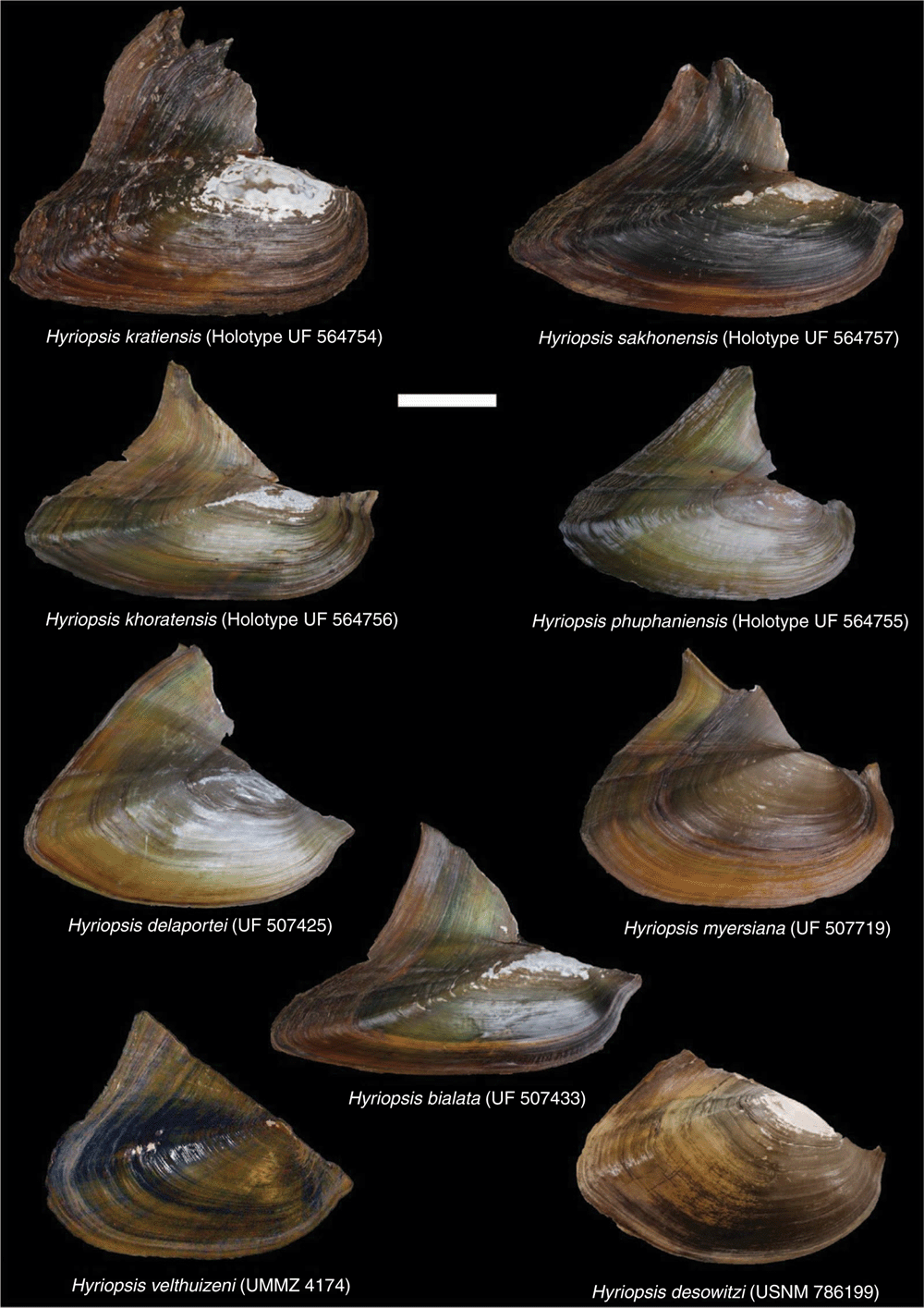
Taxonomic revision of a radiation of South-east Asian freshwater mussels (Unionidae : Gonideinae : Contradentini+Rectidentini)
John M. Pfeiffer A E , Daniel L. Graf B , Kevin S. Cummings C and Lawrence M. Page D
A E , Daniel L. Graf B , Kevin S. Cummings C and Lawrence M. Page D
A Department of Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue NW, Washington, DC 20560, USA.
B Biology Department, University of Wisconsin – Stevens Point, 800 Reserve Street, Stevens Point, WI 54481, USA.
C Illinois Natural History Survey, Prairie Research Institute, University of Illinois, 1816 S. Oak Street, Champaign, IL 61820, USA.
D Florida Museum of Natural History, University of Florida, 1659 Museum Road, Gainesville, FL 32611, USA.
E Corresponding author. Email: pfeifferj@si.edu
Invertebrate Systematics 35(4) 394-470 https://doi.org/10.1071/IS20044
Submitted: 28 May 2020 Accepted: 23 September 2020 Published: 10 May 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
The tribes Contradentini and Rectidentini (Unionidae) comprise a diverse clade of freshwater mussels endemic to South-east Asia. Our understanding of the diversity and phylogeny of this radiation has improved dramatically in recent years, but this systematic transformation has not yet benefited from comprehensive museum sampling or phylogenomic methods. A synthetic taxonomic revision of the Contradentini+Rectidentini that leverages these useful and accessible methods is needed. We set out to (1) generate a phylogenomic reconstruction of the supraspecific relationships of the Contradentini+Rectidentini using anchored hybrid enrichment, (2) revise the taxonomy and geographic boundaries of the generic and species-level diversity of the radiation, and (3) identify patterns of freshwater mussel diversity and distribution in this clade and discuss the processes that may have precipitated them. Our phylogenomic reconstruction using over 1600 loci, with a total alignment length of over a half a million nucleotides, recovers a well supported phylogeny of the clade that resolves four independent multispecies radiations endemic to the Mekong drainage. We examined, digitised, and imaged 1837 records from 15 natural history museums that provided the necessary data to document the morphological variation and geographic distributions of the focal taxa. We also analysed 860 COI sequences, 519 of which were generated in this study, to better understand the species boundaries and geographic distributions of the recovered clades. We recognise 54 valid species in the tribes Contradentini and Rectidentini, including 9 described herein as new to science. Out of this revision emerged several interesting biogeographic patterns that appear to have resulted from recent stream capture, historical confluence, and intradrainage barriers to dispersal. We hypothesise that these phenomena shaped the diversity and distribution of the Contradentini+Rectidentini, contributing to the formation of several characteristic freshwater mussel provinces in South-east Asia.
Introduction
The freshwater mussel fauna of South-east Asia is a phylogenetically diverse assemblage, with native representatives in at least four subfamilies and nine tribes of the Unionidae (Zieritz et al. 2018a; Pfeiffer et al. 2019). The tribes Contradentini and Rectidentini are an exclusively South-east Asian radiation, and they are common and diverse in the region’s freshwater communities. Portions of this clade have received considerable molecular systematic attention in recent years, and our appreciation for the phylogeny, distribution, and diversity of this radiation has improved dramatically (Pfeiffer and Graf 2013, 2015; Bolotov et al. 2017a, 2017b, 2018, 2019b, 2020; Konopleva et al. 2017, 2019a, 2019b; Lopes-Lima et al. 2017; Zieritz et al. 2018a, 2018b, 2020; Pfeiffer et al. 2018a, 2019; Jeratthitikul et al. 2019; Muanta et al. 2019).
The rapid influx of systematic knowledge in this radiation has been prompted, in large part, by many recent successful collecting expeditions in the region and Sanger sequencing of the freshly collected material. To put this rapid progress in perspective, at the start of 2015 there was a single cytochrome ‘c’ oxidase subunit I (COI) sequence available on GenBank for the entirety of this radiation. In this study, we analysed 860 COI sequences from 42 Contradentini+Rectidentini species (341 previously published sequences, 519 generated herein). The abundance of these recently collected specimens and their associated molecular data have helped to characterise the genetic and morphological variation of the sampled individuals and delimit the geographic distributions of the recovered clades.
However, this approach has often incompletely leveraged the abundance of museum records available to more completely characterise the systematics, morphological variation, and geographic boundaries of the focal taxa. This is an unfortunate and unnecessary omission especially considering the usefulness of several accessible digital resources designed to facilitate collections-based research (Global Biodiversity Information Facility, see https://www.gbif.org/; Integrated Digitized Biocollections, see https://www.idigbio.org/; and especially relevant to freshwater mussel biodiversity, the MUSSEL Project Database, see http://mussel-project.uwsp.edu/). Examining the relevant museum records is laborious but it is essential to construct biologically useful classifications, characterise intra- and interspecific variation, determine biogeographic details, and estimate historical baselines for determining conservation status.
Furthermore, the handful of molecular markers applied to reconstructing phylogenies of the Contradentini+Rectidentini (or parts thereof) are well known to recover supraspecific relationships that are unstable or inconsistent with previous topologies based on morphological and phylogenomic data (Graf and Cummings 2006a; Pfeiffer et al. 2019). Resolving the supraspecific relationships of this radiation are necessary to identifying macroevolutionary patterns and processes, including trait evolution, lineage diversification, and biogeographic history.
We aimed to overcome these shortcomings by taking advantage of the abundance of specimen records in multiple major natural history collections and more broadly sampling the genomes of the focal taxa. Specifically, we (1) generated a phylogenomic reconstruction of the Contradentini+Rectidentini using anchored hybrid enrichment, (2) revised the genus- and species-level taxa by synthesising data from museum records and COI sequences, and (3) described patterns of freshwater mussel diversity and distribution in South-east Asia and the processes that may have generated them.
Methods
Biogeographic methods
The geographic distribution of each taxon was determined by examining, digitising, and imaging South-east Asian freshwater mussel records at 15 natural history museums. Institutional abbreviations follow Sabaj (2016), with the number of lots digitised and the date they were integrated into the MUSSEL Project Database given in parentheses: ALMNH, Alabama Museum of Natural History, Tuscaloosa (6 lots; 2011); ANSP, Academy of Natural Sciences at Drexel University, Philadelphia (129 lots; 2011); CAS, California Academy of Sciences, San Francisco (4 lots; 2014); FMNH, Field Museum of Natural History, Chicago (67 lots; 2011); INHS, Illinois Natural History Survey, Champaign (15 lots; 2017); MCZ, Museum of Comparative Zoology, Harvard University, Cambridge (61 lots; 2012); MNHN, Museum National d’Histoire Naturelle, Paris (300 lots; 2012); NCSM, North Carolina Museum of Natural Sciences, Raleigh (30 lots; 2017); NHMUK, Natural History Museum, London (116 lots; 2012); SMF, Senckenberg Forschungsinstitut und Naturmuseum, Frankfort (415 lots; 2014); SMRL, Applied Malacology Center and Mollusk Museum, Bangkok (46 lots; 2016); UF, Florida Museum of Natural History, Gainesville (296 lots; 2017); UMMZ, University of Michigan Museum of Zoology, Ann Arbor (121 lots; 2011); USNM, National Museum of Natural History, Smithsonian Institution, Washington, DC (217 lots; 2011); ZMB, Museum für Naturkunde, Berlin (4 lots; 2006).
Images of specimens and the associated textual information (e.g. catalogue number, locality, collector, species identification) were converted into a standardised form and incorporated into the MUSSEL Project Database (MUSSELpdb) following methods described in previous papers (Graf and Cummings 2006b, 2009, 2011). Species identifications were reviewed for all records and revised as necessary. Precise collection locations were georeferenced using Google Maps (https://www.google.com/maps), while more vague locations (e.g. water bodies, cities, divisions) were estimated using standardised coordinates in the GEOnet Names Server (see https://geonames.nga.mil/gns/html/index.html). The voucher number, species identification, geocoordinates, drainage, and ecoregion assignments for all digitised records are in the ‘Materials examined’ (Table S1 of the Supplementary material). More information about the data model, management, and dissemination of the MUSSELpdb is available at the MUSSEL Project Web Site (www.mussel-project.net/).
Dot distribution maps were produced using ArcMap (ver. 10.2.2, see http://www.esri.com). Digitised records refer to those records that have been fully incorporated into the MUSSELpdb (i.e. specimens and labels imaged, textual information normalised). Barcoded records refer to specimen records for which COI sequence and locality data are publicly available on GenBank (see https://www.ncbi.nlm.nih.gov/genbank/). Freshwater ecoregions follow Abell et al. (2008), with the exception that the Inle Lake ecoregion was treated as a part of the Lower & Middle Salween ecoregion, rather than a small, independent ecoregion nested within the larger Lower & Middle Salween ecoregion.
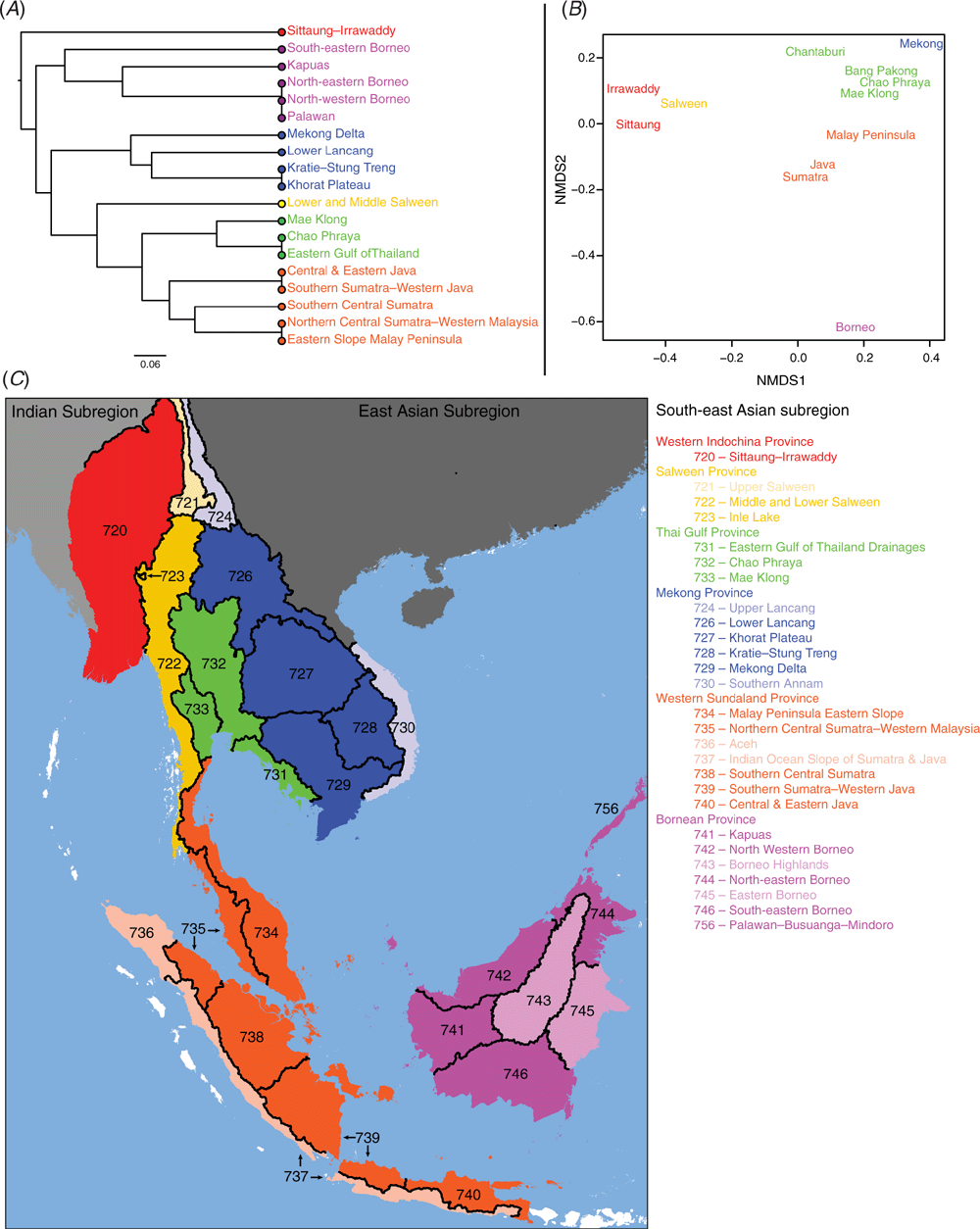
The unweighted pair-group method using arithmetic averages (UPGMA) was used to generate a 50% majority rule consensus dendrogram of ecoregions based on species presence or absence data using the recluster.cons function in the R package ‘recluster’ (ver. 2.8, see https://cran.r-project.org/web/packages/recluster/index.html; Dapporto 2013). Various metrics of phylogenetic diversity were measured using the R package ‘picante’ (ver. 1.8, see https://cran.r-project.org/web/packages/picante/index.html; Kembel 2010) including measures of Faith’s phylogenetic diversity (pd), mean pairwise diversity (ses.mpd), and mean nearest taxon distance (ses.mntd) for the major drainages in Indochina (i.e. Irrawaddy, Sittaung, Salween, Mae Klong, Chao Phraya, Bang Pakong, Chantaburi, Mekong) and the modern landmasses of Sundaland (i.e. Malay Peninsula, Java, Sumatra, Borneo, Palawan). Phylogenetic diversity among drainages and landmasses was measured using the fraction of unique branch lengths among regions (Unifrac) and visualised using non-metric multidimensional scaling (metaMDS). The resultant dendrogram and multidimensional scaling were used to delimit freshwater mussel provinces, similar to those established for freshwater mussels in the United States (Haag 2010, 2012).
Phylogenetic methods
We selected individuals representing 20 species of the Contradentini+Rectidentini for anchored hybrid enrichment (AHE) using the Unioverse probe set (Pfeiffer et al. 2019). DNA was isolated from mantle tissue using a QIAamp DNA Mini Kit (Qiagen, Inc.) and quantified using PicoGreen. Library preparation, enrichment, and Illumina sequencing were done at RAPiD GENOMICS (Gainesville, FL). Libraries were constructed by shearing DNA to an average length of 300 bp followed by an end-repair reaction and ligation of an adenine residue to the 3′-end of the blunt-end fragments. Barcoded adapters were ligated to the libraries followed by PCR amplification. Libraries were pooled into groups of up to 16 samples and the SureSelectxt Target Enrichment System for Illumina Paired-End Multiplexed Sequencing Library protocol (AgilentTechnologies, Santa Clara, CA, USA) was followed for solution-based target enrichment of the Unioverse probe regions. An Illumina HiSeq 3000 was used to generate 100 bp, paired-end reads. Previously published AHE data were downloaded from GenBank’s Short Read Archive (Bioproject PRJNA515912) to include an appropriate outgroup (Lamprotula cornuumlunae) and supplement ingroup taxon sampling.
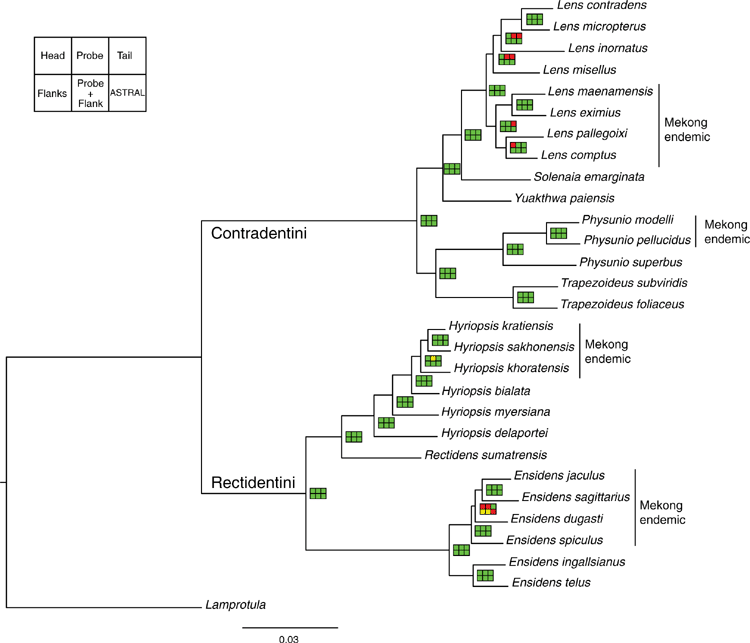
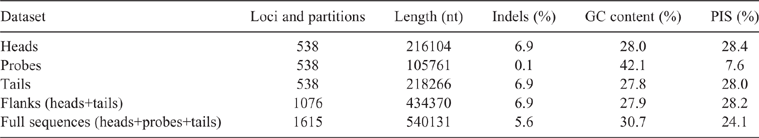
The AHE reads were processed following the AHE data processing pipeline developed by Breinholt et al. (2018) and are described below (step-by-step instructions and scripts are available at https://github.com/mudlark54/Unioverse). TRIM GALORE! (ver. 0.4.0, see www.bioinformatics.babraham.ac.uk/projects/trim_galore/) was used to remove Illumina data with a minimum read size of <30 nt and Phred quality score <20. Loci were assembled using the iterative bait assembly script (IBA.py) of Breinholt et al. (2018) using the Unioverse reference probe regions as baits and modifying the userach target coverage parameters for the ‘blast_command_ nal’ and ‘blast_ lter’ commands to 0.60 and 0.50 respectively. The Unioverse reference matrix was added to the AHE assemblies and aligned using MAFFT (ver. 7.294, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013). Gene orthology was determined using two BLASTn-based python scripts designed to identify loci with single hits to the genome and map their location (i.e. s_hit_checker.py and ortholog_filter.py; Breinholt et al. 2018). If more than one sequence existed for an individual at a particular locus the sequence with the greatest coverage was retained using a custom python script (play_for_keeps.py). To reduce the amount of missing data in our datasets, only loci that had a minimum of 70% taxon occupancy were included. The full-length reads containing the probe regions with more than 70% taxon occupancy were then trimmed using a minimum column density threshold of 60% and a maximum column entropy of 1.5 (Breinholt et al. 2018 – alignment_DE_trim.py). To remove potentially problematic regions on either side of the probe region we used the filtering procedure described by Breinholt et al. (2018) (flank_dropper.py), which removed columns with a nucleotide density of less than 60% and a nucleotide entropy of >1.5. The processed full sequences were then split into three separate loci – the head, the probe region, and the tail (Breinholt et al. 2018 – extract_probe_region.py). FASconCAT-G_v1.04.pl (ver. 1.04, see https://github.com/PatrickKueck/FASconCAT-G; Kück and Longo 2014) was used to make the concatenated matrices and partition files.
Maximum likelihood (ML) reconstruction of the concatenated datasets was conducted in IQ-TREE (ver. 1.6, see http://www.iqtree.org/; Nguyen et al. 2015) using linked partitioned models (Chernomor et al. 2016), ModelFinder (MFP option: Kalyaanamoorthy et al. 2017), and nodal support measured by 1000 ultrafast bootstraps (UFB) (Hoang et al. 2018). IQ-TREE was also used to determine model use and estimate an unpartitioned ML gene tree (MFP option) for each locus using 1000 UFB replicates for subsequent estimation of a species tree using ASTRAL (ver. 5.6.1, see https://github.com/smirarab/ASTRAL; Zhang et al. 2018). Bipartitions with <10 UFB were removed using Newick Utilities (Junier and Zdobnov 2010) before species tree estimation as this has been shown to improve species tree accuracy (Zhang et al. 2017). Nodal support and gene tree conflict were measured in the ASTRAL analysis using local posterior probabilities. The proportion of bipartitions shared between the concatenated topology and each gene tree was measured using SortaDate (Smith et al. 2018), and these values were compared to various alignment statistics measured by AMAS (Borowiec 2016).
COI sequences were generated from representatives of the Contradentini+Rectidentini to evaluate intraspecific genetic variation and help identify the geographic boundaries of the species-level clades. Primers for polymerase chain reaction (PCR) and sequencing were COI – dgLCO-1490, GGTCAACAAATCAT AAAGAYATYGG, and dgHCO-2198, TAAACTTCAGGGT GACCAAARAAYCA (Meyer 2003). PCR was performed in 25-μL reactions using the following reagents and volumes: H20 (17.75 μL), 5× MyTaq Reaction Buffer (5 μL; Bioline), primers (0.5 μL), MyTaq Red DNA polymerase (0.25 μL) and DNA template (1 μL). Bidirectional Sanger sequencing was performed at the University of Florida Interdisciplinary Center for Biotechnology Research. Raw chromatograms were assembled into bidirectional consensus sequences and edited using Geneious (ver. 6.1.2, see http://www.geneious.com; Kearse et al. 2012). Novel sequences were combined with previously published COI sequences to generate COI gene trees for all available representatives of the Contradentini and Rectidentini. Codon-partitioned COI gene trees were generated using IQ-TREE with model testing and partitioning scheme determined using the MFP+MERGE option, a perturbation strength of 0.2, 1000 UFB, and a minimum support threshold of 0.5.
Biogeographic results
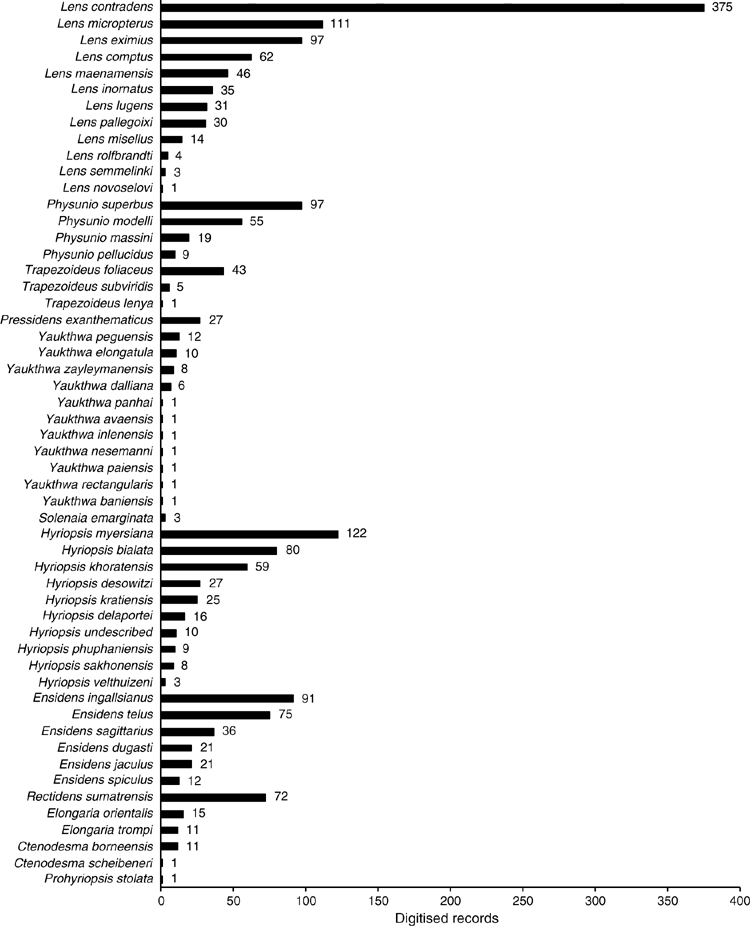
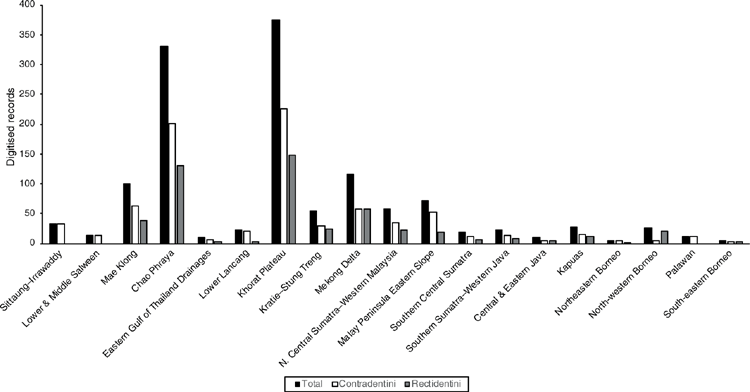
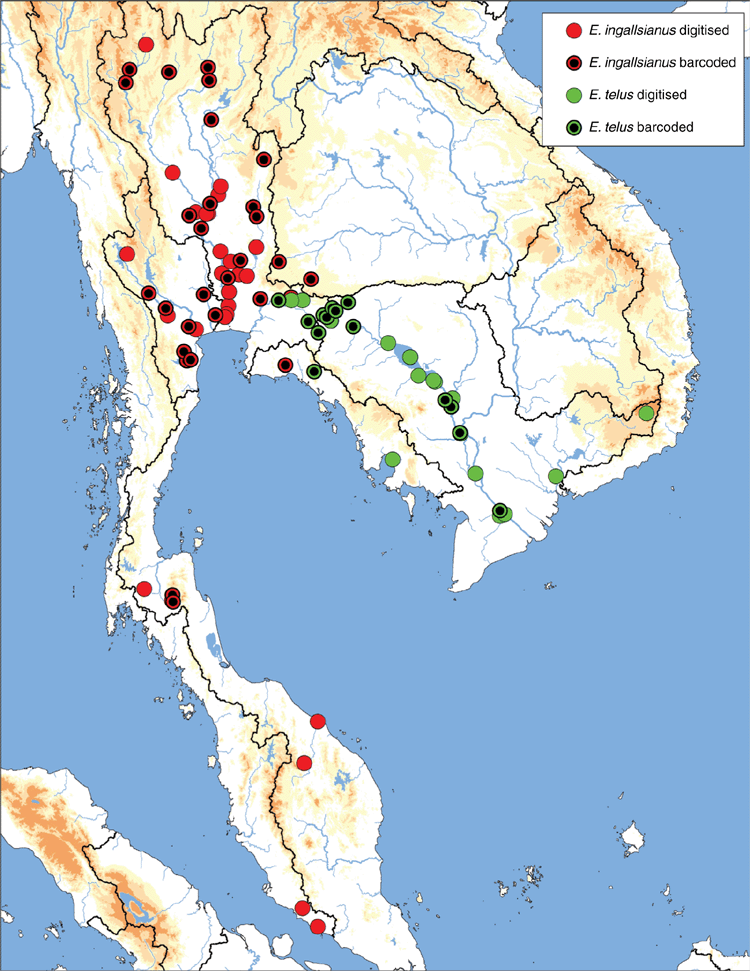
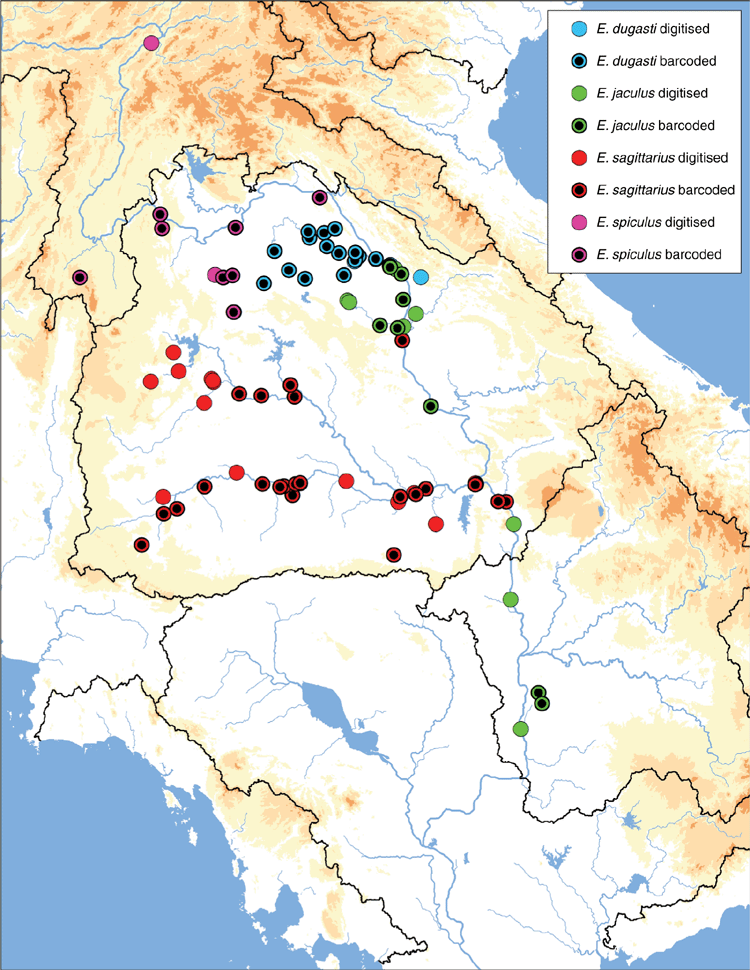
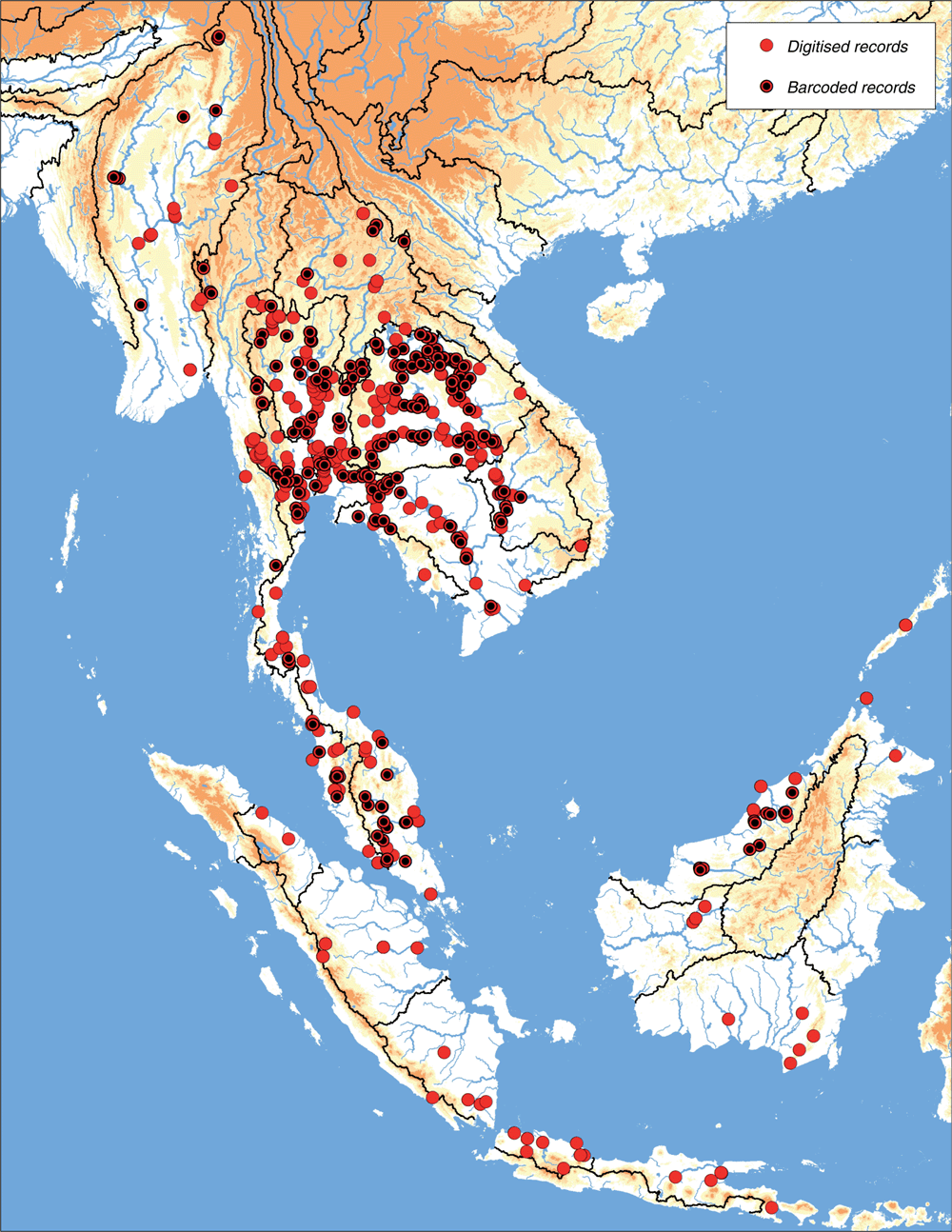
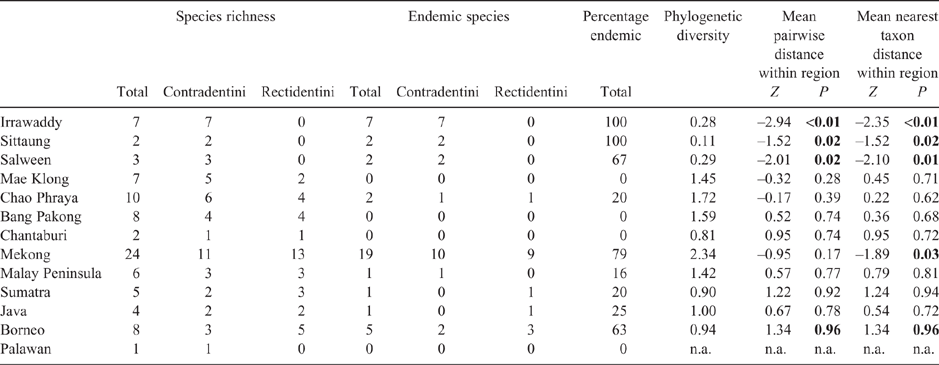
The MUSSELpdb contained 1837 digitised and imaged records from the tribes Contradentini and Rectidentini. Textual information associated with each of these records is in our ‘Material examined’ (Table S1) and the images are available on the MUSSEL Project webpage. The MUSSELpdb has digitised records for each of the recognised species of the Contradentini (32 spp.) and Rectidentini (22 spp.) with Lens contradens and Hyriopsis myersiana having the greatest number of records (375 and 122 respectively) (Fig. 1). Of the 1837 records, 1306 could be assigned to an ecoregion (71%) and 1241 could be georeferenced (67%). The digitised records are distributed across 19 ecoregions (Fig. 2), with the Khorat Plateau (374) and Chao Phraya (330) ecoregions having the greatest number of records (Fig. 3). The distribution of each species across the major drainages of Indochina and the landmasses of Sundaland are reported in Table 1. Various metrics of biodiversity (species richness, endemism, and phylogenetic diversity) for the major drainages of Indochina and landmasses of Sundaland are reported in Table 2.
