Cladistic analysis of the Brazilian troglobitic harvestmen genus Iandumoema Pinto-da-Rocha (Opiliones : Gonyleptidae) with the description of three new species: a brief exercise over the use of troglomorphisms in cladistic analysis
Ludson Neves de Ázara A D , Marcos Ryotara Hara B and Rodrigo Lopes Ferreira C
A D , Marcos Ryotara Hara B and Rodrigo Lopes Ferreira C
A Laboratório de Aracnologia, Departamento de Invertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, 20.940-040, Rio de Janeiro, RJ, Brazil.
B Escola de Artes, Ciências e Humanidades, Universidade de São Paulo, Avenida Arlindo Béttio 1000, Ermelino Matarazzo, 03828-000, São Paulo, SP, Brazil.
C Centro de Estudos em Biologia Subterrânea, Departamento de Biologia, Setor de Zoologia Geral, Universidade Federal de Lavras, Lavras, MG, Brazil.
D Corresponding author. Email: ludsonazara@yahoo.com.br
Invertebrate Systematics 34(5) 474-503 https://doi.org/10.1071/IS19037
Submitted: 3 July 2019 Accepted: 17 January 2020 Published: 26 June 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
From an ecological and evolutionary standpoint, troglobitic organisms are of special interest because they have evolved in, and are restricted to, the subterranean environment. Iandumoema Pinto-da-Rocha, 1997 stands out for being the only Brazilian harvestmen genus with more than one troglobitic species, with three species described from caves in Minas Gerais state. Traditionally, testing the monophyly of troglobitic groups is more challenging than testing groups that do not include troglobites. Many of their shared features might be the result of convergence or parallelism imposed by the cave environment, such as the absence of light, limited and infrequent availability of food resources and low population density, among others. In the case of Iandumoema, this becomes even more difficult because the genus is currently included in the species-rich and polyphyletic subfamily Pachylinae. This study tested the monophyly of this troglobitic genus and proposed the first phylogenetic hypothesis for Iandumoema based on cladistic analysis using morphological data. The analysis included all described species of Iandumoema and three new troglobitic species: I. cuca, sp. nov. (type locality: Itacarambi, Gruta da Água do João Ferreira); I. gollum, sp. nov. (type locality: Presidente Juscelino, Lapa D’Água); and I. stygia, sp. nov. (type locality: Montes Claros, Gruta do Cedro). The matrix comprises 79 characters and 28 terminal taxa: six species of Iandumoema; 14 of Pachylinae; six from other Gonyleptidae subfamilies; one species of Cosmetidae; and one of Metasarcidae. The cladistic analysis resulted in one parsimonious tree (339 steps, consistency index = 0.35, retention index = 0.56). Iandumoema is a monophyletic and well supported genus, nestled among Brazilian ‘Pachylinae’. Three new species are described and an identification key and ecological remarks for all six species of the genus Iandumoema is provided.
Additional keywords: Arachnida, caves, Neotropical fauna, taxonomy.
Introduction
Gonyleptidae Sundevall, 1833 is the largest family of harvestman in the Neotropical region, with 16 subfamilies and 830 species in 272 genera (Kury 2013, 2016). Pachylinae is the richest subfamily, including ~400 species (Kury 2016) with four scutal areas and lacking the diagnostic characters of the other subfamilies (Hara et al. 2018). It is polyphyletic, and most of the genera are poorly described for modern standards (Pinto-da-Rocha et al. 2014), which hinders comparative studies, including phylogenetic ones. Pachylinae also includes most of the Brazilian cave-dwelling species (more than 50 from 155 recorded species; de Ázara and Ferreira 2018).
The only non-monotypic cave-restricted Pachylinae genus is Iandumoema Pinto-da-Rocha, 1997, which is restricted to limestone caves in Minas Gerais state, Brazil. It was proposed by Pinto-da-Rocha (1997) to include I. uai from Gruta Olhos D’Água Cave, Itacarambi, Minas Gerais, and was the third-known troglobitic harvestmen species from Brazil (the first being Spaeleoleptes spaeleus H. Soares, 1966, and the second, Pachylospeleus strinatii Šilhavý, 1974). Pinto-da-Rocha (1997) tentatively placed it in the Pachylinae based on a classification proposed by Roewer (1913). However, he refrained from proposing further phylogenetic relationships owing to the lack of data on the genital features of other Pachylinae genera and the unarmed nature of the dorsal scutum and free tergites. Twelve years later, Hara and Pinto-da-Rocha (2008) described I. setimapocu from Lapa do Zú Cave, São João da Lagoa (erroneously cited as Coração de Jesus in the original description), Minas Gerais. In that article, the authors proposed that the distribution of the genus was restricted to northern Minas Gerais. In 2015, Pinto-da-Rocha et al. described the first eyeless species of the genus, I. smeagol, from Toca do Geraldo Cave and Lapa do Santo Antônio Cave, both in Monjolos, Minas Gerais. They suggested that I. smeagol and I. setimapocu might be closely related, but stressed the need for a proper cladistic analysis to corroborate this relationship. Later papers related to Iandumoema focused mainly on ecological and conservation aspects, highlighting their vulnerability to anthropogenic actions.
Currently, Iandumoema is composed of three species (Pinto-da-Rocha et al. 2015), all of them troglobitic, that is, restricted to subterranean environments. Despite the small number of species, the phylogenetic relationships and monophyly of Iandumoema remain untested.
One difficulty in including troglobitic taxa in cladistic analysis might be related to the apparent controversy about using troglomorphisms (or troglobiomorphisms, i.e. apomorphic character states related to the hypogean life; Trajano 2012) as characters. According to Marques and Gnaspini (2001), the inclusion of characters subject to parallel evolution would result in error and should be treated in a different manner analytically. Desutter-Grandcolas et al. (2003), as well as other researchers, heavily criticised the approach of Marques and Gnaspini (2001). The criticism was based on the different treatment of those characters before analysis, as well as some technical flaws (Harris et al. 2003). Desutter-Grandcolas et al. (2003) recommended evaluating the outcome of analysis including v. excluding the characters associated with troglomorphism.
