Use of the Fatigue Severity Scale to assess clinically reliable temporal changes in post-stroke fatigue by stroke type and subtype
Suzanne Barker-Collo A * , Rita Krishnamurthi B , Valery Feigin B , Balakrishnan Nair B , Alan Barber C , Amanda G. Thrift D , Anna Ranta E , Derrick Bennett F , Jeroen Douwes G , El-Shadan Tautolo H , Dominique A. Cadilhac D , Varsha Parag I and Bruce Arroll J §
A * , Rita Krishnamurthi B , Valery Feigin B , Balakrishnan Nair B , Alan Barber C , Amanda G. Thrift D , Anna Ranta E , Derrick Bennett F , Jeroen Douwes G , El-Shadan Tautolo H , Dominique A. Cadilhac D , Varsha Parag I and Bruce Arroll J §
A
B
C
D
E
F
G
H
I
J
§ All authors writing on behalf of the ARCOS-5 steering committee.
Handling Editor: Julia Schmidt
Abstract
A recent consensus statement on post-stroke fatigue noted the Fatigue Severity Scale (FSS) should be the primary outcome measure in post-stroke fatigue research. It also noted that data to calculate clinically reliable changes on the FSS have not been established for stroke. We present FSS data collected at 1 and 12 months post stroke, allowing the assessment of clinically reliable change by stroke type and subtype for ischaemic stroke (IS).
The sample included all participants of the fifth Auckland Region Community Outcomes of Stroke study (ARCOS-V) who consented and had FSS data (n = 338). Stroke type was recorded (IS, intracerebral haemorrhage (ICH), and subarachnoid haemorrhage (SAH)), and IS subtypes were defined using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classifications. ‘Clinically reliable change’ between 1 and 12 month FSS scores was calculated using Jacobsen and Traux’s updated formula.
Participants with ICH had the highest FSS scores at 1 month. Across IS subtypes, those with small vessel disease had the highest FSS scores at 1 month, and this increased at 12 months. Statistically significant reductions in mean FSS were found for patients with IS of other aetiology and SAH. Regarding clinically reliable changes, the greatest proportion of individuals had no clinically reliable change in FSS, up to 20% experienced reliable reductions, and 0–11% experienced reliable increases in FSS scores.
Although most participants had no clinically reliable change in fatigue between 1 and 12 months, statistically significant reductions in FSS were identified for patients with IS and SAH. Of those who did experience reliable change, the majority had reductions in fatigue over time.
Keywords: clinically reliable change, cut-offs for reliable change, fatigue, ischaemic subtype, longitudinal, stroke.
Introduction
Fatigue management is a critical unmet need that survivors of stroke identify as a high-priority area for research (Hill et al. 2022). This is unsurprising given that approximately half of survivors of stroke experience post-stroke fatigue (pooled prevalence estimate across 31 studies = 48% (95% confidence interval (CI) = 42–53%)) (Alghamdi et al. 2021). Fatigue is a major disabling condition and is a substantial barrier to engaging in rehabilitation and other activities that improve quality of life (Glader et al. 2002; van de Port et al. 2007; Duncan et al. 2012). The World Stroke Organization recommends providing people with stroke and their families/carers with information, education, and strategies to manage fatigue (Johansen Skogestad et al. 2021). Yet, there are no proven treatments for post-stroke fatigue (Ponchel and Bordet 2015).
A recent consensus statement on post-stroke fatigue (English et al. 2024) addressed central questions to research, including its biological causes, how fatigue and its potential causes should be identified in clinical practice, the most promising interventions for management, and the best available outcome measure for research. The Fatigue Severity Scale-7 item version (FSS-7) was recommended as the primary outcome measure for fatigue in all stroke research. The 7-item version of the scale was suggested for research purposes as Lerdal and Kottorp (2011) found that items 1 and 2 in the FSS-9, which reflect the impact of fatigue on motivation and the impact of physical exercise on fatigue, should not be used in a mean score because the FSS-7 shows better validity and reliability and is likely more sensitive for measuring change in fatigue. Although the FSS-7 did show better validity, in Lerdal and Kottorp (2011) baseline Cronbach’s alpha for the FSS-9 and FSS-7 did not vary (0.86 and 0.87, respectively), though alpha was better for the FSS-7 over time. Furthermore, it is noted that the FSS-7 is suggested as an outcome for research purposes. As the aim of this study was to provide data required to calculate reliable change within clinical settings, and the first two items of the FSS provide important information for clinical rehabilitation, the FSS-9 was retained.