|
|
|
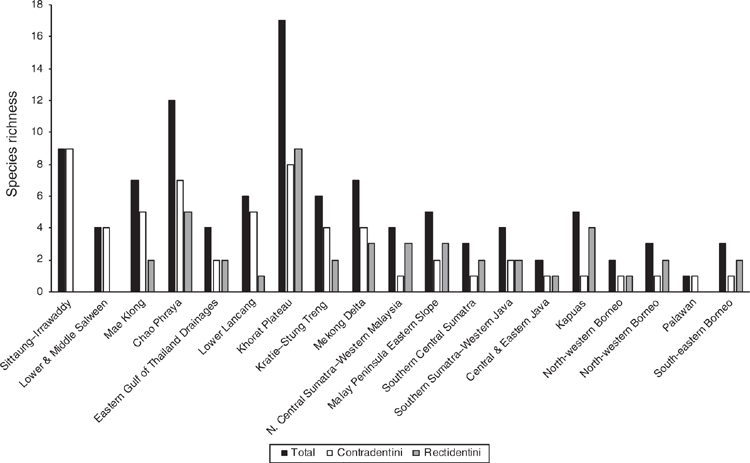
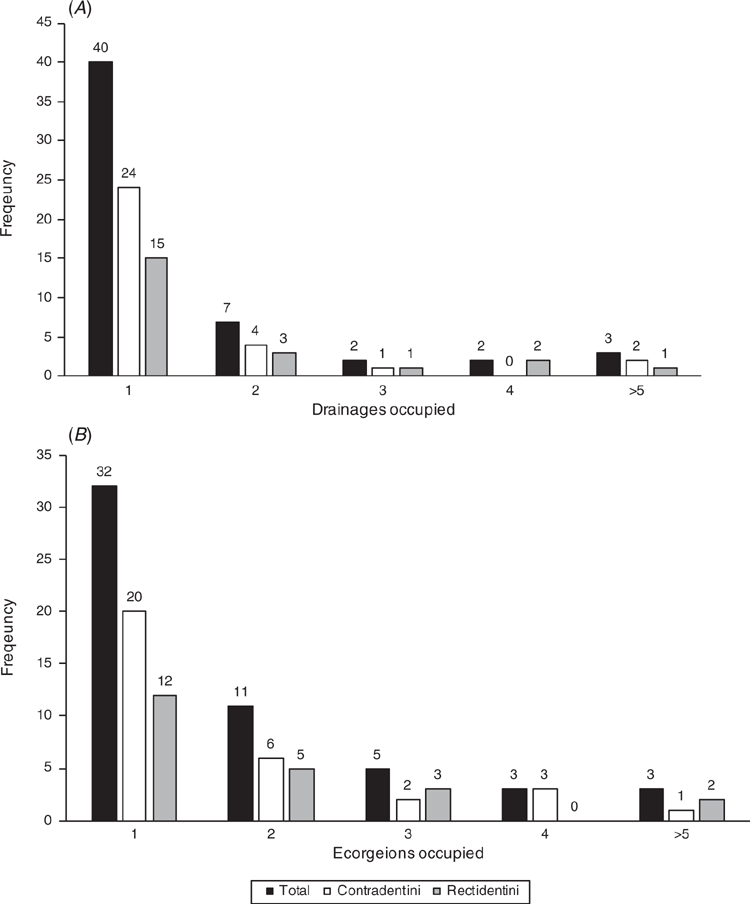
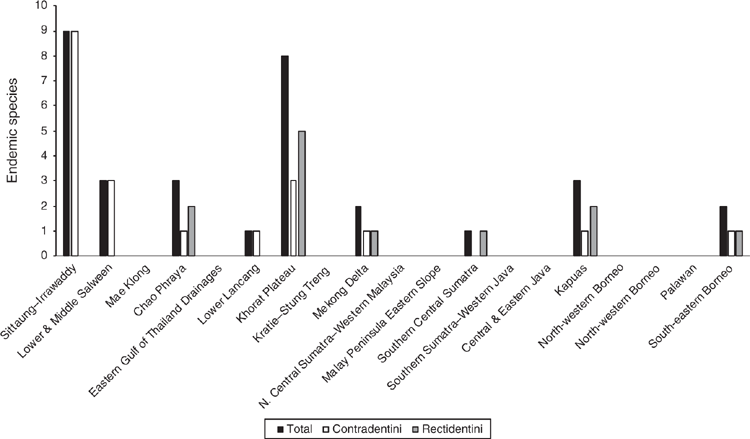
The Khorat Plateau is the most species-rich ecoregion (17 spp.), followed by the Chao Phraya (12) (Fig. 4). The Contradentini has its greatest species richness in the Sittaung–Irrawaddy (9) and Khorat Plateau ecoregions (8), followed closely by the Chao Phraya (7), Mae Klong (5), and Lower Lancang (5). The Rectidentini has its greatest diversity in the Khorat Plateau (9), followed by the Chao Phraya (5) and Kapuas (4). Of the 54 species of the Contradentini and Rectidentini, 40 are endemic to a single drainage and 32 species are endemic to a single ecoregion (Fig. 5). The Sittaung–Irrawaddy (9) and Khorat Plateau (8) ecoregions have the greatest number of endemic species (Fig. 6).The UPGMA dendrogram of species presence or absence data per ecoregion and NMDS of phylogenetic diversity per drainage and landmass is largely consistent with recognising six zoogeographic provinces in the South-east Asian Subregion (Fig. 7).
|
Phylogenetic results
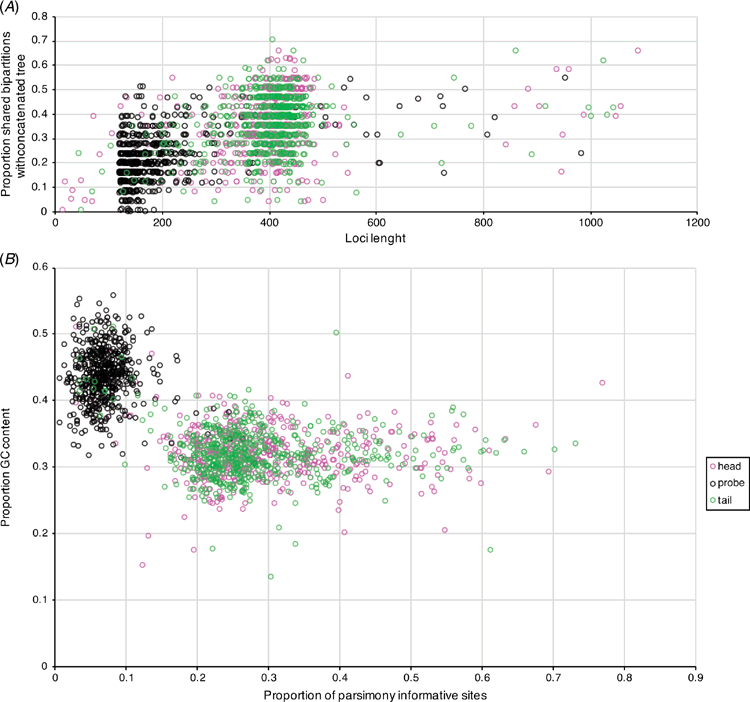
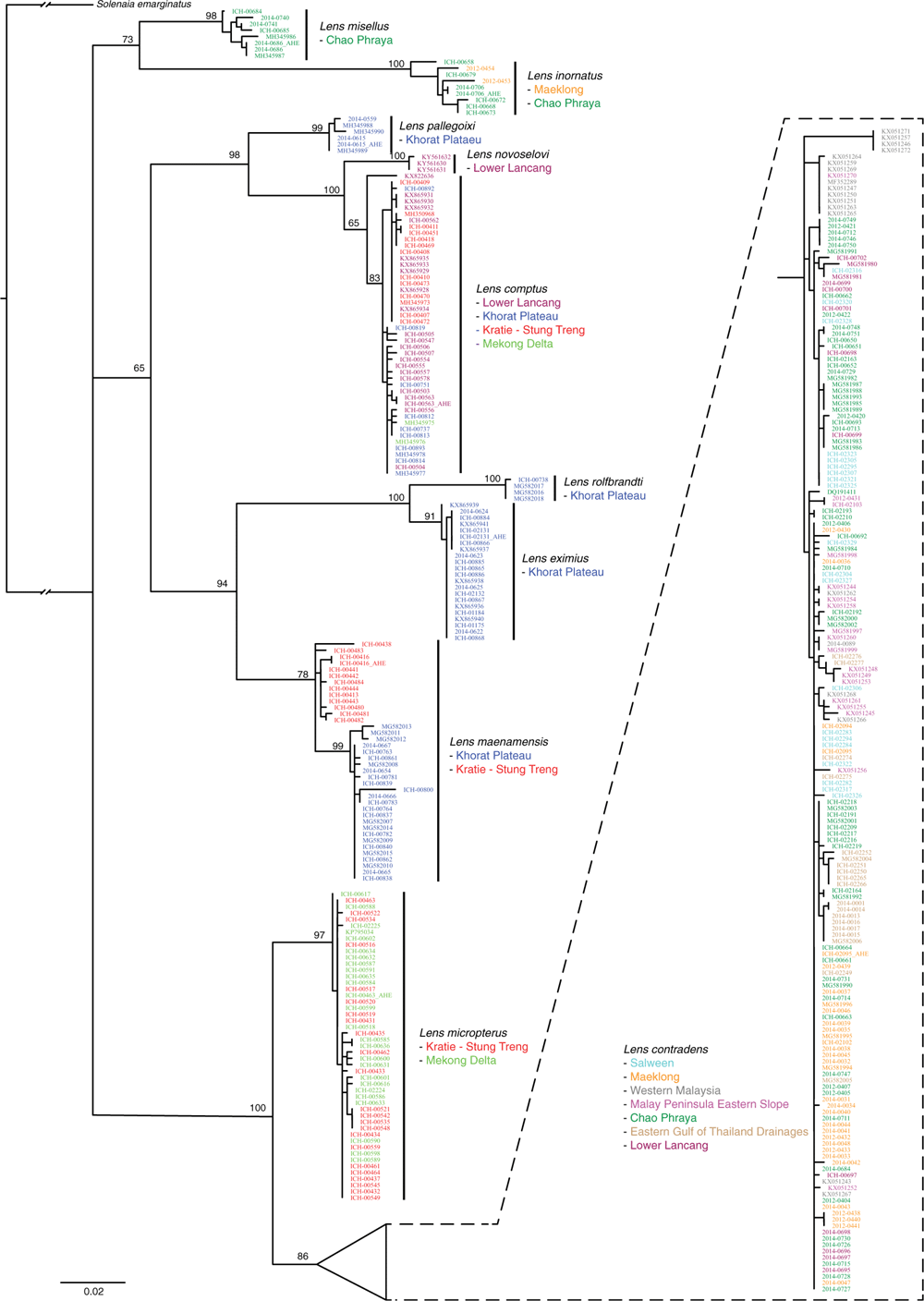
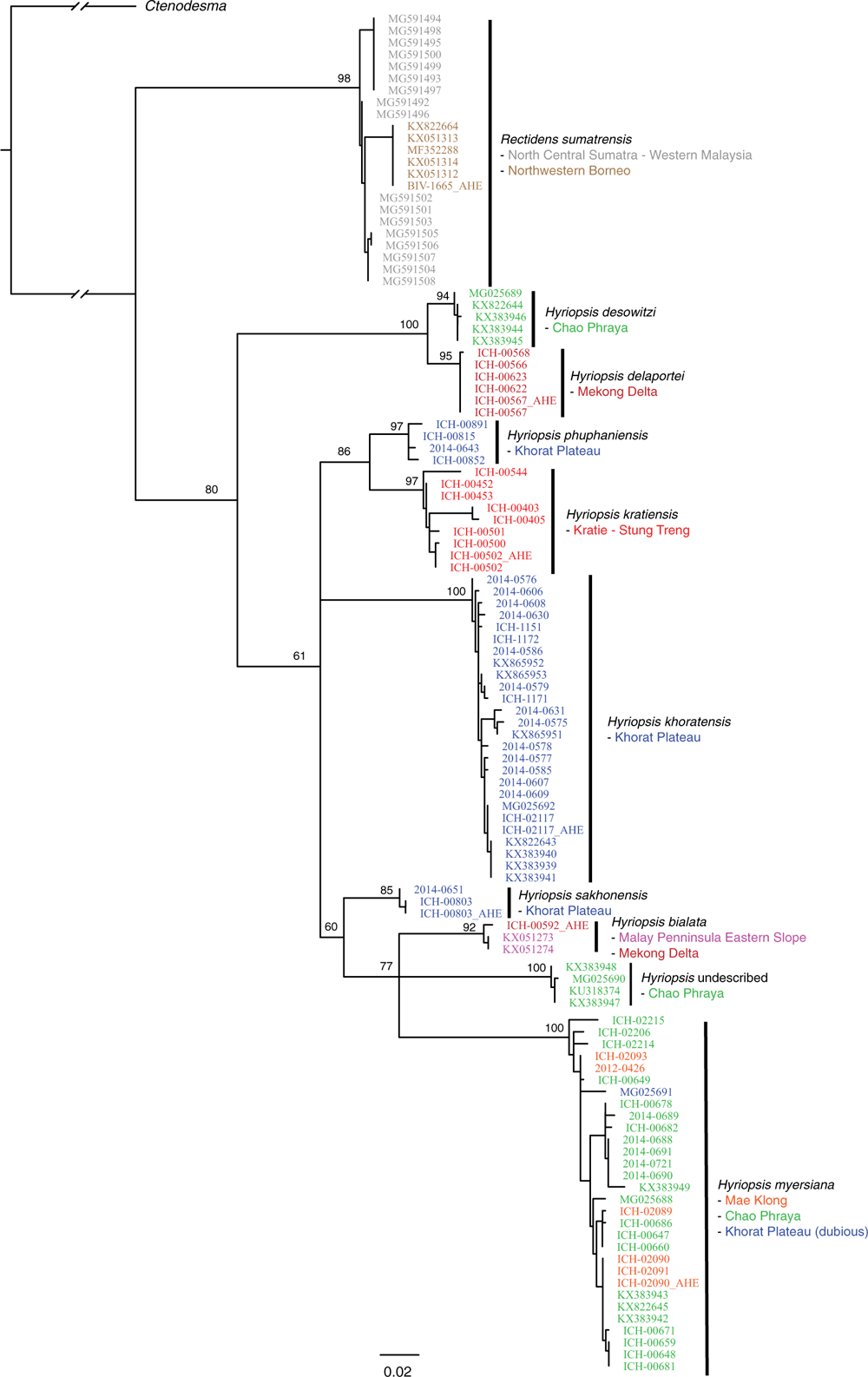
Our final AHE matrices included 29 individuals: Contradentini, 15; Rectidentini, 13; and Lamprotula cornuumlunae (Table 3). Of the 811 targeted probe regions, 538 had a taxon occupancy greater than 70%. We were able to include the head and tail regions (i.e. flanking regions) associated with each of 538 probe regions for a total of 1614 loci in our fully concatenated dataset (Table 4). In comparison to the probe regions, the flanking regions (i.e. heads and tails) were longer (P < 0.01), contained a greater proportion of parsimony informative sites (P < 0.01), had less GC content (P < 0.01), and shared a greater proportion of bipartitions with the fully concatenated tree (Fig. 8).
|
|
Table 4 describes the summary statistics of our five concatenated datasets. Topologies and support values between the five concatenated ML analyses and the ASTRAL analysis were strongly congruent (Fig. 9). The fully concatenated topology (head+probe+tail) and the ASTRAL topology were identical, except with respect to the sister group of Ensidens dugasti. Maximum likelihood topologies generated using the less inclusive datasets (head only, probe only, tail only, and flanks only) and the ASTRAL analysis are available in Fig. S1–S5 of the Supplementary material. Despite the head and tail regions having very similar molecular properties (Table 4, Fig. 8), the two regions recovered incongruent topologies with respect to the relationships within Lens (Fig. 9). However, when the head and tail regions were analysed together (i.e. flanks) the topology was identical to the fully concatenated matrix and the ASTRAL analysis (except the sister group to E. dugasti). In total, 519 COI sequences were generated in this study and were analysed with 341 previously published COI sequences (Table S2 of the Supplementary material) to generate several more taxonomically and geographically inclusive gene trees and better understand the more fine-scale phylogenetic and biogeographic patterns. Tissue samples of the specimens sequenced for these analyses are all available in the Florida Museum’s Genetic Resource Repository to ensure open access and facilitate future research (see https://www.floridamuseum.ufl.edu/grr/).
Taxonomy
Family UNIONIDAE Rafinesque, 1820
Subfamily GONIDEINAE Ortmann, 1916
CONTRADENTINI+RECTIDENTINI
Several recent molecular phylogenetic studies have recovered representatives of the tribes Contradentini and Rectidentini as sister taxa and have referred to that clade as the subfamily Rectidentinae (Pfeiffer and Graf 2015; Lopes-Lima et al. 2017; Bolotov et al. 2018; Konopleva et al. 2019a). However, recent phylogenomic reconstructions have shown that the Rectidentinae is deeply nested within the Gonideinae, rendering the latter subfamily paraphyletic (Pfeiffer et al. 2019). We are not philosophically opposed to recognising paraphyletic taxa but only if doing so communicates something useful about the evolutionary history of the paraphyletic clade. Given the uncertainty surrounding the phylogenetic relationships of the tribes of the Gonideinae (Pfeiffer et al. 2019) and the lack of synapomorphies uniting the higher-level taxa (Lopes-Lima et al. 2017), we see little justification, besides ‘branchyness’, for recognising a subfamily Rectidentinae nested within a paraphyletic subfamily Gonideinae.
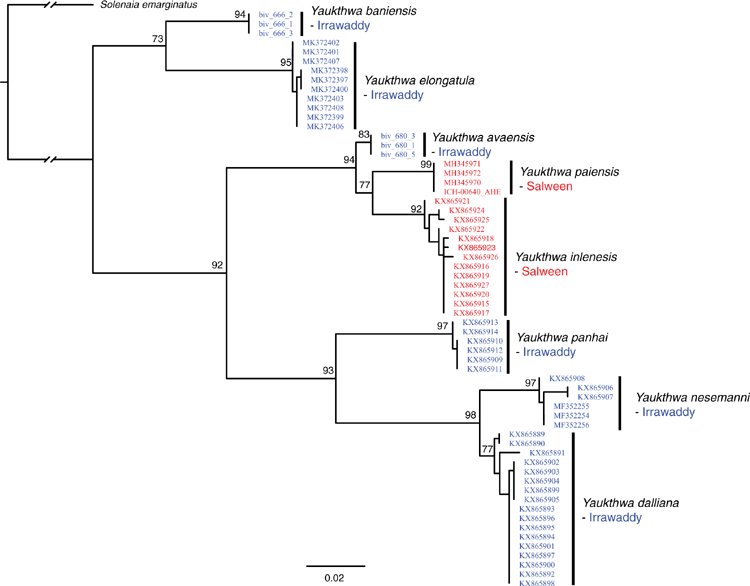
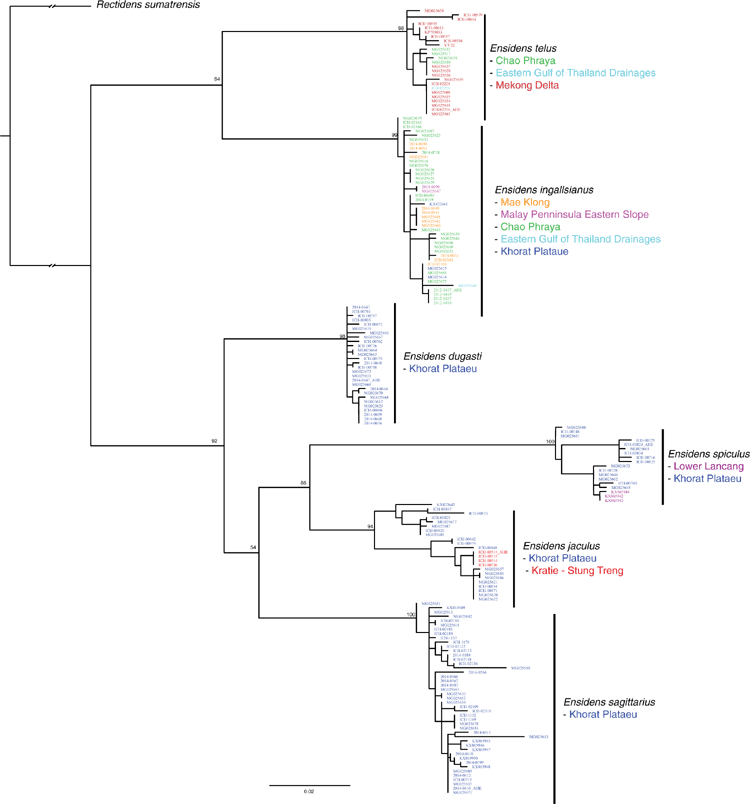
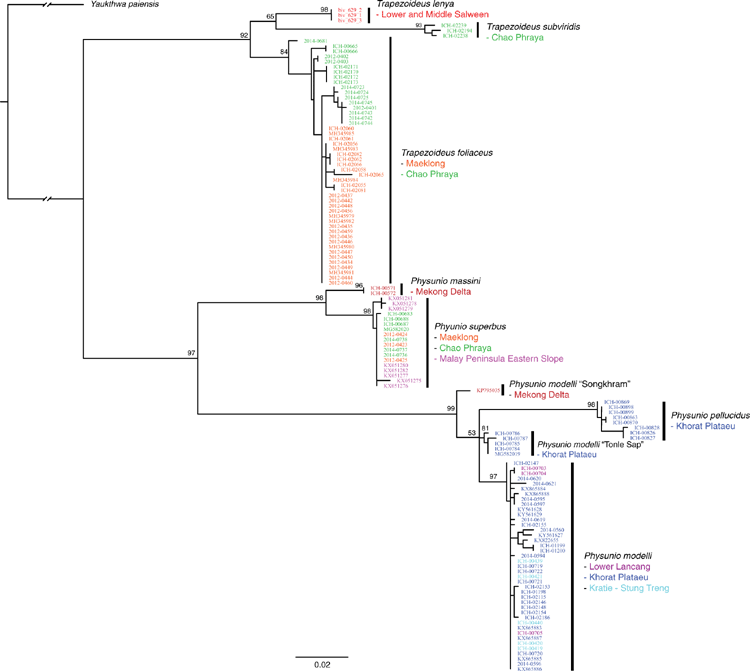
Among this radiation we recognise 54 species, 9 of which are described herein (http://zoobank.org/urn:lsid:zoobank.org:pub:D37EE4AF-C761-403E-BE76-00D0B91E5B33). We included 28 Contradentini+Rectidentini species in our phylogenomic analyses (Fig. 9) and 43 species in our COI gene trees. The 11 species from which we were unable to obtain sequence data are restricted to either the Malay Archipelago (8) or the drainages west of the Salween (3). Within the Contradentini+Rectidentini we observed four independent multispecies radiations endemic to the Mekong drainage – each of which is concentrated in the Khorat Plateau ecoregion (Fig. 9). These four multispecies Mekong radiations are distributed across the phylogeny of the Contradentini (Lens and Physunio) and Rectidentini (Ensidens and Hyriopsis) and share several molecular, morphological and biogeographic patterns, suggesting that common processes shaped the evolution of these clades.
CONTRADENTINI Modell, 1942
Type: Contradens Haas, 1911.
Diagnosis
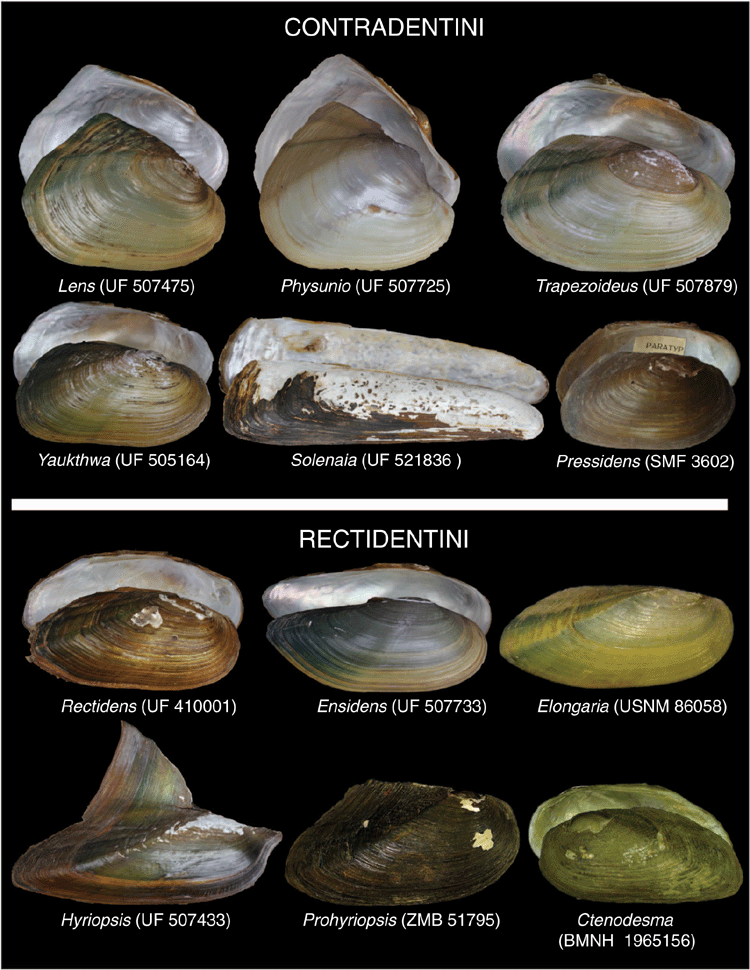
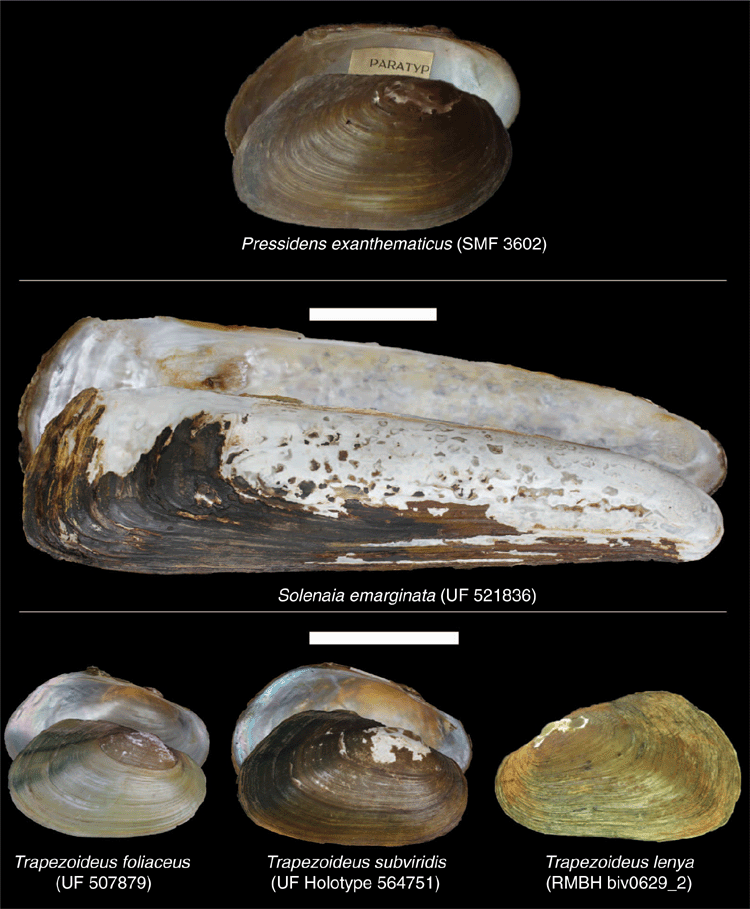
Bilaterally asymmetrical glochidia is a clear synapomorphy of the Contradentini and distinguishes that clade from all other freshwater mussels (Pfeiffer and Graf 2015). Morphologically, the Contradentini most closely resembles its sister tribe, the Rectidentini. In comparison to the Rectidentini, which are more elongate and have a more bluntly pointed posterior end, the Contradentini are more circular or rectangular and have a broadly rounded or square posterior end (Fig. 10). The Contradentini also have a more rounded posterior ridge and longer posterior slope, whereas the Rectidentini tend to have a sharper posterior ridge and a shorter posterior slope (excluding the wing of Hyriopsis). The Contradentini may also be characterised by its generally smooth and shiny, yellow to brown periostracum, often with green rays on the posterior slope (although considerable variation exists among these traits). These green rays typically take the form of two or three green lines corresponding to oblique ridges on the slope. This green pattern on the posterior slope also occurs in other lineages in South-east Asia (e.g. Rectidentini and Indochinellini), but are generally less defined in comparison to the Contradentini. Several taxa also have wrinkles on the posterior slope that run more or less perpendicular to the dorsal shell margin (e.g. Lens, Yaukthwa).