The few examples of cladistic analysis including troglobitic Neotropical harvestmen using morphological data are Pinto-da-Rocha (2002) (with Pachylospeleus strinatii Šilhavý, 1974) and, recently, Acosta (2019) (with Otilioleptes marcelae Acosta, 2019). Unfortunately, Pinto-da-Rocha (2002) did not provide the matrix or the tree search routine; hence, his treatment of troglomorphisms is unknown. Acosta (2019), on the other hand, included a matrix that is a different version of that made by Kury and Villarreal (2015). Nevertheless, no character related to troglomorphism was explicitly included or received special concern, since his focus was to elucidate the placement of the new taxon in Gonyleptoidea. In the present study, we used the Desutter-Grandcolas et al. (2003) approach to test the monophyly of Iandumoema and proposed a phylogeny using the cladistic methodology for the first time. Herein, we provide a diagnosis and ecological remarks for all Iandumoema species and describe three new species.
Materials and methods
Abbreviations of the repositories cited include the following.
ISLA, Coleção de Invertebrados Subterrâneos de Lavras, Universidade Federal de Lavras, Lavras, Minas Gerais
ISNB, Institut Royal des Sciences Naturelles de Belgique
MNRJ, Museu Nacional da Universidade Federal do Rio de Janeiro, Rio de Janeiro
MZSP, Museu de Zoologia, Universidade de São Paulo, São Paulo
SMF, Naturmuseum Senckenberg Sektion Arachnologie, Frankfurt am Main.
Abbreviations used in tables include: Fe, femur; Mt, metatarsus; Pa, patella; Ta, tarsus; Ti, tibia; and Tr, trochanter. The following abbreviations are used in synonymies: cat, catalogue; cit, citation; dis, notes on geographical distribution; and sys, systematic note. We use the notation #X(Y) in results, where #X means the number of the character, and (Y), the character state.
All types designated here as destroyed were lost in a fire on 2 September 2018 along with the bulk of the arachnological collection of MNRJ (Kury et al. 2018). There is no formal impediment according to International Commission on Zoological Nomenclature rules to describing a species for which the holotype was lost before publication. Article 73.1.4. (International Commission on Zoological Nomenclature 1999) states that ‘Designation of an illustration of a single specimen as a holotype is to be treated as designation of the specimen illustrated; the fact that the specimen no longer exists or cannot be traced does not of itself invalidate the designation’. The International Commission on Zoological Nomenclature (2017) provides more recommendations and explanation regarding this matter. For further discussion on this topic, see Marshall and Evenhuis (2015), Krell and Marshall (2017) and references therein.
We used the standard names and numbers of the 267 colour centroids of the National Bureau of Standards Inter-Society Colour Council Colour System (see http://people.csail.mit.edu/jaffer/Color/Dictionaries#nbs-iscc, accessed 2 November 2018) in the (re)descriptions as described in Kury and Orrico (2006). Scanning electron microscopy was carried out with a JSM-6390LV (JEOL, Tokyo) at the Center for Scanning Electron Microscopy of Museu Nacional, Universidade Federal do Rio de Janeiro, with an accelerating voltage of 10 kV after sputter-coating with gold–palladium.
Topological nomenclature and integumentary ornamentation follows Acosta et al. (2007). Terminology for the outline of the dorsal scutum follows Kury and Medrano (2016) and for the macrosetae and microsetae of the penis ventral plate follows Kury and Villarreal (2015) and Kury (2016). Only characteristics different from those of males are mentioned in the female descriptions. Tarsal segmentation: order is given from left to right when individual counts are provided for the numbers of tarsomeres in tarsus I to IV. All measurements are in millimetres (except for penis). Setiferous tubercles (i, small; I large) on pedipalps are given from proximal to distal order. Geographic coordinates from our localities are in decimal degrees (datum: World Geodetic System WGS84), whereas those from literature are provided verbatim.
Photographs were taken with a Cybershot DSC-V1 camera (Sony, Tokyo, Japan) attached to the stereomicroscope. The software package CombineZP (see http://www.hadleyweb.pwp.blueyonder.co.uk/CZP/News.htm, accessed 1 November 2018) by Alan Hadley was used to create composite images with extended depth of field by combining several images taken at different focal planes. The resulting images were edited with Adobe Photoshop CS5 (Adobe, San Jose, CA). The plates were prepared in CorelDraw X7 for photographs (see https://www.coreldraw.com).
Taxon sampling
The ingroup is composed of the three described species of Iandumoema plus the three new species described herein. The outgroup is composed of 20 species representing eight subfamilies of Gonyleptidae, one species of Cosmetidae and one species of Metasarcidae, both families close to Gonyleptidae (Pinto-da-Rocha et al. 2014). The list of species used as outgroup in the analysis is shown in Table 1. The outgroup selection is based on Pinto-da-Rocha et al. (2014) as well as taking into account (1) morphological resemblance to Iandumoema and (2) morphological diversity of the revised and unrevised subfamilies.

|
Cladistic analysis
The character matrix is composed of 79 characters (Appendix 1) for 28 taxa. It was constructed in MESQUITE (ver. 3.5, W. P. Maddison and D. R. Maddison, see http://www.mesquiteproject.org, accessed 10 December 2018). Winclada (ver. 1.00.08, K. C. Nixon, see http://www.cladistics.com, accessed 30 January 2019) was used for characters state optimisations and CorelDraw X7 (M. Bouillon and P. Beirne, Corel Corporation, Canada; see https://www.coreldraw.com) for tree editing. The annotated list of characters is shown in Box 1. Characters in the matrix correspond to males except where otherwise stated, because females are morphologically more homogeneous than males, frequently lacking the diagnostic dimorphic features present in males, such as leg IV armature (Mendes 2011). Character statements follow the logic of neomorphic and transformational structure proposed by Sereno (2007). The characters were treated as unordered, and the polarisation was indicated upon rooting the diagrams from the outcome of the parsimony analysis including all taxa (Nixon and Carpenter 1993).
| Box 1. List of characters and their states used in the cladistic analysis Italic formatting indicates the character proposition | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Trees were searched in TNT (ver. 1.5, see http://www.lillo.org.ar/phylogeny/tnt/, accessed 30 January 2019; Goloboff et al. 2008) using parsimony under equal and implied weights (Goloboff 1993) with the traditional search option. Heuristic searches consisted of 1000 replicates of random addition sequences followed by tree bisection–reconnection branch swapping holding 100 trees per iteration. Two measures of group support were calculated: Goodman–Bremer support (GBS) (Goodman et al. 1982; Bremer 1988; Grant and Kluge 2008) and symmetric resampling (1000 replicates, cut = 50, change probability = 33) (Goloboff et al. 2003). We used space plots (‘Navajo rugs’) to summarise the monophyly (or not) of a given clade in the trees retrieved from the analysis under implied weight with different concavity values (k = 1, 3, 6, 9, 11, 15 and 20). These plots work similarly to sensitivity analysis, aiding to evaluate the stability of the results (see Mendes 2011 and references therein).