The consensus statement also noted that data required to calculate minimum clinically reliable change on the FSS are not available in stroke. The issue of minimum clinically reliable differences in outcomes acknowledges that a statistically non-significant outcome does not automatically imply that a treatment has not been clinically effective, particularly as small sample sizes and measurement variability in this area of research can influence statistical results (Batterham and Hopkins 2006). Also noted by Page (2014), statistically significant differences (or lack thereof) may not always result in an appropriate change in clinical practice. Clinical interpretation of research using calculation of clinically reliable change to describe treatment outcomes is important because of its more direct translation to clinical decision making. As such, Page recommends that authors provide results relative to clinically meaningful differences or clinically reliable changes (Page 2014). The data required to calculate clinically reliable changes in post-stroke fatigue would support clinical decision making and measures of effectiveness for interventions designed to reduce fatigue.
The aim of this study was to describe fatigue as measured by the FSS at 1 month and 12 months post stroke using data from the population-based fifth Auckland Region Community Outcomes of Stroke Study (ARCOS-V), compare fatigue progression on the basis of the FSS by stroke subtype over time, and provide tabulated data for clinicians to determine if changes in fatigue in their own patients are clinically reliable by stroke type (i.e. ischaemic stroke (IS), intracerebral haemorrhage (ICH) and subarachnoid haemorrhage (SAH)), and IS subtype (using Trial of Org 10172 in Acute Stroke Treatment (TOAST) classifications; Adams et al. 1993).
Methods
ARCOS-V is the fifth in a series of prospective population-based studies of stroke incidence, prevalence, and outcomes in the Auckland region of New Zealand. The methods are described in detail elsewhere (Krishnamurthi et al. 2014). Each study included all incident strokes in residents of the Auckland region aged ≥16 years, with complete case ascertainment assured through multiple overlapping sources of information on all new hospitalised or non-hospitalised cases. The ARCOS-V study follows the recommendations for ideal stroke studies presented by Sudlow and Warlow (1996).
All public hospital admissions data were searched daily, with weekly checks of hospital discharge and outpatient clinic data. Regular checks of private hospitals, rest homes, and community health services were also conducted. New Zealand Ministry of Health data of all fatal and nonfatal stroke cases in the study population and residents meeting the inclusion criteria but who had their stroke while outside the Auckland region were also included.
Stroke was defined according to the World Health Organization as ‘rapidly developing clinical signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death, with no apparent cause other than of vascular origin’ (Aho et al. 1980, p. 114). A diagnostic review committee comprising neurologists confirmed the diagnosis and classification of all stroke cases using medical history, hospital discharge summaries, clinical and laboratory findings, imaging (including vascular and cardiac), or necropsy results when available. Cases without imaging or pathological necropsy confirmation of subtype were classified as stroke of undetermined type. Stroke type (i.e. IS, ICH, and SAH) was recorded, and those with IS subtypes were subclassified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classifications (i.e. large artery atherosclerosis (LAA), cardioembolism, small-vessel occlusion, stroke of other determined aetiology, and stroke of undetermined aetiology) (Adams et al. 1993).
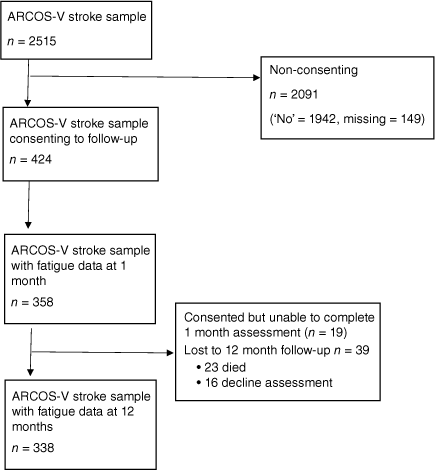
The analyses described in this paper included all ARCOS-V cases who: (1) provided written informed consent for follow-up; and (2) had outcome data from 1 month assessments, the majority of whom also had 12 month data (see Fig. 1). Individuals who consented to the study but who were too unwell to complete the baseline assessment were approached at 12 months to determine willingness and ability to participate in the follow-up assessment. Each assessment took approximately 1 hour to complete and was conducted via telephone by a trained research assistant. Assessments reported here occurred 1 month and 12 months post stroke.