Distribution
Widespread in Indochina from the Irrawaddy drainage in the west to the Mekong drainage in the east. Also occurs on the Malay Peninsula and the islands of Sumatra, Java, Borneo, and Palawan.
Genera
We recognise six genera in the Contradentini. Several of these genera are morphologically quite similar and there are few known characters capable of clearly diagnosing them. The lack of known diagnostic characters for these genera is an unfortunate and all too common shortcoming in freshwater mussel systematics. Nevertheless, these names are useful in that they communicate the monophyly of a collection of species.
-
Lens Simpson, 1900.
-
Physunio Simpson, 1900.
-
Pressidens Haas, 1910.
-
Solenaia Conrad, 1869.
-
Trapezoideus Simpson, 1900.
-
Yaukthwa Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019.
Remarks
The position of Pressidens among the Contradentini remains untested. The umbo sculpture of Pressidens is quite unusual for the Contradentini but this genus shares several other similarities with the clade including shell outline, dentition, and periostracum colour and texture.
Key to the genera of the ContradentiniThe practicality of this key is complicated by considerable intrageneric and intraspecific variation, but it may serve as a useful starting point.
|
1. Circular, ovate, rhomboidal, trapezoidal shell outline |
|
2. Elongate, posterior end <2× taller than anterior |
|
3. Fine zig-zag umbo sculpture Nodulous umbo sculpture |
|
4. Rhomboidal, trapezoidal shell outline |
Genus Lens Simpson, 1900 stat. rev.
Diagnosis
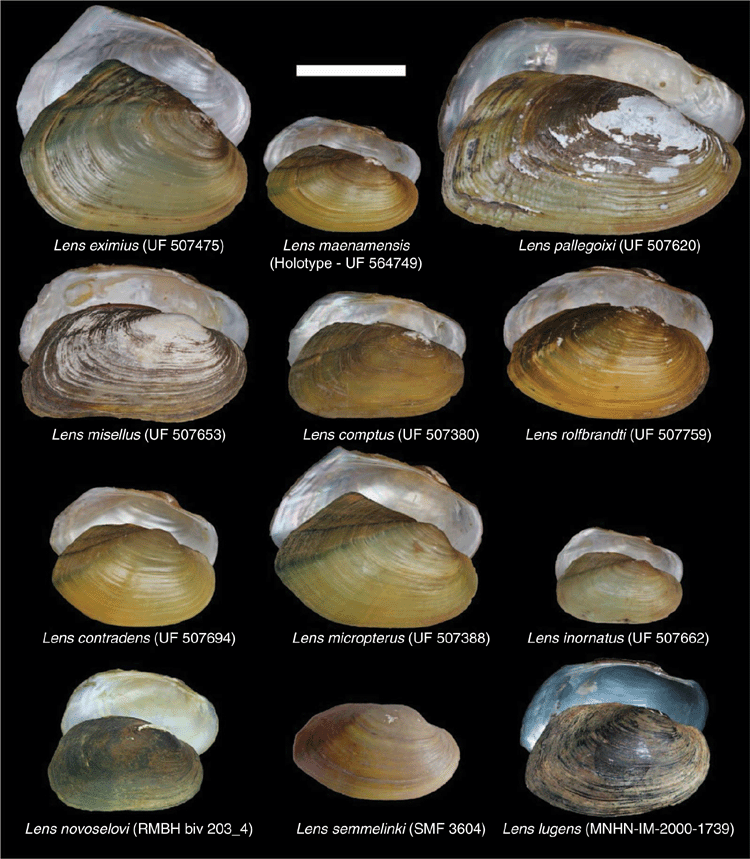
Lens is a morphologically diverse clade (Fig. 11) but is distinguished from its sister taxon, Solenaia, by being much more circular or ovate, as opposed to being ultra-elongate (Fig. 10). Lens often has some form of fine zig-zag umbo sculpture, distinguishing it from Pressidens (thick and wavy), and Yaukthwa, Physunio and Trapezoideus (faintly nodulous).
|
Distribution
Widespread in Indochina from the Salween drainage in the west to the Mekong drainage in the east. Also occurs on the Malay Peninsula and the islands of Sumatra, Java and Borneo.
Taxa
We recognise 12 valid species in the genus Lens (Fig. 11).
-
Lens eximius (Lea, 1856).
-
Lens comptus (Deshayes in Deshayes and Jullien 1876), comb. nov.
-
Lens contradens (Lea, 1838), comb. nov.
-
Lens inornatus (Lea, 1856), comb. nov.
-
Lens lugens (Drouët & Chaper, 1892), comb. nov.
-
Lens maenamensis, sp. nov.
-
Lens micropterus (Morelet, 1866), comb. nov.
-
Lens misellus (Morelet, 1865), comb. nov.
-
Lens pallegoixi (Sowerby, 1867), comb. nov.
-
Lens rolfbrandti (Jeratthitikul & Panha in Jeratthitikul et al. 2019), comb. nov.
-
Lens semmelinki (von Martens, 1891), comb. nov.
-
Lens novoselovi (Konopleva, Bolotov, Spitsyn, Kondakov, Gofarov & Vikhrev, 2019), comb. nov.
Remarks
Specimens representative of the taxon Unio eximius Lea, 1856 were recently recovered within the genus Contradens and the nominal species was revised to Contradens eximius (Lea, 1856) (Bolotov et al. 2017b). However, Unio eximius Lea, 1856 is the type species of Lens Simpson, 1900, and that genus has priority over Contradens Haas, 1911. Although resurrecting the generic status of Lens introduces many novel combinations, it is clearly the senior synonym of Contradens.
Uniandra subcircularis Brandt, 1974 has often been attributed to Contradens (=Lens) (Brandt 1974; Graf and Cummings 2007; Zieritz et al. 2018a; Jeratthitikul et al. 2019), but based on its strongly circular shape and robust cardinal teeth this species may belong to Leoparreysia (Parreysiinae: Leoparreysiini) (Pfeiffer et al. 2018a)
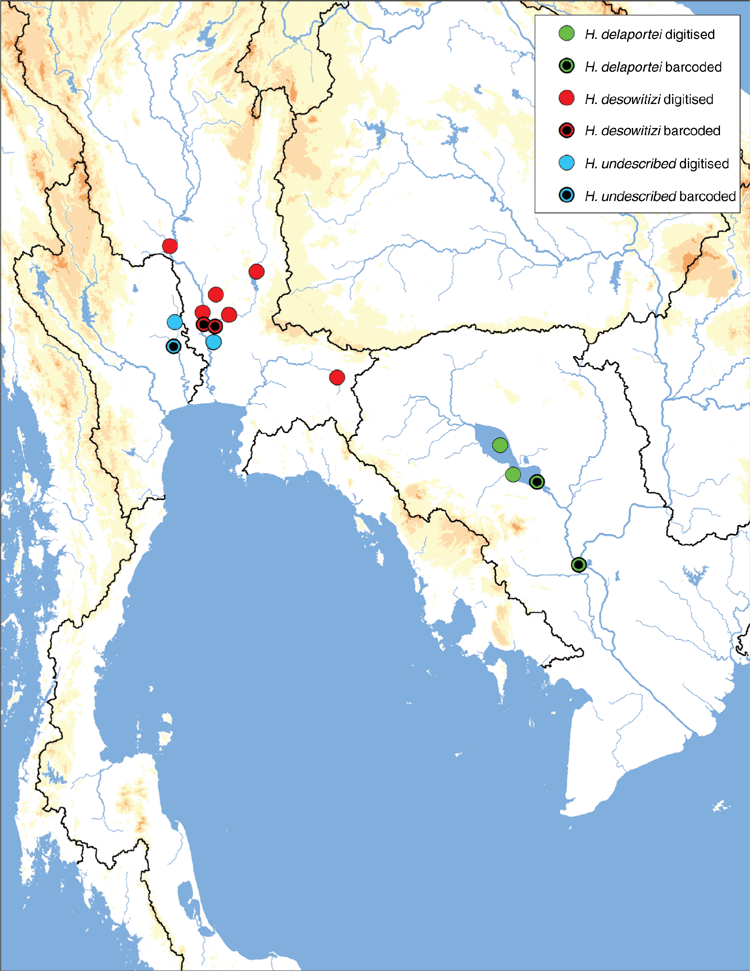
Similar to our phylogenomic reconstruction (Fig. 9) but with greater taxon sampling, our COI phylogeny shows a diverse radiation of at least six Lens species, all of which are endemic to the Mekong drainage (Fig. 12). This pattern of high genetic divergence in and around the Khorat plateau is a recurring pattern and is found in at least three other clades of the Contradentini+Rectidentini. In contrast to this geographically restricted and genetically divergent Mekong radiation is L. contradens, which is distributed across much of western South-east Asia and shows very little geographic structuring (Fig. 12). These clades are closely related but have dramatically different phylogeographic patterns.
Lens eximius (Lea, 1856)
(Tables 1, 3; Fig. 1, 9–13; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
97 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by being very laterally compressed, having a broadly rounded or square posterior end, and a posterior wing that extends well above the umbo.
Distribution
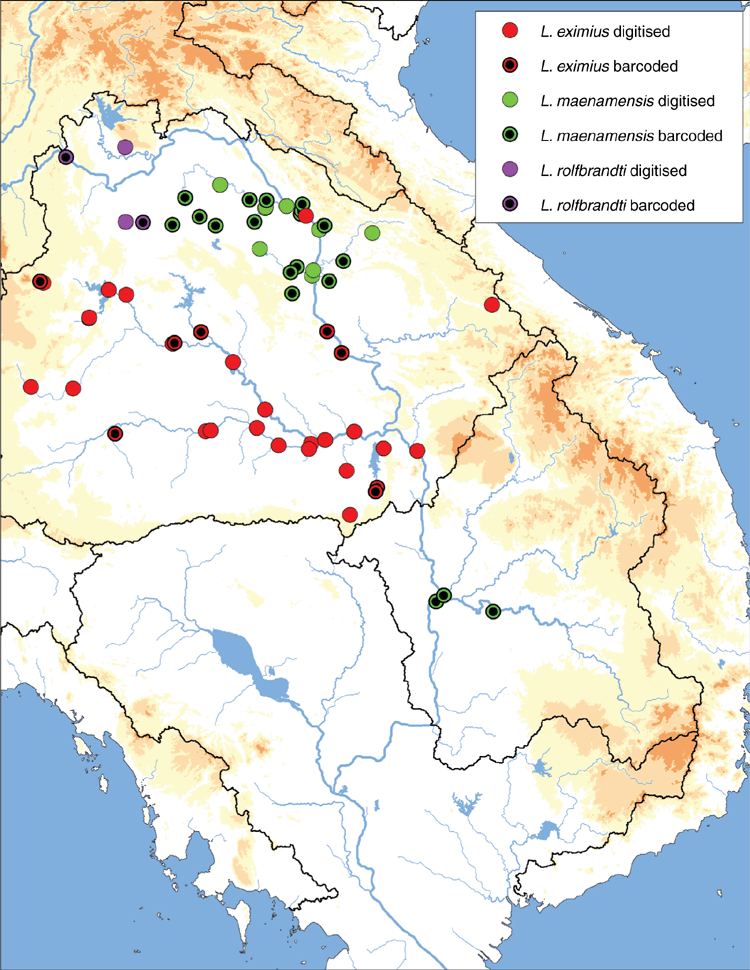
Mekong drainage in eastern Thailand, central Laos, and west-central Vietnam (Fig. 13).
Ecoregion
Khorat Plateau
Remarks
Most records are from the Mun watershed but extralimital populations occur as far north as Nakhon Phanom, Thailand, and as far east as Lao Bao, Vietnam. These geographically divergent populations are morphologically similar to the Mun River populations, but they have not been included in a molecular phylogeny. Research focused on these extralimital populations is warranted. This species has been referred to commonly as the Half Square (Sowerby 1866).
Lens comptus (Deshayes in Deshayes and Jullien 1876), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 14; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
62 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by its rectangular outline and finely sculptured posterior wing. Most similar to L. pallegoixi but has a less trapezoidal shell outline and lacks wrinkles on the ventral edge of the shell disc.
Distribution
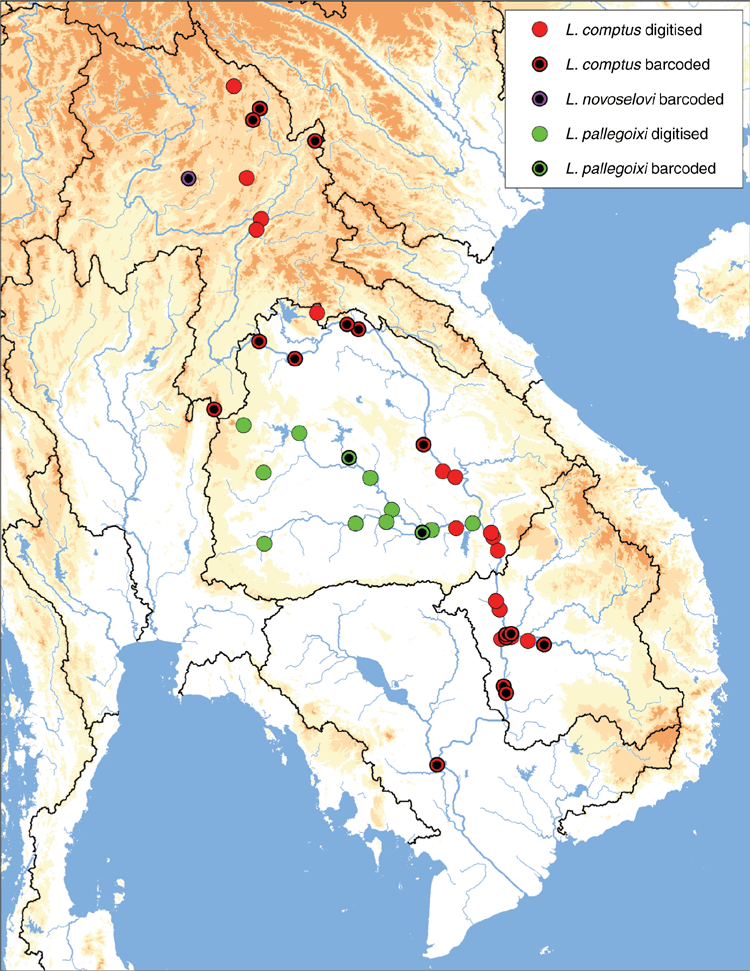
Mekong drainage in eastern Thailand, Laos, north-western Vietnam, and Cambodia (Fig. 14).
Ecoregions
Lower Lancang, Khorat Plateau, Kratie–Stung Treng, and Mekong Delta.
Remarks
This species is geographically widespread and is typically found in or near the Mekong River proper and its larger tributaries (although see exceptions in northern Laos and north-western Vietnam) (Fig. 14). This type of distribution is reminiscent of patterns observed in some North American taxa commonly referred to as ‘large river specialists’ (Haag 2012). Although very little is known about the ecology of L. comptus, its distribution (Fig. 14) and limited genetic diversity (Fig. 12) suggest that this species may also have adaptations associated with a large river ecology, like being a host generalist or relying on highly vagile, large river host fishes (Berg et al. 2007; Roe and Boyer 2015; Pfeiffer et al. 2018b).
Lens contradens (Lea, 1838), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 15; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
375 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by its ovate shell outline, moderate inflation, and a tendency to have thick concentric wrinkles across the shell disc. Most similar to L. rolfbrandti but is less subtriangular and elongate, and more ovate and truncate.
Distribution
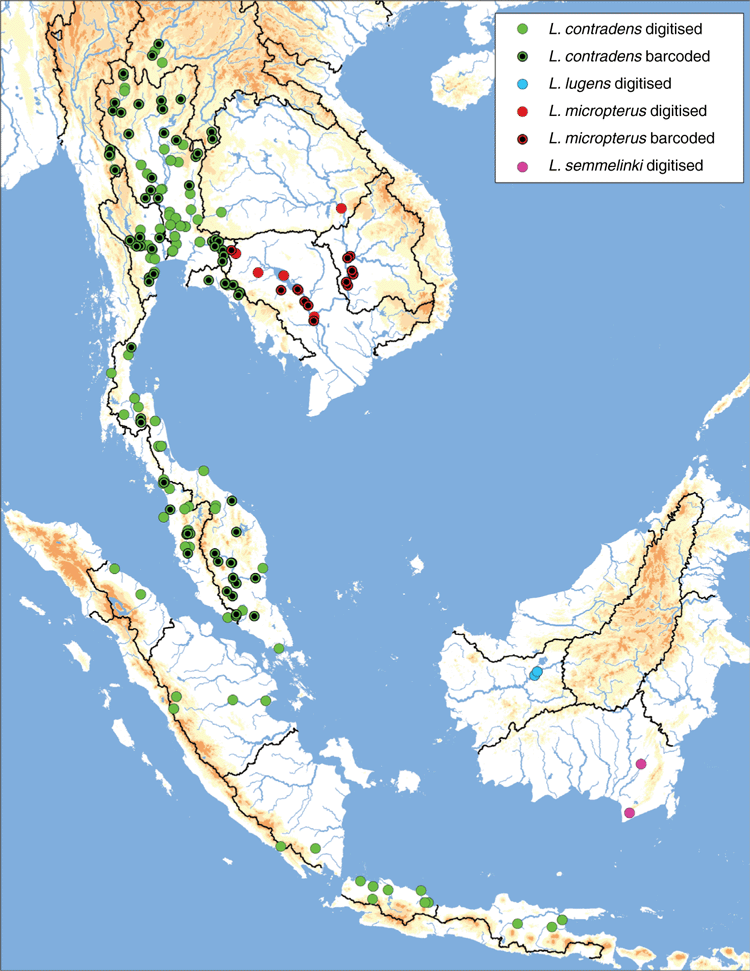
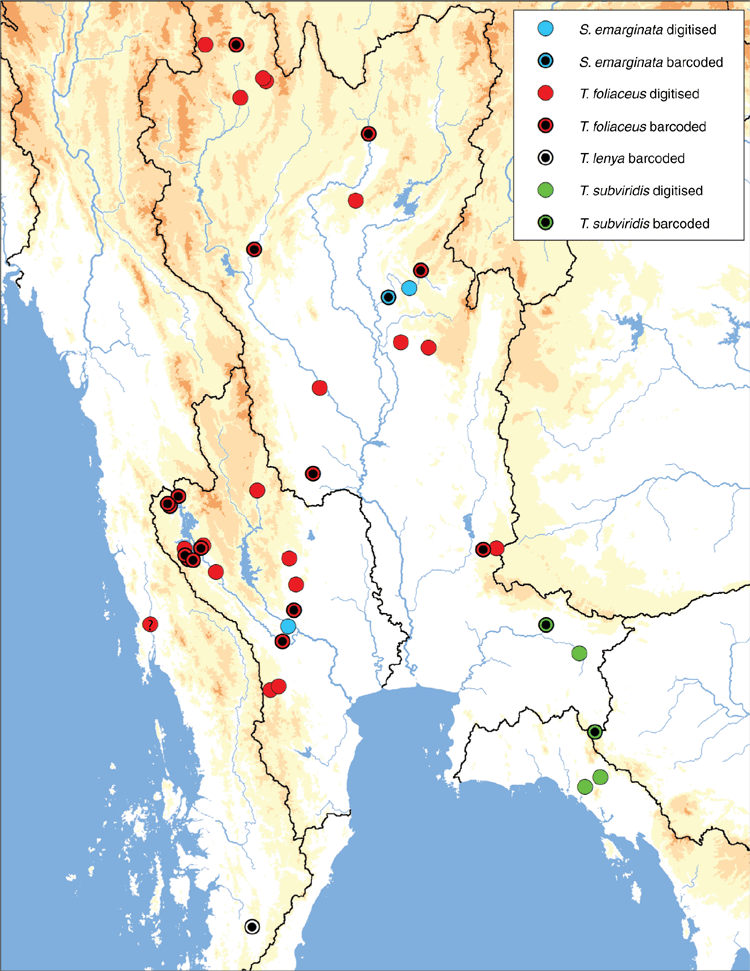
Widespread in the Chao Phraya and Mae Klong drainages in western and central Thailand, as well as the smaller coastal drainages of south-eastern Thailand, Malay Peninsula, Sumatra, and Java (Fig. 15). Nearly absent from the Mekong and Salween drainages; known only from a few geographically disjunct populations in the Mekong (Kok, Ing, Loei watersheds) and Salween (Moei watershed).
Ecoregions
Lower & Middle Salween, Mae Klong, Chao Phraya, Eastern Gulf of Thailand Drainages, Lower Lancang, Northern Central Sumatra–Western Malaysia, Malay Peninsula Eastern Slope, Southern Central Sumatra, Southern Sumatra & Western Java, and Central & Eastern Java.
Remarks
We consider the nominal species U. cambodiensis Lea, 1856 a junior synonym of L. contradens based on its strongly similar shell characteristics. This taxonomic hypothesis is somewhat problematic in that U. cambodiensis was described from ‘Tackrong River at Korat, Cambodia’ and with some interpretation most likely refers to Lam Takhong near Nakhon Ratchasima, Thailand, which is in the Mun watershed (Mekong drainage) and outside the known distribution of L. contradens (Fig. 15 – dot with ‘?’). However, the type locality of U. cambodiensis is unexpectedly precise in comparison to the other type material sent to Issac Lea from William Haines, and it may be dubious. The type of U. cambodiensis Lea, 1856 and at least 15 other type specimens were sent to Issac Lea by William Haines and the locality for each of these types, except Unio cambodiensis, was ‘Siam’. The surprising precision of the type locality of U. cambodiensis raises suspicion about its accuracy. However, some of the material sent to Lea by Haines can be deduced to have come from the Mekong drainage because several of these nominal species are endemic to that drainage (e.g. U. eximius, U. phaselus, U. humilis, U. sagittarius).
Lens contradens is one of the most geographically widespread Contradentini in South-east Asia, occurring in at least 11 ecoregions. Its current distribution (Fig. 15) and limited genetic diversity (Fig. 12) appears to be driven, at least in part, by the increased hydrological connectivity associated with Pleistocene sea level lowering and the formation of several large palaeodrainages on Sundaland (Voris 2000). The Paleo Siam River joined many drainages of the modern Gulf of Thailand (e.g. Mae Klong, Chao Phraya, Bang Pakong, Chantaburi, smaller coastal rivers of eastern Malay Peninsula) and represents most of the L. contradens distribution. The species also appears to have been influenced by the Paleo Malacca Straits River and Paleo East Sunda River, although these populations have not been thoroughly assessed in a phylogenetic context.
Despite its wide distribution, the species is nearly absent from two of the largest drainages in modern-day South-east Asia, the Mekong and Salween, both of which are not typically associated with the Paleo Siam drainage. In both the Salween and Mekong drainages, L. contradens is restricted to just a small fraction of these much larger drainages. These four disjunct populations appear to be the products of stream capture events between these adjacent drainages (see arrows in Fig. 15). Each of the isolated populations in the Mekong (Kok, Ing, Loei) and Salween (Moei) drainages are restricted to relatively small watersheds that generally flow from south to north, the opposite direction in comparison to the drainage’s general flow, creating drainage network geometries that are often characteristic of stream capture (Bishop 1995). All three of the Mekong tributaries, the Kok, Ing and Loei, have been previously hypothesised as areas of drainage rearrangement (Hutchison 1989; Rainboth et al. 2012), and the Moei has recently been hypothesised to have captured the upper reaches of the Mae Klong (Bohlen et al. 2020a, 2020b). The molecular divergences between the Mekong and Chao Phraya samples and Salween and Chao Phraya samples are very shallow, suggesting that the stream captures are recent (Fig. 12). The Lower Lancang and Middle & Lower Salween ecoregions are much more poorly sampled in comparison to other parts of the distribution of L. contradens (Fig. 3) so increased sampling in those ecoregions will be useful in better understanding the distribution of this species in the Mekong and Salween and the number of putative stream-capture events.
Lens contradens is only one of two species in the Contradentini thought to occur in both mainland South-east Asia and the Malay Archipelago (the other being Physunio superbus). However, no L. contradens population from the Malay Archipelago has been included in a phylogenetic analysis (Fig. 15) and their conspecific nature is based solely on their shared morphological similarities. Including putative L. contradens individuals from the Malay Archipelago in a phylogenetic analysis is an important research priority, especially considering the type locality of Unio contradens is ‘Java’ and the importance of these populations in understanding palaeodrainage evolution. Furthermore, there are two putative Lens species endemic to the Malay Archipelago, L. lugens and L. semmelinki, that are also morphologically very similar to L. contradens and may be junior synonyms, but these taxa have also not been included in a molecular phylogeny.
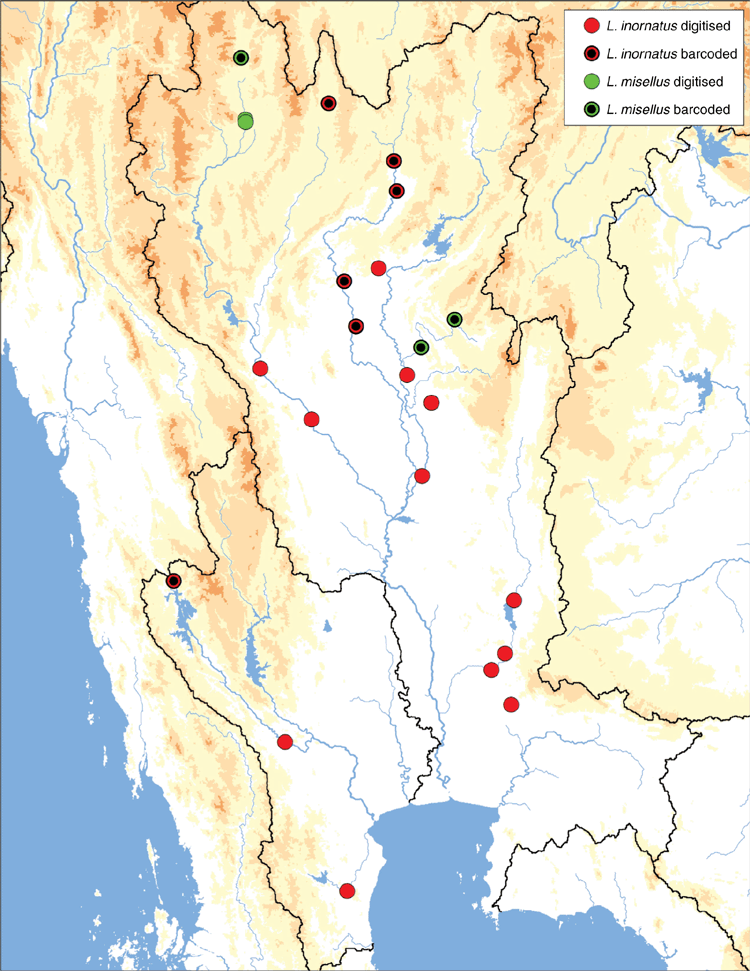
Lens inornatus (Lea, 1856), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 16; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
25 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by having a very thin shell and delicate teeth. Most similar to Trapezoideus foliaceus but is more elongate, dorso-ventrally shorter, and has a more round posterior end.
Distribution
Mae Klong, Chao Phraya, and Bang Pakong drainages in western and central Thailand (Fig. 16).
Ecoregions
Mae Klong, Chao Phraya.
Remarks
Occurs sympatrically with Trapezoideus foliaceus and can be very difficult to distinguish morphologically. Trapezoideus foliaceus tends to be the more common of the two species and is dorso-ventrally taller. Despite the large geographic distances between the sequenced individuals in the Chao Phraya and Mae Klong drainages, the sampled populations are genetically very similar, suggesting recent population expansion that may be associated with the Paleo Siam River (Fig. 12, 16).
Lens lugens (Drouët & Chaper, 1892), comb. nov.
(Table 1; Fig. 1, 11, 15; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
31 lots (Table S1).
Diagnosis
Unknown
Distribution
Kapuas drainage in Borneo (Fig. 15).
Ecoregion
Kapuas.
Remarks
The validity of this taxon is uncertain – it may be a junior synonym of L. contradens. The species has not yet been included in a molecular phylogeny.
Lens maenamensis, sp. nov.
(Tables 1, 3; Fig. 1, 9, 11–13; Tables S1, S2; Fig. S1–S5)
Type locality
Tonle Srepok River, Highway 78 bridge between Stung Treng and Ban Lung, Stung Treng Province, Cambodia, 13.54504, 106.01353.
Types
Holotype, UF 564749. Paratypes, UF 507392 (n = 5), same locality as the holotype.
Taxonomic opinions since Haas (1969a)
Material examined
46 lots (Table S1).
Diagnosis
Shell characteristics similar to L. contradens but tends to be more elongate and bluntly pointed.
Description
Shell morphology highly variable, compressed, ovate, and thin-shelled (especially in southern populations) to inflated, circular, and thick-shelled (especially in northern populations). Periostracum, yellowish-brown to brown, often with 2–3 green rays on the posterior slope. Faint, wide, wavy sculpturing on the posterior slope and shell disc (especially in northern populations). Pseudocardinal and lateral teeth nearly parallel, blade-like. Left valve with two lateral teeth, long, slightly curved, diverging. Left valve with one long pseudocardinal tooth, small triangular swelling at posterior end. Right valve with one lateral tooth, long, slightly curved. Right valve with two pseudocardinal teeth, ventral tooth much larger than dorsal, with small triangular swelling at posterior end. Umbo shallow. Nacre iridescent, very faintly purplish-blue, and translucent. Pallial fusion between supra-anal and excurrent larger than supra-anal aperture. Excurrent aperture smooth, shorter than incurrent. Incurrent aperture with multiple rows of long simple papillae. Demibranchs perforated. Interior demibranch attached to visceral mass only anteriorly. Ectobranchous brooding, marsupium laterally swollen, blush when glochidia are fully developed. Bilaterally asymmetrical glochidia.