Results
Cladistic analysis
The cladistic analysis using all the characters under equal weights retrieved one most parsimonious tree (length (L) = 339 steps, consistency index (CI) = 35, retention index (RI) = 56) (Fig. 1). Iandumoema is recovered as a monophyletic genus with high support values (resampling measures = 82, Goodman–Bremer support = 8) (Fig. 2). The genus is supported by 12 unambiguous synapomorphies; five of them being non-homoplastic synapomorphies, namely: frontal hump on anterior margin of dorsal scutum as an oval shaped (in dorsal view) high protuberance (comparable to ocularium height) [#2(2)]; absence of eye pigmentation [#4(0)]; ocularium unpaired armature apex curved backwards [#7(1)]; trochanter IV with basal–central retro-lateral row of tubercles roughly organised in a row [#45(1)]; and apex of ventral process of penis spatula-like, with serrate distal margin [#71(2)]. Of the five non homoplastic synapomorphies, four are not related to modification to hypogean life style, thus corroborating that Iandumoema monophyly does not rely on putative troglomorphism in this study. Indeed, the analysis excluding characters that might be related to troglomorphism (#3 and #4) resulted in the same tree as the one retrieved under equal weights (Fig. 1). The single most parsimonious tree recovered excluding characters #3 and #4 has the same CI and RI values and fewer steps (L = 335; CI = 35; RI = 56).
The analyses using implied weights resulted in five different trees with different topologies, but all of them corroborate the monophyly of Iandumoema. Table 2 summarises those results, including their respective length, CI and RI as well as their fit. The retrieved clades resulting from the analysis under different concavities are summarised in sensitivity plots in Fig. 1. Iandumoema is nested among the Brazilian ‘Pachylinae’ and it is a sister-group of a clade including Pachylinae (except for G. chagasi, E. catharinensis and D. inermis) + K92. Pachylinae sensu stricto that includes the type genus Pachylus Koch, 1839 (type species P. granulatus Kollar in Koch, 1839) (Pinto-da-Rocha et al. 2014) (represented by two species) and clade K92 (represented by one species of each of the five subfamilies) are also recovered as monophyletic in all analyses.

|
Systematics
Genus Iandumoema Pinto-da-Rocha
Type species: Iandumoema uai Pinto-da-Rocha, 1997, by monotypy.
Diagnosis
Gonyleptid with scutum length of ~3–5 mm; slender and elongated legs. Dorsal scutum (DS) type α (Fig. 3A, C, 6A, C, 9A, C); anterior margin of DS bearing a prominent frontal hump, its height comparable to the ocularium (Fig. 3E, F, 6E, F, 9E, F) and not projected anteriorly (elyptical in dorsal view). Frontal hump bearing a main pair of paramedian tubercles. Ocularium roughly placed in the centre of carapace, with a large spiniform apophysis, its apex curved backward. Eyes depigmented (except I. stygia, sp. nov. and I. smeagol). Four unarmed scutal areas or each with a paramedian pair of tubercles. Scutal area I divided into right and left halves. Dorsal scutum with pointed granules. Cheliceral segment I as long as the carapace maximum length, with bulla. Pedipalpal patella connected obliquely to tibia in lateral view. Coxae I–III each with one prodorsal and one retro-dorsal apophyses; the apex of retro-dorsal apophysis of coxa II fused to the apex of prodorsal apophysis of coxa III. Male coxa IV hypertelic, bearing a robust prolateral apophysis smoothly curved in dorsal view (except I. uai, with abrupt curvature) (Fig. 3A, B, E, 6A, B, E, 9A, B, E) and as a shorter spiniform apophysis in females (Fig. 3C, D, F, 6C, D, F, 9C, D, F). Male trochanter IV longer than wide, with a short, blunt prolateral apophysis on basal third and 2–4 pointed tubercles roughly organised in a row on basal–central retro-lateral face (unarmed on prolateral face in females) (Fig. 4K–M, 7K–M, 10K–M). Male femur IV approximately twice the DS length, with variable armature and curvature in dorsal view; pointed tubercles on distal quarter of proventral row.
|

|
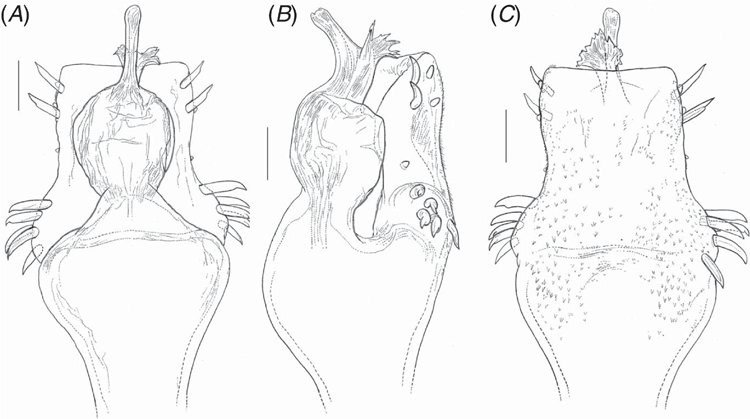
Male genitalia. Ventral plate subrectangular (Fig. 8A, B, 11A, C) to pyriform (Fig. 5A, C), distal border straight, with 3–5 pairs of macrosetae (MS) A, 1 pair of MS B, 2–4 pairs of distal MS C, 1 pair of MS D, 0–3 pairs of MS E. Glans with ventral process, stem short and as thick as stylus and apex spatula-like with serrate distal margin (in I. smeagol, narrowing apically, without lateral projections, and in I. cuca, sp. nov. and I. gollum, sp. nov., hook-like apex directed ventrally with serrate narrow lateral projections). Stylus with ventral median trichomes (Fig. 8C, D, 11B) (except I. uai, I. setimapocu and I. cuca, sp. nov.).

|
Included species
Iandumoema uai Pinto-da-Rocha, 1997 (type species), I. setimapocu Hara & Pinto-da-Rocha, 2008, I. smeagol Pinto-da-Rocha et al., 2015, I. cuca, sp. nov., I. gollum, sp. nov. and I. stygia, sp. nov.