Assessments
The FSS contains nine items (e.g. Exercise brings on my fatigue) to assess physical fatigue, with each statement rated from 1 (strongly disagree) to 7 (strongly agree) (Krupp et al. 1989). FSS total scores are the mean score calculated across all statements, with a mean of >3.9 reflecting moderate-to-severe fatigue (Choi-Kwon 2005), and higher FSS scores indicating greater fatigue. The FSS has demonstrated excellent internal consistency in subjects with stroke (Cronbach’s alpha: 0.928), test–retest reliability across 1 week (intraclass coefficient correlation (ICC): 0.742, CI: 0.512–0.863, P < 0.001) is good, and correlations to measures of quality of life are moderate (Ozyemisci-Taskiran et al. 2019).
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) from baseline hospital records. Each of the 11 items scores a specific ability from 0 to 4, where 0 indicates normal function and higher scores indicate some impairment. The NIHSS was designed to be a standardised assessment post stroke for large multicentre clinical trials (Brott et al. 1989) and has been repeatedly validated for assessing stroke severity and predicting stroke outcomes (Muir et al. 1996; Frankel et al. 2000).
Analyses
Descriptive statistics (means and s.d.s) are provided for 1 and 12 month assessments for total strokes and each stroke type/subtype. Statistical significance of within-subject change was examined using within-subject repeated measures ANOVA. In addition, the standard deviation of the difference between measurements taken at 1 and 12 months is presented. These tabulated data include all the figures required for clinicians to calculate if change observed in their own clients from 1 to 12 months reaches a level of being clinically reliable. Clinically reliable change in fatigue was calculated for each participant in the sample using an updated Jacobson and Truax’s (1991) formula. Change was examined from 1 to 12 months post stroke, calculated for each individual in the sample as:
where X1 and X2 are the scores an individual patient obtained at 1 and 12 months. M1 and M2 are the group mean scores at 1 and 12 months, and SDD is the standard deviation of the difference between 1 and 12 month scores. These were calculated separately for all strokes combined, by stroke type, and for IS by subtype. The correction for practice effects is the addition of the constant that is based on group level average change (Sveen et al. 2010). Following the convention of using 5% as the threshold for statistical significance, a Reliable Change Index calculated as greater than ±1.96 was considered clinically significant. Thus, individual participant reliable change scores on the FSS that are 1.96 or greater indicate reliable increases, and those that are −1.96 or lower indicate reliable decreases in FSS scores. This aligns with a z-score of ±1.96, which corresponds to the 97.5th/2.5th percentile of a normal distribution; in other words, 95% of all z-standardised values in a normal distribution are smaller or equal to ±1.96. The proportion of individuals for each stroke type/subtype whose reliable change scores met criteria for a significant increase, significant reduction, or no significant change on the FSS are presented. Using the above formula and data presented in Table 1, the minimum amount of change required to be considered a reliable increase or decrease were also calculated for each stroke type/subtype.
| Demographics | Consenting and providing 1 month data (n = 358) | Non-consenting (n = 2157) | Significance of difference | |||
|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |||
| Age, mean | 67.5 | 13.74 | 71.78 | 15.43 | F = 17.950, P < 0.001 | |
| NIHSS | 5.48 | 4.645 | 8.64 | 5.283 | F = 0.229, P = 0.084 | |
| Gender | n | % | n | % | χ2 = 18.226, P < 0.001 | |
| Male | 225 | 62.8 | 1073 | 50.5 | ||
| Female | 133 | 37.2 | 1053 | 49.5 | ||
| Ethnicity | χ2 = 34.634, P < 0.001 | |||||
| European | 252 | 70.4 | 1164 | 54.8 | ||
| Māori | 22 | 6.1 | 190 | 8.9 | ||
| Pacific | 29 | 8.1 | 330 | 15.5 | ||
| Asian | 51 | 14.2 | 264 | 12.4 | ||