Distribution
Mekong drainage in Laos, Thailand and Cambodia. Populations concentrated near the confluences of the Songkhram and the Mekong, and the Sekong and the Mekong (Fig. 13).
Ecoregions
Khorat Plateau, Kratie–Stung Treng.
Remarks
Jeratthitikul et al. (2019) recently recovered this molecular clade and resurrected the name Contradens crossei. However, other than the type specimen of U. crossei, Jeratthitikul et al. (2019) did not consider any Contradens (=Lens) specimens from the southern half of the Mekong drainage (see their Fig. 2). The omission of the available specimen data (i.e. museum specimens and molecular data) from this region compromised several of their hypotheses. More inclusive sampling of Lens from the Mekong drainage suggests that U. crossei is a junior synonym of L. micropterus (Morelet, 1866), and C. crossei sensu Jeratthitikul et al. (2019) represents a previously undescribed species (=L. maenamensis).
Jeratthitikul et al. (2019) resurrected C. crossei for a divergent molecular clade restricted to north-eastern Thailand. However, the type locality of Unio crossei Deshayes in Deshayes and Jullien 1876 is ‘Cambodge’ and is likely quite geographically distant from their closest samples in north-eastern Thailand. We collected Lens specimens from across Cambodia and found many specimens resembling the types of U. crossei and U. micropterus Morelet, 1866, which were molecularly very similar. Based on these findings we hypothesise that U. crossei is a junior synonym of Unio micropterus and the clade referred to as C. crossei in Jeratthitikul et al. (2019) represents a new species, L. maenamensis. Furthermore, our more inclusive taxon sampling of Lens demonstrates that L. rolfbrandti and L. maenamensis are quite distantly related to L. contradens, and the reported monophyly of these taxa is entirely artificial and a product of incomplete taxon sampling. As such, the biogeographic interpretation predicated on that hypothesis should be reconsidered.
Lens maenamensis is composed of two molecularly divergent clades, one is restricted to the Khorat Plateau and the other to the Kratie–Stung Treng ecoregion (Fig. 12). These two geographically disjunct clades appear to have some morphological disparities – the southern clade is more elongate and has a bluntly pointed posterior end while the northern population is more circular and broadly rounded and tends to have wide, wavy sculpturing on the shell disc. These divergent populations may represent distinct species, but further research is necessary.
Lens micropterus (Morelet, 1866), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 15; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
111 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by its moderate sized wing, straight ventral margin, and moderate inflation.
Distribution
Mekong drainage in Thailand, Cambodia and Laos. Majority of records from the Cambodian Mekong (Fig. 15).
Ecoregion
Khorat Plateau, Kratie–Stung Treng, Mekong Delta.
Remarks
In the westernmost part of the L. micropterus distribution (i.e. Tonle Sap watershed) some specimens look morphologically identical to its sister species, L. contradens. Despite the strong morphological similarities to L. contradens these specimens maintain L. micropterus haplotypes. The Tonle Sap watershed is thought to be outside of the native range of L. contradens but given the economic importance of fisheries in the Tonle Sap (Hap et al. 2006), including non-native molluscs (Ngor et al. 2018), L. contradens may have been introduced and could be hybridising with L. micropterus. Further research is needed.
Lens misellus (Morelet, 1865), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 16; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
14 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by its strongly reduced dentition in adults. Solenaia also has dramatically reduced dentition but is easily distinguished from L. misellus by its ultra-elongate shell shape.
Distribution
Chao Phraya drainage in northern Thailand (Fig. 16).
Ecoregion
Chao Phraya.
Remarks
In adult specimens of L. misellus the pseudocardinal and lateral teeth are greatly reduced or absent, while young specimens have very fine but distinct teeth. This reduction in dentition is unusual among the Contradentini but also occurs in Solenaia. Lens misellus is also unusual in that it is the only Contradentini endemic to the Chao Phraya drainage.
Lens novoselovi (Konopleva, Bolotov, Spitsyn, Kondakov, Gofarov & Vikhrev, 2019), comb. nov.
(Table 1; Fig. 1, 11, 12, 14; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Lens novoselovi is thought to be distinguished from its sister species, L. comptus, by its smaller size, more ovate shell shape, more broadly rounded anterior margin, and less sculpturing on the posterior slope (Konopleva et al. 2019b).
Distribution
Known only from the type locality in the Mekong drainage, near Vieng Phou Kha, north-western Laos (Fig. 14).
Ecoregion
Lower Lancang.
Remarks
Lens novoselovi is the only species of the Contradentini or Rectidentini thought to be endemic to the Lower Lancang ecoregion. However, the Lower Lancang ecoregion is poorly characterised in comparison to the other Mekong ecoregions (Fig. 2, 3) and continued sampling in the region is likely to reveal greater species diversity (e.g. see Contradentini sp. account in problematic taxa section).
Lens pallegoixi (Sowerby, 1867), comb. nov.
(Tables 1, 3; Fig. 1, 9, 11, 12, 14; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
30 lots (Table S1).
Diagnosis
Distinguished from other species of Lens by strong lateral compression, trapezoidal outline and, commonly, wrinkles on the ventral margin of the shell disc. Most similar to L. comptus but is less rectangular and more trapezoidal in shell outline.
Distribution
Mekong drainage in eastern Thailand (Fig. 14).
Ecoregion
Khorat Plateau.
Remarks
Despite many sampling events in the nearby Mekong River proper (Fig. 2), this species was never recovered and appears to be restricted to the Mun River watershed (Fig. 14).
Lens rolfbrandti (Jeratthitikul & Panha in Jeratthitikul et al. 2019), comb. nov.
(Table 1; Fig. 1, 11–13; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
4 lots (Table S1).
Diagnosis
Morphologically very similar to L. contradens and L. maenamensis but tends to have a more elongate shell outline.
Distribution
Known from a few localities in the Mekong drainage near Vientiane, Laos (Fig. 13).
Ecoregion
Khorat Plateau.
Remarks
Lens rolfbrandti was previously thought to be restricted to its type locality (Jeratthitikul et al. 2019); recent field and museum sampling has expanded the known distribution of the species to include several localities in the northern part of the Khorat Plateau ecoregion (Fig. 13).
Lens semmelinki (von Martens, 1891), comb. nov.
(Table 1; Fig. 1, 11, 15; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
3 lots (Table S1).
Diagnosis
Unknown.
Distribution
Coastal drainages of southern Borneo (Fig. 15).
Ecoregion
South-eastern Borneo.
Remarks
The validity of this species is uncertain, and it may be a synonym of L. contradens. The species has not yet been included in a molecular phylogeny.
Genus Physunio Simpson, 1900
Diagnosis
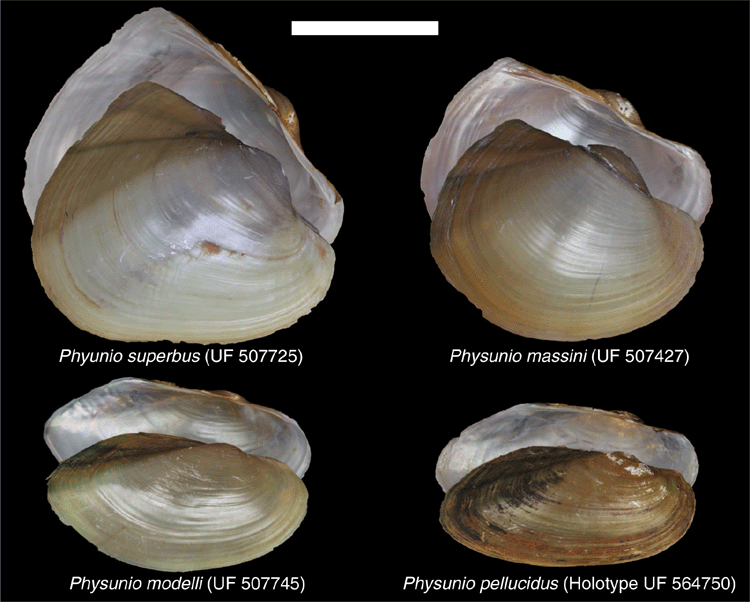
This taxon can be distinguished from its sister taxon Trapezoideus by its generally larger and thinner shells. Species of Physunio tend to have a more circular (P. superbus and P. massini) or elongate (P. modelli and P. pellucidus) shell outline (Fig. 17), in comparison to the more rhomboidal or trapezoidal shape of Trapezoideus.
Distribution
From the Mae Klong drainage in the west to the Mekong drainage in the east. Also occurs in the Malay Peninsula and a few records from the islands of Sumatra and Java.
Species
We recognise four valid species in the genus Physunio (Fig. 17).
-
Physunio superbus (Lea, 1843).
-
Physunio massini (Morelet, 1864), stat. rev.
-
Physunio modelli Brandt, 1974.
-
Physunio pellucidus, sp. nov.
Remarks
Velunio Haas, 1919 has occasionally been considered a junior synonym or subgenus of Physunio (Modell 1964; Haas 1969a; Subba Rao 1989; Ramakrishna and Dey 2007) but its type species, Unio velaris Sowerby 1868, from ‘Assam’ does not resemble most Contradentini and more likely belongs to Lamellidens Simpson, 1900 (Parreysiinae: Lamellidentini). However, this point is mostly unnecessary because Velunio Haas, 1919 is not an available name because its type species Unio velaris Sowerby, 1868 is a misidentified reference to Unio velaris Hanley, 1856 and is therefore not available, and by extension neither is Velunio Haas, 1919.
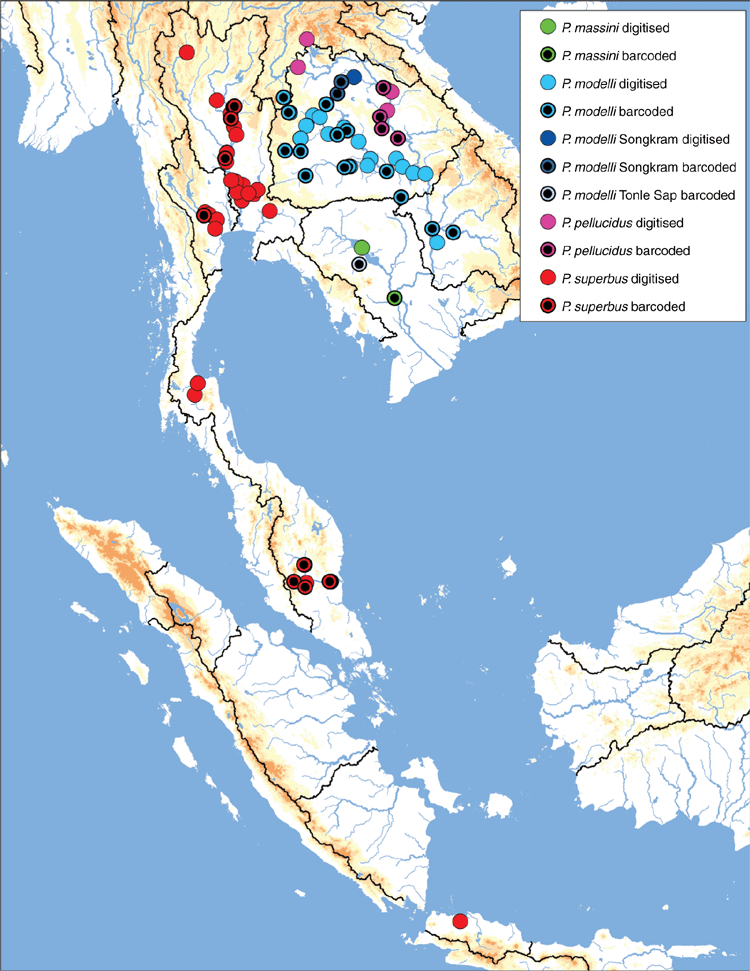
There are two morphologically divergent clades within Physunio (Fig. 18): a clade of strongly inflated and circular mussels (P. superbus and P. massini) and a clade of much more compressed and elongate mussels (P. modelli and P. pellucidus).
|
Physunio superbus (Lea, 1843)
(Tables 1, 3; Fig. 1, 9, 10, 17–19; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
97 lots (Table S1).
Diagnosis
Morphologically very similar to P. massini but tends to be less inflated and has a less prominent umbo sculpture.
Distribution
Widespread in the Mae Klong, Chao Phraya, and Bang Pakong drainages in eastern and central Thailand, as well as the Malay Peninsula. Also known from Sumatra and Java (Fig. 19).
Ecoregions
Mae Klong, Malay Peninsula Eastern Slope, Southern Sumatra–Western Java, Chao Phraya.
Remarks
Haas (1969a) and Brandt (1974) considered Unio macropterus Dunker, 1846 a junior synonym of P. superbus but the type locality of that nominal species is from Brazil. We follow Simone (2006) and consider Unio macropterus Dunker, 1846 a junior synonym of Prisodon obliquus Schumacher, 1817. Despite occupying several geographically divergent drainages, all the sampled P. superbus individuals are molecularly very similar (Fig. 18). Physunio superbus is one of the two Contradentini species thought to occur in both mainland South-east Asia and the Malay Archipelago (the other being L. contradens). Physunio superbus populations on the Malay Archipelago are known from only a few records and while some specimens strongly resemble mainland South-east Asia populations (e.g. UMMZ 110123), others are much more strongly compressed and elongate (e.g. SMF 14736) and may represent an unrecognised species. Sampling putative P. superbus populations from the Malay Archipelago will be necessary to understanding its species boundaries and the historical freshwater connections between mainland south-east Asia and the Malay Archipelago.
Local common names for this species are loosely translated in English to ‘rice soup mussel’ or ‘cow nose mussel’ (Nabhitabhata 2009).
Physunio massini (Morelet, 1864), stat. rev.
(Table 1; Fig. 1, 17–19; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
19 lots (Table S1).
Diagnosis
Morphologically very similar to P. superbus but tends to be more inflated and has a more nodulous umbo sculpture.
Distribution
Mekong drainage in Cambodia (Fig. 19).
Ecoregion
Mekong Delta.
Remarks
Physunio massini is herein resurrected from synonymy to recognise that the Mekong populations previously identified as P. superbus represent a well supported and phylogenetically divergent lineage distinct from P. superbus. Unlike its sister species, P. superbus, which is geographically widespread, P. massini is known only from the Mekong Delta ecoregion (Fig. 19).
Physunio modelli Brandt, 1974
(Tables 1, 3; Fig. 1, 9, 17–19; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
55 lots (Table S1).
Diagnosis
Morphologically very similar to its sister species, P. pellucidus, but tends to be taller and have a higher posterior wing. Very thin shell and delicate teeth in comparison to other Contradentini of its size.
Distribution
Mekong drainage in Thailand, Laos, and Cambodia. Primarily found in the Mun River watershed but also occurs in the Loei, Songkhram, Stung Treng, and Tonle Sap watersheds (Fig. 19).
Ecoregions
Lower Lancang, Khorat Plateau, Kratie–Stung Treng, Mekong Delta.
Remarks
We did not recover P. modelli as monophyletic (Fig. 18). Specimens from the Songkhram river (P. modelli ‘Songkhram’) were recovered in a divergent lineage that formed a polytomy with the P. modelli specimens from the Khorat Plateau, Kratie–Stung Treng, and Lower Lancang ecoregions, and P. pellucidus. A specimen from the Tonle sap (P. modelli ‘Tonle Sap’) represents a third lineage reluctantly assigned to P. modelli. Greater geographic and molecular character sampling is necessary to circumscribe the species boundaries in this clade. The distribution of the P. modelli clade (in the strictest sense) suggests that there has been an intradrainage stream-capture event; where the headwaters of the Mun watershed were captured by the Loei watershed (Fig. 19). This is unlike all the other hypothesised stream captures in that the faunal exchange occurred between two adjacent watersheds of the same drainage, and not between two watersheds of different drainages.
Physunio pellucidus, sp. nov.
(Tables 1, 3; Fig. 1, 9, 17–19; Tables S1, S2; Fig. S1–S5)
Type locality
Thuai River, Rt. 212 bridge S of Tha Uthen, Nakhon Phanom Province, Thailand, 17.56200, 104.60900.
Type
Holotype, UF 564750. Paratype, UF 507799 (n = 2), same locality as the holotype.
Taxonomic opinions since Haas (1969a)
Material examined
9 lots (Table S1).
Diagnosis
Morphologically very similar to its sister species, P. modelli, but tends to be narrower and have a less prominent posterior wing. Very thin shell and delicate teeth in comparison to other Contradentini of its size.
Description
Shell elongate, thin, translucent. Anterior end short, broadly rounded. Posterior end long, tapering, bluntly pointed. Dorsal margin straight. Ventral margin slightly curved to straight. Posterior ridge broadly rounded. Posterior slope steep, especially near umbo. Periostracum smooth, yellowish-brown, occasionally dull grey-green. Posterior slope often with two green rays. Umbo shallow. Nacre iridescent, very faintly purpleish-blue, translucent. Pseudocardinal and lateral teeth parallel, blade-like, delicate. Left valve with two lateral teeth, dorsal tooth much shorter than ventral. Left valve with one pseudocardinal, long, taller than laterals. Right valve, with one lateral tooth, long, straight. Left valve with two long pseudocardinal. Supra-anal aperture short. Pallial fusion between supra-anal and excurrent 3× times larger than supra-anal aperture. Excurrent aperture smooth, equal in length to incurrent. Incurrent aperture with multiple rows of long simple papillae. Demibranch perforated. Interior demibranch attached to visceral mass only anteriorly. Ectobranchous brooding, marsupium laterally swollen, blush when glochidia are fully developed. Bilaterally asymmetrical glochidia.
Distribution
Mekong drainage in Thailand and Laos (Fig. 19).
Ecoregions
Lower Lancang, Khorat Plateau.
Remarks
This species is split out of P. modelli to recognise that there are two morphologically similar but well supported and phylogenetically divergent lineages occurring in the Khorat Plateau ecoregion of the Mekong drainage. Interestingly, this species is found primarily in or near the Mekong River proper and is largely absent from the Mekong’s larger watersheds. The opposite pattern is true for its sister species, P. modelli, which is found primarily in the Mekong’s larger watersheds and absent from the Mekong proper (Fig. 19).
Genus Pressidens Haas, 1910
Diagnosis
Thick wrinkled umbo sculpture distinguishes this taxon from all other taxa in the Contradentini.
Distribution
The islands of Borneo, Banguey Island, and Palawan.
Species
We recognise one valid species in the genus Pressidens (Fig. 20).
|
-
Pressidens exanthematicus (Küster, 1861)
Remarks
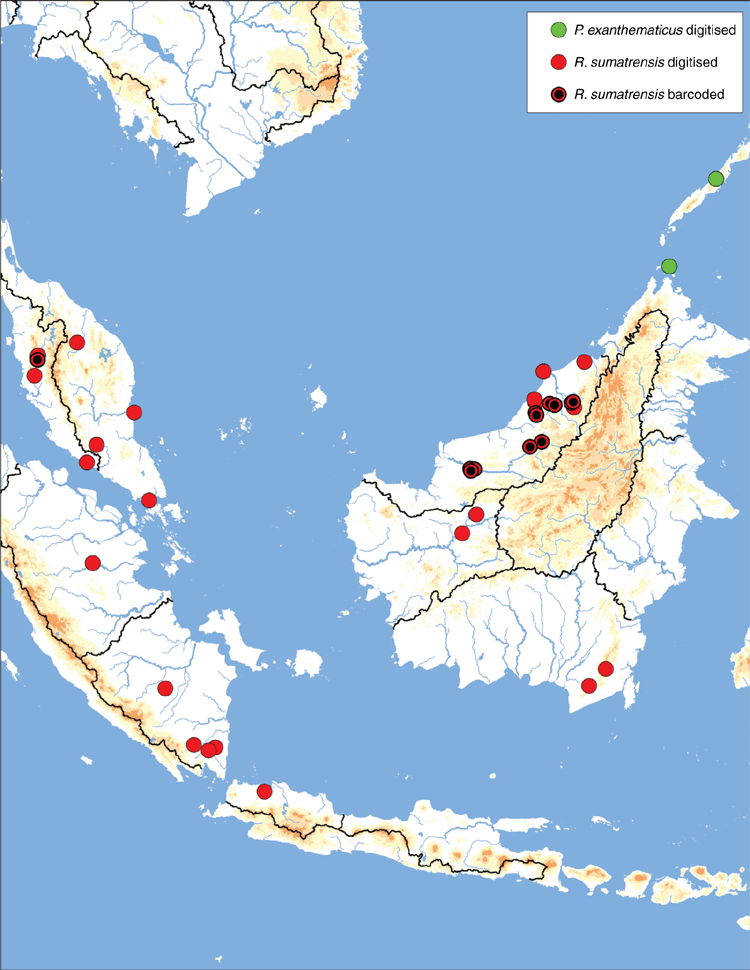
Like many of the freshwater mussels of the Malay Archipelago this hypothesised taxon is poorly understood. Its position among the Contradentini remains untested but it is similar to other members of the tribe in that it has a thin shell, delicate teeth, and a broadly rounded posterior slope (Fig. 20). However, the strongly wrinkled umbo is unlike most other members of the Contradentini. Resolving the systematic position of this taxon will be critical to a better understanding of freshwater mussel biogeography in South-east Asia.
Pressidens exanthematicus (Küster, 1861)
(Table 1; Fig. 1, 10, 20, 21; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
27 lots (Table S1).
Diagnosis
Same as the genus.
Distribution
Known from the islands of Borneo, Banguey and Palawan (Fig. 21).
Ecoregions
North-eastern Borneo, North-western Borneo, and Palawan.
Remarks
In the recent past this species has been treated as three distinct species: P. exanthematicus (Borneo), P. insularis (Borneo and Palawan), and P. moellendorffi (Palawan) (see taxonomic opinion section above). Rather than three species with partially overlapping ranges on three separate islands, we hypothesise that Pressidens is monotypic. While there is considerable morphological variation in the examined material, it does not appear to vary as a function of the three nominal species or the islands in which they are thought to occur. Given the available data, this conservative hypothesis of a single Pressidens species seems more useful. The species has not yet been included in a molecular phylogeny.
Genus Solenaia Conrad, 1869
Diagnosis
Solenaia can be distinguished from all other Contradentini by its ultra-elongate shell shape and the lack of dentition (at least in adults) (Fig. 10).
Distribution
Western and central Thailand.
Species
We recognise one species in the genus Solenaia (Fig. 20).
-
Solenaia emarginata (Lea, 1860).
Remarks
Solenaia was previously thought to be a quite diverse and geographically widespread Asian genus (5 species, Graf and Cummings 2007). The genus was recently hypothesised to be polyphyletic based on the presence of divergent larval morphologies between its putative species (bilaterally symmetrical and bilaterally asymmetrical glochidia) and a strongly disjunct reported range (India, Thailand, China) (Pfeiffer and Graf 2015). Recent molecular phylogenies have supported that hypothesis – recovering Solenaia species in three divergent clades of the Gonideinae (Lopes-Lima et al. 2017; Huang et al. 2019; Pfeiffer et al. 2019). Huang et al. (2019) partially revised Solenaia sensu latu by describing the new genus, Parvasolenaia Huang & Wu in Huang et al. (2019), that included P. rivularis, P. triangularis and P. neotriangularis. However, Huang et al. (2019) suggested that the ‘other’ Chinese Solenaia (S. oleivora [=S. iridinea] and S. carinata) would be recovered in a clade with the type species of Solenaia, S. emarginata, from Thailand. However, that hypothesis is untenable because S. oleivora [=S. iridinea] and S. carinata belong to the Gonideini and have unhooked glochidia (Huang et al. 2013; Huang et al. 2019) while S. emarginata belongs to the Contradentini and has bilaterally asymmetrical glochidia (Deein et al. 2008; Pfeiffer and Graf 2015). As such, ‘S.’ oleivora (=‘S’. iridinea) and ‘S.’ carinata do not belong to Solenaia or the Contradentini and appear to belong to a separate, currently unrecognised genus in the tribe Gonideini.
A similar morphologically untenable hypothesis was recently proposed for the Indian taxa formally attributed to Solenaia. Balwantia Prashad, 1919 has been considered a junior synonym of Solenaia since Subba Rao (1989) but was recently recognised as a valid genus in the Contradentini and is thought to consist of three species – B. soleniformis, B. baniensis and B. elongatula (Bolotov et al. 2020). However, the type species of Balwantia, Anodonta soleniformis Benson, 1836, has unhooked glochidia (Prashad 1919, Pfeiffer and Graf 2015) and is very unlikely to belong to the Contradentini because that taxon is diagnosed by having bilaterally asymmetrical glochidia. Whereas, ‘B.’ baniensis, and ‘B.’ elongatula are clearly part of the Contradentini and closely related to Yaukthwa (Fig. 25) and those taxa are herein transferred to Yaukthwa. See remarks in the taxonomic account of Yaukthwa for further details regarding that hypothesis.
We recognise Solenaia as monotypic. The Chinese taxa recently attributed to Solenaia (i.e. ‘S.’ oleivora [=‘S’. iridinea] and ‘S.’ carinata) appear to belong to a currently unrecognised genus of the Gonideini, while the Indian species formally attributed to Solenaia (‘S.’ soleniformis) is likely to represent the genus Balwantia – whose systematic position remains largely unknown, but based on our understanding of synapomorphy is not part of Contradentini and may belong to the Parreysiinae (Pfeiffer and Graf 2015).
Solenaia emarginata (Lea, 1860)
(Tables 1, 3; Fig. 1, 9, 10, 12, 20, 22; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
3 lots (Table S1).
Diagnosis
Same as for genus.
Distribution
Mae Klong and Chao Phraya drainages of western and central Thailand (Fig. 22).
|
Ecoregions
Mae Klong, Chao Phraya.
Remarks
We consider Solenaia khwaenoiensis Panha & Deein in Deein et al. (2004) a junior synonym of Mycetopus emarginatus Lea, 1860. The type locality of Mycetopus emarginatus Lea, 1860 is ‘Siam’ and is too vague to determine what drainage the holotype was collected from. Despite this ambiguity, Solenaia emarginata was recently thought to be restricted to only western Thailand because at least one Solenaia record was known from the Mae Klong drainage (Deein et al. 2004) and a recently discovered population in the adjacent Chao Phraya drainage was thought to represent a new species, Solenaia khwaenoiensis Panha & Deein in Deein et al. (2004). This suggests that Solenaia emarginata is endemic to the Mae Klong and Solenaia khwaenoiensis is endemic to the Chao Phraya. However, this biogeographic hypothesis is unlike any other in the Contradentini or Rectidentini, in that no member of either clade is known to be endemic to the Mae Klong. That is, the entire Contradentini+Rectidentini fauna in the Mae Klong drainage is shared with the adjacent Chao Phraya drainage. We consider Solenaia khwaenoiensis a junior synonym of Solenaia emarginata on the basis that there are very few morphological differences between the two nominal species and the overwhelming biogeographic evidence suggests that no members of the Contradentini+Rectidentini are endemic to the Mae Klong.
Genus Trapezoideus Simpson, 1900
Diagnosis
Distinguished from its sister taxon Physunio by being smaller and having a more trapezoidal shell outline, as opposed to circular (P. superbus and P. massini) or elongate (P. modelli and P. pellucidus) (Fig. 10).
Distribution
Western, central, and south-eastern Thailand, and north-western Malay Peninsula in Myanmar.
Species
We recognise three valid species in the genus Trapezoideus (Fig. 20).
-
Trapezoideus foliaceus (Gould, 1843).
-
Trapezoideus lenya Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020.
-
Trapezoideus subviridis, sp. nov.
Remarks
Trapezoideus is one of only two genera of the Contradentini+Rectidentini to occur in the Lower Salween ecoregion and the ecoregions to the east, the other genus being Lens. The supraspecific relationships in this genus are poorly supported in our COI gene tree (Fig. 18) and generating a more robust phylogeny of the group will be necessary to understanding the biogeographic history of this clade, including its dispersal across the influential drainage divides between the Salween Province and the Thai Gulf Province (Fig. 7).
Trapezoideus foliaceus (Gould, 1843)
(Tables 1, 3; Fig. 1, 9, 10, 20, 22; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
43 lots (Table S1).
Diagnosis
Conchologically very similar to T. subviridus but often has a less green posterior slope.
Distribution
Mae Klong and Chao Phraya drainages in western and central Thailand (Fig. 22).
Ecoregions
Mae Klong, Chao Phraya.
Remarks
The type locality of Unio foliaceus Gould, 1843 is ‘Tavoy, Burmah’ but the accuracy of this locality has been questioned (Konopleva et al. 2017, 2019a). The exact locality is never explicitly stated in the description but has been assumed as such because the specimens were sent to Augustus A. Gould by Rev. Francis Mason, a missionary living in Tavoy. Specimens morphologically similar to the type of Unio foliaceus are common and widespread in the upper reaches of Mae Klong drainage, which are very near the headwaters of the adjacent Tavoy drainage (Lower & Middle Salween ecoregion) (Fig. 22). It remains unclear whether the species occurs in the Tavoy drainage and is conspecific with populations in the Mae Klong and Chao Phraya, or the type locality is dubious; further sampling of the Tavoy drainage is needed.
Trapezoideus lenya Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020
(Table 1; Fig. 1, 20, 22; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Tables S1–S2, Fig. S1–S5).
Diagnosis
Distinguished from T. foliaceus and T. subviridis by having a narrower anterior and wider posterior.
Distribution
Known only from the type locality in the Lenya River, north-western Malay Peninsula in Myanmar (Fig. 22).
Ecoregion
Lower and Middle Salween.
Remarks
Trapezoideus lenya is the only species of the Contradentini+Rectidentini that has a distribution that is thought to terminate near the Isthmus of Kra, although the species is known only from its type locality so little can be said about its geographic scope. The Isthmus of Kra region, and more specifically the Tanintharyi–Lenya drainage divide, has recently been shown to be a significant biogeographic barrier for freshwater mussels (Bolotov et al. 2020), but its importance to the distribution and diversity of the Contradentini+Rectidentini appears to be limited.
Trapezoideus subviridis, sp. nov.
(Tables 1, 3; Fig. 1, 9, 20, 22; Tables S1, S2; Fig. S1–S5)
Type locality
Patong River, downstream of Khao Soi Dao Wildlife Sanctuary, Soi Dao District, Chanthaburi Province, Thailand, 13.10511, 102.19388.
Types
Holotype, UF 564751. Paratypes, UF 507543 (n = 8), same locality as the holotype.
Material examined
5 lots (Table S1).
Diagnosis
Conchologically very similar to T. foliaceus but often has a greener posterior slope.
Description
Shell trapezoidal, very thin, compressed. Anterior end narrow, broadly rounded. Posterior end squarish to rounded with moderate wing. Dorsal and ventral margins straight. Posterior ridge broadly rounded to biangulate, often with two green rays. Periostracum yellowish-brown, often with green posterior. Umbo shallow. Nacre iridescent, faintly purplish-blue, translucent, orangish in umbo. Pseudocardinal and lateral teeth nearly parallel, blade-like. Left valve with two lateral teeth, slightly curved, diverging. Left valve with one pseudocardinal, long, blade-like, more rounded near umbo. Right valve with one lateral tooth, long, slightly curved. Right valve with two pseudocardinal teeth, dorsal much smaller, occasionally reduced to swelling. Supra-anal aperture short. Pallial fusion between supra-anal and excurrent 2× longer than supra-anal aperture. Excurrent aperture smooth, slightly smaller than incurrent. Incurrent aperture with multiple rows of simple papillae. Demibranch perforated. Interior demibranch attached to visceral only anteriorly. Ectobranchous brooding, marsupium with moderate lateral inflation with unripe glochidia.
Distribution
Bang Pakong and Chantaburi drainages of south-eastern Thailand (Fig. 22).
Ecoregions:
Chao Phraya, Eastern Gulf of Thailand.
Remarks
Only species of the Contradentini+Rectidentini endemic to the coastal rivers of South-east Thailand.
Genus Yaukthwa Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019
Diagnosis
Distinguished from morphologically similar genera of Lens and Trapezoideus by its more rectangular shape, including a wider anterior and straighter ventral margin (Fig. 10).
Distribution
Widespread in Myanmar, known from one locality in north-western Thailand.