Geographical distribution
Brazil: Minas Gerais State: caves in the municipalities of Itacarambi, Monjolos, Montes Claros, Presidente Juscelino and São João da Lagoa.
Key to males of Iandumoema (modified from Pinto-da-Rocha et al. 2015)|
1. Ocularium without eyes (Fig. 15C, D) |
|
2. Ocularium with a spiniform tubercle (approximately twice or three times larger than the surrounding tubercles) (Fig. 6E); and coxa IV with retro-lateral apical apophysis (Fig. 6A, B, 7K, M) |
|
3. Proapical apophysis on coxa IV with abrupt curvature (Fig. 17C) |
|
4. Trochanter IV with a retro-apical robust spiniform apophysis (length longer than the podomere middle width) (Fig. 9A, B, E, 10K, M) |
|
5. Trochanter IV dorsal face with median central intumescence (Fig. 3A, E, 4K, L), without prolateral keel; and femur IV strongly curved on basal 1/4 (Fig. 4A–D) |
Iandumoema cuca, sp. nov.
Material examined
Holotype. BRAZIL. Minas Gerais: Iracarambi, Gruta da Água do João Ferreira (15°00′35.0″S, 44°07′55.5″W), 29.xi.2016, L.M. Rabelo et al. leg. (ISLA 59444, destroyed).
Paratypes. BRAZIL. Minas Gerais: Iracarambi, same locality as holotype, 2♀, 29.xi.2016, L.M. Rabelo et al. leg. (ISLA 59445, destroyed); same locality, 1♂, 25.i.2015, R.L. L.M. Rabelo leg. (ISLA 13147, destroyed); same data, 2♀ (ISLA 44197, destroyed).
Diagnosis
Iandumoema cuca, sp. nov. can be distinguished from other species of the genus by the combination of the following characters: ocularium with reduced and depigmented eyes, bearing a high, straight spiniform apophysis subapically curved backwards; scutal areas unarmed; male coxa IV with smoothly curved and subapically bifid (ventral branch shortest), very long (length longer than the coxa IV apical width) proapical apophysis and unarmed on retro-lateral face; trochanter IV with dorso-central subapical bulge.
Description
Male holotype (ISLA 59444)
Dorsum (Fig. 3A, E). Dorsal scutum length, 4.2; dorsal scutum width, 3.2; prosoma length, 1.5; prosoma width, 1.9. Measurements of pedipalps and legs are in Table 3. Dorsal scutum outline type α. Carapace and scutal areas with pointed granules. Anterior margin of carapace with prominent frontal hump bearing a main pair of paramedian tubercles. Eyes reduced and depigmented. Ocularium with a high, straight spiniform apophysis, its apex curved backwards. Scutal areas I–IV unarmed. Scutal area I divided into right and left halves. Lateral margin of DS with an external row of tubercles and an internal irregular row of granules. Posterior margin of DS and free tergites I–III each with a row of tubercles. Anal opercle irregularly tuberculate.

|

|
Venter (Fig. 3B). Coxa I with scattered granules plus an irregular row of five setiferous tubercles; II–III with scattered granules; IV and stigmatic area irregularly granulate. Posterior margin of genital sternite and free sternites each with a row of low tubercles.
Chelicerae (Fig. 3A). Segment I elongated (three times longer than wide), bulla with scattered granules. Segment II fixed finger and segment III toothed.
Pedipalps (Fig. 4I, J). Slightly elongated (~1.5 times the DS length). Trochanter with two dorsal tubercles and two ventral apical (ventral mesal largest) setiferous tubercles. Femur with one ventro-basal tubercle, median region with a ventral row of five granules and one mesal subapical setiferous tubercle. Patella smooth. Tibial setation: ectal and mesal IiiIi. Tarsal setation: ectal and mesal IiIi.
Legs (Fig. 4A–D, G, H, K–M). Coxae I–III with prodorsal and retro-dorsal apophyses; apex of retro-dorsal apophysis on coxa II fused with prodorsal apophysis on coxa III. Coxa IV granulated, with long, spiniform proapical apophysis (length similar to the coxa IV apical width), this smoothly curved (on dorsal view) and bifid (ventral branch shortest). Trochanters I–III with scattered granules. Trochanter IV with short, conical, blunt prolateral sub-basal apophysis, this bearing a dorsal intumescence; dorsal face with a dorso-median central intumescence; retro-lateral face with 2–4 basal–central tubercles roughly organised in a row and one apical pointed tubercle (its length approximately one-quarter to less of the podomere middle width). Femora, tibiae–metatarsi I–IV with granules roughly organised in six longitudinal rows (prodorsal, retro-dorsal, pro- and retro-lateral, proventral and retro-ventral rows); femora I–III approximately straight and unarmed. Femur IV strongly curved on basal 1/4, with one retro-basal and two ventro-basal large tubercles; dorso-apical face with two spiniform tubercles, retro-lateral one largest and pointing retro-laterally; two ventral rows of granules becoming tubercles (approximately twice the size of the granule) apically; ventro-apical face with a pair of spiniform large tubercles. Patella IV with ventral high tubercles. Tibia IV with a retro-lateral row of pointed tubercles; ventral face with two rows of granules increasing in size apically, becoming high, pointed tubercles; ventro-apical face unarmed. Tarsal counts: 7(3), 16(3), 6, 6.
Penis (Fig. 5). Ventral plate approximately pyriform with basal portion wider than apical one; distal margin approximately straight; basal lobes conspicuous. Ventral plate with four pairs of MS A, one pair of MS B (placed more ventrally), two pairs of straight MS C, one pair of MS D, two pairs of short MS E (placed more ventrally). Microsetae type 1 occupying all of ventral plate, with a lower density in the apical portion. Glans without dorsal process; ventral process stem short (less than a quarter of stylus length) and thick (comparable to that of stylus), apex flabellum-like with tip curved ventrally. Stylus smooth.
Colouration (male, in alcohol) (Fig. 3, 4). Body and appendages background vivid orange yellow (66), prolateral apical apophysis of coxa IV and trochanter IV strong orange (50), median to apical portion of femur IV, patella and pedipalps pale yellow (89).
Female (ISLA 59445; Fig. 3C, D, F, 4E, F)
Dorsal scutum length, 3.9; dorsal scutum width, 2.7; prosoma length, 1.4; prosoma width, 1.8. Measurements of pedipalps and legs are in Table 4. Coxa IV reaching scutal groove IV, with a short proapical apophysis (length ~1/2 of the coxa IV apical width). Trochanter IV prolateral and retro-lateral faces unarmed. Femur IV basal curvature attenuated, without tubercles except for dorso-apical ones. Tibia IV unarmed.

