Integrative taxonomy of the stick insect genus Austrocarausius Brock, 2000 (Phasmatodea: Lonchodidae) reveals cryptic species in remnant Queensland rainforests
Braxton R. Jones A * , Paul D. Brock B , Barbara Mantovani C , Perry Beasley-Hall A D E , David K. Yeates F and Nathan Lo A
A * , Paul D. Brock B , Barbara Mantovani C , Perry Beasley-Hall A D E , David K. Yeates F and Nathan Lo A
A School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW 2006, Australia.
B The Natural History Museum, Cromwell Road, London, SW7 5BD, UK.
C Department of Biological, Geological and Environmental Sciences, University of Bologna, I-40126 Bologna, Italy.
D School of Biological Sciences, The University of Adelaide, Adelaide, SA 5000, Australia.
E South Australian Museum, Adelaide, SA 5000, Australia.
F Australian National Insect Collection, CSIRO PO Box 1700, Canberra, ACT 2601, Australia.
Invertebrate Systematics 36(9) 849-873 https://doi.org/10.1071/IS21076
Submitted: 12 November 2021 Accepted: 28 July 2022 Published: 21 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Austrocarausius Brock, 2000 is a stick insect (Phasmatodea: Lonchodidae) genus containing two species restricted to the tropical rainforests of northern Queensland. Recent specimen collections between the two species’ type localities, Lizard Island and Rockhampton, have suggested that Austrocarausius might represent more than the two nominal species. Here, we apply morphological and molecular analyses to revise the taxonomy of this genus. Using both field-collected and historic museum samples, we developed morphological species hypotheses and descriptions. Genetic sequencing of mitochondrial COI and 16S were undertaken for species delimitation and phylogenetic analysis, including an estimate of the evolutionary timescale of the genus. Based on these results, we propose nine new Austrocarausius species, increasing the number of species in the genus to eleven: A. nigropunctatus (Kirby, 1896), A. mercurius (Stål, 1877), A. coronatus sp. nov., A. decorus sp. nov., A. eirmosus sp. nov., A. gasterbulla sp. nov., A. tuberosus sp. nov., A. macropunctatus sp. nov., A. truncatus sp. nov. A. waiben sp. nov. and A. walkeri sp. nov. Our results suggest Austrocarausius species diversified over the last c. 25–70 Ma, resulting in the now endemic distributions in the tropical rainforests of the central and northern Queensland coasts. This is the first integrative systematic study of an Australian phasmid genus, combining morphological, molecular and biogeographical methods. Additional species of Austrocarausius likely remain undescribed as can be inferred from methodical sampling of rainforest patches along the Queensland coast.
Keywords: biogeography, dispersal, integrative taxonomy, molecular dating, morphological analysis, phylogenetic, systematics, taxonomy.
Introduction
The Lonchodidae is the largest family of Phasmatodea (stick insects), comprising two subfamilies, four tribes and approximately 1200 species that represent 35% of known phasmids (P. D. Brock, T. Büscher and E. Baker, Phasmida Species File Online, ver. 5.0/5.0, see http://phasmida.speciesfile.org/HomePage/Phasmida/HomePage.aspx). These insects are typically slender and have long antennae, and are comparatively small to medium in size, with bodies ranging from 5 to 15 cm long (Robertson et al. 2018). Lonchodids can largely be found throughout Asia and Australasia. The family Lonchodidae has been proposed to accommodate the two subfamilies Lonchodinae, all the members of which are wingless and the often-winged Necrosciinae (Robertson et al. 2018). Lonchodinae is found mainly in Asia, Australasia and the Seychelles (Cliquennois 2020).
The tribe Lonchodini comprises 39 genera of which three are endemic to Australia: Austrocarausius Brock, 2000; Denhama Werner, 1912; and Hyrtacus Stål, 1875. Austrocarausius was erected by Brock (2000) to accommodate Australian species from Carausius Stål, 1875 and Lonchodes Gray, 1835 that were Carausius-like but more elongate. The genus comprises wingless phasmids that have simple ‘stick-like’ body forms (Fig. 1a, b). Two species, A. mercurius (Stål, 1877) and A. nigropunctatus (Kirby, 1896), are currently known from the genus. These have very similar morphologies, differing only in some minor head and genitalic structures. These insects have been observed feeding on Eucalyptus, Arecaceae, Dendrocnide moroides and Acacia spp. as host plants (Brock and Hasenpusch 2009; P. D. Brock, pers. obs.). Austrocarausius mercurius and A. nigropunctatus are found in two very different regions of Queensland: the Rockhampton–Peak Downs area and the Lizard Island–Cooktown area respectively (Brock 2000; Brock et al., see http://phasmida.speciesfile.org/HomePage/Phasmida/HomePage.aspx). Several samples collected along the coast that separates these two regions (spanning ~1000 km) have been assigned to this genus, although species assignment has been difficult due to a lack of defining morphological characters (Brock and Hasenpusch 2009).
As these are all wingless, Austrocarausius species are expected to have poor dispersal ability. The distribution across an ~1000-km stretch of north-eastern Australia in patchy rainforest areas raises the possibility that this genus is more species-rich than currently considered. We focused this systematic study on Austrocarausius because of the likelihood of additional undescribed species occupying separate rainforest patches and the fact that many more specimens of the genus have accumulated in collections.
Integrative taxonomy is a process in which species are delimited and described using a combination of morphological, biogeographical and molecular data (Dayrat 2005; Yeates et al. 2010). More robust, biologically meaningful taxa can be delimited by employing multiple lines of evidence (Will et al. 2005; Yeates et al. 2010). In this study, we integrate morphological and evolutionary species concepts to assess the species diversity of Austrocarausius before revising the taxonomy of the genus.
We have undertaken detailed anatomical analysis of numerous specimens collected during extensive fieldwork and where possible, generated relevant molecular sequence data. Here, we also estimate the evolutionary timescale of Austrocarausius and use quantitative species delimitation methods to delimit putative undescribed species. In this study we identify and describe nine new species and provide egg descriptions, where available, to assist with species identification. Species keys are also provided to identify all species, focusing on diagnostic morphological characters.
Methods
Specimen collection
Specimens were collected from the field by Paul Brock, Braxton Jones, Jack Hasenpusch, Jerome Constant and Linda Semeraro or borrowed from museums (Supplementary Tables S1, S2). Specimens collected in Queensland by B. R. Jones were obtained under the permit number WITK18701717 and other ABRS collecting and export permits for PB. Specimens donated by J. Constant and L. Semeraro were financially supported under the Leopold III Funds project ‘Fulgoridae et Eurybrachidae d’Australie: (I) Taxa de la côte SE du Queensland’.
Eggs were collected from live insects and specimens mounted, with the right midleg stored in 100% ethanol for downstream molecular analysis. Specimens used in this paper can be found in the Queensland Museum, Brisbane, Australia under registration numbers T250900–T250927 and T258567–T258572 (Supplementary Table S2).
Abbreviations used
Australian National Insect Collection, Canberra, Australia (ANIC); Natural History Museum, London, UK (NHMUK); Naturhistorisches Museum Wien, Vienna, Austria (NHMW); Naturhistoriska Riksmuseet, Stockholm, Sweden (NHRS); Queensland Museum, Brisbane, Australia (QM); Zoologisches Institut und Zoologisches Museum, Universität von Hamburg, Hamburg, Germany (ZMUH).
Morphological analysis and imaging
Fifty specimens were examined, of which 25 were used for morphological measurements (Supplementary Table S1). Morphological analysis was performed by B. R. Jones. Images were taken with a Canon 7D mark I with a 100-mm macro lens. Images were processed in Adobe Photoshop 2020 (ver. 21.2.0) and, when needed, photos were stacked either in Adobe Photoshop 2020 (ver. 23.3, Adobe, see https://www.adobe.com/au/) or ZereneStacker (ver. 1.04, Zerene Systems, see http://zerenesystems.com/cms/home). Species measurements and diagnoses were performed prior to molecular analysis to avoid bias when inferring morphological groupings.
Molecular and species delimitation analysis
For all molecular and species delimitation analyses, DNA sequence data were obtained from 31 representatives from eight of the eleven species described in this study (ranging from one to seven individuals per species; Supplementary Table S1). DNA extraction and PCR were attempted on the remaining three species, however, the museum specimens were too degraded to allow successful PCR. Specimens used in molecular analyses were collected in the field by P. D. Brock, B. R. Jones, J. Constant and L. Semeraro. Among all sampled species, pairwise distance data were obtained for cytochrome oxidase subunit I (COI) (Supplementary Table S3).
DNA was extracted using a High Pure Roche Kit, following the manufacturer’s instructions. The primers LCO1490 (5′ GTCAACAAATCATAAAGATATTGG 3′) and HCO2198 (5′ TAAACTTCAGGGTGACCAAAAAATCA 3′) (Folmer et al. 1994) were used to amplify COI. For PCR of 16S ribosomal RNA we used LR-J-12961 (5′ TTACGCTGTTATCCTAA 3′) and LR-N-13398 (16SAR)F (5′ CACCTGTTTAACAAAAACAT 3′) (Simon et al. 1994). PCR amplification was performed in a BIO RAD MyCycler Thermal Cycler as follows (as per Velonà et al. 2015): initial denaturation at 94°C for 1 min, five cycles of 94°C for 30 s, annealing at 45°C for 40 s and extension at 72°C for 1 min, followed by 30 cycles of 94°C for 30 s, 51°C for 40 s and 72°C for 1 min, with a final extension at 72°C for 10 min. PCR products were sent to Macrogen (South Korea) for Sanger sequencing. Sequences were edited in Genious Prime (ver. 2020.2.2, see https://www.geneious.com/; Kearse et al. 2012) by aligning the raw forward and reverse reads. These were trimmed and reviewed for nucleotide ambiguities. Sequences were aligned with Muscle (ver. 3.2, see http://www.drive5.com/muscle/; Edgar 2004) and checked for correct reading frame in Seqotron (ver. 1.0.1, see https://github.com/4ment/seqotron; Fourment and Holmes 2016).