| Other | 4 | 1.1 | 178 | 8.4 | ||
| Completed high school A | ||||||
| Yes | 282 | 79.6 | – | – | ||
| No | 39 | 10.9 | – | – | ||
| Missing | 34 | 9.5 | – | – | ||
| Marital status | χ2 = 3.002 P = 0.085 | |||||
| Married/de facto | 241 | 67.3 | 1194 | 56.2 | ||
| Never married | 32 | 8.9 | 169 | 7.9 | ||
| Divorced/widowed | 73 | 20.4 | 638 | 30.0 | ||
| Missing | 9 | 2.5 | 125 | 5.9 | ||
| Stroke type/subtype | F = 38.591, P < 0.001 | |||||
| Intracerebral haemorrhage | 19 | 5.3 | 376 | 17.7 | ||
| Subarachnoid haemorrhage | 15 | 4.2 | 103 | 4.8 | ||
| Ischaemic stroke | 324 | 90.5 | 1633 | 76.8 | ||
| Ischaemic subtypes | ||||||
| Large artery atherosclerosis B | 45 | 12.6 | 226 | 13.7 | ||
| Cardioembolism | 73 | 20.4 | 468 | 28.3 | ||
| Small vessel occlusion | 152 | 42.5 | 647 | 39.2 | ||
| Other aetiology | 12 | 3.4 | 51 | 3.1 | ||
| Unknown | 42 | 11.7 | 260 | 15.7 | ||
Results
Overall, 358 of the original ARCOS-V sample of 2515 participants had FSS data at 1 month, and 338 had FSS data at 12 months (Fig. 1). Descriptive data for the sample at 1 month are provided in Table 1. These are contrasted with those who did not consent or provide data at 1 month. As can be seen in the table, those who participated were significantly younger and more likely to be male, to be European, and to have experienced an IS. The two samples did not differ in terms of stroke severity (NIHSS) or marital status.
Moderate-to-severe fatigue was present in 60.3% of participants at 1 month and 51.1% of participants at 12 months. At 1 month post stroke, people with ICH had the highest FSS scores; the stroke categories with the next highest scores were SAH and then IS (Table 2). Within IS subtypes, those with small vessel occlusion had the highest initial levels of fatigue. The greatest reductions in FSS mean scores between 1 and 12 months seen in Table 2 were for IS of other aetiology and SAH. Increases in fatigue over time were found for those with IS from undetermined aetiology (mean increase 0.01) and small vessel occlusion (mean increase 0.68), whereas all other groups had reductions. Within-subject improvements in fatigue for those with SAH reached statistical significance (mean reduction 0.81). Although within-subject change for IS also reached significance, the group mean only reduced by 0.26. As can be seen in Table 2, of those comparisons that were statistically significant, only that for SAH was quite large, whereas those for total stroke and all IS were small-to-moderate. Though not statistically significant, the effect size for LAA was large, and that for undetermined IS was quite large.
| All Strokes | Ischaemic stroke | ICH | SAH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | LAA | CE | SVO | Other | Undetermined | |||||
| 1 month | ||||||||||
| n | 358 | 324 | 45 | 73 | 152 | 12 | 42 | 19 | 15 | |
| Mean (s.d.) | 4.22 (1.60) | 4.14 (1.61) | 4.14 (1.71) | 4.05 (1.51) | 4.25 (1.61) | 4.16 (1.69) | 3.87 (1.68) | 5.18 (1.42) | 4.70 (1.30) | |
| 12 months | ||||||||||
| n | 338 | 287 | 39 | 61 | 133 | 10 | 44 | 27 | 24 | |
| Mean (s.d.) | 3.94 (1.62) | 3.88 (1.61) | 4.04 (1.56) | 3.79 (1.63) | 4.93 (1.66) | 3.16 (1.18) | 3.88 (1.66) | 4.97 (1.52) | 3.89 (1.60) | |
| Mean change | 0.28 | 0.26 | 0.10 | 0.26 | −0.68 | 1.0 | −0.01 | 0.21 | 0.81 | |
| SDD differenceA | ||||||||||
| n | 338 | 287 | 39 | 61 | 133 | 10 | 44 | 19 | 15 | |
| SDD | 1.56 | 1.53 | 1.71 | 1.31 | 1.66 | 1.47 | 1.78 | 1.99 | 1.40 | |
| Significance of within-subject difference | ||||||||||
| F | 10.314 | 7.658 | 3.581 | 2.279 | 2.324 | 3.977 | 0.000 | 0.176 | 6.323 | |
| P | 0.002 | 0.006 | 0.069 | 0.140 | 0.131 | 0.093 | 1.00 | 0.684 | 0.033 | |
| 0.049 | 0.041 | 0.117 | 0.058 | 0.030 | 0.399 | 0.00 | 0.017 | 0.413 | ||
CE, cardioembolism; ICH, intracerebral haemorrhage; LAA, large artery atherosclerosis; SAH, subarachnoid haemorrhage; SDD, standard deviation of the difference; SVO, small vessel occlusion; , partial eta squared where 0.01 = small effect, 0.06 = moderate effect, 0.14 = large effect. Bolded data is used to highlight presence of significant within-subject change.