|
Etymology
Noun in apposition from the fictional character Cuca, created by the Brazilian writer José Bento Renato Monteiro Lobato based on the Brazilian folklore. This character lives in a cave and has the appearance of an alligator. It appears in the book ‘O Saci’ and in the television show called ‘Sítio do Pica-Pau Amarelo’ that is based on Lobato’s series of books.
Ecological remarks
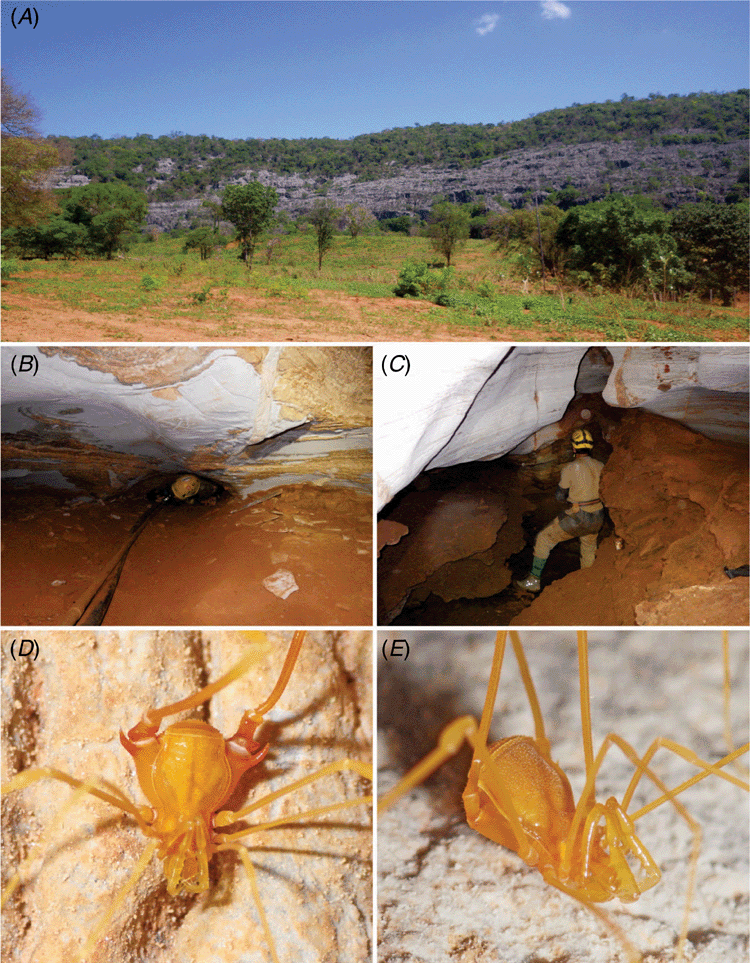
The cave where Iandumoema cuca, sp. nov. was collected (Lapa D’Água do João Ferreira Cave, Itacarambi, Minas Gerais) (Fig. 12A–C) is in a huge limestone outcrop of the Bambuí formation (Neoproterozoic limestone) surrounded by a well preserved deciduous forest. The cave is located in the transitional area between Cerrado (Brazilian savannah) and Caatinga (Velloso et al. 2002) and harbours a single conduit of 150 m of horizontal projection, traversed by a perennial stream in its final portion. Thus, despite the entrance being extremely dry, deeper parts of the cave have a high humidity. A constriction ~15 m from the entrance also helps keep the atmosphere more stable from this point on. Local farmers drain the cave water using a hose, gravitationally dragging water for domestic consumption and crop irrigation. Since they do not use pumps, the impact of water drainage seems to be small.
Specimens of I. cuca, sp. nov. were observed on the cave walls deep inside the cave. Only six specimens were found during the two visits to the cave (three in each visit), thus indicating an apparently low density of the population. We should point out that there are parts of the cave that are inaccessible to humans, such as the low ceiling at the water entrance and exit. Notwithstanding, the habitat for the harvestmen extends, accompanying the drainage. Other troglobitic species found in the cave include the whip-spider Charinus sp. (Amblypygi: Charinidae) and the isopod Xangoniscus sp. (Styloniscidae). The external environment surrounding the cave is only partially protected because, although the cave itself is located within the Cavernas do Peruaçu National Park, the entrance is not. The entrance is located outside the Park (which coincides with the limestone outcrop border), in an area where the original vegetation has been replaced by pastures and monocultures.
Finally, it is important to point out that the Lapa D’Água do João Ferreira is located not far from the caves where Iandumoema uai occurs, namely the Lapa do Cipó Cave and Olhos D’Água Cave. The distance between the Lapa D’Água do João Ferreira and the latter caves is 8.4 and 12.2 km in a straight line respectively. The regional topography indicates some potential discontinuities in the limestone outcrops between the area of Lapa D’Água do João Ferreira and the areas where I. uai occurs (with clear signs of drainages in between both areas). Furthermore, the underground streams flow in the opposite direction in each area: WSW–ENE in the Olhos D’Água Cave, WNW–ESE in the Lapa do Cipó Cave and ENE–WSW in the Lapa D’Água do João Ferreira Cave. This condition could indicate that the systems were formed by distinct drainages, supported (from the trophic point of view) by distinct flows. These conditions might prevent migrations (or at least make it difficult) between both systems. Do Monte et al. (2015) suggested that Lapa do Cipó and Olhos D’Água Caves are connected either by a complex system of subterranean microspaces (such as cracks and fissures) or that they were a single system in the past, being currently separated (at least, for humans). However, these hypotheses do not include the Lapa D’Água do João Ferreira owing to the visible discontinuities in the external landscape and the opposite flow direction of the subterranean stream.
Discussion
The main goal achieved in this article was to test the monophyly of a genus only known from caves. This is important because many genera are still recognised based on the Roewerian system (a classification system that lasted for more than half a century that was based on a limited set of characters that did not take intraspecific variation into account in most cases, obscuring phylogenetic inferences; see Pinto-da-Rocha (2002), Hara and Pinto-da-Rocha 2010 and Mendes 2011, just to name a few), despite the enormous efforts to revert this scenario in the past four decades. The uncertainty of the monophyly of a given group considerably hinders the meaningfulness of comparative, evolutionary studies, as further inferences are not possible or highly doubtful. As Iandumoema monophyly is corroborated, it is now possible to tackle other comparative studies (such as ecological, behavioural, chronobiological, just to name a few) in a cave-restricted group under a phylogenetic perspective for the first time in Brazil.