Mitochondrial COI and 16S were sampled from a total of 30 and 26 Austrocarausius individuals respectively. A total of 23 outgroup taxa were used to estimate the Austrocarausius timescale (COI from 12 individuals, 16S from 22 individuals; Supplementary Table S4, Supplementary File S1). Outgroup taxa were downloaded from GenBank where sequence data were available.
Phylogenetic trees were constructed using Bayesian inference and maximum likelihood, and partitioned by 16S and the 1st, 2nd and 3rd codon positions for COI. Bayesian inference analyses were performed in BEAST (ver. 2.6.3, see https://www.beast2.org/; Bouckaert et al. 2014). The bModelTest package (ver. 1.3.0, see https://github.com/BEAST2-Dev/bModelTest; Bouckaert and Drummond 2017) was used in BEAST to estimate substitution models, invariant sites and rate heterogeneity with default settings. Maximum likelihood analyses were performed using the IQ-TREE web server (ver. 1.6.12, see http://www.iqtree.org/; Trifinopoulos et al. 2016) with 1000 bootstrap replicates. The inbuilt ModelFinder (ver. 1, see http://www.iqtree.org/ModelFinder/) was used to specify models; 16S GTR+F+G4, COI 1st codon TIM2+F+G4, COI 1st codon HKY+F+I, COI 3rd codon TN+F+G4. Output for both trees were visualised in Figtree (ver. 1.4.4, see http://tree.bio.ed.ac.uk/software/figtree/, accessed 13 May 2021).
To infer the evolutionary timescale using Bayesian inference, crown groups were assigned representing five fossils, Eophyllium messelense at a minimum age of 47 Ma (Wedmann et al. 2007), Clonistria sp. at 20 Ma (Poinar 2011), Eophasma spp. at 44 Ma (Poinar 2011), Anisomorphini at 44 Ma (Sellick 1994) and Malacomorpha sp. at 20 Ma (Poinar 2011) (Supplementary Table S5). Fossil calibration nodes, including soft upper bounds of 110 Ma for each fossil were selected based on recent studies of Phasmatodea (Robertson et al. 2018; Simon et al. 2019). A lognormal relaxed clock model was selected for our analysis and we selected a birth-death tree beforehand. A lognormal prior distribution was selected for each fossil calibration. The resulting tree was checked for adequate sampling and convergence in TRACER (ver. 1.7, see https://github.com/beast-dev/tracer/releases/tag/v1.7; Rambaut et al. 2018). The maximum clade credibility tree was produced in TreeAnnotator (ver. 2.6.3, see https://www.beast2.org/treeannotator/; Bouckaert et al. 2014) with 10% burn-in.
Species delimitation was performed on the browser-based implementation of the Automatic Barcode Gap Discovery (ABGD) program (ver. 2.1, see https://bioinfo.mnhn.fr/abi/public/abgd/; Puillandre et al. 2012). Our alignment of COI was submitted under the JC69 model. The following settings were used: Pmin 0.001, Pmax 0.1, steps 10 and Nb bins 20. Generalised Mixed Yule Coalescent (GMYC) (Kapli et al. 2017) analyses were performed in RStudio (ver. 1.1.463, RStudio, Inc., Boston, MA, USA, see http://www.rstudio.com/) using the BEAST output tree file with the ‘splits’ R package (ver. 1.2, T. Ezard, T. Fujisawa and T. G. Barraclough, see https://rdrr.io/rforge/splits/) downloaded from R-Forge.
Data accessibility
Molecular data for this project can be found in GenBank under the following accession numbers. For COI: HM425710.1, HM425711.1, HM425716.1, HM425725.1–HM425728.1, KJ201995.1, KJ202015.1, KJ202026.1, KJ202027.1, ON077343–ON077348, ON101990–ON102002; and for 16S: OM992332–OM992357 (Supplementary Table S2).
Ethics and permits
All material was collected under permits with approved ethics, under the ABRS collecting and export permits for P. D. Brock, and in Queensland protected areas under the Entomological Society of Queensland Scientific Purpose Permit for National Parks and CYPAL WITK18701717-3 and State Forests WITF18701717 with Authority Holder Christine Lambkin for B. R. Jones.
Results
Phylogenetic analyses
Phylogenetic relationships among Austrocarausius spp. were congruent between our Bayesian inference and maximum likelihood trees (Fig. 2, Supplementary Fig. S1, S3). GMYC and ABGD analyses both divided the taxa examined into eight putative species (Fig. 2), in agreement with our morphological examinations (see Taxonomy section below).
Individuals from each putative species were typically found in a restricted area and displayed high endemism. In some cases, two sympatric species were found in one area, e.g. A. decorus and A. nigropunctatus from the Daintree rainforest, and A. macropunctatus and A. walkeri from the Whitsundays region.
The earliest divergence in the genus, occurring at c. 53.8 Ma (95% HPD 46.12–62.49 Ma), separates the group comprising A. waiben and A. nigropunctatus from the rest of the genus (Fig. 2); A. waiben and A. nigropunctatus diverged at c. 39.9 Ma (95% HPD 33.37–46.99 Ma). A second major divergence occurred at c. 48.6 Ma (95% HPD 41.56–56.77 Ma), separating A. decorus and A. coronatus from A. macropunctatus, A. eirmosus, A. walkeri and A. mercurius; A. decorus and A. coronatus diverged at c. 32.9 Ma (95% HPD 26.91–38.91 Ma). The latter represent the most southerly distributed species. Austrocarausius macropunctatus and A. eirmosus diverged from A. walkeri and A. mercurius at c. 41.3 Ma (95% HPD 35.05–48.53 Ma); A. macropunctatus and A. eirmosus diverged at c. 25.2 Ma (95% HPD 20.42–30.39 Ma), and A. walkeri and A. mercurius diverged at c. 35.5 Ma (95% HPD 29.72–41.94 Ma).
In our Bayesian analyses, all node support values had >0.95 posterior probability support (Fig. 2), except for the node uniting A. walkeri and A. mercurius (0.70 posterior probability). In our maximum likelihood analyses (Supplementary Fig. S2), the divergence between A. waiben, A. nigropunctatus, A. decorus, and A. coronatus (northern clade) and A. macropunctatus, A. eirmosus, A. walkeri and A. mercurius (southern clade) had >95% bootstrap and >95 ESS values.
Taxonomy
Family LONCHODIDAE Brunner, 1893
Subfamily LONCHODINAE Brunner, 1893
Tribe LONCHODINI Brunner, 1893
Genus Austrocarausius Brock
Description
Described in Brock (2000, p. 52).
Species included
Austrocarausius coronatus sp. nov. (northern Qld: Garradunga; ZooBank number: CC454399-B83A-4648-BA43-DE5B9311F5C5).
Austrocarausius decorus sp. nov. (far northern Qld: Cow Bay to Cape Tribulation; ZooBank number: 6E563988-FC74-4B69-BA2B-FB3FD14AC9CB).
Austrocarausius eirmosus sp. nov. (central Qld: Whitsunday Islands National Park; ZooBank number: 94FDB207-785C-46A1-BFBC-C0620D194111).
Austrocarausius gasterbulla sp. nov. (central Qld: Mt Macartney; ZooBank number: DA8F455B-9606-4861-8670-068DE9BEE5C6).
Austrocarausius macropunctatus sp. nov. (central Qld: Shute Harbour; ZooBank number: 4B75CBB7-DB6F-4C5F-BF60-6686008066FD).
Austrocarausius mercurius (Stål, 1877) (central Qld: Rockhampton to Byfield; ZooBank number: 7DE90F0E-3B91-4205-9598-5D19B55E3312).
Austrocarausius nigropunctatus (Kirby, 1896) (northern Qld: Cow Bay to Cooktown and Lizard Island; ZooBank number: FDBFAAF1-B21D-462A-A636-63A3387AD7C8).
Austrocarausius truncatus sp. nov. (far northern Qld: Iron Range; ZooBank number: CD533028-371A-4158-BDD2-8D2BA5F97D3C).
Austrocarausius tuberosus sp. nov. (far northern Qld: Mount Hemmant; ZooBank number: 09B01C1E-F81A-4D5D-9F7A-9EF99872F210).
Austrocarausius waiben sp. nov. (far northern Qld: Thursday Island; ZooBank number: B90F0632-863E-446A-9993-691E339555E6).
Austrocarausius walkeri sp. nov. (central Qld: Mount Dryander; ZooBank number: D8EB61F2-AD3A-4EB5-B997-DAAAC82D64E9).
Key to adult Austrocarausius femalesUnknown females: A. truncatus, A. tuberosus and A. walkeri.
