Impact of crop load on nitrogen uptake and reserve mobilisation in Vitis vinifera
Thibaut Verdenal A E , Jorge E. Spangenberg B , Vivian Zufferey A , Ágnes Dienes-Nagy A , Olivier Viret C , Cornelis van Leeuwen D and Jean-Laurent Spring A
A E , Jorge E. Spangenberg B , Vivian Zufferey A , Ágnes Dienes-Nagy A , Olivier Viret C , Cornelis van Leeuwen D and Jean-Laurent Spring A
A Agroscope Institute, Avenue Rochettaz 21, 1009 Pully, Switzerland.
B Institute of Earth Surface Dynamics, University of Lausanne, 1015 Lausanne, Switzerland.
C Direction générale de l’agriculture, de la viticulture et des affaires vétérinaires, 1110 Morges, Switzerland.
D Ecophysiologie et Génomique Fonctionnelle de la Vigne (EGFV), Bordeaux Sciences Agro, Institut national de la recherche pour l’agriculture, l’alimentation et l’environnement (INRAE), Univ. Bordeaux, Institut des Sciences de la Vigne et du Vin (ISVV), 33882 Villenave d’Ornon, France.
E Corresponding author. Email: thibaut.verdenal@agroscope.admin.ch
Functional Plant Biology 47(8) 744-756 https://doi.org/10.1071/FP20010
Submitted: 9 January 2020 Accepted: 16 March 2020 Published: 12 June 2020
Journal Compilation © CSIRO 2020 Open Access CC BY
Abstract
Nitrogen deficit affects both crop production and composition, particularly in crops requiring an optimal fruit N content for aroma development. The adaptation of cultural practices to improve N use efficiency (NUE) (i.e. N uptake, assimilation and partitioning) is a priority for the sustainable production of high-quality crops. A trial was set on potted grapevines (Vitis vinifera L. cv. Chasselas) to investigate the potential of crop limitation (via bunch thinning) to control plant NUE and ultimately fruit N composition at harvest. A large crop load gradient was imposed by bunch thinning (0.5–2.5 kg m–2) and N traceability in the plant was realised with an isotope-labelling method (10 atom % 15N foliar urea). The results indicate that the mobilisation of root reserves plays a major role in the balance of fruit N content. Fertiliser N uptake and assimilation appeared to be strongly stimulated by high-yielding conditions. Fertilisation largely contributed to fulfilling the high fruit N demand while limiting the mobilisation of root reserves under high yield conditions. Plants were able to modulate root N reserve mobilisation and fertiliser N uptake in function of the crop load, thus maintaining a uniform N concentration in fruits. However, the fruit free amino N profile was modified, which potentially altered the fruit aromas. These findings highlight the great capacity of plants to adapt their N metabolism to constraints, crop thinning in this case. This confirms the possibility of monitoring NUE by adapting cultural practices.
Additional keywords: crop thinning, foliar urea, grapevine, isotope labelling, N partitioning, reserve mobilisation.
Introduction
Fruit composition, though partly determined by genotype and uncontrolled environmental conditions, can be managed to some extent through the optimisation of agricultural practices, such as vineyard floor management, fertilisation, canopy management and crop thinning, before and during fruit development (Masclaux-Daubresse et al. 2010; Sweetman et al. 2014; Alem et al. 2019). The effect of crop load on C assimilation and partitioning has been extensively studied (Chaves 1984; Morinaga et al. 2003; Dai et al. 2010). Although a higher crop load reduces root and trunk C reserves, it does not appear to affect the photoassimilation rate (Chaumont et al. 1994; Dayer et al. 2016; Reeve et al. 2016). In contrast to carbohydrates, it is still unclear how crop load influences N accumulation in fruits, even though N is essential for fruitfulness (number of bunches per shoot) and aroma development (Wang et al. 2007; Ojeda-Real et al. 2009; Schreiner et al. 2014). In grapevine (Vitis vinifera L.), the berry N concentration – particularly, yeast-assimilable N (YAN), including ammonium NH4+ and free amino N (FAN) – is a determining parameter for wine making, affecting both the alcoholic fermentation kinetics and the wine’s organoleptic profile (Bell and Henschke 2005; Hannam et al. 2016).