Species
We recognise 11 valid species in the genus Yaukthwa (Fig. 23).
-
Yaukthwa nesemanni (Konopleva, Vikhrev & Bolotov in Bolotov et al. 2017b).
-
Yaukthwa avaensis Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020.
-
Yaukthwa baniensis (Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020), comb. nov.
-
Yaukthwa dalliana (Frierson, 1913).
-
Yaukthwa elongatula Bolotov, Konopleva, Vikhrev, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Gofarov, Tomilova & Kondakov, 2019, comb. res.
-
Yaukthwa inlenensis Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019.
-
Yaukthwa paiensis Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019.
-
Yaukthwa panhai (Konopleva, Bolotov & Kondakov in Bolotov et al. 2017b).
-
Yaukthwa peguensis (Anthony, 1865).
-
Yaukthwa rectangularis (Tapparone-Canefri, 1889), comb. nov.
-
Yaukthwa zayleymanensis (Preston, 1912).

|
Remarks
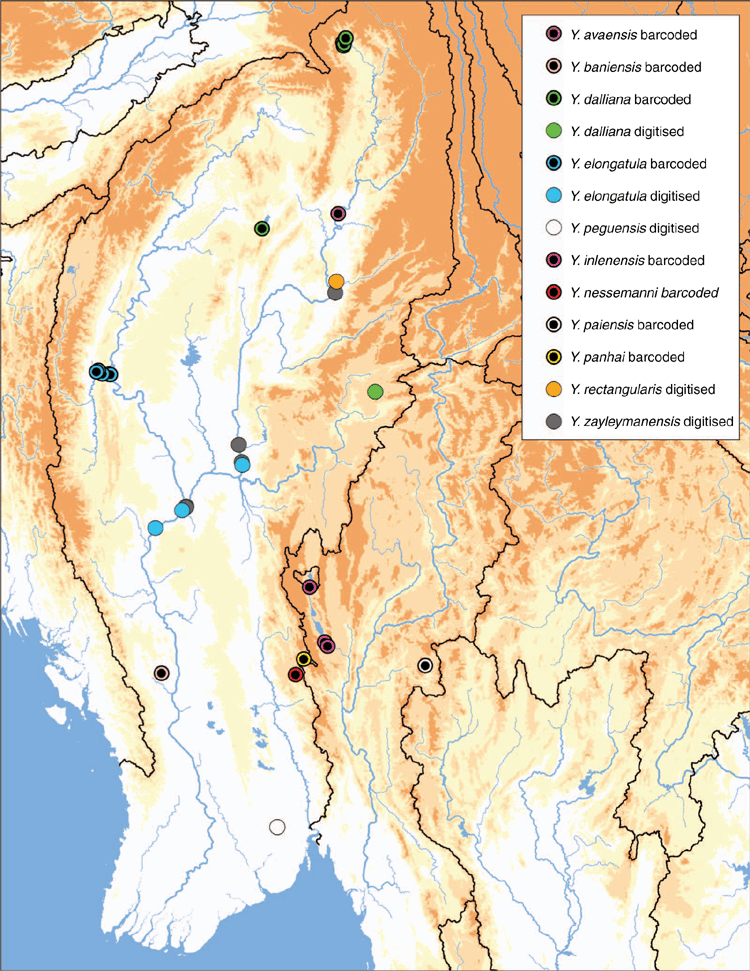
Recent studies have substantially increased our phylogenetic understanding of this clade, including the description of the genus and seven new species (Bolotov et al. 2017b, 2019b, 2020; Konopleva et al. 2019a). Despite being quite species-rich, the genus is apparently restricted to a narrow geographic range (Fig. 24).The genus is also known from relatively few records (Fig. 1) and six of the 11 species are known only from their type localities. Three of the five Yaukthwa species described before 2017 have not been included in a molecular phylogenetic analysis (Y. peguensis, Y. rectangularis and Y. zayleymanensis). These three species are the only Contradentini+Rectidentini species from Indochina not yet included in a molecular phylogeny (Fig. 25). Including representatives of these older named species in a phylogenetic analysis will be important to better understanding the systematics, species diversity, and biogeographic history of this clade.
The genus Balwantia was recently resurrected from synonymy under Solenaia for a clade hypothesised to contain Anodonta soleniformis Benson, 1836 (type species of Balwantia), Yaukthwa elongatula Bolotov, Konopleva, Vikhrev, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Gofarov, Tomilova & Kondakov, 2019, and Balwantia baniensis Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020 (Bolotov et al. 2020). Balwantia elongatula and B. baniensis are clearly part of Contradentini and were recovered as sister to Yuakthwa (Bolotov et al. 2020). However, the type species of Balwantia, Anodonta soleniformis Benson, 1836, has not been included in a phylogenetic analysis but based on our understanding of synapomorphy in freshwater mussels that species is distantly related to any member of the Contradentini including ‘B.’ elongatula and ‘B.’ baniensis. Bilaterally asymmetrical glochidia is an unambiguous synapomorphy of the Contradentini and Balwanatia solenifomis has unhooked glochidia (Prashad 1919; Pfeiffer and Graf 2015) and thus Balwantia sensu Bolotov et al. (2020) is unlikely to be monophyletic.
As such, we transfer Balwantia elongatula (Bolotov, Konopleva, Vikhrev, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Gofarov, Tomilova & Kondakov, 2019) back to its originally described position, Yaukthwa elongatula Bolotov, Konopleva, Vikhrev, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Gofarov, Tomilova & Kondakov, 2019, comb. res., and move Balwantia baniensis Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020 to Yuakthwa baniensis (Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020), comb. nov. We recognise Balwantia as a valid monotypic genus whose subfamily-level position remains untested but, given its larval morphology and distribution, it may be a member of the Parreysiinae (Pfeiffer and Graf 2015).
Yaukthwa nesemanni (Konopleva, Vikhrev & Bolotov in Bolotov et al. 2017b)
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Similar to Y. panhai but differs by its thinner shell and shallow adductor muscle scars (Bolotov et al. 2017b).
Distribution
Known only from the type locality in the Sittaung drainage, south-central Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Yaukthwa avaensis Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Distinguished from other Yaukthwa by its more ovate shell outline and more pointed posterior (Bolotov et al. 2020).
Distribution
Known only from the type locality in the Irrawaddy drainage, northern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Yaukthwa baniensis (Bolotov, Konopleva, Vikhrev, Gofarov, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Tomilova, Tanmuangpak, Tumpeesuwan & Kondakov, 2020), comb. nov.
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Yaukthwa baniensis is distinguished from all other members of Yaukthwa, except its sister species Y. elongatula, by its strongly reduced dentition. It is thought to be distinguished from Y. elongatula by having a more rostrate anterior margin, a more inflated shell, and bars on the posterior slope (Bolotov et al. 2020).
Distribution
Known only from the type locality in the Irrawaddy drainage, southern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
This species is herein transferred from Balwantia to Yaukthwa based on the divergent larval morphologies between Balwantia and members of the Contradentini (Pfeiffer and Graf 2015).
Yaukthwa dalliana (Frierson, 1913)
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
6 lots (Table S1).
Diagnosis
Yaukthwa dalliana has much more robust teeth in comparison to other representatives of the genus.
Distribution
Irrawaddy drainage in northern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
There is considerable morphological variation in this putative species. Several records, including the lectotype, are morphologically divergent (robust lateral and pseudocardinal teeth, strongly sculptured umbo) and geographically distant from barcoded records (Fig. 24). As suggested by Konopleva et al. (2019a), including specimens from near the type locality will be important to better understanding the species’ boundaries of this taxon.
Yaukthwa elongatula Bolotov, Konopleva, Vikhrev, Lopes-Lima, Bogan, Lunn, Chan, Win, Aksenova, Gofarov, Tomilova & Kondakov, 2019, comb. res.
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
9 lots (Table S1).
Diagnosis
Yaukthwa elongatula is distinguished from all other members of Yaukthwa, except its sister species Y. baniensis, by its strongly reduced dentition. It is thought to be distinguished from Y. baniensis by having a less rostrate anterior margin, a less inflated shell, and the absence of bars on the posterior slope (Bolotov et al. 2020).
Distribution
Irrawaddy drainage in western and central Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
Some specimens identified here as Y. elongatula, including one collected very near the type locality (FMNH 120070), look more similar to the holotype of Y. baniensis than the holotype of Y. elongatula. The fact that these species are relatively thin-shelled and are often deeply buried in the substrate (Bolotov et al. 2020) may create a great deal of non-heritable variation in shell shape and is likely contributing to difficulties identifying historical specimens and delimiting species’ geographic boundaries.
Yaukthwa inlenensis Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Yaukthwa inlenensis is morphologically very similar to Y. paiensis but has a larger and more sculptured umbo (Konopleva et al. 2019a).
Distribution
Inle and Moebyel lakes (Salween drainage) in eastern Myanmar (Fig. 24).
Ecoregion
Lower and Middle Salween (including Inle Lake).
Yaukthwa paiensis Konopleva, Pfeiffer, Vikhrev, Kondakov, Gofarov, Aksenova, Lunn, Chan & Bolotov, 2019
(Tables 1, 3; Fig. 1, 9, 10, 18, 23–25; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
2 lots (Table S1).
Diagnosis
Yaukthwa paiensis is morphologically very similar to Y. inlenensis but has less prominent and sculptured umbo (Konopleva et al. 2019a).
Distribution
Known only from the type locality in the Salween drainage, north-western Thailand (Fig. 24).
Ecoregion
Lower and Middle Salween.
Remarks
Only Yaukthwa species known from Thailand.
Yaukthwa panhai (Konopleva, Bolotov & Kondakov in Bolotov et al. 2017b)
(Table 1; Fig. 1, 23–25; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Yaukthwa panhai is morphologically very similar to T. nesemanni but has a thicker shell and more developed hinge and umbo (Bolotov et al. 2017b).
Distribution
Known only from the type locality in the Sittaung drainage, eastern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Yaukthwa peguensis (Anthony, 1865)
(Table 1; Fig. 1, 23, 24; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
12 lots (Table S1).
Diagnosis
Yaukthwa peguensis is distinguished from other members of the genus by being more trapezoidal and laterally compressed.
Distribution
Irrawaddy drainage in southern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
The species has not yet been included in a molecular phylogeny.
Yaukthwa rectangularis (Tapparone-Canefri, 1889), comb. nov.
(Table 1; Fig. 1, 23, 24; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Closely resembles other members of the genus, especially Y. paiensis, but is less elongate and more laterally compressed.
Distribution
Known only from the type locality in the Irrawaddy drainage, northern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
This species was recently hypothesised to be a member of the genus Indonaia (Parreysiinae: Indochinellini) based on shell shape, elevated umbo and dentition (Bolotov et al. 2019b). However, this species shares several traits that are common among the Contradentini, and especially the genus Yaukthwa, including a rectangular shell outline, smooth yellow periostracum, oblique green ridges on the posterior slope, and wrinkles on the posterior slope that are largely perpendicular to the shell margin. The wrinkles on the posterior slope described as ‘a unique feature’ and ‘never seen in other species’ by Bolotov et al. (2019b) is a common trait in many members of the Contradentini including Yaukthwa (see fig. 4 in Konopleva et al. 2019a; Fig. 23). As such, we hypothesise that this species belongs to the genus Yaukthwa. The species has not yet been included in a molecular phylogeny.
Yaukthwa zayleymanensis (Preston, 1912)
(Table 1; Fig. 1, 23, 24; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
8 lots (Table S1).
Diagnosis
Yaukthwa zayleymanensis is distinguished from other members of the genus by being more delicate and more dorso-ventrally narrow.
Distribution
Irrawaddy drainage in central and northern Myanmar (Fig. 24).
Ecoregion
Sittaung–Irrawaddy.
Remarks
The species has not yet been included in a molecular phylogeny.
RECTIDENTINI Modell, 1942
Type: Rectidens Simpson, 1900.
Diagnosis
The Rectidentini is distinguished from its sister group the Contradentini by having bilaterally symmetrical glochidia. Conchologically, the Rectidentini are more elongate and have a bluntly pointed posterior end in comparison to the Contradentini, which are generally more circular or rectangular and have a more broadly rounded posterior. The Rectidentini also tend to have a sharper posterior ridge and a shorter posterior slope (excluding the wing of Hyriopsis and Prohyriopsis) than the Contradentini (Fig. 10).
Distribution
Widespread in Indochina from the Mae Klong in the west and to the Mekong drainage in the east. Also occurs on the Malay Peninsula and the islands of Sumatra, Java and Borneo.
Genera
We recognise six genera in the Rectidentini (Fig. 10).
-
Rectidens Simpson, 1900.
-
Ctenodesma Simpson, 1900.
-
Elongaria Haas, 1911.
-
Ensidens Frierson, 1911.
-
Hyriopsis Conrad, 1853.
-
Prohyriopsis Haas, 1914.
Remarks
The position of Elongaria and Prohyriopsis among the Rectidentini remains untested. However, the type species of Prohyriopsis is morphologically very similar to Hyriopsis, and the type species Elongaria is morphologically very similar to Rectidens, so their positions among the Rectidentini seem likely. The systematic position of ?Elongaria trompi among the Rectidentini is much more tenuous (Pfeiffer et al. 2018a) and is further described in the taxonomic account for that species.
Key to the genera of the RectidentiniThe practicality of this key is complicated by the fact that several of these genera may not be valid or monophyletic (e.g. Elongaria, Ctenodesma).
|
1. Wing absent |
|
2. Wing small, sculpturing present |
|
3. Gradual posterior slope, ventral margin longer than dorsal |
|
4. Little to no sculpture *?Elongaria trompi also has sculpture but that species may not belong to the Rectidentini – see remarks in the species account. |
Genus Rectidens Simpson, 1900
Diagnosis
Rectidens is the most elongate genus of the Rectidentini. Rectidens is morphologically very similar to Elongaria orientalis but tends to have a sharper posterior ridge and is often more biangulate (Fig. 26). Rectidens also tends to have a darker periostracum (brown to black) in comparison to E. orientalis (greenish-yellow to brown). Rectidens can be distinguished from Ctenodesma borneensis by its larger size and smoother shell disc.

|
Distribution
Malay Peninsula, and the islands of Sumatra, Java and Borneo.
Species
We recognise one valid species in the genus Rectidens (Fig. 26).
-
Rectidens sumatrensis (Dunker, 1852)
Remarks
Distinguishing Rectidens sumatrensis from Elongaria orientalis using shell characters is difficult or impossible. Affandi et al. (2017) suggested the two species can be distinguished by the presence of wrinkles ventral to the pallial line in Rectidens. However, only one Rectidens specimen was included in their molecular assessment and the sequence data referenced in the paper remains unavailable at the time of this publication. Furthermore, the presence of wrinkles ventral to the pallial line is not unique to Rectidens and are quite common in many Rectidentini.
Rectidens sumatrensis (Dunker, 1852)
(Tables 1, 3; Fig. 1, 9, 10, 21, 26, 27; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
70 lots (Table S1).
Diagnosis
Same as for genus.
Distribution
Malay Peninsula and the islands of Sumatra, Java and Borneo (Fig. 21).
Ecoregions
Malay Peninsula Eastern Slope, Northern Central Sumatra–Western Malaysia, Southern Central Sumatra, Southern Sumatra–Western Java, South-eastern Borneo, Kapuas, North-western Borneo.
Remarks
Rectidens sumatrensis is the only Rectidentini known to occur on both mainland South-east Asia and the Malay Archipelago. The low genetic divergences between mainland South-east Asian populations and those on Borneo (Fig. 27) suggest that the species has undergone a large and relatively recent geographic expansion (Zieritz et al. 2020). Among the Contradentini, the only species known to occur on mainland South-east Asia and the Malay Archipelago are L. contradens and P. superbus.
Genus Ctenodesma Simpson, 1900
Diagnosis
This taxon is unlikely to be monophyletic and we are unable to provide a useful generic diagnosis.
Distribution
Borneo.
Species
We recognise two valid species in the genus Ctenodesma (Fig. 26).
-
Ctenodesma borneensis (Issel, 1874).
-
?Ctenodesma scheibeneri Haas, 1927.
Remarks
The monophyly of this genus is untested and unlikely. Morphologically, Ctenodesma borneensis resembles other Rectidentini and recent molecular phylogenetic reconstructions recover that taxon as sister to the rest of the Rectidentini, albeit with limited support (Zieritz et al. 2020). ?Ctenodesma scheibeneri is morphologically quite different from C. borneensis and most other Rectidentini in that it has strongly fractured pseudocardinal teeth (v. lamelliform), distinct green rays (v. blurry), and more circular shell outline (v. elongate) (Fig. 26).
Ctenodesma borneensis (Issel, 1874)
(Table 1; Fig. 1, 10, 26–28; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
11 lots (Table S1).
Diagnosis
Ctenodesma borneensis resembles Rectidens sumatrensis but has yellow periostracum and has more sculpturing on the shell disc and posterior slope.
Distribution
Western and northern Borneo (Fig. 28).
|
Ecoregions
Kapuas, North-eastern Borneo, North-western Borneo.
Remarks
Von Martens (1867) listed this species from north-western Borneo, coastal areas near Mempawah, and Danau Seriang. However, the specimens from Mempawah and Danau Seriang were not located in our museum sampling and therefore are not included in the map (Fig. 28). Issel (1874) listed north-east Borneo in the distribution of this species but this appears to be an incorrect translation of von Martens (1867).
Despite significant recent sampling efforts in northern Borneo this species has been found at only one site (Zieritz et al. 2018b, 2020).
?Ctenodesma scheibeneri Haas, 1927
(Table 1; Fig. 1, 10, 26, 28; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
?Ctenodesma scheibeneri is diagnosed by its strongly fractured pseudocardinal teeth, fine and distinct green rays, sculptured shell disc, and more circular shell outline.
Distribution
Known only from the type locality in the Sampit drainage, southern Borneo (Fig. 28).
Ecoregion
South-eastern Borneo.
Remarks
The species has not yet been included in a molecular phylogeny.
Genus Elongaria Haas, 1911
Diagnosis
This taxon is unlikely to be monophyletic, and we are unable to provide a useful generic diagnosis.
Distribution
Known only from Borneo and Java.
Species
We recognise two valid species in the genus Elongaria (Fig. 26).
-
Elongaria orientalis Haas, 1911.
-
?Elongaria trompi (Drouët & Chaper, 1892).
Remarks
The monophyly of this genus is untested and unlikely. Elongaria orientalis is morphologically very similar to Rectidens and appears to be closely related to that genus (or is potentially a junior synonym of that genus) (Fig. 26). ?Elongaria trompi has shell sculpturing that is unlike all other Rectidentini, and its position among the Rectidentini has recently been questioned (Pfeiffer et al. 2018a).
Elongaria orientalis (Lea, 1840)
(Table 1; Fig. 1, 10, 26, 28; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
17 lots (Table S1).
Diagnosis
Distinguishing Elongaria orientalis from Rectidens sumatrensis using shell characters is very difficult but E. orientalis tends to have a brighter periostracum (greenish-yellow to brown) than R. sumatrensis (brown to black).
Distribution
Restricted to Java (Fig. 28).
Ecoregions
Southern Sumatra–Western Java, Central & Eastern Java.
Remarks
Elongaria orientalis is the only Contradentini or Rectidentini thought to be endemic to the island of Java. However, the validity of this taxa is uncertain, and it may be a junior synonym of Rectidens sumatrensis. The species has not yet been included in a molecular phylogeny.
?Elongaria trompi (Drouët & Chaper, 1892)
(Table 1; Fig. 1, 26, 28; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
11 lots (Table S1).
Diagnosis
?Elongaria trompi is distinguished from other Rectidentini by its slightly concave ventral margin and sculpturing on the shell disc.
Distribution
Kapuas drainage in western Borneo (Fig. 28).
Ecoregion
Kapuas.
Remarks
This taxon shares several traits with representatives of the tribe Indochinellini (Subfamily Parreysiinae), including its small size, slightly concave ventral margin, and fine zig-zag sculpturing on the shell disc, and may belong to that tribe (Pfeiffer et al. 2018a). If so, the generic name Nannonaia Haas, 1913 may be valid, and the Nannonaiini Modell, 1942 would have priority over the Indochinellini Bolotov, Pfeiffer, Vikhrev & Konopleva in Bolotov et al. (2018). The Indochinellini is distributed across much of Indochina and the Malay Peninsula, but is not yet known to occur on the Malay Archipelago. Testing the phylogenetic position of ?Elongaria trompi has important biogeographic and nomenclatural implications. The species has not yet been included in a molecular phylogeny.
Genus Ensidens Frierson, 1911
Diagnosis
Ensidens can be distinguished from other representatives of the Rectidentini by its very short and steep posterior slope. The posterior end tends to terminate more dorsally in Ensidens, whereas in Rectidens the posterior tends to terminate more ventrally (Fig. 10).
Distribution
Widespread in Indochina. Uncommon in the Malay Peninsula, and absent from Malay Archipelago.
Species
We recognise six valid species in the genus Ensidens (Fig. 29).
-
Ensidens ingallsianus (Lea, 1852).
-
Ensidens dugasti (Morlet, 1892), stat. rev.
-
Ensidens jaculus (Rochebrune, 1882), stat. rev.
-
Ensidens sagittarius (Lea, 1895).
-
Ensidens spiculus, sp. nov.
-
Ensidens telus, sp. nov.
Remarks
Ensidens is a morphologically conserved clade (Fig. 29) but is phylogenetically quite diverse (Fig. 30). Muanta et al. (2019) recently demonstrated that the species-level diversity of Ensidens is underestimated and a synthetic taxonomic revision of the clade is needed.
The genus can be divided into two clades, one of which is restricted to the Mekong drainage (and largely to just the Khorat Plateau) (Fig. 32), the other clade also occurs in the Mekong but is further distributed in the drainages to the west, and south down the Malay Peninsula (Fig. 31). These six species are largely allopatric but in a relatively short stretch of the Mekong River (~150 miles), near the confluence of the Songkhram River, there are four phylogenetically distinct but morphologically similar species of Ensidens. Distinguishing the Ensidens spp. from this area using shell characters is difficult to impossible, as indicated when comparing the diagnoses (or lack thereof) for each species.
Ensidens is unusual in comparison to most other Contradentini+Rectidentini in that we have observed it to be tetragenous or ectobranchous. Tetrageny is the more common of the brooding conditions in Ensidens, and it may be that ‘ectobranchous’ individuals were observed at an early or late developmental stage.
Many Ensidens species are named for their resemblance to various ranged weapons like arrows, spears, javelins, darts (latin – jaculum, spiculum, tellum, sagitta).
Ensidens ingallsianus (Lea, 1852)
(Tables 1, 3; Fig. 1, 9, 10, 29–31; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
90 lots (Table S1).
Diagnosis
Ensidens ingallsianus has a posterior end that terminates more dorsally than that of other congeners. Ensidens ingallsianus also tends to be slightly more elongate and compressed in comparison to its sister species, E. telus. However, all Ensidens species are morphologically very similar and are distinguished most easily by their cohesive geographic and genetic patterns.
Distribution
Widespread in the Mae Klong, Chao Phraya, and Bang Pakong drainages of western and central Thailand, as well as the coastal drainages of the Malay Peninsula. Disjunct populations also occur in the headwaters of the Mun watershed (Mekong drainage) (Fig. 31)
Ecoregions
Mae Klong, Chao Phraya, Eastern Gulf of Thailand Drainages, Khorat Plateau, North Central Sumatra–Western Malaysia, Malay Peninsula Eastern Slope.
Remarks
The type localities of Unio ingallsianus Lea, 1852 and Unio sagittarius Lea, 1856 are both ‘Siam’. This ambiguity complicates determining what name belongs to what clade. This is especially unclear because the collector of these type specimens, William Haines, is known to have sampled from both the Chao Phraya and Mekong drainages and listed almost all specimens as simply being from ‘Siam’ (See L. contradens account for further discussion regarding this problem). It is our opinion that the holotype of Unio ingallsianus shares more similarities to samples collected from the Chao Phraya, including their more ovate shell shape and a posterior end that terminates dorsally. Whereas the lectotype of Unio sagittarius shares more similarities to samples collected from the Mun River watershed (Mekong drainage) including their more elongate shell shape and a posterior end that terminates centrally or ventrally.
This species is absent from much of the Mekong drainage and is restricted to two localities along the western edge of the Khorat Plateau ecoregion. This range disjunction and the shallow genetic divergences suggests that the Mun watershed may have recently captured portions of the Chao Phraya or the Bang Pakong drainages, or portions of both (Fig. 30, 31).
Ensidens dugasti (Morlet, 1892), stat. rev.
(Tables 1, 3; Fig. 1, 9, 29, 30, 32; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
20 lots (Table S1).
Diagnosis
Ensidens dugasti is very similar to E. spiculus and we did not observe any traits that were capable of distinguishing the two species. Ensidens dugasti is distinguished from E. spiculus most easily by their cohesive geographic and genetic patterns.
Distribution
Mekong drainage in Thailand and Laos. Populations concentrated in the Songkhram watershed but also occurs in other nearby watersheds of the Mekong (Fig. 32).
Ecoregion
Khorat Plateau.
Remarks
Ensidens dugasti appears to be the most geographically restricted and genetically homogenous Ensidens species.
Ensidens jaculus (Rochebrune, 1882), stat. rev.
(Tables 1, 3; Fig. 1, 9, 29, 30, 32; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
21 lots (Table S1).
Diagnosis
Ensidens jaculus may be slightly less elongate than its sister species, E. spiculus. However, all Ensidens species are morphologically very similar and are distinguished most easily by their cohesive distributional and genetic patterns.
Distribution
Mekong drainage in Thailand, Laos, and Cambodia (Fig. 32).
Ecoregion
Khorat Plateau, Kratie–Stung Treng.
Remarks
Majority of records are from the Mekong River proper or nearby tributaries.
Ensidens sagittarius (Lea, 1856)
(Tables 1, 3; Fig. 1, 9, 29, 30, 32; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
36 lots (Table S1).
Diagnosis
Ensidens sagittarius is typically more elongate than E. ingallsianus and E. telus but we unable to use morphological characters to distinguish this species from the other Mekong endemic Ensidens. All Ensidens species are morphologically very similar and are distinguished most easily on the basis cohesive of their cohesive geographic and genetic patterns
Distribution
Mekong drainage in Thailand and Laos (Fig. 32).
Ecoregion
Khorat Plateau.
Remarks
Most records are from the Mun watershed, but the species does occur in the Mekong proper, including one geographically divergent population >150 river miles upstream of the nearest population.
Ensidens spiculus, sp. nov.
(Tables 1, 3; Fig. 1, 9, 29, 30, 32; Tables S1, S2; Fig. S1–S5)
Type locality
Mekong River, along Rt. 211, Sangkhom District, Nong Khai Province, Thailand, 18.11106, 102.22679.
Types
Holotype, UF 564752. Paratype, UF 507755 (n = 1), same locality as the holotype.
Taxonomic opinions since Haas (1969a)
Material examined
12 lots (Table S1).
Diagnosis
Ensidens spiculus is very similar to E. dugasti, and we did not observe any traits that were capable of distinguishing the two species. Ensidens spiculus is distinguished from E. dugasti most easily on the basis of cohesive geographic and genetic patterns.
Description
Shell elongate, moderately thick, somewhat inflated. Anterior end narrow, broadly rounded. Posterior end elongate, bluntly pointed. Dorsal and ventral margins slightly curved. Posterior slope very steep, especially near the umbo. Periostracum light yellow to brown. Umbo not elevated above the hinge line. Nacre iridescent, faintly blueish-purple, opaque, strong striations. Pseudocardinal and lateral teeth in slight arch, blade-like, coarse texture. Left valve with two lateral teeth, curved, slightly sinuous in large specimens. Left valve with one pseudocardinal tooth, triangular accessory tooth at the posterior end. Right valve with one lateral tooth, slightly curved. Right valve with two pseudocardinal teeth, ventral tooth much larger than dorsal. Supra-anal aperture, excurrent aperture and pallial fusion between of similar length. Excurrent aperture smooth, slightly smaller than incurrent. Incurrent aperture with multiple rows of short simple, conical-shaped papillae. Tetragenous brooding (occasionally ectobranchous). Gills laterally swollen when gravid. Septa with small perforations. Unhooked glochidia.
Distribution
Mekong drainage in Thailand and Laos. (Fig. 32).
Ecoregions
Lower Lancang, Khorat Plateau.
Remarks
This is the only species of Ensidens known to occur in the Lower Lancang ecoregion.
Ensidens telus, sp. nov.
(Tables 1, 3; Fig. 1, 9, 29–31; Tables S1, S2; Fig. S1–S5)
Type locality
Tonle Sap River, near Phumi Trâpeang Préal, Kampong Chhnang Province, Cambodia, 12.05848, 104.77307.
Types
Holotype, UF 564753. Paratypes, UF 507435 (n = 37), same locality as the holotype.
Taxonomic opinions since Haas (1969a)
Material examined
46 lots (Table S1).
Diagnosis
Ensidens telus is the least elongate species of Ensidens. However, all Ensidens species are morphologically very similar and are distinguished most easily by cohesive biogeographic and genetic patterns.
Description
Shell elongate oval, moderately thick-shelled, somewhat inflated. Anterior end narrow, broadly rounded. Posterior end elongate, bluntly pointed. Dorsal and ventral margins rounded. Posterior slope steep, especially near the umbo. Umbo slightly elevated above the hinge line. Nacre iridescent, faintly blueish-purple, opaque, strong striation, tan to orangish tint near umbo. Left valve with two lateral teeth, curved, coarse texture. Left valve with one pseudocardinal tooth, occasionally with accessory dentition at the posterior end. Right valve with one lateral tooth, curved. Right valve with two pseudocardinal teeth, ventral tooth longer than dorsal. Supra-anal aperture short. Pallial fusion between supra-anal and excurrent 2× longer than supra-anal aperture. Excurrent aperture smooth, smaller than incurrent. Incurrent aperture with multiple rows of short simple, conical-shaped papillae. Tetragenous brooding. Marsupium laterally swollen and slightly blush when glochidia fully developed. Perforated septa, perforation width approximately equal to connection width. Semielliptical unhooked glochidia
Distribution
Widespread in Mekong drainage in western Cambodia and southern Vietnam. Also known from the Bang Pakong and Chantaburi drainages of south-eastern Thailand and Soai Rap drainage of southern Vietnam (Fig. 31).
Ecoregions
Chao Phraya, Eastern Gulf of Thailand Drainages, Mekong Delta.
Remarks
This species is known primarily from the Mekong drainage but it is also known from a few localities along the western edge of the Chao Phraya ecoregion. This range disjunction and the shallow genetic divergences suggest that the Bang Pakong may have recently captured portions of the Mekong (Fig. 30, 31).
Genus Hyriopsis Conrad, 1853
Diagnosis
Hyriopsis can be distinguished from all other Contradentini+Rectidentini by its large posterior wing (Fig. 10).
Distribution
Widespread in mainland South-east Asia. From the Mae Klong drainage in the west to the Mekong drainage in the east, and south across the Malay Peninsula. A few records from Sumatra (2) and Borneo (1).
Species
We recognise nine valid species and one undescribed species in the genus Hyriopsis (Fig. 33).
-
Hyriopsis bialata Simpson, 1900.
-
Hyriopsis delaportei (Crosse & Fischer, 1876).
-
Hyriopsis desowitzi Brandt, 1974.
-
Hyriopsis myersiana (Lea, 1856).
-
Hyriopsis kratiensis, sp. nov.
-
Hyriopsis phuphaniensis, sp. nov.
-
Hyriopsis khoratensis, sp. nov.
-
Hyriopsis sakhonensis, sp. nov.
-
Hyriopsis undescribed.
-
Hyriopsis velthuizeni (Schepman, 1896).
Remarks
Several recent multilocus phylogenies have recovered Hyriopsis as non-monophyletic (Zieritz et al. 2016, 2020; Lopes-Lima et al. 2017; Pfeiffer et al. 2018a). However, our phylogenomic reconstruction strongly supports the monophyly of the genus (Fig. 9). Our phylogenomic reconstruction also finds strong support for a multispecies Mekong radiation including H. kratiensis, H. khoratensis and H. sakhonensis (H. phuphaniensis also belongs to this clade but was not included in our phylogenomic analysis). This Mekong radiation is not recovered in our COI gene tree (Fig. 27).