|
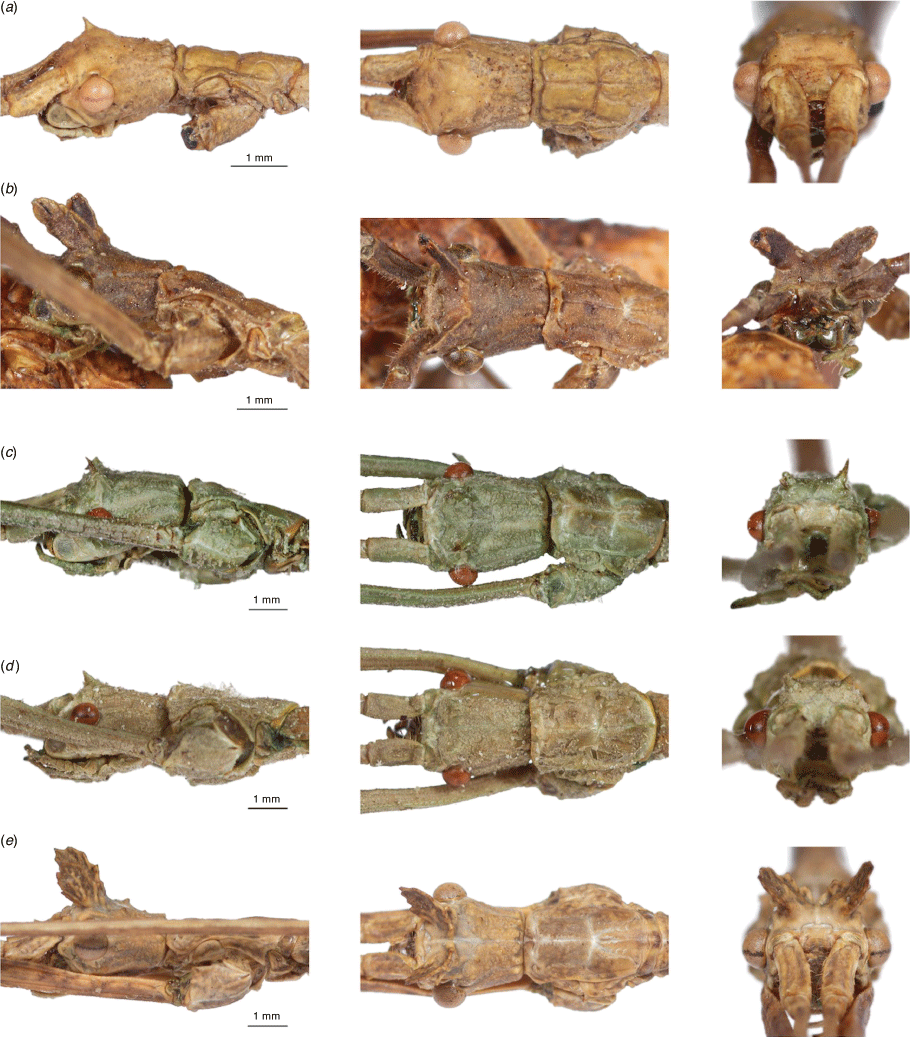
1 Disjointed black ventral stripe on pronotum, mesonotum and metanotum, pair of spines between eyes, dark coloured tubercles over entire body, short tumescence protruding at end of abdominal segments 1–6, white bands at end of each femora (Fig. 3f, 4f, 5f, Supplementary Fig. S3i, S4f) |
|
2 Large black dots surrounding tubercles on mesonotum, black- and brown-tipped tubercles over dorsal and lateral body surface, two black spines between eyes, white banding present at ends of all femora (Fig. 3e, 4e, 5e, Supplementary Fig. S3h, S4e) |
|
3 Lateral black stripe on head behind eyes, dorsal and lateral black-tipped tubercles across body, pair of spines between eyes (Fig. 3a, 4a, 5a, Supplementary Fig. S3b, S4a) |
|
4 Preopercular organ truncate, not extending into segment 8, uniform dorsal and lateral body tubercles, some black-tipped, medium-sized head protuberances between eyes, 6th abdominal segment swollen, most segments blotched with black markings, mostly on ventral surface of metanotum and abdomen, short tumescence protruding at end of each abdominal segment (Fig. 3d, 4d, 5d, Supplementary Fig. S3g, S4d) |
|
5 Pair of spines between eyes, black granulations across dorsal and ventral body surface |
|
6 Fore tibiae or tarsi with dorsal lobe protrusions, pair of large flange-like protuberances between eyes, three pairs of small spines behind these protuberances, each decreasing in size, black-tipped tubercles dorsally and laterally across body, ventral tubercles only on metanotum, dorsal bumps at the end of each abdominal segment (Fig. 3b, 4b, 5b, Supplementary Fig. S3e, S4b) |
|
7 Abdominal segment 6 not swollen, black-tipped tubercles dorsally and laterally across body, ventral surface of metanotum has black-tipped tubercles, large flange-like protuberance between eyes, preopercular organ spinelike with two pairs of appendage-like structures, dorsal bumps at the end of each abdominal segment (Fig. 3c, 4c, 5c, Supplementary Fig. S3f, S4c) |

|

|

|
|
1 Genital claspers truncate, approximately equal in length to 9th abdominal segment |
|
2 No dorsal bumps at the end of any abdominal segments |
|
3 Black-tipped tubercles on mesonotum and metanotum, sparser on metanotum, two spines between eyes, genital claspers rounded (Fig. 6d, 7d, 8d, Supplementary Fig. S3) |
|
4 Two spines between eyes, sparse lateral tubercles on mesonotum, short tumescence protruding at end of abdominal segments 1–7 (Fig. 7i, 9d, 10d, Supplementary Fig. S3i) |
|
5 Dark lateral dots on thorax, two spines between eyes (Fig. 7h, 9c, 10c, Supplementary Fig. S3h) |
|
6 Two large protuberances between eyes, body smooth throughout, no ornamentation (Fig. 7g, 9b, 10d, Supplementary Fig. S3g) |
|
7 No spines between eyes, genital claspers distinctly straight and sharply pointed at end (Fig. 6b, 7b, 8b, Supplementary Fig. S3b) |
|
8 Genital claspers rounded apically |
|
9 White bands on apices of all femora, pair of spines between eyes (Fig. 6a, 7a, 8a, Supplementary Fig. S3a) |
|
10 Genital claspers rounded but not duck bill-shaped, two spines between eyes (Fig. 7f, 9a, 10a, Supplementary Fig. S3f) |

|

|
|

|
|
1 Egg without lateral depressions |
|
2 Egg angular and elongate, lateral pointed bulge above micropylar plate, two large oval lateral depressions, capitulum stem thin (Fig. 11b) |
|
3 Egg surface is smooth with network of granulation in the centre, singular ridge running along the outside of the egg on the lateral side, starting and ending at the operculum in a ‘U’-like shape (Fig. 11d) |
|
4 Egg oval, lateral bulge above micropylar plate, surface uniformly rough, micropylar plate more round than oval (Fig. 11c) |
|
5 Egg oval, lateral bulge slightly pointed, protruding further out than the top of the micropylar plate, micropylar plate ovular |
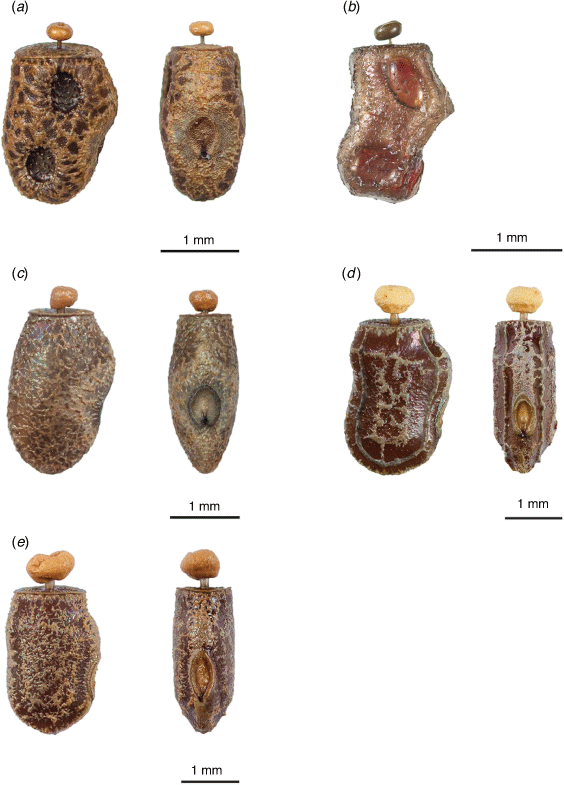
|
Austrocarausius nigropunctatus (Kirby, 1896)
(Black-spotted Stick-insect)
Description
Female
Described in Brock (2000, p. 52) (NHMUK).
Male
Described in Brunner von Wattenwyl (1907, p. 275) (as the synonym Carausius macerrimus).
Egg
Described in Brock (2000, p. 52) (NHMUK) (Fig. 11a).
Specimens examined
♂ Quandong Road, Cow Bay, Qld,16°13′51″S, 145°27′15″E, 30.i.2019, B. Jones and N. Tweed, BJ-240 (QM; Registration number: T258567; GenBank numbers: ON101990, OM992332); ♂ same data, BJ-242 (QM; Registration number: T258569; GenBank numbers ON101991, OM992334); ♂ same data, BJ-241 (QM; Registration number: T258568; GenBank number: OM992333); ♂ same data, BJ-243 (QM; Registration number: T258570; GenBank numbers: ON101992, OM992335); ♀ same data, BJ-245 (QM; Registration number: T258571; GenBank numbers: ON077343, OM992337); ♀ same data, BJ-246 (QM; Registration number: T258572; GenBank numbers: ON101994, OM992338).
Austrocarausius mercurius (Stål, 1877)
(Plain Stick-insect)
Diagnosis
Pair of short spines between eyes. Body smooth, with no flanges (Fig. 6a, 7a, 8a, Supplementary Fig. S3a, Supplementary Table S3).
Description
Female
Described in Brock and Hasenpusch (2007) (NHRS).
Male
Uniform brown with reddish tinge on the thorax. White bands present on apices of all femora.
Head. Elongate, width 0.6× length. Antennae long, 62 segments, extending past forelegs, ~2.5× length of fore femora. Pair of spines present between eyes (Fig. 6a).
Thorax. Pronotum slightly shorter than head, mesonotum 8× length of pronotum, metanotum 0.5× length of mesonotum (Fig. 7a).
Abdomen. Segments 2–5 are equal in length, 2× length of median segment. Segments 6 and 7 equal in length, slightly shorter than segments 2–5. Segments 8 and 9 equal in length, ~0.5× length of segment 10. Cerci concealed, 0.2× length of 10th abdominal segment (Fig. 8a). Genital claspers pointed.
Legs. Without ornamentation (Supplementary Fig. S3a).
Egg
Described in Brock (2000, p. 52) (NHMUK).
Measurements (mm)
For 2 males. Body length: 82.4–92. Head: 2.1–2.8. Antennae: 62 segments. Pronotum: 2.3–2.6. Mesonotum: 19.9–22.1. Metanotum: 11.0–12.4. Median segment: 3.2–3.5. Femora: fore, mid, hind: 22.4–24.4, 17.1, 19.7–21.4. Tibiae: fore, mid, hind: 24.8–26.9, 18.3, 24.1–27.1.
Variation among paratypes
Females uniform dark brown or heavily mottled with grey and dark brown. Dorsal surface of head behind eyes sometimes light brown. Thick, white, dorsal longitudinal stripe on last two abdominal segments.