Many studies have demonstrated that overcropping can delay fruit ripening (i.e. carbohydrate accumulation and acid degradation) and hence reduce fruit quality (Petrie and Clingeleffer 2006; Rutan et al. 2018). Therefore, crop thinning (i.e. limiting crop load by removing a proportion of fruits early in the season) has become a common practice to increase the source : sink ratio and enhance fruit maturation. Several studies have explained the impact of crop thinning on fruit composition, considering the leaf : fruit ratio as an indicator of balanced plants (Jackson and Lombard 1993; Keller et al. 2005; Mawdsley et al. 2018; Wang et al. 2018). In grapevines, a sufficient leaf : fruit ratio (above ~1 m2 of exposed leaf area per kg of fruit) promotes fruit development and maturation by providing a nonlimiting source of photosynthetic carbohydrates (Kliewer and Dokoozlian 2005; Zufferey et al. 2015). However, an oversized canopy (caused by higher trimming height rather than higher vigour) modifies N partitioning in the plant and thus might induce a deficient N concentration in the fruits, despite proper resources being provided to the plant (Spring et al. 2012; Verdenal et al. 2016). It is known that under specific conditions, the pathways of C and N accumulation in some fruits are different. For example, under restricted water conditions, carbohydrates continue to accumulate in fruits through (partial) remobilisation of root reserves, whereas N concentration declines (Chaves 1984; Rossouw et al. 2017).
Predicting and modulating plant N status in perennial fruit crops requires an understanding of the seasonal movement of N within the plant. In the case of grapevine, 90% of the C reserves (mainly starch) and 75% of the N reserves (mainly amino acids) are stored in the roots of dormant vines (Bates et al. 2002; Zapata et al. 2004). C and N uptake is low for several weeks after bud burst. As a consequence, the root starch content decreases until early flowering and only then increases, with the photosynthetic carbohydrates provided from the leaves (Zapata et al. 2004). Similar to C, the root N reserves are the major source of N mobilised early in the season to support early shoot growth until root N uptake is sufficient to maintain growth near the flowering stage (Zapata et al. 2004; Schreiner 2016). Whole-vine N uptake is maximal before flowering (Schreiner 2016). The refilling of N reserves usually starts before fruit maturity and lasts until leaf senescence (Zufferey et al. 2015; Rossouw et al. 2017).
Plant N status depends on both N use efficiency (NUE) and N availability (Porro et al. 2010). NUE is the combination of the assimilation efficiency (which includes uptake and assimilation) and the utilisation efficiency (allocation and remobilisation) (Kant et al. 2011). NUE strongly depends on environmental and genetic factors. Plant growth is often limited in the natural environment by N availability, which restricts plant development (Hachiya and Sakakibara 2017). Such N restriction limits the accumulation of N in fruits, changing the fruits’ FAN profile (Schreiner et al. 2014). Foliar urea application during veraison (i.e. the onset of fruit ripening when fruit starts accumulating total soluble sugars) increases fruit N content, thus improving their organoleptic character (Alem et al. 2019), without affecting plant vigour (Nisbet et al. 2014; Hannam et al. 2016; Gutiérrez-Gamboa et al. 2019). However, fertilisation efficiency largely depends on NUE. It has been estimated that 50–70% of N provided to crops is generally lost by leaching and volatilisation, depending on the conditions (Masclaux-Daubresse et al. 2010). Similar losses are reported for soil fertilisation, foliar fertilisation or both (Kant et al. 2011; Verdenal et al. 2016).
Therefore, improving NUE through the adaptation of agricultural practices is critical to enhance productivity and minimise N losses to the environment. In particular, assessment of the effect of crop load on the plant N source : sink relationship and fruit composition is essential for improving fruit quality, NUE and climate change adaptability (Boss et al. 2014; González-Barreiro et al. 2015). In this context, the aims of the study were (i) to identify how crop load strategies influence fruit N accumulation and composition, and (ii) to examine the impacts of crop load on fertiliser use efficiency and on the functional N balance between roots and fruits. These aims were accomplished by testing a large gradient of crop loads and by applying foliar 15N-labelled urea to the potted white grapevine cultivar Chasselas.
Materials and methods
Experimental site
The experiment was conducted in 2017 at the Agroscope research station in Pully, Switzerland (46°30′45.8″N 6°40′05.7″E). The low-calcareous colluvial soil at the station developed on upper Oligocene (Chattian) molasse sedimentary rocks and is composed of clay (15wt %), silt (38wt %), sand (47wt %) and carbonates (4.3wt % equivalent CaCO3). The soil pH was 7.9 and the humus content was 1.75%. Phosphorus (8.2 mg kg–1), K (25.2 mg kg–1) and Mg (11.4 mg kg–1) were not deficient for vine growing. This soil was used as the growth medium in the pots. The soil water-holding capacity in the pot was 11 L. The climate in this region is classified as warm and temperate (Köppen–Geiger classification Cfb; Peel et al. 2007). During the grapevine growing season (April–October), the daily mean temperature ranged between 4.3°C (19 April) and 27.6°C (3 August), averaging 16.6°C; the total precipitation during that period was 562 mm (data from the Swiss meteorological station in Pully). An important amount of precipitation (252 mm) was received between 25 April and 6 June during the early stage of plant growth (before flowering). The plant water potential was measured regularly with a pressure chamber (Model 600, PMS Instruments) to prevent eventual water restriction (Scholander et al. 1965). The vines were drip-irrigated (6 L per plant) twice in July (10 and 17 July) when the stem water potential was below –0.8 MPa.