We observed overall significant within-subject improvements for the following items: ‘My motivation is lower when I am fatigued’, ‘My fatigue prevents sustained physical functioning’, ‘Fatigue is among my most disabling symptoms’, and ‘Fatigue interferes with my work, family, or social life’ (Table 3). None of the other items on the FSS showed detectable changes over time.
| FSS item | 1 month | 12 months | Within-subject change (DF1 = 1, DF2 = 218) | |||
|---|---|---|---|---|---|---|
| n = 358 | n = 338 | |||||
| Mean | s.d. | Mean | s.d. | |||
| My motivation is lower when I am fatigued | 5.00 | 1.964 | 4.69 | 1.943 | F = 4.501, P = 0.035 | |
| Exercise brings on my fatigue | 4.53 | 1.932 | 4.32 | 2.025 | F = 1.706, P = 0.193 | |
| I am easily fatigued | 4.35 | 2.082 | 4.30 | 2.087 | F = 0.113, P = 0.738 | |
| Fatigue interferes with my physical functioning | 4.29 | 2.055 | 4.09 | 2.061 | F = 3.653, P = 0.057 | |
| Fatigue causes frequent problems for me | 3.55 | 1.962 | 3.57 | 1.987 | F = 0.059, P = 0.808 | |
| My fatigue prevents sustained physical functioning | 4.43 | 2.094 | 4.09 | 2.109 | F = 11.885, P < 0.001 | |
| Fatigue interferes with carrying out certain duties and responsibilities | 3.89 | 2.146 | 3.64 | 2.055 | F = 3.681, P = 0.056 | |
| Fatigue is among my most disabling symptoms | 4.21 | 2.205 | 3.65 | 2.088 | F = 13.342, P < 0.001 | |
| Fatigue interferes with my work, family, or social life | 3.89 | 2.104 | 3.45 | 2.024 | F = 21.243, P < 0.001 | |
| FSS total mean score | 4.21 | 1.604 | 3.98 | 1.667 | F = 10.314, P = 0.002 | |
Individual item ratings range from 1 (strongly disagree) to 7 (strongly agree). Total scores range from 9 to 63. Higher scores indicate poorer outcomes. Bolded data is used to highlight significant differences.
The greatest proportion of individuals in each stroke type and IS subtype (72.7%–90%) had no reliable change in fatigue (Table 4). Of those who did experience reliable change, more people had reliable reductions in fatigue (2–14 fold) than reliable increases in fatigue. The exception to this was for those with IS of undetermined aetiology, where the proportion of those whose scores reliably improved was the same as the proportion whose scores deteriorated. Reliable change was not significantly correlated with baseline stroke severity as measured by the NIHSS (r = −0.140, P > 0.05).
| Stroke type/subtype | Change required for reliable changeA | % Reliably reduced | % No change | % Reliably increased | ||
|---|---|---|---|---|---|---|
| Reliable increase | Reliable reduction | |||||
| Total strokes | ≥2.77 | ≤−3.34 | 10.84 | 83.1 | 6.1 | |
| Ischaemic stroke | ≥2.74 | ≤−3.34 | 10.4 | 83.4 | 6.2 | |
| Large artery atherosclerosis | ≥3.25 | ≤−3.45 | 14.3 | 78.6 | 7.1 | |
| Cardioembolism | ≥2.31 | ≤−2.83 | 13.2 | 84.2 | 2.6 | |
| Small vessel occlusion | ≥3.94 | ≤−2.57 | 20.4 | 74.2 | 5.4 | |
| Other | ≥1.88 | ≤−3.88 | 14.3 | 85.7 | 0 | |
| Undetermined | ≥3.50 | ≤−3.48 | 11.1 | 77.8 | 11.1 | |
| Intracerebral haemorrhage | ≥3.70 | ≤−4.11 | 18.2 | 72.7 | 9.1 | |
| Subarachnoid haemorrhage | ≥1.93 | ≤−3.55 | 10 | 90 | 0 | |
Reliable changes in FSS over time were calculated as ((X2 − X1) − (M2 − M1))/SDD using M1, M2, and SDD for the stroke type/subtype provided in Table 2. Scores obtained using this formula below −1.96 were used to categorise ‘reliably reduced’ fatigue, whereas scores above +1.96 were used to categorise ‘reliably increased’ fatigue. ‘No change’ refers to those with changes in the FSS between −1.96 and +1.96.