Specimens examined
Paratypes: ♂ Australia: Qld, Byfield 19.xi.2011 P. Brock and N. Tweed, PB-0078 (QM; Registration number: T250901; GenBank number: KJ202015.1); ♂ same data, PB-0079 (QM; Registration number: T250900; GenBank numbers: KJ202027.1, OM992352).
Specimens used in DNA only: PB-0081 (GenBank numbers: KJ201995.1, OM992353); PB-0080 (GenBank number: KJ202026.1).
Austrocarausius waiben sp. nov.
(Waiben Stick-insect)
Diagnosis
Females with black-tipped tubercles sparsely scattered across head, thorax and first three segments of abdomen (Fig. 3a, 4, 5a, Supplementary Fig. S3b, S4a, Supplementary Table S3). Two small spines between eyes. Males uniformly smooth (Fig. 6b, 7b, 8b, Supplementary Fig. S3b). Genital claspers distinctly straight and apically pointed.
Description
Female
Body uniform brown. Lateral black stripe on head behind eyes.
Head. Small pair of spines between eyes (Fig. 3a). Head smooth, with sparse black-tipped tubercles. Head slightly longer than wide. Antennae reaching end of forelegs, 54 segments, ~2× length of fore femora.
Thorax. Pronotum slightly shorter than head, mesonotum 6× length of pronotum, metanotum 0.5× length of mesonotum. Metanotum sparsely covered in dorsal only black-tipped tubercles (Fig. 4a).
Abdomen. First three segments sparsely tuberculate. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of 2–6. Segments 9 and 10 equal in length and 0.6× length of segment 8. Tip of operculum rounded, sub-acute (Fig. 5a). End of supra-anal plate forming a rounded bump. End of operculum and supra-anal plate level. Supra-anal plate ~0.3× length of anal segment. Cerci mostly concealed, approximately equal in length to the supra-anal plate. End of preopercular organ consists of two outer lobes either side of a spine, almost reaching end of segment 8 (Fig. 5a).
Legs. Without ornamentation (Supplementary Fig. S3b).
Male
Uniform green.
Head. Longer than wide (Fig. 6b). Antennae long, reaching fore tarsi, 74 segments, 2× length of fore femora.
Thorax. Smooth (Fig. 7b). Pronotum 0.75× head length, mesonotum 7× length of pronotum length. Metanotum 0.5× length of mesonotum.
Abdomen. Segments 2–6 equal in length, 2× length of median segment. Segment 7 is slightly shorter than segments 2–6. Segments 8 and 9 equal in length and ~0.5× length of segment 10. Cerci visible, 0.3× length of 10th abdominal segment (Fig. 8b). Genital claspers very straight and apically pointed.
Legs. Without ornamentation (Supplementary Fig. S3b).
Egg
Small, flat, somewhat angular and elongate with lateral bulge above micropylar plate that comes to a sharp point (Fig. 11b). Egg capitulate, connected by a thin stem. Egg surface is uniformly coarse. Two large oval lateral depressions. Micropylar plate and dorsal view too damaged to make any observations.
Measurements (mm)
For 3 females and 1 male. Body length: female 79–92, male 66. Head: female 4.2–4.5, male 3.1. Antennae: female 54 segments, male 74 segments. Pronotum: female 3.1–3.7, male 2.4. Mesonotum: female 18.6–22.2, male 16.4. Metanotum: female 8.5–9.8, male 7.8. Median segment: female 3.2–4.5, male 3.0. Femora: fore, mid, hind: female 21.1–22.3, 16.5–17.6, 19.6–20.6, male 19.5, 14.6, 16.9. Tibiae: fore, mid, hind: female 20.3–22.2, 15.9–17.6, 20.0–21.6, male 21.2, 16.1, 21.1. Eggs: capsule length 2.49, height 1.44, width too damaged for accurate measurements.
Variation among paratypes
Females sometimes reddish over dorsal and ventral surfaces of thorax and median segment. May also have black dorsal stripe running either head to thorax or entire length of body.
Specimens examined
Holotype: ♀ Qld, Thursday Island, 28 Sep 2020, Dominic Funnell, BJ-324 (QM; Registration number: T250904). Paratypes: ♂ same data, BJ-323 (QM; Registration number: T250903); ♀ same data, BJ-327 (QM; Registration number: T250906); ♀ same data, BJ-326 (QM; Registration number: T250905).
Specimens used in DNA only: ♀ Qld, Thursday Island, 28 Sep 2020, Dominic Funnell, BJ-322 (QM; Registration number: T250902; GenBank numbers: ON102002, OM992351).
Etymology
Waiben is the local indigenous name for Thursday Island, the type locality of the species. To be treated as a noun in apposition.
Austrocarausius truncatus sp. nov.
(Truncate Stick-insect)
Diagnosis
Males notably shorter than in other species (Fig. 6c, 7c, 8c, Supplementary Fig. S3c). Without ornamentation. Apices of male genital claspers truncate, distinct from other species.
Description
Female
Not known.
Male
Uniform brown.
Head. Elongate, width 0.6× length (Fig. 6c). Antennae long, 68 segments, extend past forelegs, ~2.5× length of fore femora.
Thorax. Pronotum 0.6× head length, mesonotum 8× length of pronotum, metanotum 0.5× length of mesonotum (Fig. 7c).
Abdomen. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of 2–6. Segments 8–10 equal in length. Cerci concealed, 0.25× length of 10th abdominal segment (Fig. 8c). Apices of genital claspers truncate.
Legs. Without ornamentation (Supplementary Fig. S3c).
Egg
Not known.
Measurements (mm)
For 1 male. Body length: 71. Head: 3.2. Antennae: 68 segments. Pronotum: 2.5. Mesonotum: 19.3. Metanotum: 8.9. Median segment: 2.8. Femora: fore, mid, hind: 21.8, 15.3, 20.2. Tibiae: fore, mid, hind: 25.6, 18.4, 26.5.
Specimens examined
Holotype; ♂ Gordon’s Mine Area, Iron Range, N. Qld. 12–19.ii.1996, G. B. Monteith, Rainforest (QM; Registration number: T250907).
Etymology
The species name is derived from the shape of the genital claspers.
Austrocarausius tuberosus sp. nov.
(Tuber Stick-insect)
Diagnosis
Males lack foreleg flanges and have small spines between eyes (Fig. 6d, 7d, 8d, Supplementary Fig. S3d). Black tipped dorsal and lateral tubercles on mesonotum and metanotum, sparser on metanotum.
Description
Female
Not known.
Male
Uniform brown.
Head. Slightly longer than wide (Fig. 6d). Antennae long, 35 segments, reaching beyond end of forelegs, ~2.25× length of fore femora.
Thorax. Pronotum and head equal in length, mesonotum 7× length of pronotum, metanotum 0.5× length of mesonotum (Fig. 7d).
Abdomen. Short tumescence protruding at end of abdominal segments 1–3. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segments 8 and 9 equal in length and ~0.6× length of segment 10. Cerci short, 0.3× length of segment 10 (Fig. 8d). Genital claspers short and rounded.
Legs. Without ornamentation (Supplementary Fig. S3d).
Egg
Not known.
Measurements (mm)
For 1 male. Body length: 84. Head: 3. Antennae: 35 segments. Pronotum: 3.3. Mesonotum: 22.5. Metanotum: 10.2. Median segment: 3.8. Femora: fore, mid, hind: 23.3, 16.6, 19.1. Tibiae: fore, mid, hind: 26.3, 18.0, 24.0.
Specimens examined
Holotype: ♂ north-eastern Qld: 16°07′S, 145°25′E, Mt Hemmant, 1050 m, 25–27.xi.1993, Monteith, Cook, Janetzki and Roberts (QM; Registration number: T250908).
Etymology
The species name is derived from the black tipped protuberances on the thorax.
Austrocarausius decorus sp. nov.
(Flanged Stick-insect)
Diagnosis
Females with black tipped tubercles over body, ventral tubercles only on metanotum (Fig. 3b, 4b, 5b, Supplementary Fig. S3e, S4b, Supplementary Table S3). Pair of large flange-like protuberances between eyes, with three smaller pairs caudally, each reducing in size. Fore tibiae and tarsi have dorsal flange like ridge, fore tibiae with two dorsal lobes and tarsi with one. Short tumescence protruding at end of each abdominal segment. Males smooth, with two small spines between eyes (Fig. 6e, 7e, 8e, Supplementary Fig. S3e). Fore tarsi dorsally flanged. Thorax with few black dots sparsely covering dorsal surface.
Description
Female
Uniform brown, fore tibiae flanges green.
Head. Pair of large flange-like protuberances between eyes (Fig. 3b). Two pairs of blunt spines and rounded tubercles behind horns. Head slightly longer than wide. Antennae long, 71 segments, extending past forelegs, ~2× length of fore femora.
Thorax. Black tipped tubercles are present dorsally and laterally, ventral tubercles only on metanotum (Fig. 4b). Pronotum slightly shorter than head, mesonotum 8× length of pronotum. Metanotum ~0.5× length of mesonotum.
Abdomen. Covered in dorsal and ventral black tipped tubercles. Short tumescence protruding at end of each abdominal segment. Operculum surpassing last abdominal segment and in line with the supra-anal plate (Fig. 5b). Operculum terminating ventrally in a point. End of operculum and supra-anal plate truncate. Cerci short, reaching end of last abdominal segment. Segments 2–6 equal in length, 2× length of median segment. Segment 7 slightly shorter than segment 2–6. Segments 9 and 10 equal in length and 0.2× shorter than segment 8. Preopercular organ extending to 0.5× length of segment 8, from midpoint abruptly becoming constricted and spinelike (Fig. 5b).
Legs. Fore tibiae and tarsi highly flanged, with two dorsal lobes on tibiae and one on tarsi; flange like ridge present between lobes (Supplementary Fig. S3e).
Male
Dark brown.
Head. Pair of spines between eyes, with two smaller pairs caudally (Fig. 6e). Head slightly longer than wide. Antennae reach beyond forelegs, 76 segments, ~2.5× length of fore femora.