We addressed the issue regarding the use of troglomorphic characters in cladistic analysis using the approach of Desutter-Grandcolas et al. (2003). In this study, we took a rather conservative approach, reducing the characters potentially associated to cave life to a minimum of two, namely the presence or absence of eyes and presence or absence of eye pigmentation (#3 and #4). We are aware that pigmentation of the eyes is rather a continuous character. However, we notice that there was no need to treat the pigmentation of the eyes as such based on the taxon sampling, i.e. we did not notice the need to create a character depicting the degree of pigmentation. The analysis under equal weights excluding these two characters resulted in the same topology as including them (Fig. 1), but with fewer steps and the same consistency and retention indexes values (L = 335 steps; CI = 35; RI = 56). This analysis indicates that Iandumoema is a monophyletic genus, regardless of the inclusion of characters that are associated with cave dwelling.
For obvious reasons, no relationship among the species of Iandumoema had been addressed until Pinto-da-Rocha et al. (2015). According to their hypothesis, I. setimapocu and I. smeagol are closely related. In the present analysis, I. gollum, sp. nov. is the sister-species of I. smeagol. In addition, I. setimapocu is the sister-species of the clade including I. stygia, sp. nov. plus I. setimapocu + I. gollum, sp. nov.
Although the monophyly of Iandumoema is undisputed under the many analyses, its placement among Brazilian taxa is still an unsettled issue, as discussed below. Upon its description, Pinto-da-Rocha (1997) related Iandumoema to Gyndulus Roewer, 1929, Acanthopachylus Roewer, 1913 (=Heteropachyloidellus Mello-Leitão, 1927), Platygyndes Roewer, 1943, Progyndes Roewer, 1917, Pseudoacrographinotus Soares, 1966 and Pucrolia Sørensen, 1895 based on the Roewerian system. The knowledge about Neotropical harvestmen has improved considerably since that time and, despite not including taxa from those genera in the present analysis, we can rule out Platygyndes, as it is a Cosmetidae (Pinto-da-Rocha et al. 2012) and Acanthopachylus, which is closely related to Pachylus (Hara et al. 2012; Pinto-da-Rocha et al. 2014). Gyndulus and Pseudoacrographinotus are difficult to include in the analysis because the type species of those genera are females and most of the characters in the present analysis are based on male features, such as penis and the dimorphic armature on the leg IV. The type species of Pucrolia is a male and, based on its description, it most likely belongs to Eusarcus Perty, 1833, possibly related to or even a synonym of E. hastatus Sørensen, 1884 (unpubl. data). Other species of Pucrolia are only known from females.
In the present analysis, the sister-group of Iandumoema is the clade including Pachylinae (except for G. chagasi, E. catharinensis and D. inermis) + K92 (Fig. 1). Considering that Pachylinae is polyphyletic and the most taxon-rich subfamily in the Gonyleptidae (with ~100 genera), we are aware of the sampling issue. We composed the outgroup seeking to include the main subfamilies of Gonyleptidae and as much of the morphological diversity in Pachylinae as possible, given the aim of this manuscript. Considering these, the sister-taxon relationship must be taken with care, as we included just over 10% of the known Pachylinae genera. In the present analysis, we can infer with certain confidence that it is not closely related to Pachylinae sensu stricto, but possibly close to the other Brazilian ‘Pachylinae’ lineage. As the taxonomic knowledge of the Brazilian ‘Pachylinae’ increases, it shall be dismantled in many monophyletic groups (Pinto-da-Rocha et al. 2014). Hence, the sister-taxon of Iandumoema shall be gradually understood with more confidence, and this study is the first step towards that goal. Ideally, we wish that future phylogenetic studies including Iandumoema would be based on the present matrix, being further improved by addition of more taxa with proper sampling in some genera (as some might include over 50 species, such as Discocyrtus Holmberg, 1878) as well as more morphological characters, eventually also using different types of data (e.g. molecular). This should corroborate (or not) the present sister-taxon of Iandumoema and further enhance understanding of this cave-restricted genus.
Geographical distribution
Iandumoema is restricted to the limestone caves of the Bambuí group from northern to almost central Minas Gerais, corroborating previous authors (Hara and Pinto-da-Rocha 2008; Pinto-da-Rocha et al. 2015; Fig. 18). I. gollum, sp. nov. expands the genus distribution towards the southern part of the karstic formation, and we agree with Pinto-da-Rocha et al. (2015) that the eastern limit seems to be limited by quartzites from the Serra do Espinhaço. All Iandumoema species are associated with the deeper chambers or conduits of limestone caves with some kind of drainage, except for I. stygia, sp. nov. The lack of data regarding the conduits and microconduits that Iandumoema species are able to use in the hypogean system, as well as the difficulty for humans to actually check them to find subterranean barriers, considerably hinders further inferences regarding the speciation process in the genus. A biogeographical study is beyond the scope of this manuscript, but such a study considering all the geomorphological data available is desirable in the near future to understand subterranean speciation processes.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
We are grateful to Vale SA and Agência de Desenvolvimento Econômico e Social de Itabirito – ADESITA for financial support (Public Call Notice number 01/2015). L. N. de Ázara was supported by the scholarship from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (Process E-26/200.605/2018 (236032). M. R. Hara thanks the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Process 2018/07193-2). R. L. Ferreira thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant number 308334/2018-3). M. R. Hara also thanks FAPESP for funding Open Access publication (#2018/07193-2).
Acknowledgements
Marconi Souza Silva from Universidade Federal de Lavras was the ADESITA project coordinator and helped in many field trips. Thanks to Centro Nacional de Pesquisa e Conservação de Cavernas (CECAV). Luiz Felipe M. Iniesta for the help with ArcMap and Gilson Argolo for the help with some aspects of the descriptions. We thank Lucas M. Rabelo for gently sending photos of some live specimens. We thank Cristina Anne Rheims for the language review.