Discussion
The findings of this study provide the data needed to calculate minimum clinically reliable differences on the recommended FSS post stroke, thereby filling a gap in knowledge noted by a recent consensus statement (English et al. 2024). Between 1 and 12 months post stroke, the majority of patients (72%–90%) did not experience any reliable change in fatigue, 10%–20% experienced improvements in fatigue, and only 0–11% experienced reliable increases in fatigue. Although most individuals experienced no clinically reliable change in fatigue, the proportions of individuals who did experience reliable increases or decreases varied widely across stroke type and IS subtypes. The level of fatigue experienced within the sample warrants intervention, with over half of participants experienced moderate-to-severe fatigue at both 1 month and 12 months.
Examinations of changes in post-stroke fatigue should be considered by stroke type/IS subtype. This is supported by our observation that mean levels of fatigue and changes in fatigue over time varied by stroke type and IS subtype. Statistically significant reductions in FSS were only found for IS of other aetiology and SAH, and not any other stroke type/subtype. Of those that were statistically significant, only the SAH group had a large effect size. Though not statistically significant, the effect size for LAA was large, and the effect size for undetermined IS was quite large. These discrepancies were likely affected by different sample sizes and lack of power.
Use of minimum clinically reliable differences acknowledges that a statistically non-significant outcome does not automatically mean a treatment has not been clinically effective, particularly as research in this area often has small sample sizes and large measurement variability (Batterham and Hopkins 2006). The findings presented here support this assertion with individuals experiencing clinically significant change where statistically significant changes were absent at a group level. The absence of statistically significant changes in a group may mask the effectiveness of an intervention present in a sub-sample (Page 2014). The calculation of a ‘clinically reliable change’ to describe treatment outcomes is important because of its more direct translation to clinical decision making. Reliable change should therefore be reported in clinical trials (Page 2014).
The data presented here will allow clinicians and researchers who examine this issue to calculate clinically reliable change by stroke type and by IS subtype. For example, if a clinician were working with an individual who had experienced an SAH who had been assessed with the FSS and received scores of 4.9 at 1 month and 3.88 at 12 months post stroke and wanted to know if that drop was clinically reliable, this would be calculated as (X2 − X1) − (M2 − M1)/SDD or ((3.88 − 4.9) − (3.89 − 4.7))/1.40 = −0.157 where M1, M2, and the SDD in Table 2 are 4.7, 3.89, and 1.40. Thus, although the individual has experienced a drop in FSS score (−1.02), it does not meet criteria (±1.96) for a reliable reduction. For clinicians, looking at Table 4, this drop of −1.02 falls between the critical values for a reliable increase/decrease, so would not be considered a reliable change. In applying the data, it must be remembered that the degree of change presented here is for a period from 1 month to 12 months post stroke. A limitation to the clinical utility of the data is that only these two measurement points are presented, and a clinician might not have access to FSS scores for these timepoints. It should be noted that the extend of change within this sample is likely to exceed that for clinical data for shorter timeframes. We therefore suggest caution if applying these data to clinical cases that have data from differing timeframes. Recent work on adaption of reliable change calculations to allow for more flexibility in applications related to timing of the data is underway (Helmich 2024).
Limitations
The greatest limitation of this study was sample size. Although the initial incidence sample was large, very few ARCOS-V participants consented to take part in this sub-study, leaving few individuals with 1 and/or 12 month data. The numbers of participants used here are relatively large when compared with other published studies on post-stroke fatigue, but it is possible that those who consented might differ from those excluded from these analyses. It is likely that those who declined to participate would have had greater levels of disability and higher levels of fatigue. Thus, any generalisation of the findings must be made with caution. With regard to generalisability, it must be noted that those who provided data were significantly younger and more likely to be male, to be European, and to have experienced an IS than those who did not. A related limitation due to small sample size was the inability to explore ethnicity-specific differences. In New Zealand, there is an increased burden of stroke in Māori and Pacific communities (Feigin et al. 2015). It might therefore be necessary to explore alternative strategies for studies such as this to pursue focused recruitment of minority populations to ensure culturally nuanced experiences are captured. In relation to this, some participants may be reluctant to engage in this type of research for a variety of factors, and cultural safety must be ensured. For example, in New Zealand the use of Kaupapa Māori research approaches needs to be considered.