Thorax. Smooth with few black dots sparsely covering dorsal surface (Fig. 7e). Pronotum and head equal in length, mesonotum 9× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Segments 2–5 equal in length, 2× length of median segment. Segment 6 slightly shorter than segments 2–5, segment 7 slightly shorter than segment 6. Segments 8 and 9 equal in length and ~0.5× length of segment 10. Cerci concealed, 0.2× length of 10th abdominal segment (Fig. 8e). Genital claspers rounded, bend outwards.
Legs. Fore tarsi dorsally flanged (Supplementary Fig. S3e).
Egg
Small, flat, oval in shape with a lateral bulge above the micropylar plate (Fig. 11c). Egg capitulate, connected by enlarged stem. Egg surface rough throughout. Micropylar plate low and round.
Measurements (mm)
For 3 females and 3 males. Body length: female 85–130, male 76–94. Female can occur in lichen form at a significantly reduced body size.
Head. Female 2.8–4.4, male 2–2.4. Antennae: female 71 segments, male 76 segments. Pronotum: female 2.4–3.9, male 2.2–2.9. Mesonotum: female 18.5–30.1, male 18.1–23.0. Metanotum: female 13.5–18.1, male 11.1–15.4. Median segment: female 3.5–4.6, male 2.7–3.5. Femora: fore, mid, hind: female 19.3–29.9, 13.4–21.4, 15.1–23.0, male 21.6–24.1, 13.6–17.6, 16.2–20.3. Tibiae: fore, mid, hind: female 19.0–32.3, 14.1–21.2, 18.1–27.0, male 21.6–29.5, 15.4–19.9, 21.8–27.1. Eggs: capsule length 2.25, height 1.58, width 1.29.
Variation among paratypes
Females may have some mottling of darker brown over body. Males can be uniform green. Females may have reduced dorsal head protuberances and swollen 6th abdominal segments. Male tarsi can be found without dorsal flange.
Specimens examined
Holotype: ♀ Cape Tribulation Road, Thornton Beach, Qld, 16°10′24″S, 145°26′27″E, 3.ii.2018. B. Jones and N. Tweed, BJ-294 (QM; Registration number: T250911; GenBank numbers: ON101995, OM992340). Paratypes: ♂, Quandong Road, Cow Bay, Qld, 16°13′51″S, 145°27′15″E, 30J.i.2019, B. Jones and N. Tweed, BJ-244 (QM; Registration number: T250909; GenBank numbers: ON101993, OM992336). ♀ Cape Tribulation Rd, Cape Tribulation, Qld, 16°05′25″S, 145°27′52″E, 19.v.2019, B. Jones BJ-295 (QM; Registration number: T250912; GenBank numbers: ON077345, OM992341). ♀ Cape Tribulation Rd, Cape Tribulation, Qld, 16°05′25″S, 145°27′52″E, 19.v.2019, B. Jones BJ-298 (QM; Registration number: T250915; GenBank numbers: ON077347, OM992344). ♂ Cape Tribulation Rd, Cape Tribulation, Qld, 16°05′25″S, 145°27′52″E, 19.v.2019, B. Jones BJ-296 (QM; Registration number: T250913; GenBank numbers: ON101996, OM992342). ♂ Cape Tribulation Rd, Cape Tribulation, Qld, 16°05′25″S, 145°27′52″E, 19.v.2019, B. Jones BJ-297 (QM; Registration number: T250914; GenBank numbers: ON077346, OM992343).
Etymology
The species is named after the Latin decorus, meaning beautiful or ornament, in reference to the flanged fore legs and head protuberance of this species.
Notes
Females may have unflanged tarsi and tibiae. Males and females observed to feign death when disturbed. Female lichen form was observed to hang vertically holding branch with only the two midleg tarsi, whereas all legs were straightened lengthwise along body.
Austrocarausius coronatus sp. nov.
(Crowned Stick-insect)
Diagnosis
Females have tubercles across body, black-tipped on thorax (Fig. 3c, 4c, 5c, Supplementary Fig. S3f, S4c, Supplementary Table S3). Pair of large flange-like protuberances between eyes, notably larger than any other species. Dorsal flanges found on fore tarsi and tibiae. This species has black-tipped tubercles over body. Short tumescence protruding at end of each abdominal segment. Male body without ornamentation, two spines between eyes (Fig. 7f, 9a, 10a, Supplementary Fig. S3f).
Description
Female
Mottled light and dark brown with blotches of dark spots, mostly on ventral side.
Head. Pair of large flange-like protuberances between eyes (Fig. 3c). Behind this protuberance are two pairs of spines each smaller than the other. Other tubercles and smaller spines can be found on the dorsal side of the head. Head slightly longer than wide. Antennae long, 58 segments, extending past forelegs, ~1.5× length of fore femora.
Thorax. Black-tipped tubercles cover the dorsal and lateral surfaces of whole thorax, only metanotum has ventral black-tipped tubercles (Fig. 4c). Pronotum and head equal in length, mesonotum 8× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Short tumescence protruding at end of each abdominal segment. Abdomen speckled with black-tipped tubercles throughout the dorsal and ventral surface. Segments 2–5 equal in length, 2× length of median segment. Segment 6 slightly shorter than segments 2–5. Segment 7 slightly shorter than segment 6. Segments 8 and 9 equal in length and ~0.6× length of segment 10. Segments 9 and 10 equal in length and 0.3 shorter than segment 8. Operculum shape is angular but extends to end of supra-anal plate (Fig. 5c). End of operculum is round but supra-anal plate is emarginate. Cerci short, extending 0.5× length of the supra-anal plate. Preopercular organ extends to 0.5× length of segment 8, spine-like in shape, consisting of two pairs of elongated appendages on either side of the preopercular organ (Fig. 5c).
Legs. Fore tibiae and tarsi have dorsal flange like ridge (Supplementary Fig. S3f).
Male
Uniform brown, thorax has a dorsal reddish tinge.
Head. Pair of small spines between eyes, dorsal surface heavily tuberculate (Fig. 9a). Antennae reach to the end of the fore tarsi, 69 segments. Head slightly longer than wide. Antennae extends past forelegs, ~2.5× length of fore femora.
Thorax. Thorax smooth (Fig. 7f). Pronotum and head equal in length, mesonotum 9× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Segments 2–5 equal in length, 2× length of median segment. Segment 6 slightly shorter than segments 2–5. Segment 7 slightly shorter than segment 6. Segments 8 and 9 equal in length and ~0.6× length of segment 10. End of genital claspers curved (Fig. 10a). Genital claspers parallel and partly conceal cerci. Cerci short, extending 0.2× length of 10th abdominal segment.
Legs. Without ornamentation (Supplementary Fig. S3f).
Egg
Not known.
Measurements (mm)
For 1 female and 1 male. Body length: female 110, male 83. Head: female 3.8, male 2.2. Antennae: female 58 segments, male 69 segments. Pronotum: female 3.2, male 2.3. Mesonotum: female 24.9, male 20.2. Metanotum: female 14.5, male 11.5. Median segment: female 4.5, male 3. Femora: fore, mid, hind: female 23.3, 17.1, 18.8, male 21.0, 15.7, 18.6. Tibiae: fore, mid, hind: female 22.4, 17.3, 22.7, male 26.0, 16.9, 23.3.
Specimens examined
Holotype: ♀ Polly Ck, Garradunga, N. Qld, 27.xi.2009, J. Hasenpusch, JH-0055 (QM; Registration number: T250917; GenBank numbers: HM425711.1, OM992354). Paratype: ♂ Polly Ck, Garradunga, N. Qld, 27.xi.2009, J. Hasenpusch, JH-0054 (QM; Registration number: T250918; GenBank numbers: HM425710.1, OM992356).
Specimens used in DNA only: PB-0028 (GenBank number: HM425728.1), PB-0026 (GenBank number: HM425727.1), PB-0025 (GenBank numbers: HM425726.1, OM992357), PB-0024 (GenBank numbers: HM425725.1, OM992355).
Etymology
Derived from the Latin corona meaning crown, based on the distinct crown-like head structure of the female. The species was also discovered during the corona-virus pandemic in 2020.
Austrocarausius gasterbulla sp. nov.
(Gaster Stick-insect)
Diagnosis
Females have uniform tubercles across body, black-tipped on thorax (Fig. 3d, 4d, 5d, Supplementary Fig. S3g, S4d). Pair of moderately sized protuberances between eyes. Fore tibiae and tarsi are dorsally flanged. Short tumescence protruding at end of each abdominal segment. Sixth abdominal segment swollen. Females have black spots sparsely covering body. Male body without ornamentation (Fig. 7g, 9b, 10b, Supplementary Fig. S3g), pair of horns between eyes.
Description
Female
Blotched with black spots sparsely over body, mostly on ventral side. Female mottled light and dark brown.
Head. Pair of moderately sized flange-like protuberances between eyes (Fig. 3d). Heavily covered in dorsal and lateral tubercles. Head slightly longer than wide. Antennae do not extend past forelegs, 48 segments, ~1.5× length of fore femora.
Thorax. Thorax dorsally and laterally tuberculate throughout (Fig. 4d). Pronotum and head equal in length, mesonotum 6× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Dorsally and laterally tuberculate across abdomen. Abdominal segment 6 swollen and has a rough tuberculate surface (Supplementary Fig. S4d). Short tumescence protruding at end of each abdominal segment. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segments 9 and 10 0.5× length of segment 8. Tip of operculum and supra-anal plate rounded (Fig. 5d). Operculum extends past the supra-anal plate by approximately 0.3× length of the supra-anal plate. Cerci are not visible. Preopercular organ rounded and short, not extending into segment 8 (Fig. 5d).
Legs. Fore tibiae and tarsi have dorsal flange-like ridge (Supplementary Fig. S3g).
Male
Uniform brown, thorax has a dorsal reddish tinge.
Head. Slightly longer than wide (Fig. 9b). Antennae extend past forelegs, 59 segments, ~2.5× length of fore femora. Large pair of horns between eyes.
Thorax. Thorax smooth (Fig. 7g). Pronotum and head equal in length, mesonotum 6× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Segments 2–5 equal in length, 2× length of median segment. Segment 6 slightly shorter than segments 2–5. Segment 7 slightly shorter than segment 6. Segment 9 0.6× length of segment 8 and ~0.5× length of segment 10. Cerci concealed, 0.2× length of 10th abdominal segment (Fig. 10b). Genital claspers rounded.