References
Acosta, L. E. (2019). A relictual troglomorphic harvestman discovered in a volcanic cave of western Argentina: Otilioleptes marcelae, new genus, new species, and Otilioleptidae, new family (Arachnida, Opiliones, Gonyleptoidea). PLoS One 14, e0223828.| A relictual troglomorphic harvestman discovered in a volcanic cave of western Argentina: Otilioleptes marcelae, new genus, new species, and Otilioleptidae, new family (Arachnida, Opiliones, Gonyleptoidea).Crossref | GoogleScholarGoogle Scholar | 31644592PubMed |
Acosta, L. E., Pérez-González, A., and Tourinho, A. L. (2007). Chapter 15. Methods and techniques of study: methods for taxonomic study. In ‘Harvestmen, the Biology of Opiliones’. (Eds G. Machado, R. Pinto-da-Rocha and G. Giribet.) pp. 494–505. (Harvard University Press: Cambridge, MA, USA.)
Bremer, K. (1988). The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution 42, 795–803.
| The limits of amino acid sequence data in angiosperm phylogenetic reconstruction.Crossref | GoogleScholarGoogle Scholar | 28563878PubMed |
Chaimowicz, F. (1986). Observações preliminares sobre o ecossistema da gruta Olhos d’Agua, Itacarambi, MG. Espeleo-Tema 15, 65–77.
de Ázara, L. N., and Ferreira, R. L. (2018). Annotated checklist of Gonyleptoidea (Opiliones: Laniatores) associated with Brazilian caves. Zootaxa 4439, 001–107.
| Annotated checklist of Gonyleptoidea (Opiliones: Laniatores) associated with Brazilian caves.Crossref | GoogleScholarGoogle Scholar |
Desutter-Grandcolas, L., D’Haese, C., and Robillarda, T. (2003). The problem of characters susceptible to parallel evolution in phylogenetic analysis: a reply to Marquès and Gnaspini (2001) with emphasis on cave life phenotypic evolution. Cladistics 19, 131–137.
| The problem of characters susceptible to parallel evolution in phylogenetic analysis: a reply to Marquès and Gnaspini (2001) with emphasis on cave life phenotypic evolution.Crossref | GoogleScholarGoogle Scholar |
Do Monte, B. T. O., Gallão, J. E., von Schimonsky, D. M., and Bichuette, E. M. (2015). New records of two endemic troglobitic and threatened arachnids (Amblypygi and Opiliones) from limestone caves of Minas Gerais state, southeast Brazil. Biodiversity Data Journal 3, e5260.
| New records of two endemic troglobitic and threatened arachnids (Amblypygi and Opiliones) from limestone caves of Minas Gerais state, southeast Brazil.Crossref | GoogleScholarGoogle Scholar |
Goloboff, P. A. (1993). Estimating character weights during tree search. Cladistics 9, 83–91.
| Estimating character weights during tree search.Crossref | GoogleScholarGoogle Scholar |
Goloboff, P. A., Farris, J. S., Källesjö, M., Oxelman, B., Ramírez, M. J., and Szumik, C. A. (2003). Improvements to resampling measures of group support. Cladistics 19, 324–332.
| Improvements to resampling measures of group support.Crossref | GoogleScholarGoogle Scholar |
Goloboff, P. A., Farris, J. S., and Nixon, K. C. (2008). TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786.
| TNT, a free program for phylogenetic analysis.Crossref | GoogleScholarGoogle Scholar |
Goodman, M., Olson, C. B., Beeber, J. E., and Czelusniak, J. (1982). New perspectives in the molecular biological analysis of mammalian phylogeny. Acta Zoologica Fennica 169, 19–35.
Grant, T., and Kluge, A. (2008). Clade support measures and their adequacy. Cladistics 24, 1051–1064.
| Clade support measures and their adequacy.Crossref | GoogleScholarGoogle Scholar |
Hara, M. R., and Pinto-da-Rocha, R. (2008). A new species of Brazilian troglobitic harvestman of the genus Iandumoema (Opiliones: Gonyleptidae). Zootaxa 1744, 50–58.
| A new species of Brazilian troglobitic harvestman of the genus Iandumoema (Opiliones: Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Hara, M. R., and Pinto-da-Rocha, R. (2010). Systematic review and cladistic analysis of the genus Eusarcus Perty 1833 (Arachnida, Opiliones, Gonyleptidae). Zootaxa 2698, 1–136.
| Systematic review and cladistic analysis of the genus Eusarcus Perty 1833 (Arachnida, Opiliones, Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Hara, M. R., Pinto-da-Rocha, R., and Kury, A. B. (2012). Revision of Nanophareus, a mysterious harvestman genus from Chile, with descriptions of three new species (Opiliones: Laniatores: Gonyleptidae). Zootaxa 3579, 37–66.
| Revision of Nanophareus, a mysterious harvestman genus from Chile, with descriptions of three new species (Opiliones: Laniatores: Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Hara, M. R., Pinto-da-Rocha, R., and Benedetti, A. R. (2018). Redescription of Juticus furcidens Roewer, 1943 (Opiliones: Gonyleptidae), with a discussion of its relationships in the subfamily Gonyleptinae. Zootaxa 4422, 422–430.
| Redescription of Juticus furcidens Roewer, 1943 (Opiliones: Gonyleptidae), with a discussion of its relationships in the subfamily Gonyleptinae.Crossref | GoogleScholarGoogle Scholar | 30313496PubMed |
Harris, S. R., Wilkinson, M., and Marquès, A. C. (2003). Countering concerted homoplasy. Cladistics 19, 128–130.
| Countering concerted homoplasy.Crossref | GoogleScholarGoogle Scholar |
International Commission on Zoological Nomenclature (1999). ‘International Code of Zoological Nomenclature’, 4th edn. (International Trust for Zoological Nomenclature: London, UK.)
International Commission on Zoological Nomenclature (2017). Declaration 45 – addition of recommendations to Article 73 and of the term ‘specimen, preserved’ to the glossary. Bulletin of Zoological Nomenclature 73, 96–97.
| Declaration 45 – addition of recommendations to Article 73 and of the term ‘specimen, preserved’ to the glossary.Crossref | GoogleScholarGoogle Scholar |
Krell, F.-T., and Marshall, S. A. (2017). New species described from photographs: yes? no? sometimes? A fierce debate and a new declaration of the ICZN. Insect Systematics and Diversity 1, 3–19.
| New species described from photographs: yes? no? sometimes? A fierce debate and a new declaration of the ICZN.Crossref | GoogleScholarGoogle Scholar |
Kury, A. B. (2013). Order Opiliones Sundevall, 1833. Zootaxa 3703, 27–33.
| Order Opiliones Sundevall, 1833.Crossref | GoogleScholarGoogle Scholar |
Kury, A. B. (2016). Opiliones. Catálogo Taxonômico da Fauna do Brasil. Programa das Nações Unidas para o Desenvolvimento, Brasília. Available at http://fauna.jbrj.gov.br/fauna/faunadobrasil/98744 [In Portuguese, verified 6 February 2016].