Another possible limitation of this type of research is the use of participant self-reported fatigue. An alternative approach would have been to utilise a physiological measure of fatigue such as oxygenated haemoglobin level while performing an activity (e.g. walking) (Holzer et al. 2017).The primary reason for selecting self-report measure of fatigue for this study was practicality, as it was not possible to fund administration of physiological measures at each assessment to such a large sample, nor is it likely that testing haemoglobin would be used to assess fatigue in clinical practice. The weakness of self-report methodology is reliance on accurate reporting, which can be negatively affected by factors such as participant understanding of the items, ability to introspect, image management, and response bias. However, as the FSS focusses on observable behaviours (e.g. fatigue interferes with my work, family, or social life), these influences are less likely. Also, as noted in the methods, the FSS has good reliability and validity, suggesting that these issues are likely not major.
Finally, although the ARCOS-V study provides a large amount of data, which allows exploration of outcomes such as fatigue, no information was collected on the types or durations of allied therapies participants accessed (e.g. physiotherapy, occupational therapy). Information regarding allied therapies would provide a better picture of the sample and would have allowed us to determine if access to such therapies was associated with changes in fatigue.
Conclusion
Although most participants had no clinically reliable change in fatigue between 1 and 12 months post stroke, statistically significant reductions in FSS were identified in patients who had experienced IS and SAH. Across stroke type and IS subtypes, the majority of those who did experience reliable change had reductions in fatigue over time. There was wide variability in the proportions of individuals who experienced reductions and increases in fatigue, suggesting that any examination of changes in post-stroke fatigue should be considered by stroke type/IS subtype.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
The ARCOS-V project was funded by the New Zealand Health Research Council (ref# 19/691).
Ethics standard
Ethical approval was obtained from the New Zealand Health and Disability Ethics Committee (19/NTA/177) and Auckland University of Technology Ethics Committee (20/71). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All participants provided written informed consent prior to participation.
Guarantor
SBC acts as guarantor, taking responsibilty for the integrity of the work which led to this submission.
Author contributions
All authors are ARCOS-V steering committee members, contributing to study design and implementation. SBC produced the initial draft manuscript and conducted the main analyses. All named authors contributed through critical feedback on and approved the final version of the manuscript.
References
Adams H, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1), 35-41.
| Crossref | Google Scholar | PubMed |
Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T (1980) Cerebrovascular disease in the community: results of a WHO collaborative study. Bulletin of the World Health Organisation 58, 113-130.
| Google Scholar | PubMed |
Alghamdi I, Ariti C, Williams A, Wood E, Hewitt J (2021) Prevalence of fatigue after stroke: a systematic review and meta-analysis. European Stroke Journal 6(4), 319-332.
| Crossref | Google Scholar | PubMed |
Batterham A, Hopkins W (2006) Making meaningful inferences about magnitudes. International Journal of Sports Physiology and Performance 1(1), 50-57.
| Google Scholar | PubMed |
Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. (1989) Measurements of acute cerebral infarction—a clinical examination scale. Stroke 20, 864-870.
| Crossref | Google Scholar | PubMed |
Choi-Kwon (2005) Poststroke fatigue: characteristics and related factors. Cerebrovascular Disease 19, 84-90.
| Crossref | Google Scholar | PubMed |
Duncan F, Wu S, Mead G (2012) Frequency and natural history of fatigue after stroke: a systematic review of longitudinal studies. Journal of Psychosomatic Research 73, 18-27.
| Crossref | Google Scholar | PubMed |
English C, Simpson DB, Billinger SA, Churilov L, Coupland KG, Drummond A, Kuppuswamy A, Kutlubaev MA, Lerdal A, Mahmood A, Moseley GL, Pittman QJ, Riley EA, Sutherland BA, Wong CH, Corbett D, Mead G (2024) A roadmap for research in post-stroke fatigue: consensus-based core recommendations from the third Stroke Recovery and Rehabilitation Roundtable. Neurorehabilitation and Neural Repair 38, 7-18.