Legs. Without ornamentation (Supplementary Fig. S3g).
Egg
Not known.
Measurements (mm)
For 1 female and 1 male. Body length: female 112, male 92. Head: female 4, male 2.6. Antennae: female 48 segments, male 59 segments. Pronotum: female 4, male 2.7. Mesonotum: female 25.5, male 23.6. Metanotum: female 13.9, male 12.5. Median segment: female 4.7, male 3.6. Femora: fore, mid, hind: female 24.0, 16.6, 17.6, male 25.4, 17.0, 20.2. Tibiae: fore, mid, hind: female 22.7, 16.8, 20.3, male 29.8, 20.2, 27.1.
Specimens examined
Holotype; ♀ Mt Macartney, 600–850 m, Cathu State Forest, C. Qld, 20–21.iv.1979 G. B. Monteith (QM; Registration number: T250919). Paratype: ♂ Mt Macartney, 600–850 m, Cathu State Forest, C. Qld, 20–21.iv.1979 G. B. Monteith (QM; Registration number: T250920).
Etymology
The new species is a combination of the Latin gaster, meaning belly and bulla, meaning bubble, in reference to the swollen and bulbous 6th abdominal segment of the female. To be treated as a noun in apposition.
Austrocarausius macropunctatus sp. nov.
(Black-dotted Stick-insect)
Diagnosis
Females are covered in black- and brown-tipped tubercles (Fig. 3e, 4e, 5e, Supplementary Fig. S3h, S4e, Supplementary Table S3). Pair of black spines between eyes. Insects have large black dots surrounding tubercles on mesonotum. Male body without ornamentation (Fig. 7h, 9c, 10c, Supplementary Fig. S3h). Pair of spines between eyes. Dark dots present on thorax of both sexes.
Description
Female
Uniform brown, white banding present on all femora.
Head. Two large black spines between eyes, longer than wide, with several smaller black-tipped spines caudally (Fig. 3e). Antennae extend past forelegs, 104 segments, ~2.5× length of fore femora.
Thorax. Dorsal and lateral thorax sparsely covered in black-tipped and brown tubercles, on mesonotum black-tipped tubercles are surrounded by black (Fig. 4e). Pronotum and head equal in length, mesonotum 7× length of pronotum, metanotum 0.5× length of mesonotum.
Abdomen. Smooth with black-tipped tubercles. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segments 9 and 10 equal in length, 0.6× length of segment 8. End of supra-anal plate is mucronate (Fig. 5e). Overall shape of operculum curves to the supra-anal plate. The end of the operculum is rounded. Preopercular organ square and short, not extending into segment 8 (Fig. 5e).
Legs. Without ornamentation (Supplementary Fig. S3h).
Male
Light brown with green legs.
Head. Pair of large elongate spines between eyes (Fig. 9c). Head otherwise without tubercles. Head slightly longer than wide. Antennae extend past forelegs, 130 segments, 2.5× length of fore femora.
Thorax. Smooth thorax with few darker dots covering parts of the dorsal and lateral surface (Fig. 7h). Pronotum slightly longer than head, mesonotum 8× length of pronotum, metanotum slightly more than 0.5× length of mesonotum.
Abdomen. Abdominal segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segment 9 0.6× length of segment 8 and ~0.5× length of segment 10. Cerci short, 0.2× length of 10th abdominal segment (Fig. 10c). Genital claspers pointed at end, dorsal bump at half the length.
Legs. Without ornamentation (Supplementary Fig. S3h).
Egg
Small, flat, roughly oval in shape, lateral bulge above the micropylar plate (Fig. 11d). Egg capitula connected by enlarged stem. Underlying egg surface smooth with network of granulation in the centre, on both lateral and dorsal sides. Singular ridge running along the outside of the egg on the lateral side, starting and ending at the operculum in a ‘U’-like shape. Micropylar plate is low and ovular.
Measurements (mm)
For 1 female and 2 males. Body length: female 121, male 98–103. Head: female 4.6, male 2.8–3.3. Antennae: female 104 segments, male 130 segments. Pronotum: female 3.7, male 2.8–3. Mesonotum: female 26.2, male 23.7–24.9. Metanotum: female 15.3, male 14.7–15.3. Median segment: female 4.3, male 2.9–3.3. Femora: fore, mid, hind: female 31.7, 22.7, 25.3, male 30.8–34.1, 23.7–26.6, 27.1–30.7. Tibiae: fore, mid, hind: female 33.9, 25.4, 31.4, male 38.3–40.9, 30.2–32.5, 38.5–42.5. Eggs: capsule length 2.79, height 1.93, width 1.32.
Specimens examined
Holotype; ♀ Shute Harbour, Qld, 22.iv.2020, Leg J. Constant and L. Semeraro, Leopold III Funds Expedition – Qld, BJ-302 (QM; Registration number: T250921; GenBank numbers: ON102000, OM992348). Paratype: ♂ same data, BJ-304 (QM; Registration number: T250922; GenBank numbers: ON102001, OM992350); ♂ same data, BJ-303 (QM; Registration number: T250923; GenBank numbers: ON077348, OM992349).
Etymology
The new species is a combination of macro meaning large and Latin punctum meaning dot and spot, in reference to the large black dots surrounding the lateral tubercles on the mesonotum.
Austrocarausius eirmosus sp. nov.
(Black-striped Stick-insect)
Diagnosis
Females have a pair of spines between eyes and dark tubercles over body (Fig. 3f, 4f, 5f, Supplementary Fig. S3i, S4f, Supplementary Table S3). Disjointed black ventral stripe on mesonotum and metanotum, only in this species. Short tumescence protruding at end of abdominal segments 1–6. Males have a smooth body with few lateral tubercles on thorax (Fig. 7i, 9d, 10d, Supplementary Fig. S3i). Pair of spines between eyes. Some lateral tubercles on thorax. Short tumescence protruding at end of abdominal segments 1–6.
Description
Female
Uniform brown, legs lightly mottled brown with white bands on apices of all femora.
Head. Slightly longer than wide (Fig. 3f). Two black spines between eyes, longer than wide, with several smaller brown spines caudally. Antennae reach end of fore tarsi, 103 segments, 2× length of fore femora.
Thorax. Large black-tipped lateral tubercles on thorax (Fig. 4f). Disjointed black ventral stripe on mesonotum and metanotum. Pronotum and head equal in length, mesonotum 7× length of pronotum, metanotum 0.6× length of mesonotum.
Abdomen. Short tumescence protruding at end of abdominal segments 1–6. Segments 2–6 equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segments 9 and 10 0.5× length of segment 8. Cerci extends to tip of ovipositor (Fig. 5f). Supra-anal plate emarginate. Ovipositor curved and extending to end of supra-anal plate, apically pointed. Preopercular organ cordate, approximately 0.3× length of segment 8 (Fig. 5f).
Legs. Without ornamentation (Supplementary Fig. S3i).
Male
Light brown with green legs.
Head. Pair of spines between eyes, otherwise without ornamentation (Fig. 9d). Head slightly longer than wide. Antennae extend past forelegs, 111 segments, 2.5× length of fore femora.
Thorax. Smooth, with few lateral tubercles (Fig. 7i). Pronotum and head equal length, mesonotum 8× length of pronotum, metanotum just over 0.5× length of mesonotum.
Abdomen. Short tumescence protruding at end of abdominal segments 1–6. Abdominal segments 2–6 are equal in length, 2× length of median segment. Segment 7 0.6× length of segments 2–6. Segment 9 0.6× length of segment 8, and 0.5× length of segment 10. Cerci short, 0.2× length of 10th abdominal segment (Fig. 10d). Genital claspers pointed, at half the length reduce 0.5× in height.
Legs. Without ornamentation (Supplementary Fig. S3i).
Egg
Small, flat, roughly oval in shape with a slight lateral bulge above the micropylar plate (Fig. 11e). Egg capitula connected by enlarged stem. Egg surface rough throughout. Micropylar plate low and ovular.
Measurements (mm)
For 2 females and 1 male. Body length: female 135–137, male 111. Head: female 4.4–4.5, male 3. Antennae: female 103 segments, male 111 segments. Pronotum: female 4.1–4.5, male 3.3. Mesonotum: female 30.6–31.2, male 27. Metanotum: female 18.1, male 16.4. Median segment: female 4.4–4.6, male 3.3. Femora: fore, mid, hind: female 33.6–36.0, 24.1–26.0, 27.8–30.0, male 32.6, 24.8, 28.1. Tibiae: fore, mid, hind: female 35.8–40.1, 27.5–29.7, 35.7–36.8, male 28.0, 29.5, 37.0. Eggs: capsule length 3.08, height 1.72, width 1.25.
Specimens examined
Holotype: ♀ Whitsunday Islands National Park, Qld, 21–22.iv.2020, Leg J. Constant and L. Semeraro, Leopold III Funds Expedition – Qld, BJ-300 (QM; Registration number: T250924; GenBank numbers: ON101998, OM992346). Paratype: ♂ same data, BJ-301 (QM; Registration number: T250925; GenBank numbers: ON101999, OM992347); ♀ same data, BJ-299 (QM; Registration number: T250926; GenBank numbers: ON101997, OM992345).
Etymology
Derived from the Latin eirmos meaning series and train, in reference to the disjointed black ventral stripe on the mesonotum and metanotum of the female.
Austrocarausius walkeri sp. nov.
(Walker’s Austrocarausius)
Diagnosis
This species has the largest male dorsal head protuberance in the genus (Fig. 7j, 9e, 10e, Supplementary Fig. S3j, Supplementary Table S3). The body is smooth. Fore tarsi are flanged. Short tumescence protruding at end of abdominal segments 1–6.
Description
Female
Not known.
Male
Very elongated, brown with mottled body and particularly legs, the latter with whitish markings on femora and tibiae. Upper half of metanotum with reddish flush; ventral surface of mesothorax and upper half of metathorax reddish.
Head. As long as wide, pair of large flange-like protuberances between eyes, with two pairs of smaller spines caudally, decreasing in size and lateral distance from each other (Fig. 9e). Antennae extend to end of forelegs, 68 segments, 2.5× length of fore femora.