Limnoscapha Lindholm, 1932 has recently been treated as a subgenus (Kittivorachate and Yangyuen 2004; Deein et al. 2004) or junior synonym of Hyriopsis Simpson, 1900 (Graf and Cummings 2006a; Vinarski 2019) but we follow Do et al. (2018) who suggested that Limnoscapha Lindholm, 1932 represents a distantly related fossil taxon distinct from Hyriopsis.
Common names for species of Hyriopsis are loosely translated in English as ‘two-winged clam’, ‘hatchet clam’ and ‘flying boat clam’ (Nabhitabhata 2009).
Hyriopsis bialata Simpson, 1900
(Tables 1, 3; Fig. 1, 9, 10, 27, 33, 34; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
83 lots (Table S1).
Diagnosis
Hyriopsis bialata is the most elongate and dorsoventrally compressed species of Hyriopsis.
Distribution
Disjunct distribution in Mekong drainage in Cambodia and Vietnam and the southern Malay Peninsula (Fig. 34).
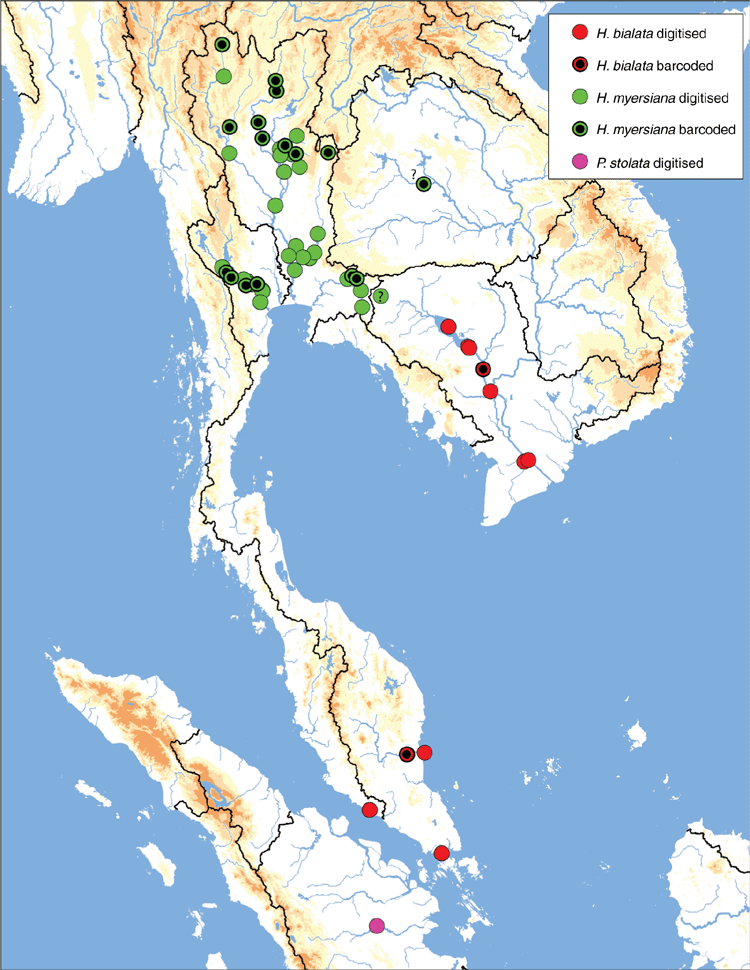
|
Ecoregions
North Central Sumatra–Western Malaysia, Malay Peninsula Eastern Slope, Mekong Delta.
Remarks
The few records of H. bialata from the Philippines (UF 270731, USNM 7664) and India (USNM 25679) are likely to have incorrect locality information and were omitted from our analyses.
Despite the large geographic distance between H. bialata populations in the Malay Peninsula and the Tonle Sap (Fig. 34), the sequenced individuals are very closely related (Fig. 27). The large geographic disjunction and limited genetic diversity observed in H. bialata is quite unusual but a similar pattern is known from at least one other freshwater mussel (Monodontina cambodjensis (Petit, 1865) – compare individuals associated with GenBank accessions KP795028 and KX051297). The Mekong drainage is not typically associated with the Paleo-Siam River but parts of western Cambodia, and perhaps parts of the modern-day Tonle Sap watershed, may have drained into the Gulf of Thailand and been connected to the Paleo-Siam River (Rainboth 1996; Attwood and Johnston 2001; Adamson et al. 2012). The species may have recently undergone a major geographic expansion associated with the Paleo-Siam River with subsequent extinction across much of its range (e.g. Eastern Gulf of Thailand, Chao Phraya, Mae Klong and most of the Malay Peninsula Eastern Slope ecoregions). An alternative explanation could be non-native introduction to the Malay Peninsula.
Hyriopsis delaportei (Crosse & Fischer, 1876)
(Tables 1, 3; Fig. 1, 9, 27, 33, 35; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
16 lots (Table S1).
Diagnosis
Hyriopsis delaportei is most similar to its sister taxon, H. desowitzi, but is less inflated and has strong concentric ridges on the umbo.
Distribution
Mekong drainage in western Cambodia (Fig. 35).
Ecoregion
Mekong Delta.
Remarks
This species exhibits substantial amounts of ontogenetic variation. Small individuals are very thin-shelled with delicate teeth and ridges on the umbo whereas adults are quite robust with strongly flattened and fractured pseudocardinals.
Hyriopsis desowitzi Brandt, 1974
(Table 1; Fig. 1, 27, 33, 35; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
27 lots (Table S1).
Diagnosis
Hyriopsis desowitzi is most similar to its sister taxon, H. delaportei, but has a much more circular shell outline and is more inflated.
Distribution
Chao Phraya and Bang Pakong drainages of south-central and south-eastern Thailand (Fig. 35).
Ecoregion
Chao Phraya.
Hyriopsis myersiana (Lea, 1856)
(Tables 1, 3; Fig. 1, 9, 27, 33, 34; Tables S1, S2; Fig. S1–S5)
Taxonomic opinions since Haas (1969a)
Material examined
122 lots (Table S1).
Diagnosis
Hyriopsis myersiana has a more ovate shell outline and a smaller posterior wing in comparison to other Hyriopsis.
Distribution
Widespread in Mae Klong, Chao Phraya, and Bang Pakong drainages in Thailand (Fig. 34).
Ecoregions
Mae Klong, Chao Phraya.
Remarks
Unio sutrangensis Morlet, 1889 is described from ‘Riviere de Sutrang (Siam)’, which is a tributary of the Tonle Sap and is one of two questionable records of H. myersiana from the Mekong drainage (Fig. 34). However, Morlet (1904) emended the name Unio sutrangensis to Unio patrangensis so that it reflected the correct collection locality, Patrang, which is part of the Bang Pakong drainage. Morlet’s (1904) emendation of Unio sutrangensis is unjustified but it is useful in that it raises further doubts concerning the presence of this species in the Mekong.
The other questionable record corresponds to a published COI sequence (MG025691) identified as H. delaportei and allegedly collected from the Mun watershed (Muanta et al. 2019). That sequence was recovered within our H. myersiana clade (Fig. 27) and it either represents a highly unusual disjunction or the metadata associated with this sequence is erroneous.
Hyriopsis kratiensis, sp. nov.
(Tables 1, 3; Fig. 1, 9, 27, 33, 36; Tables S1, S2; Fig. S1–S5)
Type locality
Mekong River, 1.4 miles upstream of Sambour, Sambour, Kratié Province, Cambodia, 12.79458, 105.97255.
Types
Holotype, UF 564754. Paratypes, UF 507415 (n = 18), same locality as the holotype.
Material examined
26 lots (Table S1).
Diagnosis
Hyriopsis kratiensis is morphologically similar to Hyriopsis phuphaniensis but has a squarer posterior end and larger wing.
Description
Shell elongately ovate to rectangular with large wing, thick-shelled, and compressed. Anterior end wide, broadly rounded. Posterior end angular. Dorsal margin consists of large, serrated wing, nearly as tall as shell disc. Ventral margin is straight to slightly curved. Biangulate posterior slope, wide, gradual. Umbo not elevated above hinge line. Nacre iridescent anteriorly, salmon towards umbo, opaque, strong striations. Left valve with two lateral teeth, slightly curved, textured margins. Left valve with one oblique tooth extending from umbo to anterior adductor muscle (in smaller individuals), otherwise strongly fractured surface. Right valve with one lateral tooth, slightly curved, textured. Right valve with two small oblique pseudocardinal teeth extending from umbo to anterior adductor muscle (in small individuals), otherwise strongly fractured surface. Supra-anal aperture greater than 5× longer than pallial fusion between excurrent and supra-anal, smooth, tall. Excurrent aperture smooth, shorter than incurrent. Incurrent with 1–2 rows of short, conical-shaped papillae. Perforated septa, connections much smaller than perforations. Ectobranchous brooding. Laterally swollen and blush with fully developed glochidia. Semielliptical unhooked glochidia.
Distribution
Mekong drainage in southern Laos and eastern Cambodia (Fig. 36).
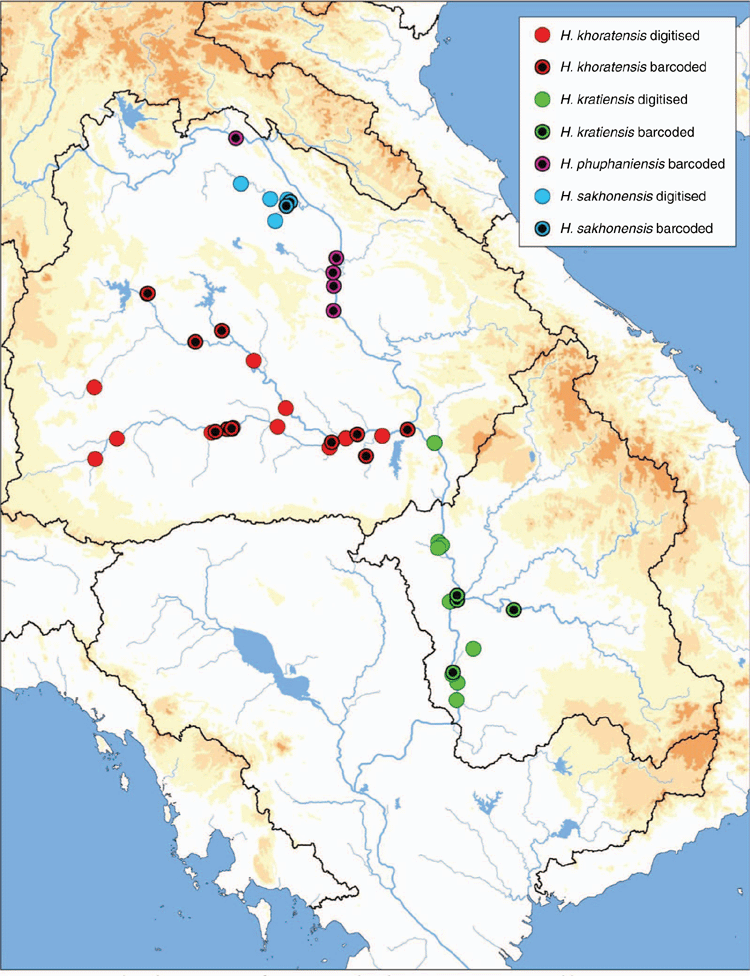
|
Ecoregions
Khorat Plateau, Kratie–Stung Treng.
Remarks
Known only from the Mekong drainage south of Mun River watershed.
Hyriopsis phuphaniensis, sp. nov.
(Table 1; Fig. 1, 27, 33, 36; Tables S1, S2)
Type locality
Mekong River, Mukdahan, at Muk River mouth, Mukdahan Province, Thailand, 16.54613, 104.73171.
Types
Holotype, UF 564755. Paratypes, UF 507832 (n = 2), same locality data as the holotype.
Material examined
9 lots (Table S1).
Diagnosis
Hyriopsis phuphaniensis is morphologically very similar to Hyriopsis kratiensis but tends to be less rectangular and more ovate.
Description
Shell elongately ovate, large wing, thick-shelled, and compressed. Anterior end wide, broadly rounded. Posterior end bluntly pointed, angular. Large wing, nearly as tall as shell disc. Ventral margin is straight to slightly curved. Biangulate posterior slope, wide, gradual. Umbo not elevated above hinge line. Nacre iridescent anteriorly, salmon-coloured towards umbo, opaque, strong striations. Left valve with two lateral teeth, curved, textured margins. Left valve with one oblique tooth extending from umbo to anterior adductor muscle (in smaller individuals), otherwise strongly fractured surface. Right valve with one lateral tooth, slightly curved, textured. Right valve with two small oblique pseudocardinal teeth extending from umbo to anterior adductor muscle (in small individuals), otherwise strongly fractured surface.
Distribution
Mekong drainage in north-eastern Thailand and west-central Laos (Fig. 36).
Ecoregion
Khorat Plateau.
Remarks
This species is known only from the Mekong River proper near and north of the Phu Phani Range (Fig. 36).
Hyriopsis khoratensis, sp. nov.
(Tables 1, 3; Fig. 1, 9, 27, 33, 36; Tables S1, S2; Fig. S1–S5)
Type locality
Pao River, Kamalasai, at Rt 214 bridge, Kamalasai District, Kalasin Province, Thailand, 16.34022, 103.57584.
Types
Holotype, UF 564756. Paratypes, UF 507618 (n = 9), same locality data as the holotype.
Taxonomic opinions since Haas (1969a)
Material examined
59 lots (Table S1).
Diagnosis
Hyriopsis khoratensis is conchologically similar to Hyriopsis bialata but is less elongate and more ovate.
Description
Shell ovate with large posterior wing, small anterior wing, thick shelled, somewhat inflated. Anterior end slightly sinuous to slightly rounded. Posterior end bluntly pointed. Dorsal margin consists of large smooth-margined wing, nearly as tall as shell disc. Ventral margin curved. Posterior slope somewhat biangulate, moderately narrow, steep. Umbo not elevated above hinge line. Nacre iridescent anteriorly, bluish white, salmon or brown towards umbo, opaque, strong striations. Left valve with two lateral teeth, diverging, textured. Left valve with one large, oblique pseudocardinal tooth extending from umbo to anterior adductor muscle, otherwise deeply fractured surface dentition. Right valve with one lateral tooth, slightly curved, textured. Right valve with two oblique pseudocardinals extending from umbo to adductor muscle (in smaller individuals), posterior pseudocardinal is modified to deeply fractured surface with multiple oblique ridges. Supra-anal aperture greater than 5× longer than pallial fusion between excurrent and supra-anal, smooth, tall. Excurrent aperture smooth, shorter than incurrent. Incurrent with 1 or 2 rows of short, conical-shaped papillae. Perforated septa, connections much smaller than perforations. Ectobranchous brooding. Laterally swollen and cream-coloured with brooding egg.
Distribution
Restricted to the Mun watershed (Mekong drainage) in eastern Thailand (Fig. 36).
Ecoregion
Khorat Plateau.
Hyriopsis sakhonensis, sp. nov.
(Tables 1, 3; Fig. 1, 9, 27, 33, 36; Tables S1, S2; Fig. S1–S5)
Type locality
Songkhram River, near confluence with Un River at bridge N of Ban Si Songkhram, Nakhon Phanom Province, Thailand, 17.63400, 104.24600.
Types
Holotype, UF 564757. Paratypes, UF 507786 (n = 6), same locality data as the holotype.
Material examined
8 lots (Table S1).
Diagnosis
Hyriopsis sakhonensis is morphologically very similar to H. phuphaniensis and H. kratiensis but tends to have a more bluntly pointed posterior end.
Description
Shell elongate with large posterior wing, small anterior wing, thick shelled. Anterior end slightly sinuous to slightly rounded. Posterior end pointed. Dorsal margin consists of large smooth-margined wing, nearly as tall as shell disc. Ventral margin curved. Posterior slope gentle, slightly biangulate. Umbo not elevated above hinge line. Nacre iridescent anteriorly, bluish or purplish white, salmon or pink towards umbo, opaque, strong striations. Left valve with two lateral teeth, straight, diverging, textured. Left valve with one small, oblique pseudocardinal tooth extending from umbo to anterior adductor muscle, otherwise a series of shallow ridges. Right valve with one lateral tooth, straight, textured. Right valve with two oblique pseudocardinals extending from umbo to adductor muscle, many shallow ridges posterior to teeth. Supra-anal aperture greater than 5× longer than pallial fusion between excurrent and supra-anal, smooth, tall. Excurrent aperture smooth, shorter than incurrent. Incurrent with 1 or 2 rows of short, conical-shaped papillae. Perforated septa, connections slightly smaller than perforations. Ectobranchous brooding. Limited lateral swelling and cream-coloured when brooding eggs.
Distribution
Restricted to the Songkhram watershed (Mekong drainage) in eastern Thailand (Fig. 36).
Ecoregion
Khorat Plateau.
Hyriopsis undescribed
(Table 1; Fig. 1, 27, 35; Tables S1, S2)
Taxonomic opinions since Haas (1969a)
Material examined
10 lots (Table S1).
Diagnosis
Not available. Shell characters very similar to H. bialata.
Distribution
Chao Phraya drainage in southern Thailand (Fig. 35).
Ecoregion
Chao Phraya.
Remarks
This undescribed species has been consistently recovered as a divergent molecular lineage in comparison to all other recognised Hyriopsis species. However, we have neither collected this species nor could we locate any voucher specimens with soft anatomy, and therefore choose not to describe it.
Hyriopsis velthuizeni (Schepman, 1896)
(Table 1; Fig. 1, 33; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
3 lots (Table S1).
Diagnosis
Hyriopsis velthuizeni is unlike all other Hyriopsis in that its posterior wing, umbo, and anterior wing form a straight line.
Distribution
Sumatra and Borneo.
Ecoregion
Kapuas.
Remarks
Geographic information available for this species is limited but suggests an unusual distribution. Of the three records we were able to examine and digitise, none could be georeferenced, and, as such, a dot distribution map could not be generated. The type locality of Unio velthuizeni, Mandai River near Nanga Kalis, can be georeferenced [0.69092, 112.91016] but the type specimens were unknown to us until just before publication and were not included in our Material Examined.
No other species of the Contradentini or Rectidentini is thought to be distributed on Borneo and Sumatra but absent from Java. This unusual distribution may be explained by the Paleo-North Sunda River which is thought to be a historical hydrological connection between these two modern landmasses (Voris 2000), but more carefully delineated species boundaries are necessary to test this hypothesis. The species has not yet been included in a molecular phylogeny.
Genus Prohyriopsis Haas, 1914
Diagnosis
Distinguished from Hyriopsis by a smaller wing and the presence of sculpturing on the posterior slope.
Distribution
Central Sumatra.
Species
We recognise one species in the genus Prohyriopsis (Fig. 26).
•Prohyriopsis stolata (von Martens, 1900).
Remarks
This taxon is known from a single specimen, the holotype of Unio stolatus von Martens, 1900 (ZMB 51795).
Prohyriopsis stolata (von Martens, 1900)
(Table 1; Fig. 1, 10, 26, 34; Table S1)
Taxonomic opinions since Haas (1969a)
Material examined
1 lot (Table S1).
Diagnosis
Same as for genus.
Distribution
Known only from the type locality in Indragiri drainage in central Sumatra (Fig. 34).
Ecoregion
Southern Central Sumatra.
Remarks
The species has not yet been included in a molecular phylogeny.
Undetermined taxa
‘Contradens fultoni’ Haas, 1930
Taxonomic opinions since Haas (1969a)
Diagnosis
NA.
Distribution
Disjunct distribution in northern Vietnam. Known from two records: SMF 3735 – 1 valve from Manson, Tonkin; NCSM 84934 – 2 specimens from Phong Tho, Vietnam.
Ecoregions
Xi Yiang, Hong Song.
Drainages
Zhujiang [Pearl River], Hong Song [Red River].
Remarks
The validity and systematic position of this species are uncertain and may have important implications if it belongs to the Contradentini as some of its morphological characters suggest. If the species is valid and belongs among the Contradentini it would be the only member of the Contradentini+Rectidentini radiation distributed east of Mekong drainage and outside of the South-east Asian subregion, and into the East Asian subregion (Fig. 7). The mussel fauna of East Asia is almost entirely different from that of South-east Asia and the presence of a member of the Contradentini in this region would be highly uncharacteristic of that regional assemblage (Zieritz et al. 2018a; Bolotov et al. 2020).
The type specimen of Unio fultoni (SMF 3735: 1 valve) is from Manson, Tonkin, which is thought to correspond to Mou Son, Vietnam (Do et al. 2018) – a mountainous region in north-eastern Vietnam draining the headwaters of the Pearl drainage. The single valve of the holotype does resemble members of the Contradentini, especially Lens, by sharing a similar shell outline, yellowish-brown periostracum with a green posterior slope, and lamellar pseudocardinal teeth nearly parallel to the lateral teeth. However, this record is geographically very distant, ~250 aerial miles from any of the confirmed Contradentini+Rectidentini records.
The other putative record of ‘C’. fultoni (NCSM 84934: 2 specimens) was recently collected from the Hong Song drainage and is much closer in proximity, ~90 aerial miles, to other confirmed Contradentini records in the Mekong drainage (but is distant from the type locality). These specimens are morphologically quite similar to L. comptus and close to this species’ northern range (Fig. 14). It may be that these specimens represent a disjunct population of L. comptus in the Song Hong drainage, suggesting a recent stream capture between the Mekong and Song Hong. However, other ‘C’. fultoni specimens (NCSM 84935: 2 specimens) collected during this same expedition and thought to be from outside the Mekong drainage are misidentified and have an incorrect drainage assignment. This record is listed as being collected from the Ma drainage but the given latitude and longitude for this record are within the Mekong drainage (Fig. 14), and the published COI sequence from this record (KX822636, Lopes-Lima et al. 2017) is recovered within our L. comptus clade (Fig. 12). Further research is needed to determine the accuracy of the two putative ‘C’. fultoni records, and validity and systematic position of that nominal species.
Undescribed Contradentini sp.
Taxonomic opinions since Haas (1969a)
Diagnosis
NA.
Distribution
Mekong drainage in northern Laos.
Ecoregions
Upper Lancang.
Remarks
This molecular lineage is known from one sequenced specimen (UMMZ 304347: GenBank accession KP795036) from Gnot Ou, Laos and is strongly divergent from all other known Contradentini. The lineage is clearly a part of the Contradentini but its position among that tribe remains uncertain. It may represent an undescribed genus. Greater sampling of northern Laos is necessary to resolving the systematic position and taxonomic status of this undescribed lineage.
Discussion
Distribution and geographic sampling bias of the Contradentini+Rectidentini
The geographic scope of this radiation is supported by 1837 digitised and imaged records from 15 natural history collections (Fig. 1, 2, Table S1, images available at http://www.mussel-project.net/). These data allowed us to more rigorously determine the distribution of each taxon but also provided a means to understand geographic sampling bias, which can be useful in determining where future sampling will be especially useful. The Contradentini+Rectidentini is distributed across much of South-east Asia from the Irrawaddy drainage in the west, to the Mekong drainage in the east, and south across the Malay Peninsula and throughout much of the Malay Archipelago, including the islands of Sumatra, Java, Borneo and Palawan.
The examined material is unevenly distributed across the 19 freshwater ecoregions occupied by the Contradentini+Rectidentini (Fig. 3). Our understanding of the total geographic distribution of this clade would benefit from greater sampling focused on less completely characterised regions. This is especially true of the northern ecoregions of the Mekong drainage, the ecoregions east of the Mekong, the ecoregions of the Salween drainage and the ecoregions to the west of the Salween, and the ecoregions of the Malay Archipelago. We take this opportunity to highlight and briefly discuss each of these poorly characterised regions.
Sampling efforts within the Mekong drainage have been strongly biased towards its southern half (Fig. 2). For example, we identified 374 museum records from the Khorat Plateau but documented only 23 records from the neighbouring upstream ecoregion, the Lower Lancang, and recorded zero lots from the ecoregion upstream of that, the Upper Lancang ecoregion (Fig. 3). The Upper Lancang and Lower Lancang ecoregions are more mountainous than the southernly ecoregions (Fig. 2), and the availability of suitable mussel habitat and access to sample that habitat appear to be driving the lack of material from this region. The paucity of records from the northern half of the most species-rich drainage in South-east Asia (Table 2) is likely to result in the underestimation of species richness in this biodiversity hotspot. Future sampling efforts focused on the more mountainous regions of the Mekong drainage should be a priority.
The ecoregions east of the Mekong drainage are also poorly sampled and this is an important area for future research. The lack of Contradentini or Rectidentini records in the Southern Annam ecoregion of central and southern Vietnam is somewhat surprising (Fig. 7). This ecoregion is separated from the Mekong by a relatively small drainage divide, and at least one other South-east Asian freshwater mussel radiation, the Pseudodontini, has dispersed into this ecoregion (e.g. UMMZ 108074, NHMUK 1901-12-12-98). The limited number of museum records from this ecoregion complicates delimiting the south-eastern distribution of the Contradentini+Rectidentini, and additional field and museum sampling effort in the region should also be a research priority. The unknown systematic position, validity, and geographic range of north Vietnamese nominal species ‘Contradens’ fultoni also obscures delimiting the Contradentini+Rectidentini north-eastern range (see ‘Contradens’ fultoni account). It remains unclear if the apparent absence of the Contradentini+Rectidentini in the non-Mekong ecoregions of Vietnam (Song Hong, Northern Annam, and Southern Annam ecoregions) is real or perceived. Greater sampling effort in these ecoregions would more completely elucidate the geographic extent of this radiation and the biogeographic barriers in the region more generally (e.g. the divide between the South-east Asian Subregion and the East Asian Subregion, Fig. 7).
The Salween ecoregion and the ecoregions to the west tend to have fewer Contradentini+Rectidentini records in comparison to most other ecoregions in mainland South-east Asia (Fig. 2, 3). While our knowledge of mussel diversity in these regions has grown dramatically in just the past few years (Bolotov et al. 2017a, 2019b, 2020; Konopleva et al. 2019a), evidence for the geographic extent of this diversity remains quite limited. Of the 13 Contradentini+Rectidentini species found in the Salween River ecoregions and the ecoregions to the west, over half are known from just their type localities. Similar to the sampling biases in the Mekong River, the upper Salween is also poorly sampled and there are no known Contradentini records from the Upper Salween ecoregion (Fig. 7). The Lower & Middle Salween ecoregion extends further south than the biogeographic divide recently proposed by Bolotov et al. (2020), who found convincing evidence for a significant faunal break at the Tanintharyi–Lenya drainage divide. This appears to be an area where fish and mussel biogeographic patterns may differ but further sampling of southern extent of the Lower & Middle Salween ecoregion is also needed (Bolotov et al. 2020).
The distribution and diversity of the Contradentini+Rectidentini of Sundaland are poorly characterised in comparison to that of mainland South-east Asia (Fig. 3). However, there has been much recent progress in sampling the Malay Peninsula and parts of the Malay Archipelago (Zieritz et al. 2016, 2018b, 2020). We know of no records of the Contradentini+Rectidentini from several of the archipelago’s southern ecoregions including Ache, Indian Ocean Slope of Sumatra and Java, Borneo Highlands, and Eastern Borneo (Fig. 7). These regions have been poorly sampled for freshwater mussels and deserve further attention. Data from these ecoregions will be useful for testing alternative hypotheses regarding the various hypothesised palaeo-drainages in Sundaland (e.g. Paleo-Malacca Straits River, Paleo-North Sunda River, Paleo-East Sunda River – see discussion below).
Individually, the two tribes have quite similar geographic distributions. The Contradentini and the Rectidentini co-occur across all of South-east Asia except the Rectidentini is absent from three ecoregions occupied by the Contradentini (Fig. 3). The Contradentini is quite diverse in the Salween and Sittaung–Irrawaddy ecoregions, whereas the Rectidentini is absent from them (Table 2). The only other area in which the Contradentini occurs but the Rectidentini does not is the island of Palawan. However, only one freshwater mussel species, Pressidens exanthematicus, occurs on Palawan and the systematic position of this taxon remains untested. We tentatively maintain it among the Contradentini (Pfeiffer et al. 2019).
Diversity and Endemism in the Contradentini and Rectidentini
The Contradentini and Rectidentini have their greatest species richness in the Mekong drainage with 24 species in total, 11 and 13 species respectively (Table 2). The Mekong is also the drainage with the greatest endemism: 19 of the 24 Contradentini+Rectidentini species in the Mekong are endemic to portions of that drainage (especially the Khorat Plateau – discussed below). The next most species-rich drainage, the adjacent Chao Phraya, is less than half as rich as the Mekong, with just 10 Contradentini+Rectidentini species (Table 2). The Contradentini typically has greater species richness than the Rectidentini in the drainages and ecoregions of mainland South-east Asia (the Mekong being the exception), whereas the Rectidentini tends to be slightly more diverse than the Contradentini in Sundaland (Table 2, Fig. 4).
Many species of the Contradentini+Rectidentini were historically thought to be geographically widespread and distributed across several of South-east Asia’s large drainages (e.g. Brandt 1974; Zieritz et al. 2018a). However, this paradigm has been recently questioned in favour of the hypothesis that most of the geographically widespread species are instead species complexes composed of several single-drainage-endemic species (Bolotov et al. 2017a). Our analysis demonstrates that the level of drainage-specific endemism in the Contradentini+Rectidentini has been recently overstated, as has also been shown for the Indochinellini (Pfeiffer et al. 2018a). All 24 Contradentini+Rectidentini species treated in Bolotov et al. (2017a) were considered to be endemic to single drainages (their supplementary table 5), while more geographically comprehensive sampling shows that eight of those species are found in at least two drainages. Of the 54 Contradentini+Rectidentini species treated in our assessment, 14 are distributed in more than one drainage (Fig. 5). This recent overestimation of drainage-specific endemism appears to be an over-reliance on geographically coarse recent sampling, narrowly defined phylogenetic species, and a lack of focus on historical sampling. More completely leveraging the publicly available data in the world’s natural history museums provides a more detailed view of South-east Asian freshwater mussel biogeography and one that more accurately reflects the dynamic geoclimatic history of the region.
Conversely, our assessment of the Contradentini+Rectidentini in the Irrawaddy, Sittaung and Salween does show very high levels of endemicity, similar to the levels proposed by Bolotov et al. (2017a). Twelve of the 13 Contradentini species in these three drainages are endemic to a single drainage, Lens contradens being the only exception. The high levels of endemism in the Irrawaddy, Sittaung and Salween are distinctly different from most other parts of South-east Asia, except for perhaps the Mekong and Borneo (Table 2). That said, the taxa thought to be endemic to the Irrawaddy, Sittaung and Salween are known from comparatively fewer records (Fig. 1) and these regions are more poorly sampled in comparison to the rest of mainland South-east Asia (Fig. 2, 3). As the sampling increases in the region we may see this level of endemism decrease, as has been the case with the other more completely sampled parts of South-east Asia (Pfeiffer et al. 2018a; this study).
Diversity and distribution patterns of the Contradentini+Rectidentini
As we revised the diversity and distribution of this radiation several interesting biogeographic patterns emerged.
Multiple Mekong radiations
We recovered four independent multispecies radiations endemic to the Mekong drainage (Fig. 9). These four radiations are distributed across the phylogeny of the Contradentini+Rectidentini and include at least 16 species, with many of these species being restricted to small portions of the Khorat Plateau ecoregion. Two of these Mekong radiations are in the Contradentini – a six-species clade in Lens (Fig. 12, 13, 14), and a 2–4-species clade in Physunio (Fig. 18, 19). The other two Mekong radiations are in the Rectidentini – a 4-species clade in Ensidens (Fig. 30, 32), and a 4-species clade in Hyriopsis (Fig. 27, 36). While the morphological diversification in these Mekong radiations is very limited (e.g. see the ‘diagnoses’ in our taxonomic accounts), they are molecularly quite divergent. And perhaps most convincingly to their recognition as biological species, is that these morphologically conserved and molecularly divergent lineages occur in the same drainage, and often occur sympatrically with their sister taxon, suggesting that these species are intrinsically reproductively isolated.