| Crossref | Google Scholar | PubMed |
Feigin V, Krishnamurthi R, Barker-Collo S, McPherson K, Barber P, Parag V, Arroll B, Bennett D, Tobias M, Jones A, Witt E, Brown P, Abbott M, Bhattacharjee R, Rush E, Suh F, Theadom A, Rathnasabapathy Y, Te Ao B, et al. (2015) 30-Year Trends in Stroke Rates and Outcome in Auckland, New Zealand (1981-2012): A Multi-Ethnic Population-Based Series of Studies. PLoS One 10(8), e0134609.
| Crossref | Google Scholar | PubMed |
Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M, Tilley BC, Broderick JP, et al. (2000) Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology 55, 952-959.
| Crossref | Google Scholar | PubMed |
Glader EL, Stegmayr B, Asplund K (2002) Poststroke fatigue: a two year follow up study of stroke patients in Sweden. Stroke 33, 1327-1333.
| Crossref | Google Scholar | PubMed |
Helmich MA (2024) The Duration-Adjusted Reliable Change Index: Defining Clinically Relevant Symptom Changes of Varying Durations. Assessment 31, 1493-1507.
| Crossref | Google Scholar | PubMed |
Hill G, Regan S, Francis R (2022) Research priorities to improve stroke outcomes. Lancet Neurology 21, 312-313.
| Crossref | Google Scholar | PubMed |
Holzer R, Yuan J, Verghese J, Mahoney JR, Izzetoglu M, Wang C (2017) Interactions of Subjective and Objective Measures of Fatigue Defined in the Context of Brain Control of Locomotion. Journal of Gerontology A, Biological Science Medical Science 72(3), 417-423.
| Crossref | Google Scholar |
Jacobson N, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy-research. Journal of Consulting and Clinical Psychology 59, 12-19.
| Crossref | Google Scholar | PubMed |
Johansen Skogestad I, Kirkevold M, Larsson P, Råheim Borge C, Indredavik B, Gay CL, Lerdal A (2021) Post-stroke fatigue: an exploratory study with patients and health professionals to develop a patient-reported outcome measure. Journal of Patient-Reported Outcomes 5, 35-46.
| Crossref | Google Scholar |
Krishnamurthi R, Jones A, Barber P, Barker-Collo S, McPherson K, Bennett D, et al. (2014) Methodology of a population-based stroke and TIA incidence and outcomes study: the Auckland Regional Community Stroke Study (ARCOS IV) 2011–2012. International Journal of Stroke 9(1), 140-147.
| Crossref | Google Scholar | PubMed |
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with Multiple Sclerosis and systemic lupus erythematosus. Archives of Neurology 46(10), 1121-1123.
| Crossref | Google Scholar | PubMed |
Lerdal A, Kottorp A (2011) Psychometric properties of the fatigue severity Scale—Rasch analyses of individual responses in a Norwegian stroke cohort. International Journal of Nursing Studies 48(10), 1258-1265.
| Crossref | Google Scholar | PubMed |
Muir KW, Weir CJ, Murray GD, Povey C, Lees KR (1996) Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 27, 1817-1820.
| Crossref | Google Scholar | PubMed |
Ozyemisci-Taskiran O, Batur EB, Yuksel S, Cengiz M, Karatas GK (2019) Validity and reliability of fatigue severity scale in stroke. Topics in Stroke Rehabilitation 26(2), 122-127.
| Crossref | Google Scholar | PubMed |
Page P (2014) Clinical Commentary. Beyond statistical significance: clinical Interpretation of rehabilitation research literature. International Journal of Sports Physical Therapy 9(5), 726-738.
| Google Scholar | PubMed |
Ponchel A, Bordet R (2015) Factors associated with poststroke fatigue: a systematic review. Stroke Research and Treatment 2015, 347920.
| Crossref | Google Scholar |
Sudlow CLM, Warlow CP (1996) Comparing stroke incidence worldwide: what makes studies comparable? Stroke 27(3), 550-558.
| Crossref | Google Scholar | PubMed |
Sveen U, Bautz-Holter E, Sandvik L, Alvsåker K, Røe C (2010) Relationship between competency in activities, injury severity, and post-concussion symptoms after traumatic brain injury. Scandinavian Journal of Occupational Therapy 17, 225-232.
| Crossref | Google Scholar | PubMed |
van de Port G, Kwakkel G, Schepers VP, Heinemans CT, Lindeman E (2007) Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health-related quality of life in chronic stroke? Cerebrovascular Diseases 23(1), 40-45.
| Crossref | Google Scholar | PubMed |