Thorax. Smooth (Fig. 7j). Pronotum slightly longer than head, mesonotum 8× length of pronotum, metanotum slightly over 0.5× length of mesonotum.
Abdomen. Short tumescence protruding at end of abdominal segments 1–6. Segment 2 slightly over 2× length of median segment. Segments 2–5 approximately equal in length. Segment 6 slightly shorter than segments 2–5. Segment 7 shorter than segment 6. Segments 8–9 combined are approximately the same as segment 7. Segment 10 0.6× longer than segment 9. Genital claspers lobe-like and splayed outwards, rounded apically (Fig. 10e). Cerci short. Poculum tapered to tip, reaching end of 9th abdominal segment.
Legs. Fore tarsi flanged (Supplementary Fig. S3j).
Egg
Not known.
Measurements (mm)
Body length: 111. Head: 2.8. Antennae: 68 segments. Pronotum: 3.4. Mesonotum: 27.5. Metanotum: 15.2. Median segment: 3.8. Femora: fore, mid, hind: 31.5, 21.5, 24.0. Tibiae: fore, mid, hind: 39.0, 26.3, 33.2.
Specimens examined
Holotype: ♂ Qld, Mt Dryander, 21.11.2009, P. D. Brock and I. O. Walker, PB-0014 (QM; Registration number: T250927; GenBank number: HM425716.1).
Etymology
Named after Ian O. Walker, a keen naturalist from Bowen with a vast knowledge of that area and Mt Dryander, who greatly assisted P. D. Brock with his trip to the area in 2009.
Discussion
Using a combination of molecular and morphological data, we have found evidence that Austrocarausius represents multiple undescribed species and has diversified at the species level within the tropical rainforests of Queensland over the last c. 25–70 Ma.
Species delimitation
Both ABGD and GMYC species delimitation methods suggest eight undescribed species among the individuals examined (Fig. 2). These species delimitation results also corroborate the species hypotheses presented in our morphological analyses. We note that for some specimens, we were unable to obtain molecular data. Among these specimens, we were able to confidently describe three new species on the basis of morphological data (A. truncatus, A. tuberosus and A. gasterbulla). Future molecular studies are required to determine the phylogenetic position among the other eight Austrocarausius species.
Morphological analysis
Close inspection reveals that this genus has some of the most ornate and unusual features seen in Australian phasmids, including dorsal head structures in the form of a flange-like protuberance, horn or spine (Fig. 3, 6, 9), and preopercular organs located ventrally on the 7th abdominal segment in females (Fig. 5). Although head structures can be variable within each Austrocarausius species (Fig. 3, 6, 9), the overall form (protuberance, horn, spine or absent) is useful for species identification. The variation in preopercular organ shape and additional appendage structures observed only in this genus, make for some of the most morphologically unique phasmid species. No intraspecific variation was observed in this morphological structure. The preopercular organ is known to be used for reproduction (Bragg 2001), however, further behavioural investigation into these organs and structures is needed to fully understand the precise function. The eggs of Austrocarausius are capitulate, like other closely related taxa such as Carausius.
Phylogenetic placement of Austrocarausius among other lonchodids
In agreement with other studies (Robertson et al. 2018, Simon et al. 2019), our Bayesian analysis places Eurycantha (Lonchodidae: Lonchodinae: Eurycanthini), Carausius (Lonchodidae: Lonchodinae: Lonchodini), and Sipyloidea (Lonchodidae: Necrosciinae) as closely related taxa to Austrocarausius (Supplementary Fig. S1). Previous studies have placed Carausius as sister to Eurycantha (Robertson et al. 2018; Simon et al. 2019). However, our maximum likelihood analysis did not recover this relationship (Supplementary Fig. S1) and our Bayesian analysis places Carausius as sister to Sipyloidea instead of Eurycantha. The support values for these relationships are notably not high among outgroup taxa. Our results regarding the closest relative of Austrocarausius should therefore be considered preliminary. Compared to our study, both Robertson et al. (2018) and Simon et al. (2019) used a significantly larger number of taxa and more molecular evidence. These authors found that Eurycantha and Carausius are consistently placed in the subfamily Lonchodinae and Sipyloidea in Necrosciinae. The lack of resolution in our phylogenetic trees is likely due to limited genetic and taxon sampling.
Austrocarausius has only been included in one previous molecular analysis (Velonà et al. 2015). In that study, Austrocarausius was grouped with Denhama Werner, 1912 (Lonchodidae: Lonchodinae: Lonchodini). Further studies including the two genera will help determine the phylogenetic position with more confidence.
Historical biogeography of Austrocarausius
The drivers of ancient speciation events within Austrocarausius are difficult to determine, since these occurred at a time when rainforests were considered to have dominated the Australian landscape (Greenwood and Christophel 2005; Byrne et al. 2008; Byrne et al. 2011). However, the formation and maintenance of ancient biogeographic barriers from the Cretaceous to late Oligocene may offer one possible explanation for speciation in Austrocarausius. For example, the Black Mountain Corridor, where A. nigropunctatus, A. decorus and A. coronatus are found, has been suggested as a geographical barrier for earthworms between the Eocene and Cretaceous periods (Moreau et al. 2015), this being a similar time period for the divergence of A. waiben and A. nigropunctatus (c. 53.8 Ma (95% HPD 46.12–62.49 Ma)), and A. decorus and A. coronatus (c. 48.6 Ma (95% HPD 41.56–56.77 Ma)). The St Lawrence Gap is a geographical barrier for assassin spiders appearing during the Eocene (Rix and Harvey 2012) and between the distributions of A. mercurius and A. macropunctatus, A. eirmosus and A. walkeri. Although these geographical barriers may play a role in the ancient speciation events of Austrocarausius, our study is only based on two mitochondrial genes and our biogeographic inferences should be regarded as preliminary.
Austrocarausius is highly endemic to specific patches of remnant rainforest, unlike other phasmids that can be found in a variety of vegetation communities. For example, Extatosoma tiaratum and Tropidoderus rhodomus can be found along most of the eastern coast of Australian between Cape York and southern New South Wales (~2500 km) (Brock and Hasenpusch 2009; Atlas of Living Australia, see http://www.ala.org.au, accessed 12 April 2021). These species have winged males and are generalist feeders (Brock and Hasenpusch 2009), and this may explain the widespread distribution. In contrast, Austrocarausius species are wingless with limited dispersal capabilities, and are restricted to tropical rainforests. Loss of these already fragmented rainforests could put such endemic species at risk of extinction. Austrocarausius nigropunctatus was given an IUCN Red List conservation status of 'Data Deficient' in 2017 (Rudolf and Brock 2017).
A previous study of the rainforest phasmid genus Parapodacanthus showed that this comprises only two species with restricted distributions (one in the border ranges near Brisbane and the other in the Daintree rainforest) (Brock and Monteith 2018). Whether members of Parapodacanthus were previously present in the patchy rainforest areas inhabited by Austrocarausius but later went extinct, or whether the current distribution is the result of long-distance dispersal, is unclear. Our study indicates that Austrocarausius has been able to survive in rainforests over tens of millions of years, during which time these vegetation communities disappeared from large parts of Queensland.
Our analyses show that morphologically different species of Austrocarausius can coexist in sympatry within the same habitats (Fig. 2). For example, A. nigropunctatus and A. decorus both live within identical habitats in the Daintree rainforest and have similar distributions. Austrocarausius walkeri and A. macropunctatus are found in similar rainforest habitat close together (Shute Harbour), although the distributions are separated by an area devoid of rainforest.
Although this study analyses specimens from different museum collections along with the addition of new material, there is still a great deal to learn. For example, no DNA was recovered from specimens of A. nigropunctatus from the type locality of Lizard Island, as no specimens were available. Additionally, A. coronatus might also live in Kuranda and Paluma (Paul Brock, pers. comm., 2022). Further research on these species will benefit from field surveys in central and northern Queensland and to discover the currently undescribed females of A. truncatus, A. tuberosus and A. walkeri, how far the distributions of each species spread, and exactly how many other species there might be in Austrocarausius.
Data availability
Molecular data for this project can be found on GenBank under the following accession numbers. For COI: HM425710.1, HM425711.1, HM425716.1, HM425725.1–HM425728.1, KJ201995.1, KJ202015.1, KJ202026.1, KJ202027.1, ON077343–ON077348, ON101990–ON102002; and for 16S: OM992332–OM992357 (Supplementary Table S2). Data used to generate this paper are available in Supplementary Tables S1–S5 and Supplementary File S1.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Funding for this project was provided by the Linnean Society of NSW, Ecological Society of Australia and a CSIRO Postgraduate Scholarship.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank the following people for helping with collections and providing specimens: Linda Semeraro, Jerome Constant, Dominic Funnell and Noelene Tweed. We also thank the Queensland Museum and Australian National Insect Collection for allowing us to borrow specimens for examination.
References
Balderson J, Rentz DCF, Roach AME (1998) Phasmatodea. In ‘Zoological Catalogue of Australia. Vol. 23. Archaeognatha, Zygentoma, Blattodea, Isoptera, Mantodea, Dermaptera, Phasmatodea, Embioptera, Zoraptera’. (Eds WWK Houston, A Wells) pp. 347–376, 451–456. (CSIRO Publishing: Melbourne, Vic., Australia)Bouckaert, RR, and Drummond, AJ (2017). bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evolutionary Biology 17, 42.
| bModelTest: Bayesian phylogenetic site model averaging and model comparison.Crossref | GoogleScholarGoogle Scholar |
Bouckaert, R, Heled, J, Kühnert, D, Vaughan, T, Wu, CH, Xie, D, Suchard, MA, Rambaut, A, and Drummond, AJ (2014). BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10, e1003537.
| BEAST 2: a software platform for Bayesian evolutionary analysis.Crossref | GoogleScholarGoogle Scholar |
Bragg PE (2001) ‘Phasmids of Borneo.’ (Natural History Publications: Kota Kinabalu, Malaysia)
Brock, PD (2000). Studies on Australian stick-insects of the Family Heteronemiidae, Subfamily Lonchodinae, including the description of a new genus. Journal of Orthoptera Research 9, 51–55.
| Studies on Australian stick-insects of the Family Heteronemiidae, Subfamily Lonchodinae, including the description of a new genus.Crossref | GoogleScholarGoogle Scholar |
Brock, PD, and Hasenpusch, J (2007). Studies on the Australian stick insects (Phasmida), including a checklist of species and bibliography. Zootaxa 1570, 1–84.
| Studies on the Australian stick insects (Phasmida), including a checklist of species and bibliography.Crossref | GoogleScholarGoogle Scholar |
Brock PD, Hasenpusch JW (2009) ‘The complete field guide to stick and leaf insects of Australia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Brock, PD, and Monteith, GB (2018). A striking new species of Parapodacanthus Brock (Phasmida: Phasmatidae) from southeastern Queensland. Australian Entomologist 45, 17–26.