In each of these independent Mekong radiations, there are consistent biogeographic patterns that suggest the presence of several significant barriers to dispersal within the modern-day Mekong drainage, most of which are in the Khorat Plateau ecoregion. Within these Mekong radiations we recovered several species-level clades that are largely endemic to the Mun watershed (Ensidens sagittarius, Hyriopsis khoratensis, Lens pallegoixi), the Songkhram watershed (Ensidens dugasti, Hyriopsis sakhonensis, Physunio modelli Songkhram clade, Lens maenamensis Songkhram clade), and, to a lesser extent, the longitudinal portion of the middle Mekong (Ensidens spiculus, Lens rolfbrandti). This specific and consistent biogeographic pattern in the Khorat Plateau suggests that there are several significant biogeographic barriers within the Mekong drainage that are driving a substantial portion of diversification in the Contradentini+Rectidentini. Our ability to detect these finer-scale biogeographic patterns may be because the Khorat Plateau is the most well sampled portion of the Mekong drainage (Fig. 3). Some of these apparent barriers within the Mekong drainage have also been observed in the Indochinellini (Kongim et al. 2015; Pfeiffer et al. 2018a). We suspect that increased sampling in other major watersheds of the Mekong may reveal additional intradrainage barriers to dispersal (e.g. Tonle Sap, Stung Treng, Xe Don, Nam Ou).
It is also worth noting that each of these Mekong radiations contain species that are commonly found in, or close to, the Mekong River proper (i.e. L. comptus, E. jaculus, H. phuphaniensis, P. pellucidus), suggesting that these taxa may be closely associated with the Mekong River mainstem ecosystem. Several taxa in the North American mussel assemblage are associated with large river ecology and are commonly referred to as ‘large-river specialists’ (Haag 2012). Although very little is known about the ecology of these Mekong River species, their large distributions and limited genetic divergences are reminiscent of other well known large river specialists in North America (Berg et al. 2007; Roe and Boyer 2015; Pfeiffer et al. 2018b). Our understanding of how ecology shapes the diversity and distribution of South-east Asian freshwater mussels is almost non-existent, and, as a consequence, the observed biogeographic patterns in South-east Asian freshwater mussels are often explained by geophysical phenomena alone (including those discussed here). It remains unclear how ecology shapes freshwater mussel diversity and distribution in South-east Asia.
Stream capture
We have documented how many Contradentini+Rectidentini species are distributed across multiple drainages, and it is worthwhile to speculate how these disjunctions may have been precipitated. Many of these disjunctions are likely the product of two different geoclimatic phenomena – stream capture and historical confluence. Stream capture is when a drainage divide migrates and causes a portion of one drainage to be redirected (victim) and integrated into an adjacent drainage (aggressor) (Willett et al. 2014). Historical confluence is when two adjacent drainages, separated by a marine ecosystem, become a single larger palaeo-connected drainage as sea level drops and the rivers converge on the recently exposed continental shelf (Dias et al. 2014).
Several species of the Contradentini+Rectidentini have disjunct distributions that may be best explained by stream capture and geodispersal. For example, Lens contradens is nearly absent from the Mekong drainage but occurs in three, isolated, low-order streams, very near the Mekong–Chao Phraya drainage divide – the Kok, Ing and Loei watersheds (Fig. 15). The absence of intervening L. contradens records from the Mekong River is consistent with these peripheral populations being integrated into the Mekong system by multiple separate stream-capture events, rather than one isolated event. However as noted before, this region of the Mekong is undersampled and further sampling efforts are needed to determine whether these populations are as isolated as the currently available data suggest. All three of these rivers (Kok, Ing, Loei) have been previously implicated in hypothesised connections between the Mekong and Chao Phraya (Hutchison 1989; Rainboth et al. 2012), and this part of the Mekong has many sharp changes in channel direction, a highly crenulated drainage divide, and many obtuse conflunce angles – each of which are common network geometries of drainage rearrangements (Bishop 1995).
Similarly, Lens contradens is absent from the Salween River except for one relatively small watershed, the Moei, very near the Salween–Chao Phraya–Mae Klong drainage divide (Fig. 15). This disjunct population of L. contradens in the Salween suggests a stream-capture event caused part of the Mae Klong or Chao Phraya to start draining to the Salween. Bohlen et al. (2020a, 2020b) hypothesised that the Salween drainage recently captured part of the Mae Klong drainage ~1.5 million years ago and that this event effected the distribution of at least two freshwater fish species. These putative stream-capture events occur in relatively small, high-gradient watersheds very near a drainage divide, which are likely areas of drainage rearrangement especially ‘bottom up’ processes such as headwater extension (Bishop 1995). These watersheds (Moei, Kok, Ing, Loei) also tend to flow in a northerly direction, opposite the general direction of the Mekong and Salween River mainstems, creating strongly obtuse confluences that are also characteristic of drainage rearrangements (Bishop 1995).
We also found disjunct distributions in two species of Ensidens that also suggest stream capture. Ensidens ingallsianus is distributed across much of eastern Indochina but the species is known only from the Mekong drainage by two isolated populations very close to the Mekong–Chao Phraya–Bang Pakong drainage divide at the south-western edge of the Khorat Plateau (Fig. 31). This disjunction and the shallow molecular divergence suggests that the Mekong drainage recently captured portions of the Chao Phraya or Bang Pakong, or perhaps both. The western edge of the Khorat Plateau is a geologically active area that is thought to have caused many minor and major stream-capture events between the Mekong and the Chao Phraya. Although the timings of these capture events are often poorly understood, the majority appear to have occurred in the Quaternary (Hutchison 1989; Heggemann et al. 1994; Parry 1996; Rainboth et al. 2012), which may be consistent with shallow genetic divergences observed here. Ensidens telus shows a somewhat inverse pattern, being distributed across much of the lower Mekong with disjunct populations in the eastern Bang Pakong drainage, suggesting that the Bang Pakong recently captured parts of the Mekong drainage. The drainage divide between these two watersheds is much less dramatic than in comparison to the other hypothesised captures (Fig. 31) and there is evidence of hydrological connectivity between the Bang Pakong and Tonle Sap (Rainboth 1996; Rainboth et al. 2012).
Evidence also exists for at least one intradrainage stream capture in the Mekong – where a portion of the Mun watershed (Khorat Plateau ecoregion) was captured by the Loei watershed (Lower Lancang ecoregion). This putative stream capture is supported by the distribution and genetic structure of P. modelli (Fig. 18, 19). We found a shallow clade of P. modelli that is largely restricted to the Khorat Plateau and Kratie–Stung Treng ecoregions; however, individuals from the Loei watershed, are nested deeply within this clade. Physunio modelli is otherwise absent from the Lower Lancang drainage and the northern portion of the Khorat Plateau. This disjunction is best explained by the Loei watershed capturing parts of the upper Mun watershed.
The disjunct distributions of these species and the geomorphology of the watersheds have many characteristics of geodispersal via stream capture, but these biogeographic hypotheses deserve further scrutiny. Too often have disjunct distributions and drainage network morphologies (e.g. capture elbows, obtuse confluence angles, crenulated drainage divides) been used as evidence of drainage rearrangement and faunal exchange without direct geological evidence (Bishop 1995) – those described here are no exception. Ideally, hypotheses of drainage rearrangement should be supported with dated geological evidence that can be demonstrably linked to palaeodrainage (Bishop 1995; Fan et al. 2018).
Historical confluence
During the last glacial maximum, much of the world’s freshwater was tied up in glaciers, which dramatically lowered global sea levels and converted many of the world’s shallow seas to large coastal plains. This geoclimatic phenomenon profoundly shaped global freshwater biodiversity as these lower sea-level stands created many palaeo-connected river systems that facilitated dispersal of many obligate freshwater taxa (Dias et al. 2014). In South-east Asia, this phenomenon exposed the entirety of the Sunda Shelf and this new landmass was drained by several great palaeo-drainages, the largest of which was the Paleo-Siam River system (Voris 2000). Today much of the Paleo-Siam River is ‘drowned’ and separated into various independent drainages including the Chao Phraya, Mae Klong, Bang Pakong, Chantaburi, and many smaller coastal drainages of the Malay Peninsula. The palaeo-connections between these modern drainages have shaped freshwater biodiversity in South-east Asia (de Bruyn et al. 2013; Dias et al. 2014) and are reflected in the distribution of many Contradentini and Rectidentini.
Several Contradentini+Rectidentini species are distributed across many independent drainages but have very limited genetic divergences. This geographic and genetic pattern is consistent with recent dispersal and connectivity associated with the Paleo-Siam River system and is found in many species, including Lens contradens (Fig.12, 15), Lens inornatus (Fig. 12, 16), Physunio superbus (Fig. 18, 19), Trapezoideus foliaceus, T. subviridus, Solenaia emarginata (Fig. 18, 22), Ensidens. ingallsianus (Fig. 30, 31), Hyriopsis myersiana (Fig. 27, 34), and H. desowitzi (Fig. 27, 35). The phylogeographic patterns of these taxa indicate that there is considerable variation within the degree of connectivity or isolation within the Paleo-Siam River system – some taxa are distributed across much of the palaeo-connected system (E. ingallsianus, L. contradens, P. superbus) whereas others are restricted to much smaller portions of the greater palaeodrainage (H. myersiana, T. foliaceus, T. subviridis). Clearly, there is an abundance of evidence suggesting that the Paleo-Siam River system has shaped Contradentini+Rectidentini diversity and distribution.
However, the importance of the other hypothesised palaeo-drainages in the region (e.g. Paleo-Malacca Straits River, Paleo-North Sunda River, Paleo-East Sunda River, Voris 2000) in shaping Contradentini+Rectidentini diversity and distribution is very limited. Lens contradens specimens collected from areas implicated in the Paleo-Siam River (e.g. Malay Peninsula eastern slope) and areas of the Paleo-Malacca River (e.g. Malay Peninsula western slope) show some limited sequence divergence that may reflect a pattern consistent with the influence of the Paleo-Malacca River (Fig. 12). Rectidens sumatrensis also shows some limited molecular divergence consistent with separation between the Paleo-Malacca River and the palaeo-drainages of northern Borneo (Fig. 27). The unusual geographic distribution of Hyriopsis velthuizeni may be consistent with the existence of the palaeo-North Sunda River system but the boundaries and validity of this putative species are too poor to make any meaningful inferences. Increased sampling of the Malay Archipelago will be necessary to better understand how palaeo-drainage evolution has affected freshwater mussel diversity and distribution in the region.
Zoogeographic regions
Several recent papers have delimited, refined and discussed various useful freshwater mussel zoogeographic regions in South-east Asia (Graf and Cummings 2007; Zieritz et al. 2018a; Konopleva et al. 2019a; Bolotov et al. 2020). However, these regions have been somewhat subjectively defined rather than relying on analysis of the underlying distributional data. Our UPGMA analysis of Contradentini+Rectidentini presence or absence across the freshwater ecoregions of South-east Asia is consistent with recognising six zoogeographic regions (Fig. 7a) and our MNDS analysis of Contradentini+Rectidentini phylogenetic diversity recovers a similar biogeographic pattern, consistent with recognising five (or six) cohesive zoogeographic provinces (Fig. 7b). For the most part, these regions are consistent with previously defined freshwater mussel regions (Graf and Cummings 2007; Zieritz et al. 2018a; Konopleva et al. 2019a; Bolotov et al. 2020), but provide more objectivity. We briefly discuss each of these provinces with respect to the diversity of the Contradentini and Rectidentini, and hope that it is useful in understanding biogeographic patterns in South-east Asian freshwater biodiversity more generally.
The Western Indochina province is the Sittaung–Irrawaddy ecoregion and is the only mussel province in South-east Asia where the Contradentini occurs but the Rectidentini does not. The absence of the Rectidentini causes these drainages to be the least phylogenetically diverse in our assessment (Table 2). Despite these two drainages sharing geographically close river mouths and having several natural interbasin connections, these systems are thought to be composed of entirely endemic Contradentini assemblages. This level of drainage-specific endemism is unlike the diversity patterns for the rest of South-east Asia (Table 2) but is consistent with the hypothesis that at least the Irrawaddy is thought to be a palaeo-disconnected drainage (Dias et al. 2014 – although Sittaung River not treated in their analysis). It remains unclear whether the level of Contradentini+Rectidentini endemism in these two adjacent drainages is real or an artefact of the paucity of records from this ecoregion and its taxa (Fig. 1, 3).
The Salween province is composed of the Upper Salween, Inle Lake, and Lower & Middle Salween ecoregions. This province appears to represent a transitionary zone between Western Indochina and the Gulf of Thailand provinces. The Salween province shares one genus of the Contradentini+Rectidentini in common with the Western Indochina (Yaukthwa) and two with the Gulf of Thailand province (Lens and Trapezoideus) and this fact strongly influences our UPGMA analysis (Fig. 7a). However, the observed phylogenetic diversity of the Salween drainage is much more similar to the drainages of Western Indochina province than it is to the drainages of the Gulf of Thailand provinces (Fig. 7b). This disparate pattern led us to classify this region as distinct from the adjacent provinces and to highlight its transitionary nature, at least with respect to the Contradentini+Rectidentini. Bolotov et al. (2020) demonstrated that there is a substantial change in freshwater mussel fauna at the Tanintharyi–Lenya drainage divide, whereas the Salween ecoregion extends much further south. This disparity may suggest that mussel and fish zoogeographic barriers in area are incongruent or the ecoregion may be in need of refinement.
The Mae Klong, Chao Phraya, and Eastern Gulf Thailand ecoregions comprise the Gulf of Thailand province. This region has quite high species and phylogenetic diversity but very low drainage-specific endemism (Table 2). The similarity in the mussel assemblages of these three ecoregions is likely a product of the historical confluences between the Mae Klong, Chao Phraya, Bang Pakong, Chantaburi, and smaller coastal drainages of the Malay Peninsula associated with the Paleo-Siam River. The Gulf of Thailand province is also faunistically similar to the South-western Sundaland province (Fig. 7); a shared similarity in these assemblages is also likely to be partially explained by the Paleo-Siam River.
The Mekong province is composed of the Upper Lancang, Lower Lancang, Khorat Plateau, Kratie–Stung Treng, and Mekong Delta ecoregions. While the diversity varies substantially among ecoregions (Fig. 4), collectively they form a cohesive zoogeographic area (Fig. 7a). For example, the Lower Lancang and Mekong Delta ecoregions have similar species richness (six and seven species respectively), but only two species are shared between them (i.e. Lens comptus and Physunio modelli). Clearly, there are several influential biogeographic barriers within this drainage, and the currently recognised ecoregions appear to do a good job at characterising these intradrainage assemblages.
The South-western Sundaland province includes the Malay Peninsula Eastern Slope, North Central Sumatra–Western Malaysia, Ache, Southern Central Sumatra, Indian Ocean Slope of Sumatra and Java, Southern Sumatra–Western Java, and Central and Eastern Java ecoregions. The Contradentini+Rectidentini fauna of this region is most similar to the Gulf of Thailand assemblage but also shares some taxa with the Bornean province. The northern extent of this province is somewhat consistent with a long recognised and well known zoogeographic transitionary zone between the Indochinese and Sundaland regions which occurs more or less near the Isthmus of Kra (Wallace 1876; Woodruff and Turner 2009), although the latitude of this boundary and how it was generated is thought to be highly taxon dependent (Lohman et al. 2011; Dejtaradol et al. 2016). Several freshwater taxa in the region have biogeographic patterns that are thought to be limited by three prominent marine transgressions (i.e. Isthmus of Kra seaway, Surat Thani–Krabi seaway, Kangar–Pattani seaway) that inundated parts of the Malay Peninsula during the Pliocene (mussel, Bolotov et al. 2020; snail, Veeravechsukij et al. 2018; prawn, de Bruyn et al. 2005; fish, Bohlen et al. 2020a). However, these historical seaways appear to have had limited effect on the modern-day distribution or genetic structure of any of the taxa analysed here – except for perhaps Trapezoideus lenya but that species is known only from its type locality. Most freshwater mussels that occur on or near the Malay Peninsula have ranges that terminate well north (Lens inornatus, Trapezoideus foliaceus, Hyriopsis myersiana) or well south (Rectidens sumatrensis) of the Isthmus of Kra. And four of the analysed taxa have populations on both sides of the Isthmus of Kra that show very limited genetic differentiation (Lens contradens, Physunio superbus, Ensidens ingallsianus, Hyriopsis bialata). It is likely that these taxa dispersed south after global sea levels decreased and the marine transgressions near the Isthmus of Kra were replaced by coastal plain and the growing Paleo-Siam River.
The Borneo province includes the Kapuas, North-western Borneo, Borneo Highlands, South-eastern Borneo, North-eastern Borneo, Eastern Borneo, and Palawan–Busuanga– Mindoro ecoregions. This region is distinct from the neighbouring South-western Sundaland province in terms of its species assemblage and phylogenetic diversity (Fig. 7). Despite including only a small fraction of the Bornean assemblage in our analysis of phylogenetic β diversity, this province is phylogenetically divergent from all others in the South-east Asian Subregion. The high level of phylogenetic diversity in this province is consistent with the divergent morphologies of many of its taxa and their widespread taxonomic distribution (Pfeiffer et al. 2019).
These provinces provide a useful step towards more objectively defining freshwater mussel regional assemblages in South-east Asia, but increasing the hydrographic resolution of the units would be useful. The previously defined ecoregions are practical in that they provide a unified zoogeographic framework for comparing biodiversity patterns across many freshwater assemblages, even if the ecoregions lack the more fine-scale resolution of other available hydrographic units (e.g. hydrosheds). For example, by leveraging the available freshwater ecoregions and their previously determined fish species richness (Abell et al. 2008), we can see that freshwater mussel species richness and fish species richness is strongly correlated across the ecoregions of South-east Asia (Fig. 37). These broader taxonomic comparisons are useful in identifying freshwater biodiversity hotspots (e.g. high fish and mussel diversity in the Khorat Plateau) or identifying taxa that do not follow regional patterns of diversity (e.g. low mussel diversity in several ecoregions of the Western Sundaland Province relative to fish diversity). That said, some of the previously defined ecoregions (e.g. Lower and middle Salween) may not accurately reflect some important freshwater mussel barriers (e.g. Tanintharyi–Lenya drainage divide, Bolotov et al. 2020). We look forward to more taxonomically comprehensive and geographically nuanced evaluations of South-east Asian freshwater biodiversity.
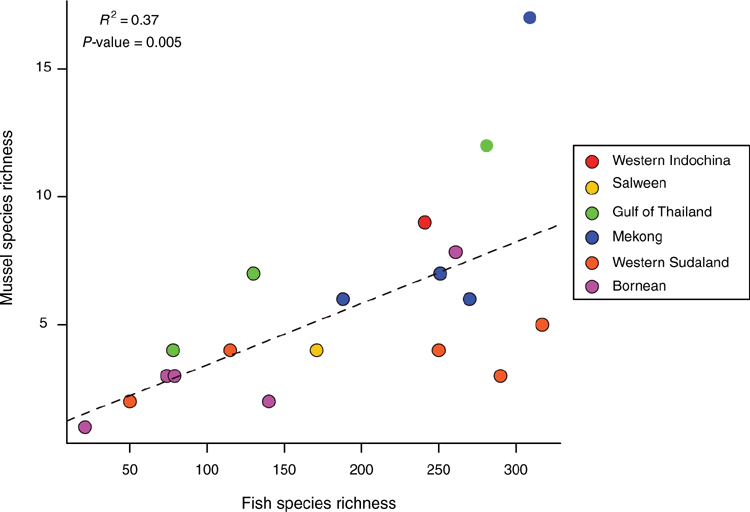
|
Conclusion
Recent efforts to better understand the systematics and biogeography of the South-east Asian freshwater mussels have relied heavily on either museum specimens or recently sequenced individuals. While both of these approaches have been tremendously useful in isolation, they are greater together than the sum of their parts. This revision unites these two approaches and provides an updated systematic and biogeographic framework that future studies can use and improve.
It is remarkable how much progress has been made recently by the freshwater mussel systematics community with respect to describing the phylogeny, distribution, and diversity of the Contradentini+Rectidentini. Despite this transformation in systematic knowledge, the basic ecological requirements of this radiation remain almost completely unknown. For example, to our knowledge host fish suitability has been assessed for only one of the 54 valid Contradentini+Rectidentini species (Hyriopsis myersiana, Arayawatanavij et al. 1992). If we are to make meaningful progress with respect to understanding South-east Asian freshwater mussel ecology, evolution and biogeography, this knowledge gap must be bridged.
Evaluating the conservation status of these taxa is also an important research priority. Many of these taxa have had no formal conservation assessment, and, given the proposed taxonomic and distributional changes, most previous assessments are in need of updating. This revision, incomplete and imperfect as it is, provides an improved framework for addressing these research priorities and many others. Equipped with clearly defined genetic and geographic species boundaries organised in a biological classification that reflects evolutionary relationships, we can more completely characterise the origin and transformation of traits, how species interact with their environment, rigorously measure changes in distribution, and better appreciate the emergent properties of South-east Asian freshwater ecosystems.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was funded in part by the USA National Science Foundation: All Cypriniformes Species Inventory Project (DEB 1022720) and a Doctoral Dissertation Improvement Grant (DEB 1701901).
Acknowledgements
Deein Gridsada (Suphan Buri Inland Fisheries Research and Development Center) and Tach Phanara and So Nam (Inland Fisheries Research and Development Institute, Fisheries Administration) assisted with collection and export permits for Thailand and Cambodia respectively. Special thanks to the world’s natural history museums and their staff for making biodiversity data accessible.
References
Abell, R., Thieme, M. L., Revenga, C., Bryer, M., Kottelat, M., Bogutskaya, N., Coad, B., Mandrak, N., Balderas, S. C., and Bussing, W. (2008). Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58, 403–414.| Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Adamson, E. A., Hurwood, D. A., and Mather, P. B. (2012). Insights into historical drainage evolution based on the phylogeography of the chevron snakehead fish (Channa striata) in the Mekong Basin. Freshwater Biology 57, 2211–2229.
| Insights into historical drainage evolution based on the phylogeography of the chevron snakehead fish (Channa striata) in the Mekong Basin.Crossref | GoogleScholarGoogle Scholar |
Affandi, M., Candra, L. A., Priatama, A. B., Irawan, B., and Soegianto, A. (2014). Diversity of the unionid freshwater mussels (Bivalvia: Unionidae) in Brantas River, east Java, Indonesia. Journal of Biological Researches 18, 111–115.
| Diversity of the unionid freshwater mussels (Bivalvia: Unionidae) in Brantas River, east Java, Indonesia.Crossref | GoogleScholarGoogle Scholar |
Affandi, M., Hariyanto, S., and Soegianto, A. (2017). The distinctive character of shell morphology Rectidens sumatrensis (Dunker, 1852) against Elongaria orientalis (Lea, 1840) (two local species, Bivalvia: Unionidae) from the Brantas River, Indonesia. AIP Conference Proceedings 1888, 020004.
| The distinctive character of shell morphology Rectidens sumatrensis (Dunker, 1852) against Elongaria orientalis (Lea, 1840) (two local species, Bivalvia: Unionidae) from the Brantas River, Indonesia.Crossref | GoogleScholarGoogle Scholar |
Araujo, R., Buckley, D., Nagel, K.-O., and Machordom, A. (2017). Potomida littoralis (Bivalvia, Unionidae) evolutionary history: slow evolution or recent speciation? Zoological Journal of the Linnean Society 179, 277–290.
Arayawatanavij, P., Nagachinta, A., and Tispijit, N. (1992). Site selection and attachment duration of freshwater pearl mussel glochidia on 4 species of freshwater fishes. Technical Paper 5, Inland Fisheries Division, Department of Fisheries, Ministry of Agriculture, Bangkok, Thailand
Attwood, S., and Johnston, D. (2001). Nucleotide sequence differences reveal genetic variation in Neotricula aperta (Gastropoda: Pomatiopsidae), the snail host of schistosomiasis in the lower Mekong Basin. Biological Journal of the Linnean Society. Linnean Society of London 73, 23–41.
| Nucleotide sequence differences reveal genetic variation in Neotricula aperta (Gastropoda: Pomatiopsidae), the snail host of schistosomiasis in the lower Mekong Basin.Crossref | GoogleScholarGoogle Scholar |
Berg, D. J., Christian, A. D., and Guttman, S. I. (2007). Population genetic structure of three freshwater mussel (Unionidae) species within a small stream system: significant variation at local spatial scales. Freshwater Biology 52, 1427–1439.
| Population genetic structure of three freshwater mussel (Unionidae) species within a small stream system: significant variation at local spatial scales.Crossref | GoogleScholarGoogle Scholar |
Bishop, P. (1995). Drainage rearrangement by river capture, beheading and diversion. Progress in Physical Geography 19, 449–473.
| Drainage rearrangement by river capture, beheading and diversion.Crossref | GoogleScholarGoogle Scholar |
Bogan, A. E., and Do, V. T. (2014). Two freshwater bivalve species new to the fauna of Vietnam (Mollusca: Bivalvia: Arcidae and Unionidae). Tropical Natural History 14, 113–116.
Bohlen, J., Dvořák, T., Šlechta, V., and Šlechtová, V. (2020a). Sea water shaping the freshwater biota: hidden diversity and biogeographic history in the Paracanthocobitis zonalternans species complex (Teleostei: Nemacheilidae) in western Southeast Asia. Molecular Phylogenetics and Evolution 148, 106806.
| Sea water shaping the freshwater biota: hidden diversity and biogeographic history in the Paracanthocobitis zonalternans species complex (Teleostei: Nemacheilidae) in western Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 32247884PubMed |
Bohlen, J., Dvořák, T., Šlechta, V., and Šlechtová, V. (2020b). Resolving an unnoticed diversity within the Schistura robertsi species complex (Teleostei: Nemacheilidae) using molecules and morphology. Molecular Phylogenetics and Evolution 151, 106894.
| Resolving an unnoticed diversity within the Schistura robertsi species complex (Teleostei: Nemacheilidae) using molecules and morphology.Crossref | GoogleScholarGoogle Scholar | 32562824PubMed |
Bolotov, I. N., Kondakov, A. V., Vikhrev, I. V., Aksenova, O. V., Bespalaya, Y. V., Gofarov, M. Y., Kolosova, Y. S., Konopleva, E. S., Spitsyn, V. M., and Tanmuangpak, K. (2017a). Ancient river inference explains exceptional Oriental freshwater mussel radiations. Scientific Reports 7, 2135.
| Ancient river inference explains exceptional Oriental freshwater mussel radiations.Crossref | GoogleScholarGoogle Scholar | 28522869PubMed |
Bolotov, I. N., Vikhrev, I. V., Kondakov, A. V., Konopleva, E. S., Gofarov, M. Y., Aksenova, O. V., and Tumpeesuwan, S. (2017b). New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Scientific Reports 7, 11573.
| New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 28912555PubMed |
Bolotov, I. N., Pfeiffer, J. M., Konopleva, E. S., Vikhrev, I. V., Kondakov, A. V., Aksenova, O. V., Gofarov, M. Y., Tumpeesuwan, S., and Win, T. (2018). A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia. Scientific Reports 8, 10030.
| A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 29968793PubMed |
Bolotov, I. N., Vikhrev, I. V., Lopes-Lima, M., Gofarov, M. Y., Konopleva, E. S., Lunn, Z., Chan, N., and Bogan, A. E. (2019a). Indonaia rectangularis (Tapparone-Canefri, 1889), comb. nov., a forgotten freshwater mussel species from Myanmar (Bivalvia, Unionidae). ZooKeys 852, 23.
| Indonaia rectangularis (Tapparone-Canefri, 1889), comb. nov., a forgotten freshwater mussel species from Myanmar (Bivalvia, Unionidae).Crossref | GoogleScholarGoogle Scholar | 31210740PubMed |
Bolotov, I. N., Konopleva, E. S., Vikhrev, I. V., Lopes-Lima, M., Bogan, A. E., Lunn, Z., Chan, N., Win, T., Aksenova, O. V., and Gofarov, M. Y. (2019b). Eight new freshwater mussels (Unionidae) from tropical Asia. Scientific Reports 9, 12053.
| Eight new freshwater mussels (Unionidae) from tropical Asia.Crossref | GoogleScholarGoogle Scholar | 31427656PubMed |
Bolotov, I. N., Konopleva, E. S., Vikhrev, I. V., Gofarov, M. Y., Lopes-Lima, M., Bogan, A. E., Lunn, Z., Chan, N., Win, T., and Aksenova, O. V. (2020). New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia. Scientific Reports 10, 6616.
| New freshwater mussel taxa discoveries clarify biogeographic division of Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 32313058PubMed |
Borowiec, M. L. (2016). AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ 4, e1660.
| AMAS: a fast tool for alignment manipulation and computing of summary statistics.Crossref | GoogleScholarGoogle Scholar | 26835189PubMed |
Brandt, R. A. (1974). The non-marine aquatic Mollusca of Thailand. Archiv für Molluskenkunde 105, 1–423.
Breinholt, J. W., Earl, C., Lemmon, A. R., Lemmon, E. M., Xiao, L., and Kawahara, A. Y. (2018). Resolving relationships among the megadiverse butterflies and moths with a novel pipeline for anchored phylogenomics. Systematic Biology 67, 78–93.
| Resolving relationships among the megadiverse butterflies and moths with a novel pipeline for anchored phylogenomics.Crossref | GoogleScholarGoogle Scholar | 28472519PubMed |
Chernomor, O., Von Haeseler, A., and Minh, B. Q. (2016). Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65, 997–1008.
| Terrace aware data structure for phylogenomic inference from supermatrices.Crossref | GoogleScholarGoogle Scholar | 27121966PubMed |
Dang, N. T., Thai, T. B., and Pham, V. M. (1980). ‘Dinh Loai Dong Vat Khong Zuong Song Nuoc Ngot Bac Viet Nam.’ (Nha Xuat Ban Knoa Hoc Va Ky Thuat: Ha Noi, Vietnam.)
Dapporto, L. (2013). recluster: an unbiased clustering procedure for beta-diversity turnover. Ecography 36, 1070–1075.
| recluster: an unbiased clustering procedure for beta-diversity turnover.Crossref | GoogleScholarGoogle Scholar |
Dautzenberg, P., and Fischer, H. (1905). Liste des mollusques reecoltes par MH Mansuy en Indo-Chine et au Yunnan et description d’especes nouvelles. Journal de Conchyliologie 53, 343–471.
de Bruyn, M., Nugroho, E., Hossain, M. M., Wilson, J. C., and Mather, P. B. (2005). Phylogeographic evidence for the existence of an ancient biogeographic barrier: the Isthmus of Kra Seaway. Heredity 94, 370–378.
| Phylogeographic evidence for the existence of an ancient biogeographic barrier: the Isthmus of Kra Seaway.Crossref | GoogleScholarGoogle Scholar | 15523504PubMed |
de Bruyn, M., Rüber, L., Nylinder, S., Stelbrink, B., Lovejoy, N. R., Lavoué, S., Tan, H. H., Nugroho, E., Wowor, D., and Ng, P. K. (2013). Paleo-drainage basin connectivity predicts evolutionary relationships across three Southeast Asian biodiversity hotspots. Systematic Biology 62, 398–410.
| Paleo-drainage basin connectivity predicts evolutionary relationships across three Southeast Asian biodiversity hotspots.Crossref | GoogleScholarGoogle Scholar | 23391942PubMed |
Deein, G., Unakornsawat, Y., Rattanadaeng, P., Sutcharit, C., Kong-Im, B.-O., and Panha, S. (2004). A new species of Solenaia from Thailand (Bivalvia: Unionidae: Ambleminae). The Natural History Journal of Chulalongkorn University 3, 53–58.
Deein, G., Pongsri, C., Rattanadeang, P., Prateepasen, R., and Panha, S. (2008). Glochiduim shell morphology of Solenaia khwaenoiensis Panha & Deein, 2003 (Bivlavia: Unionidae). Tropical Natural History 8, 61–64.
Dejtaradol, A., Renner, S. C., Karapan, S., Bates, P. J., Moyle, R. G., and Päckert, M. (2016). Indochinese‐Sundaic faunal transition and phylogeographical divides north of the Isthmus of Kra in Southeast Asian bulbuls (Aves: Pycnonotidae). Journal of Biogeography 43, 471–483.
| Indochinese‐Sundaic faunal transition and phylogeographical divides north of the Isthmus of Kra in Southeast Asian bulbuls (Aves: Pycnonotidae).Crossref | GoogleScholarGoogle Scholar |
Deshayes, G.-P., and Jullien, J. (1876). Mémoire sur les mollusques nouveaux du Cambodge: envoyés au muséum par M. le docteur Jullien. Bulletin de Nouvelles Archives du Muséum d’Histoire Naturelle 10, 115–162.
