kitahara MV, Miller DJ, Cairns SD, Mazur M, Meibom A (2011) The ancient evolutionary origins of Scleractinia revealed by azooxanthellate corals. BMC Evolutionary Biology 11, 316.
| Crossref | Google Scholar | PubMed |
Terraneo TI, Benzoni F, Baird AH, Arrigoni R, Berumen ML (2019) Morphology and molecules reveal two new species of Porites (Scleractinia, Poritidae) from the Red Sea and the Gulf of Aden. Systematics and Biodiversity 17, 491-508.
| Crossref | Google Scholar |
Terraneo TI, Benzoni F, Arrigoni R, Baird AH, Mariappen KG, Forsman ZH, Wooster MK, Bouwmeester J, Marshell A, Berumen ML (2021) Phylogenomics of Porites from the Arabian Peninsula. Molecular Phylogenetics and Evolution 161, 107173.
| Crossref | Google Scholar | PubMed |
Terrón-Sigler A, Casado-Amezúa P, Torre F (2015) Abundance and distribution of the rapid expansive coral Oculina patagonica in the Northern Alborán Sea (Western Mediterranean). Marine Biodiversity Records 8, e45.
| Crossref | Google Scholar |
Thompson JD, Gibson TJ, Plewniak F, Jeanmoughin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24, 4876-4882.
| Crossref | Google Scholar | PubMed |
Untiedt CB, Quattrini AM, McFadden CS, Alderslade PA, Pante E, Burridge CP (2021) Phylogenetic relationships within Chrysogorgia (Alcyonacea: Octocorallia), a morphologically diverse genus of octocoral, revealed using a target enrichment approach. Frontiers in Marine Science 7, 599984.
| Crossref | Google Scholar |
Vaughan TW, Wells JW (1943) Revision of the suborders, families and genera of the Scleractinia. Special Papers of the Geological Society of America 44, 1-363.
| Google Scholar |
Veron JEN, Stafford-Smith MG, Turak E, DeVantier LM (2016a) Oculina patagonica de Angelis, 1908. In ‘Corals of the World’. Version 0.01 (Beta). Available at http://www.coralsoftheworld.org/species_factsheets/species_factsheet_summary/oculina-patagonica/ [Verified 16 April 2024]
Veron JEN, Stafford-Smith MG, Turak E, DeVantier LM (2016b) Cladocora caespitosa. In ‘Corals of the World’. Version 0.01 (Beta). Available at http://www.coralsoftheworld.org/species_factsheets/species_factsheet_summary/cladocora-caespitosa/ [Verified 16 April 2024]
Verrill AE (1901) Variations and nomenclature of Bermudian, West Indian and Brazilian reef corals, with notes on various Indo-Pacific corals. Transactions of the Connecticut Academy of Arts and Sciences 11, 63-168.
| Google Scholar |
Zaquin T, Zaslansky P, Pinkas I, Mass T (2019) Simulating bleaching: long-term adaptation to the dark reveals phenotypic plasticity of the Mediterranean Sea coral Oculina patagonica. Frontiers in Marine Science 6, 662.
| Crossref | Google Scholar |
Zibrowius H (1974) Oculina patagonica, scléractiniaire hermatypique introduit en Méditerranée. Helgoländer Meeresuntersuchungen 26, 153-173 [In French with English abstract].
| Crossref | Google Scholar |
Zibrowius H (1980) Les scléractiniaires de la Méditerranée et de l’Atlantique nord-oriental. Mémoires de l’Institut océanographique (Monaco) 11, 1-284 [In French].
| Google Scholar |
Zlatarski V, Stake J (2012) The scleractinian corals: a perspective. Geologica Belgica 15, 370-375.
| Google Scholar |