Brunner von Wattenwyl K (1907) ‘Die Insektenfamilie der Phasmiden. Vol. 2. Phasmidae Anareolatae (Clitumnini, Lonchodini, Bacunculini)’. pp. 181–340. (Wilhelm Engelmann: Leipzig: German Empire)
Byrne, M, Yeates, DK, Joseph, L, Kearney, M, Bowler, J, Williams, MAJ, Cooper, S, Donnellan, SC, Keogh, JS, Leys, R, Melville, J, Murphy, DJ, Porch, N, and Wyrwoll, K-H (2008). Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular ecology 17, 4398–4417.
| Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota.Crossref | GoogleScholarGoogle Scholar |
Byrne, M, Steane, DA, Joseph, L, Yeates, DK, Jordan, GJ, Crayn, D, Aplin, K, Cantrill, DJ, Cook, LG, Crisp, MD, Keogh, JS, Melville, J, Moritz, C, Porch, N, Sniderman, JMK, Sunnucks, P, and Weston, PH (2011). Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. Journal of biogeography 38, 1635–1656.
| Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota.Crossref | GoogleScholarGoogle Scholar |
Cliquennois N (2020) Chapter 18 Ordre des Phasmatodea (Phasmes). In ‘Les Insectes du Monde Biodiversité, classification, clés de détermination des familles’. (Ed. H-P Aberlenc) Vol. 1, pp. 403–437. (Éditions Quae: France)
Dayrat, B (2005). Towards integrative taxonomy. Biological Journal of the Linnean Society 85, 407–415.
| Towards integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Drummond, AJ, and Rambaut, A (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7, 214.
| BEAST: Bayesian evolutionary analysis by sampling trees.Crossref | GoogleScholarGoogle Scholar |
Edgar, RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797.
| MUSCLE: multiple sequence alignment with high accuracy and high throughput.Crossref | GoogleScholarGoogle Scholar |
Folmer , O, Black , M, Hoeh , W, Lutz , R, and Vrijenhoek , R (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294–299.
Fourment, M, and Holmes, EC (2016). Seqotron: a user-friendly sequence editor for Mac OS X. BMC Research Notes 9, 106.
| Seqotron: a user-friendly sequence editor for Mac OS X.Crossref | GoogleScholarGoogle Scholar |
Greenwood DR, Christophel DC (2005) The origins and tertiary history of Australian ‘tropical’ rainforests. In ‘Tropical rainforests: past, present and future’. pp. 336–373. (University of Chicago Press: Chicago, IL, USA)
Hennemann, FH, and Conle, OV (2008). Revision of Oriental Phasmatodea: the tribe PharnaciiniGünther, 1953, including the description of the world's longest insect, and a survey of the family Phasmatidae Gray, 1835 with keys to the subfamilies and tribes (Phasmatodea: Anareolatae: Phasmatidae). Zootaxa 1906, 1–316.
Kapli, P, Lutteropp, S, Zhang, J, Kobert, K, Pavlidis, P, Stamatakis, A, and Flouri, T (2017). Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33, 1630–1638.
| Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo.Crossref | GoogleScholarGoogle Scholar |
Kearse, M, Moir, R, Wilson, A, Stones-Havas, S, Cheung, M, Sturrock, S, Buxton, S, Cooper, A, Markowitz, S, Duran, C, Thierer, T, Ashton, B, Meintjes, P, and Drummond, A (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649.
| Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data.Crossref | GoogleScholarGoogle Scholar |
Kirby, WF (1896). VI. On some new or rare Phasmidae in the collection of the British Museum. Transactions of the Linnean Society of London. 2nd Series. Zoology 6, 447–475.
| VI. On some new or rare Phasmidae in the collection of the British Museum.Crossref | GoogleScholarGoogle Scholar |
Kirby WF (1904) ‘A synonymic catalogue of Orthoptera. I. Orthoptera Euplexoptera, Cursoria et Gressoria).’ (British Museum Natural History: London, UK)
| Crossref |
Moreau, CS, Hugall, AF, McDonald, KR, Jamieson, BGM, and Moritz, C (2015). An ancient divide in a contiguous rainforest: endemic earthworms in the Australian Wet Tropics. PLoS One 10, e0136943.
| An ancient divide in a contiguous rainforest: endemic earthworms in the Australian Wet Tropics.Crossref | GoogleScholarGoogle Scholar |
Otte D, Brock P (2005) ‘Phasmida Species File. Catalog of the Stick and Leaf Insects of the World’, 2nd edn. (Insect Diversity Association: Philadelphia, PA, USA)
Poinar, G Jr (2011). A walking stick, Clonistria dominicana n. sp. (Phasmatodea: Diapheromeridae) in Dominican amber. Historical Biology 23, 223–226.
| A walking stick, Clonistria dominicana n. sp. (Phasmatodea: Diapheromeridae) in Dominican amber.Crossref | GoogleScholarGoogle Scholar |
Puillandre, N, Lambert, A, Brouillet, S, and Achaz, G (2012). ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21, 1864–1877.
| ABGD, Automatic Barcode Gap Discovery for primary species delimitation.Crossref | GoogleScholarGoogle Scholar |
Rainbow, WJ (1897). Description of two new Australian Phasmas, together with a synopsis of the Phasmidae in Australia. Records of the Australian Museum 3, 34–37.
| Description of two new Australian Phasmas, together with a synopsis of the Phasmidae in Australia.Crossref | GoogleScholarGoogle Scholar |
Rambaut, A, Drummond, AJ, Xie, D, Baele, G, and Suchard, MA (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901–904.
| Posterior summarization in Bayesian phylogenetics using Tracer 1.7.Crossref | GoogleScholarGoogle Scholar |
Rix, MG, and Harvey, MS (2012). Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within Tertiary refugia. Molecular Phylogenetics and Evolution 62, 375–396.
| Phylogeny and historical biogeography of ancient assassin spiders (Araneae: Archaeidae) in the Australian mesic zone: evidence for Miocene speciation within Tertiary refugia.Crossref | GoogleScholarGoogle Scholar |
Robertson, JA, Bradler, S, and Whiting, MF (2018). Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Frontiers in Ecology and Evolution 6, 216.
| Evolution of oviposition techniques in stick and leaf insects (Phasmatodea).Crossref | GoogleScholarGoogle Scholar |
Rudolf E, Brock P (2017) Plain stick-insect Austrocarausius mercurius. In ‘The IUCN Red List of Threatened Species 2017’. e.T78789295A78792052. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/species/78789295/78792052 [Verified 29 July 2022]
Sellick, JTC (1994). Phasmida (stick insect) eggs from the Eocene of Oregon. Palaeontology 37, 913–922.
Simon, C, Frati, F, Beckenbach, A, Crespi, B, Liu, H, and Flook, P (1994). Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87, 651–701.
| Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers.Crossref | GoogleScholarGoogle Scholar |
Simon, S, Letsch, H, Bank, S, Buckley, TR, Donath, A, Liu, S, Machida, R, Meusemann, K, Misof, B, Podsiadlowski, L, Zhou, X, Wipfler, B, and Bradler, S (2019). Old World and New World Phasmatodea: phylogenomics resolve the evolutionary history of stick and leaf insects. Frontiers in Ecology and Evolution 7, 345.
| Old World and New World Phasmatodea: phylogenomics resolve the evolutionary history of stick and leaf insects.Crossref | GoogleScholarGoogle Scholar |
Stål, C (1877). Espèces nouvelles de Phasmides. Annales de la Société Entomologique de Belgique, Comptes Rendues 20, 62–68.
Tepper, JGO (1903). List of described genera and species of the Australian and Polynesian Phasmidae (Spectre-insects). Transactions of the Royal Society of Southern Australia 26, 278–287.
Trifinopoulos, J, Nguyen, LT, von Haeseler, A, and Minh, BQ (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44, W232–W235.
| W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis.Crossref | GoogleScholarGoogle Scholar |
Velonà, A, Brock, PD, Hasenpusch, J, and Mantovani, B (2015). Cryptic diversity in Australian stick insects (Insecta; Phasmida) uncovered by the DNA barcoding approach. Zootaxa. 3957, 455–466.
| Cryptic diversity in Australian stick insects (Insecta; Phasmida) uncovered by the DNA barcoding approach.Crossref | GoogleScholarGoogle Scholar |
Vickery VR (1983) Catalogue of Australian stick insects (Phasmida, Phasmatodea, Phasmatoptera, or Cheleutoptera). CSIRO Division of Entomology, Technical Paper 20, CSIRO, Melbourne, Vic., Australia.
Will, KW, Mishler, BD, and Wheeler, QD (2005). The perils of DNA barcoding and the need for integrative taxonomy. Systematic Biology 54, 844–851.
| The perils of DNA barcoding and the need for integrative taxonomy.Crossref | GoogleScholarGoogle Scholar |
Wedmann, S, Bradler, S, and Rust, J (2007). The first fossil leaf insect: 47 million years of specialized cryptic morphology and behavior. Proceedings of the National Academy of Sciences 104, 565–569.
Yeates, DK, Seago, A, Nelson, L, Cameron, SL, Joseph, L, and Trueman, JWH (2010). Integrative taxonomy, or iterative taxonomy? Systematic Entomology 36, 209–217.
| Integrative taxonomy, or iterative taxonomy?Crossref | GoogleScholarGoogle Scholar |