Dias, M. S., Oberdorff, T., Hugueny, B., Leprieur, F., Jézéquel, C., Cornu, J. F., Brosse, S., Grenouillet, G., and Tedesco, P. A. (2014). Global imprint of historical connectivity on freshwater fish biodiversity. Ecology Letters 17, 1130–1140.
| Global imprint of historical connectivity on freshwater fish biodiversity.Crossref | GoogleScholarGoogle Scholar | 25039890PubMed |
Do, V. T., Tuan, L. Q., and Bogan, A. E. (2018). Freshwater mussels (Bivalvia: Unionida) of Vietnam: diversity, distribution, and conservation status. Freshwater Mollusk Biology and Conservation 21, 1–18.
| Freshwater mussels (Bivalvia: Unionida) of Vietnam: diversity, distribution, and conservation status.Crossref | GoogleScholarGoogle Scholar |
Fan, N., Chu, Z., Jiang, L., Hassan, M. A., Lamb, M. P., and Liu, X. (2018). Abrupt drainage basin reorganization following a Pleistocene river capture. Nature Communications 9, 3756.
| Abrupt drainage basin reorganization following a Pleistocene river capture.Crossref | GoogleScholarGoogle Scholar | 30217980PubMed |
Gould, A. (1843). New species of Mollusca from Tavoy, British Burmah. Proceedings of the Boston Society of Natural History 1, 139–141.
Graf, D. L. (2002). Molecular phylogenetic analysis of two problematic freshwater mussel genera (Unio and Gonidea) and a re-evaluation of the classification of Nearctic Unionidae (Bivalvia: Palaeoheterodonta: Unionoida). The Journal of Molluscan Studies 68, 65–71.
| Molecular phylogenetic analysis of two problematic freshwater mussel genera (Unio and Gonidea) and a re-evaluation of the classification of Nearctic Unionidae (Bivalvia: Palaeoheterodonta: Unionoida).Crossref | GoogleScholarGoogle Scholar |
Graf, D. L. (2007). Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoida) diversity and the Comparatory Method as a species concept. Proceedings. Academy of Natural Sciences of Philadelphia 156, 71–88.
| Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoida) diversity and the Comparatory Method as a species concept.Crossref | GoogleScholarGoogle Scholar |
Graf, D. L., and Cummings, K. S. (2006a). Palaeoheterodont diversity (Mollusca: Trigonioida + Unionoida): what we know and what we wish we knew about freshwater mussel evolution. Zoological Journal of the Linnean Society 148, 343–394.
| Palaeoheterodont diversity (Mollusca: Trigonioida + Unionoida): what we know and what we wish we knew about freshwater mussel evolution.Crossref | GoogleScholarGoogle Scholar |
Graf, D. L., and Cummings, K. S. (2006b). Freshwater mussels (Mollusca: Bivalvia: Unionoida) of Angola, with description of a new species, Mutela wistarmorrisi. Proceedings of the Academy of Natural Sciences of Philadelphia 155, 163–194.
| Freshwater mussels (Mollusca: Bivalvia: Unionoida) of Angola, with description of a new species, Mutela wistarmorrisi.Crossref | GoogleScholarGoogle Scholar |
Graf, D. L., and Cummings, K. S. (2007). Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida). The Journal of Molluscan Studies 73, 291–314.
| Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida).Crossref | GoogleScholarGoogle Scholar |
Graf, D. L., and Cummings, K. S. (2009). Actual and alleged freshwater mussels (Mollusca: Bivalvia: Unionoida) from Madagascar and the Mascarenes, with description of a new genus, Germainaia. Proceedings. Academy of Natural Sciences of Philadelphia 158, 221–238.
| Actual and alleged freshwater mussels (Mollusca: Bivalvia: Unionoida) from Madagascar and the Mascarenes, with description of a new genus, Germainaia.Crossref | GoogleScholarGoogle Scholar |
Graf, D. L., and Cummings, K. S. (2011). Freshwater mussel (Mollusca: Bivalvia: Unionoida) richness and endemism in the ecoregions of Africa and Madagascar, based on comprehensive museum sampling. Hydrobiologia 678, 17–36.
| Freshwater mussel (Mollusca: Bivalvia: Unionoida) richness and endemism in the ecoregions of Africa and Madagascar, based on comprehensive museum sampling.Crossref | GoogleScholarGoogle Scholar |
Haag, W. R. (2010). A hierarchical classification of freshwater mussel diversity in North America. Journal of Biogeography 37, 12–26.
| A hierarchical classification of freshwater mussel diversity in North America.Crossref | GoogleScholarGoogle Scholar |
Haag, W. R. (2012). ‘North American Freshwater Mussels: Natural History, Ecology, and Conservation.’ (Cambridge University Press: Cambridge, UK.)
Haas, F. (1913). Die Unioniden. Neubearbeitung und Fortsetzung der Küsterschen und Clessinschen Monographien von Unio und Anodonta. Systematisches Conchylien-Cabinet 9, 137–184.
Haas, F. (1914). Die Unioniden. Neubearbeitung und Fortsetzung der Küsterschen und Clessinschen Monographien von Unio und Anodonta. Systematisches Conchylien-Cabinet 9, 185–256.
Haas, F. (1919). Die Unioniden. Neubearbeitung und Fortsetzung der Küsterschen und Clessinschen Monographien von Unio und Anodonta. Systematisches Conchylien-Cabinet 9, 257–288.
Haas, F. (1940). A tentative classification of the Palearctic unionids. Zoological Series of Field Museum of Natural History 24, 115–141.
| A tentative classification of the Palearctic unionids.Crossref | GoogleScholarGoogle Scholar |
Haas, F. (1969a). ‘Superfamilia Unionacea. Das Tierreich, Lief. 88.’ (Walter de Gruyter and Co.: Berlin) 663.
Haas, F. (1969b). Superfamily Unionacea Fleming, 1828. In ‘Treatise on Invertebrate Paleontology, Part N, Mollusca 6: Bivalvia. Vol. 1’. (Ed. R. C. Moore.) pp. N411–N467. (The Geological Society of America, Inc. and the University of Kansas: Lawrence, KS, USA.)
Hap, N., Seng, L., and Chuenpagdee, R. (2006). Socioeconomics and livelihood values of Tonle Sap Lake fisheries. (Inland Fisheries Research and Development Institute, IFReDI: Phnom Penh, Cambodia.) Available at http://pubs.iclarm.net/resource_centre/socoeconomic.synopsis_eng.pdf
He, J., and Zhuang, Z. (2013). ‘The Freshwater Bivalves of China.’ (ConchBooks: Harxheim, Germany.)
Heggemann, H., Helmcke, D., and Tietze, K. (1994). Sedimentary evolution of the Mesozoic Khorat Basin in Thailand. Zentralblatt für Geologie und Paläontologie 1, 1267–1285.
Hoang, D. T., Chernomor, O., Von Haeseler, A., Minh, B. Q., and Vinh, L. S. (2018). UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518–522.
| UFBoot2: improving the ultrafast bootstrap approximation.Crossref | GoogleScholarGoogle Scholar | 29077904PubMed |
Hoeh, W. R., Bogan, A. E., Heard, W. H., and Chapman, E. G. (2009). Palaeoheterodont phylogeny, character evolution and phylogenetic classification: a reflection on methods of analysis. Malacologia 51, 307–317.
| Palaeoheterodont phylogeny, character evolution and phylogenetic classification: a reflection on methods of analysis.Crossref | GoogleScholarGoogle Scholar |
Huang, X. C., Rong, J., Liu, Y., Zhang, M. H., Wan, Y., Ouyang, S., Zhou, C. H., and Wu, X. P. (2013). The complete maternally and paternally inherited mitochondrial genomes of the endangered freshwater mussel Solenaia carinatus (Bivalvia: Unionidae) and implications for Unionidae taxonomy. PLoS One 8, e84352.
| The complete maternally and paternally inherited mitochondrial genomes of the endangered freshwater mussel Solenaia carinatus (Bivalvia: Unionidae) and implications for Unionidae taxonomy.Crossref | GoogleScholarGoogle Scholar | 24358356PubMed |
Huang, X.-C., Su, J.-H., Ouyang, J.-X., Ouyang, S., Zhou, C.-H., and Wu, X.-P. (2019). Towards a global phylogeny of freshwater mussels (Bivalvia: Unionida): species delimitation of Chinese taxa, mitochondrial phylogenomics, and diversification. Molecular Phylogenetics and Evolution 130, 45–59.
| Towards a global phylogeny of freshwater mussels (Bivalvia: Unionida): species delimitation of Chinese taxa, mitochondrial phylogenomics, and diversification.Crossref | GoogleScholarGoogle Scholar | 30308278PubMed |
Hutchison, C. S. (1989). ‘Geological Evolution of South-east Asia.’ (Clarendon Press: Oxford, UK.)
Issel, A. (1874). ‘Molluschi Borneensi: Illustrazione delle Specie Terrestri e d’Acqua Dolce Raccolte nell’Isola di Borneo dai Signori G. Doria e O. Beccari.’ (Tipografia del R. Istituto Sordo-Muti)
Jeratthitikul, E., Phuangphong, S., Sutcharit, C., Prasankok, P., Kongim, B., and Panha, S. (2019). Integrative taxonomy reveals phenotypic plasticity in the freshwater mussel Contradens contradens (Bivalvia: Unionidae) in Thailand, with a description of a new species. Systematics and Biodiversity 17, 134–147.
| Integrative taxonomy reveals phenotypic plasticity in the freshwater mussel Contradens contradens (Bivalvia: Unionidae) in Thailand, with a description of a new species.Crossref | GoogleScholarGoogle Scholar |
Johnson, R. I. (1956). The types of naiades (Mollusca: Unionidae) in the Museum of Comparative Zoology. Bulletin of the Museum of Comparative Zoology 115, 101–142.
Johnson, R. I. (1964). The recent Mollusca of Augustus Addison Gould. Bulletin – United States National Museum 239, 1–182.
| The recent Mollusca of Augustus Addison Gould.Crossref | GoogleScholarGoogle Scholar |
Johnson, R. I. (1971). The types and figured specimens of Unionacea (Mollusca: Bivalvia) in the British Museum (Natural History). Bulletin of the British Museum (Natural History) – Historical Series 20, 75–108.
Johnson, R. I. (1972). Illustrations of all of the mollusks described by Lorraine Screven Frierson. Occasional Papers on Mollusks, Museum of Comparative Zoology, Harvard University 41, 137–173.
Johnson, R. I. (1974). Lea’s unionid types: or, recent and fossil Taxa of Unionacea and Mutelacea introduced by Isaac Lea, including the location of all the extant types. Special Occasional Publication, Museum of Comparative Zoology, Harvard University.
Johnson, R. I. (1980). The types of Unionacea (Mollusca: Bivalvia) in the Academy of Natural Sciences of Philadelphia: additions and corrections. Proceedings. Academy of Natural Sciences of Philadelphia 132, 277–278.
Johnson, R. I., and Baker, H. B. (1973). The types of Unionacea (Mollusca: Bivalvia) in the Academy of Natural Sciences of Philadelphia. Proceedings of the Academy of Natural Sciences of Philadelphia 125, 145–186.
Junier, T., and Zdobnov, E. M. (2010). The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics 26, 1669–1670.
| The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell.Crossref | GoogleScholarGoogle Scholar | 20472542PubMed |
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., von Haeseler, A., and Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587.
| ModelFinder: fast model selection for accurate phylogenetic estimates.Crossref | GoogleScholarGoogle Scholar | 28481363PubMed |
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780.
| MAFFT multiple sequence alignment software version 7: improvements in performance and usability.Crossref | GoogleScholarGoogle Scholar | 23329690PubMed |
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., and Thierer, T. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649.
| Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data.Crossref | GoogleScholarGoogle Scholar | 22543367PubMed |
Kembel, S. W. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464.
| Picante: R tools for integrating phylogenies and ecology.Crossref | GoogleScholarGoogle Scholar | 20395285PubMed |
Kittivorachate, R., and Yangyuen, C. (2004). Molluscs in the Ubolratana Reservoir, Khon Kaen. Witthayasan Kasetsat Witthayasat 38, 131–139.
Kongim, B., Sutcharit, C., and Panha, S. (2015). Cytotaxonomy of unionid freshwater mussels (Unionoida, Unionidae) from northeastern Thailand with description of a new species. ZooKeys 514, 93–110.
| Cytotaxonomy of unionid freshwater mussels (Unionoida, Unionidae) from northeastern Thailand with description of a new species.Crossref | GoogleScholarGoogle Scholar |
Konopleva, E. S., Bolotov, I. N., Vikhrev, I. V., Gofarov, M. Y., and Kondakov, A. V. (2017). An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae). Systematics and Biodiversity 15, 204–217.
| An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae).Crossref | GoogleScholarGoogle Scholar |
Konopleva, E. S., Pfeiffer, J. M., Vikhrev, I. V., Kondakov, A. V., Gofarov, M. Y., Aksenova, O. V., Lunn, Z., Chan, N., and Bolotov, I. N. (2019a). A new genus and two new species of freshwater mussels (Unionidae) from western Indochina. Scientific Reports 9, 4106.
| A new genus and two new species of freshwater mussels (Unionidae) from western Indochina.Crossref | GoogleScholarGoogle Scholar | 30858440PubMed |
Konopleva, E. S., Bolotov, I. N., Spitsyn, V. M., Kondakov, A. V., Gofarov, M. Y., and Vikhrev, I. V. (2019b). A new Contradens from Laos (Bivalvia: Unionidae: Contradentini). Ecologica Montenegrina 24, 25–31.
| A new Contradens from Laos (Bivalvia: Unionidae: Contradentini).Crossref | GoogleScholarGoogle Scholar |
Kück, P., and Longo, G. C. (2014). FASconCAT-G: extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Frontiers in Zoology 11, 81.
| FASconCAT-G: extensive functions for multiple sequence alignment preparations concerning phylogenetic studies.Crossref | GoogleScholarGoogle Scholar | 25426157PubMed |
Lamarck, J. B. (1835). Histoire naturelle des animaux sans vertebraes. Tome sixieme. In ‘Histoire des mollusques. Deuxième Edition. (les Nayades)’. pp. 524–574. (J.-B. Baillière, Libaire, Paris, France.)
Lea, I. (1838). Description of new freshwater and land shells. Transactions of the American Philosophical Society 2, 1–152.
Lea, I. (1840). Descriptions of new fresh water and land shells. Proceedings of the American Philosophical Society 1, 284–289.
Lea, I. (1842). ‘Observations on the Genus Unio, Together with Descriptions of New Species in the Families Naiades, Conchae, Colimacea, Lymnaeana, Melaniana and Peristomiana.’ (Printed for the Author: Philadelphia, PA, USA.)
Lea, I. (1843). Description of twelve new species of Uniones. Proceedings of the American Philosophical Society 4, 11.
Lea, I. (1852). ‘A Synopsis of the Family of Naïades.’ (T.K. and P.G. Collins Printers: Philadelphia, PA, USA.)
Lea, I. (1857). Descriptions of exotic genera and species of the family Unionidae. Journal of the Academy of Natural Sciences of Philadelphia 3, 289–321.
Lea, I. (1860a). Descriptions of exotic Unionidae. Journal of the Academy of Natural Sciences of Philadelphia 4, 235–273.
Lea, I. (1860b). Descriptions of three new species of exotic Unionidæ. Proceedings. Academy of Natural Sciences of Philadelphia 12, 307–308.
Lea, I. (1866). Description of five new species of the genus Unio. Proceedings of the Academy of Natural Sciences of Philadelphia 18, 13–3.
Lea, I. (1868). New Unionidae, Melanidae, etc., chiefly of the United States. Journal of the Academy of Natural Sciences of Philadelphia 6, 249–302.
Lohman, D. J., de Bruyn, M., Page, T., von Rintelen, K., Hall, R., Ng, P. K., Shih, H.-T., Carvalho, G. R., and von Rintelen, T. (2011). Biogeography of the Indo-Australian archipelago. Annual Review of Ecology, Evolution, and Systematics 42, 205–226.
| Biogeography of the Indo-Australian archipelago.Crossref | GoogleScholarGoogle Scholar |
Lopes-Lima, M., Froufe, E., Ghamizi, M., Mock, K. E., Kebapçı, Ü., Klishko, O., Kovitvadhi, S., Kovitvadhi, U., Paulo, O. S., and Pfeiffer, J. M. (2017). Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): defining modern subfamilies and tribes. Molecular Phylogenetics and Evolution 106, 174–191.
| Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): defining modern subfamilies and tribes.Crossref | GoogleScholarGoogle Scholar | 27621130PubMed |
Lopes-Lima, M., Hattori, A., Kondo, T., Lee, J. H., Kim, S. K., Shirai, A., Hayashi, H., Usui, T., Sakuma, K., Toriya, T., Sunamura, Y., Ishikawa, H., Hoshino, N., Kusano, Y., Kumaki, H., Utsugi, Y., Yabe, S., Yoshinari, Y., Hiruma, H., Tanaka, A., Sao, K., Ueda, T., Sano, I., Miyazaki, J.-I., Gonçalves, D. V., Klishko, O. K., Konopleva, E. S., Vikhrev, I. V., Kondakov, A. V., Gofarov, M. Y., Bolotov, I. N., Sayenko, E. M., Soroka, M., Zieritz, A., Bogan, A. E., and Froufe, E. (2020). Freshwater mussels (Bivalvia: Unionidae) from the rising sun (Far East Asia): phylogeny, systematics, and distribution. Molecular Phylogenetics and Evolution 146, 106755.
| Freshwater mussels (Bivalvia: Unionidae) from the rising sun (Far East Asia): phylogeny, systematics, and distribution.Crossref | GoogleScholarGoogle Scholar | 32028028PubMed |
Marwoto, R. M. (1987). Beberapa Jenis Kerang Kijing (Suku: Unionidae) Dan Keong Di Tepi Perairan Sungai Rengas Dan Sungkai, Propinsi Jambi. Berita Biologi 3, 306–309.
Meyer, C. P. (2003). Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biological Journal of the Linnean Society. Linnean Society of London 79, 401–459.
| Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics.Crossref | GoogleScholarGoogle Scholar |
Modell, H. (1964). Das natürliche System der Najaden. Archiv für Molluskenkunde 93, 71–129.
Morelet, A. (1864). Description de coquilles inédites. Journal de Conchyliologie 12, 286–290.
Morelet, A. (1866). Description ďespèces appartenant à la faune malacologique de ľIndo-Chine. Journal de Conchyliologie 14, 62–64.
Morelet, L. (1875). ‘Séries Conchyliologiques Comprenant l’Enumération de Mollusques Terrestres et Fluviatiles Recueilles pendant le Cours de Différents Voyages ainsi que la Description de Plusieurs Espèces Nouvelles.’ (Chez Klincksieck Libraire-Editeur: Paris, France.)
Morlet, L. (1893). Descriptions d’espèces nouvelles provenant de l’Indo-Chine (suite). Journal de Conchyliologie 41, 153–157.
Morlet, L. (1904). Descriptions de mollusques nouveaux recueillis par M A Pavie en Indo-chine. In ‘Mission Pavie Inochine 3’. pp. 351–450. (E. Leroux: Paris, France.)
Mousson, A. (1849). ‘Die Land- und Süßwassermollusken von Java.’ (Druck und Verlag von Friedrich Schulthess: Zürich, Switzerland.)
Muanta, S., Jeratthitikul, E., Panha, S., and Prasankok, P. (2019). Phylogeography of the freshwater bivalve genus Ensidens (Unionidae) in Thailand. The Journal of Molluscan Studies 85, 224–231.
| Phylogeography of the freshwater bivalve genus Ensidens (Unionidae) in Thailand.Crossref | GoogleScholarGoogle Scholar |
Nabhitabhata, J. (2009). ‘Checklist of Mollusca Fauna in Thailand.’ (Office of Natural Resources and Environmental Policy and Planning: Bangkok, Thailand.)
Ngor, P. B., Sor, R., Prak, L. H., So, N., Hogan, Z. S., and Lek, S. (2018). Mollusc fisheries and length–weight relationship in Tonle Sap flood pulse system, Cambodia. Annales de Limnologie – International Journal of Limnology 54, 34–44.
| Mollusc fisheries and length–weight relationship in Tonle Sap flood pulse system, Cambodia.Crossref | GoogleScholarGoogle Scholar |
Nguyen, L.-T., Schmidt, H. A., Von Haeseler, A., and Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268–274.
| IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies.Crossref | GoogleScholarGoogle Scholar | 25371430PubMed |
Parry, J. (1996). The high terrace gravels, northeast Thailand. A re-evaluation and an integrated theory of their origin. Zeitschrift für Geomorphologie 40, 145–175.
Pfeiffer, J. M., and Graf, D. L. (2013). Re-analysis confirms the polyphyly of Lamprotula Simpson, 1900 (Bivalvia: Unionidae). The Journal of Molluscan Studies 79, 249–256.
| Re-analysis confirms the polyphyly of Lamprotula Simpson, 1900 (Bivalvia: Unionidae).Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., and Graf, D. L. (2015). Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae). Zoological Journal of the Linnean Society 175, 307–318.
| Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae).Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., Graf, D. L., Cummings, K. S., and Page, L. M. (2018a). Molecular phylogeny and taxonomic revision of two enigmatic freshwater mussel genera (Bivalvia: Unionidae incertae sedis: Harmandia and Unionetta) reveals a diverse clade of Southeast Asian Parreysiinae. The Journal of Molluscan Studies 84, 404–416.
| Molecular phylogeny and taxonomic revision of two enigmatic freshwater mussel genera (Bivalvia: Unionidae incertae sedis: Harmandia and Unionetta) reveals a diverse clade of Southeast Asian Parreysiinae.Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., Sharpe, A. E., Johnson, N. A., Emery, K. F., and Page, L. M. (2018b). Molecular phylogeny of the Nearctic and Mesoamerican freshwater mussel genus Megalonaias. Hydrobiologia 811, 139–151.
| Molecular phylogeny of the Nearctic and Mesoamerican freshwater mussel genus Megalonaias.Crossref | GoogleScholarGoogle Scholar |
Pfeiffer, J. M., Breinholt, J. W., and Page, L. M. (2019). Unioverse: a phylogenomic resource for reconstructing the evolution of freshwater mussels (Bivalvia, Unionoida). Molecular Phylogenetics and Evolution 137, 114–126.
| Unioverse: a phylogenomic resource for reconstructing the evolution of freshwater mussels (Bivalvia, Unionoida).Crossref | GoogleScholarGoogle Scholar | 30797940PubMed |
Prashad, B. (1919). Studies on the anatomy of Indian Mollusca. 3. The soft parts of some Indian Unionidae. Records of the Indian Museum 16, 289–296.
| Studies on the anatomy of Indian Mollusca. 3. The soft parts of some Indian Unionidae.Crossref | GoogleScholarGoogle Scholar |
Preston, H. (1912). A catalogue of the Asiatic naiads in the collection of the Indian Museum, Calcutta, with descriptions of new species. Records of the Indian Museum 7, 279–308.
Rainboth, W. J. (1996). ‘Fishes of the Cambodian Mekong.’ (FAO.)
Rainboth, W. J., Vidthayanon, C., and Yen, M. D. (2012). Fishes of the greater Mekong ecosystem with species list and photographic atlas. Miscellaneous Publications of the Museum of Zoology. University of Michigan 201, 1–294.
Ramakrishna, and Dey, A. (2007). ‘Handbook on Indian Freshwater Molluscs.’ (Zoological Survey of India: Calcutta, India.)
Razak, N., Supramaniam, C., and Zieritz, A. (2019). A dichotomous PCR–RFLP identification key for the freshwater mussels (Bivalvia: Unionida) of Peninsular Malaysia. Conservation Genetics Resources 11, 457–464.
| A dichotomous PCR–RFLP identification key for the freshwater mussels (Bivalvia: Unionida) of Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Rochebrune, A. (1882). Documents sur la faune malacologique de la Cochinchine et du Cambodge. Bulletin de la Société Philomathique de Paris 7, 35–74.
Roe, K. J., and Boyer, S. L. (2015). A comparison of genetic diversity between sympatric populations of the endangered winged-mapleleaf (Quadrula fragosa) and the pimpleback (Amphinaias pustulosa) in the St Croix River, USA. American Malacological Bulletin 33, 52–60.
| A comparison of genetic diversity between sympatric populations of the endangered winged-mapleleaf (Quadrula fragosa) and the pimpleback (Amphinaias pustulosa) in the St Croix River, USA.Crossref | GoogleScholarGoogle Scholar |
Sabaj, M. (2016). Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 6.5 (16 August 2016). (American Society of Ichthyologists and Herpetologists: Washington, DC, USA.) Available at http://www.asih.org/
Schepman, M. (1896). Zoological results of the Dutch Scientific Expedition to central Borneo. Notes from the Leyden Museum 17, 145–162.
Simone, L. R. L. (2006). ‘Land and Freshwater Molluscs of Brazil.’ (EGB, Fapesp: São Paulo, Brazil.)
Simpson, C. T. (1900). Synopsis of the Naiades, or pearly fresh-water mussels. Proceedings of the United States National Museum 22, 501–1044.
| Synopsis of the Naiades, or pearly fresh-water mussels.Crossref | GoogleScholarGoogle Scholar |
Smith, S. A., Brown, J. W., and Walker, J. F. (2018). So many genes, so little time: a practical approach to divergence-time estimation in the genomic era. PLoS One 13, e0197433.
| So many genes, so little time: a practical approach to divergence-time estimation in the genomic era.Crossref | GoogleScholarGoogle Scholar | 29772020PubMed |
Solem, A. (1967). New molluscan taxa and scientific writings of Fritz Haas. Fieldiana. Zoology 53, 71–144.
Sowerby, G. B. (1866). Monograph of the genus Unio. Conchologia Iconica 16, 31–54.
Sowerby, G. B. (1867). Monograph of the genus Anodon. Conchologica Iconica 17, 2–19.
Sri-aroon, P., Butraporn, P., Limsoomboon, J., Kaewpoolsri, M., Chusongsang, Y., Charoenjai, P., Chusongsang, P., Numnuan, S., and Kiatsiri, S. (2007). Freshwater mollusks at designated areas in eleven provinces of Thailand according to the water resource development projects. The Southeast Asian Journal of Tropical Medicine and Public Health 38, 294.
| 17539279PubMed |
Starobogatov, Y. I. (1970). ‘Fauna Mollyuskov i Zoogeograficheskoe Raionirovanie Kontinental’nykh Vodoemov Zemnogo Shara.’ (Nauk SSSR: Leningrad, USSR.)
Subba Rao, N. V. (1989). ‘Handbook. Freshwater Molluscs of India.’ (Zoological Survey of India: Calcutta, India.)
Veeravechsukij, N., Krailas, D., Namchote, S., Wiggering, B., Neiber, M. T., and Glaubrecht, M. (2018). Molecular phylogeography and reproductive biology of the freshwater snail Tarebia granifera in Thailand and Timor (Cerithioidea, Thiaridae): morphological disparity versus genetic diversity. Zoosystematics and Evolution 94, 461–493.
| Molecular phylogeography and reproductive biology of the freshwater snail Tarebia granifera in Thailand and Timor (Cerithioidea, Thiaridae): morphological disparity versus genetic diversity.Crossref | GoogleScholarGoogle Scholar |
Vinarski, M. V. (2019). WA Lindholm: a bibliography of his malacological publications with the catalogue of molluscan taxa described by him or named in his honour. Труды Зоологического института РАН 323, 187–213.
von Martens, E. (1867). Ueberlick der Najadeen des indischen Archipels. Malakozoologische Blätter 14, 10–17.
von Martens, E. (1891). Süsswasser-Mollusken des malayischen Archipels im Allgemeinen und einen neuen Unio aus Borneo insbesondere. Sitzungs-Berichte der Gesellschaften naturforschender Freunde zu Berlin 16, 109–112.
von Martens, E. (1900). Ueber Land- und Süsswasser-Schnecken aus Sumatra. Nachrichtsblatt der Deutschen Malakozoologischen Gesellschaft 32, 3–18.
Voris, H. K. (2000). Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography 27, 1153–1167.
| Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations.Crossref | GoogleScholarGoogle Scholar |
Wallace, A. R. (1876). ‘The Geographical Distribution of Animals: with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface.’ (Cambridge University Press.)
Whelan, N. V., Geneva, A. J., and Graf, D. L. (2011). Molecular phylogenetic analysis of tropical freshwater mussels (Mollusca: Bivalvia: Unionoida) resolves the position of Coelatura and supports a monophyletic Unionidae. Molecular Phylogenetics and Evolution 61, 504–514.
| Molecular phylogenetic analysis of tropical freshwater mussels (Mollusca: Bivalvia: Unionoida) resolves the position of Coelatura and supports a monophyletic Unionidae.Crossref | GoogleScholarGoogle Scholar | 21827862PubMed |
Willett, S. D., McCoy, S. W., Perron, J. T., Goren, L., and Chen, C.-Y. (2014). Dynamic reorganization of river basins. Science 343, 1248765.
| Dynamic reorganization of river basins.Crossref | GoogleScholarGoogle Scholar | 24604204PubMed |
Woodruff, D. S., and Turner, L. M. (2009). The Indochinese–Sundaic zoogeographic transition: a description and analysis of terrestrial mammal species distributions. Journal of Biogeography 36, 803–821.
| The Indochinese–Sundaic zoogeographic transition: a description and analysis of terrestrial mammal species distributions.Crossref | GoogleScholarGoogle Scholar |
Woodward, F. (1969). The morphology of Ensidens ingallsianus (Lea, 1852) and Scabies crispata (Gould, 1843) (Bivalvia, Unionidae). Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening 132, 49–62.
Zhang, C., Sayyari, E., and Mirarab, S. (2017). ASTRAL-III: increased scalability and impacts of contracting low support branches. In ‘RECOMB International Workshop on Comparative Genomics’, 4-6 October 2017, Barcelona, Spain. (Eds J. Meidanis and L. Nakhleh.) pp. 53–75. (Springer.)
Zhang, C., Rabiee, M., Sayyari, E., and Mirarab, S. (2018). ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinformatics 19, 153.
| ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees.Crossref | GoogleScholarGoogle Scholar | 29745866PubMed |
Zieritz, A., Lopes-Lima, M., Bogan, A. E., Sousa, R., Walton, S., Rahim, K. A. A., Wilson, J.-J., Ng, P.-Y., Froufe, E., and McGowan, S. (2016). Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. The Science of the Total Environment 571, 1069–1078.
| Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar | 27473771PubMed |
Zieritz, A., Bogan, A. E., Froufe, E., Klishko, O., Kondo, T., Kovitvadhi, U., Kovitvadhi, S., Lee, J. H., Lopes-Lima, M., Pfeiffer, J. M., Sousa, R., Do, V. T., Vikhrev, I., and Zanatta, D. T. (2018a). Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia 810, 29–44.
| Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia.Crossref | GoogleScholarGoogle Scholar |
Zieritz, A., Bogan, A. E., Rahim, K. A. A., Sousa, R., Jainih, L., Harun, S., Razak, N. F. A., Gallardo, B., McGowan, S., and Haasan, R. (2018b). Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biological Conservation 219, 126–137.
| Changes and drivers of freshwater mussel diversity and distribution in northern Borneo.Crossref | GoogleScholarGoogle Scholar |
Zieritz, A., Taha, H., Lopes-Lima, M., Pfeiffer, J., Sing, K. W., Sulaiman, Z., McGowan, S., and Rahim, K. A. (2020). Towards the conservation of Borneo’s freshwater mussels: rediscovery of the endemic Ctenodesma borneensis and first record of the non-native Sinanodonta lauta. Biodiversity and Conservation 29, 2235–2253.
| Towards the conservation of Borneo’s freshwater mussels: rediscovery of the endemic Ctenodesma borneensis and first record of the non-native Sinanodonta lauta.Crossref | GoogleScholarGoogle Scholar |
Zilch, A. (1967). Die Typen und Typoide des Natur-Museums Senckenberg, 39: Mollusca, Unionacea. Archiv für Molluskenkunde 97, 45–154.
Zilch, A. (1983). Die typen und typoide des Natur-Museums Senckenberg, 72: Mollusca: Unionacea (Nachträgezu Teil 39). Archiv für Molluskenkunde 114, 77–92.