Kury, A. B., and Alonso-Zarazaga, M.-A. (2011). Addenda and corrigenda to the ‘Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones)’. Zootaxa 3034, 47–68.
| Addenda and corrigenda to the ‘Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones)’.Crossref | GoogleScholarGoogle Scholar |
Kury, A. B., and Medrano, M. A. (2016). Review of terminology for the outline of dorsal scutum in Laniatores (Arachnida, Opiliones). Zootaxa 4097, 130–134.
| Review of terminology for the outline of dorsal scutum in Laniatores (Arachnida, Opiliones).Crossref | GoogleScholarGoogle Scholar | 27394531PubMed |
Kury, A. B., and Orrico, V. G. D. (2006). A new species of Lacronia Strand, 1942 from the highlands of Rio de Janeiro (Opiliones, Gonyleptidae, Pachylinae). Revista Ibérica de Aracnología 13, 147–153.
Kury, A. B., and Villarreal, O. M. (2015). The prickly blade mapped: establishing homologies and a chaetotaxy for macrosetae of penis ventral plate in Gonyleptoidea (Arachnida, Opiliones, Laniatores). Zoological Journal of the Linnean Society 174, 1–46.
| The prickly blade mapped: establishing homologies and a chaetotaxy for macrosetae of penis ventral plate in Gonyleptoidea (Arachnida, Opiliones, Laniatores).Crossref | GoogleScholarGoogle Scholar |
Kury, A. B., Giupponi, A. P. L., and Mendes, A. C. (2018). Immolation of Museu Nacional, Rio de Janeiro – unforgettable fire and irreplaceable loss. The Journal of Arachnology 46, 556–558.
| Immolation of Museu Nacional, Rio de Janeiro – unforgettable fire and irreplaceable loss.Crossref | GoogleScholarGoogle Scholar |
Marques, A. C., and Gnaspini, P. (2001). The problem of characters susceptible to parallel evolution in phylogenetic reconstructions: suggestion of a practical method and its application to cave animals. Cladistics 17, 371–381.
| The problem of characters susceptible to parallel evolution in phylogenetic reconstructions: suggestion of a practical method and its application to cave animals.Crossref | GoogleScholarGoogle Scholar |
Marshall, S. A., and Evenhuis, N. L. (2015). New species without dead bodies: a case for photobased descriptions, illustrated by a striking new species of Marleyimyia Hesse (Diptera, Bombyliidae) from South Africa. ZooKeys 525, 117–127.
| New species without dead bodies: a case for photobased descriptions, illustrated by a striking new species of Marleyimyia Hesse (Diptera, Bombyliidae) from South Africa.Crossref | GoogleScholarGoogle Scholar |
Mendes, A. C. (2011). Phylogeny and taxonomic revision of Heteropachylinae (Opiliones: Laniatores: Gonyleptidae). Zoological Journal of the Linnean Society 163, 437–483.
| Phylogeny and taxonomic revision of Heteropachylinae (Opiliones: Laniatores: Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Nixon, K. C., and Carpenter, J. M. (1993). On outgroups. Cladistics 9, 413–426.
| On outgroups.Crossref | GoogleScholarGoogle Scholar |
Pinto-da-Rocha, R. (1997). Iandumoema uai, a new genus and species of troglobitic harvestman from Brazil (Arachnida, Opiliones, Gonyleptidae). Revista Brasileira de Zoologia 13, 843–848.
| Iandumoema uai, a new genus and species of troglobitic harvestman from Brazil (Arachnida, Opiliones, Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Pinto-da-Rocha, R. (2002). Systematic review and cladistic analysis of the Caelopyginae (Opiliones: Gonyleptidae). Arquivos de Zoologia do Estado de São Paulo 36, 357–464.
Pinto-da-Rocha, R., Benedetti, A. R., Vasconcelos, E. G., and Hara, M. R. H. (2012). New systematic assignments in Gonyleptoidea (Arachnida, Opiliones, Laniatores). ZooKeys 198, 25–68.
| New systematic assignments in Gonyleptoidea (Arachnida, Opiliones, Laniatores).Crossref | GoogleScholarGoogle Scholar |
Pinto-da-Rocha, R., Bragagnolo, C., Marques, F. P. L., and Antunes-Junior, M. (2014). Phylogeny of harvestmen family Gonyleptidae inferred from a multilocus approach (Arachnida: Opiliones). Cladistics 30, 519–539.
| Phylogeny of harvestmen family Gonyleptidae inferred from a multilocus approach (Arachnida: Opiliones).Crossref | GoogleScholarGoogle Scholar |
Pinto-da-Rocha, R., Fonseca-Ferreira, R., and Bichuette, M. E. (2015). A new highly specialized cave harvestman from Brazil and the first blind species of the genus: Iandumoema smeagol sp. n. (Arachnida, Opiliones, Gonyleptidae). ZooKeys 537, 79–95.
| A new highly specialized cave harvestman from Brazil and the first blind species of the genus: Iandumoema smeagol sp. n. (Arachnida, Opiliones, Gonyleptidae).Crossref | GoogleScholarGoogle Scholar |
Roewer, C. F. (1913). Die Familie der Gonyleptiden der Opiliones–Laniatores. Archiv für Naturgeschichte 79, 1–256.
Sereno, P. C. (2007). Logical basis for morphological characters in phylogenetics. Cladistics 23, 565–587.
| Logical basis for morphological characters in phylogenetics.Crossref | GoogleScholarGoogle Scholar |
Trajano, E. (2012). Ecological classification of subterranean organisms. In ‘Encyclopedia of Caves’, 2nd edn. (Eds W. B. White and D. C. Culver.) pp. 275–277. (Academic Press: Oxford, UK.)
Velloso, A. L., Sampaio, E. V. S. B., and Pareyn, F. G. C. (Eds.) (2002). ‘Ecorregiões – Propostas para o bioma caatinga.’ (Associação Plantas do Nordeste/Instituto de Conservação Ambiental The Nature Conservancy do Brasil: Recife, Brazil.) [In Portuguese].
Appendix 1. Data matrix for Iandumoema and outgroup species
Polymorphisms: A = 0/1, B = 1/2. ‘-’ inapplicable data; ‘?’, missing data

|