Plant material
Vitis vinifera L. cv. Chasselas cultivars were grafted onto 3309C rootstock and planted in 2013 in 90-L underground pots with a planting density of 8330 vines ha–1 (1.5 × 0.8 m). The pot size allowed the unconstrained development of the roots. Planting in pots was chosen to ensure good recovery of the root biomass. The vines were grown with a vertical shoot positioning system (single Guyot) with a trunk height of 60 cm and seven shoots per plant. In 2017, the phenological stages of bud burst (phenological stage 01 on the BBCH-scale, Lancashire et al. 1991), flowering (BBCH 65) and veraison (BBCH 85) occurred on the days of year (DOY) 84, 164 and 214 respectively. The canopy was trimmed to a height of 1.2 m and the lateral shoots were removed from the bunch area following common practices. Harvest was performed on DOY 257. Three out of the 24 vines were discarded from the experiment because of outlier behaviour, such as unusually low fruitfulness, low berry set and small bunches, and poor plant development (vigour). These outlier vines had extremely low total N (TN) content (<0.5% DW) and YAN (<90 mg L–1).
Crop load and 15N labelling treatments
The plot was divided in two homogeneous blocks of 12 vines, namely the control and fertilised blocks. Each block consisted of three rows of four vines. Buffer vines separated the blocks to minimise fertiliser cross-contamination. In each block, three crop load conditions (one per row) were set by crop thinning at bunch closure (phenological stage BBCH 77, DOY 193, which is a standard time for crop thinning), maintaining two, five or eight bunches per plant, with the aim of building a large crop load gradient. For statistical purposes, the vines from each block were gathered in two groups of six vines each: low-yielding conditions (LYC) and high-yielding conditions (HYC), based on the yield per vine at harvest. Each vine was considered as a replicate. The threshold used to separate the two groups was set at 1.3 kg m−2, which represents an average crop load for Chasselas in the region. The vines of the fertilised block received N during veraison (onset of maturation, BBCH 85) in four applications (DOY 199, 208, 214 and 226), for a total of 20 kg N ha–1 of 15N-labelled urea (10 atom % 15N, Sigma-Aldrich). The labelled foliar urea was carefully applied on both sides of the canopy with two hand-sprayers (Birchmeier). Besides the urea application in the fertilised treatment, the soil was the only source of nutrients.
Field measurements and sample preparation
For each vine row per treatment, the average chlorophyll index, the average light-exposed leaf area and average leaf mineral content were determined. The chlorophyll index was determined on primary leaves from the medial part of the canopy (n = 30, DOY 227) with an N-tester (Yara International) (Spring and Zufferey 2000). The leaf mineral content (i.e. total N, P, K, Ca and Mg) was determined by analysing the powder obtained from eight dried leaves (two per vine) sampled on DOY 236. Total N was determined by the Kjeldahl method (Method 5.3.2MV004, Sol-Conseil) and the other elements were determined by inductively coupled plasma–optical emission spectroscopy after acid digestion (Methods 5.3.2MV005, -6 and -7). The concentrations were expressed as % DW.
The light-exposed leaf area (m2 m–2 of ground) was calculated on DOY 237 from the measured canopy height, width and porosity via the method of Carbonneau (1995) only once per treatment, since the percentage of holes could not be estimated for each vine separately. For each vine, the total leaf area was assessed via a nondestructive approach, based on the strong correlation between shoot length and total leaf area (Mabrouk and Carbonneau 1996). The correlation equation in the context of our experiment was determined as follows. Fifteen shoots from 15 different buffer plants were collected on DOY 206. The total shoot length (TSL, main shoot + laterals) was measured and the total leaf area was determined with a leaf area meter (LI-3100C, Li-Cor Biosciences). As a result, Eqn 1 allowed the estimation of the total leaf area (TLA) from the TSL:

Leaf gas exchange was measured for one fully expanded leaf per vine on sunny days approximately every 10 days from flowering (BBCH 65, DOY 164) to harvest (BBCH 89, DOY 257). Photosynthesis (µmol m–2), transpiration (mol m–2 s–1), stomatal conductance (mol m–2 s–1), ambient CO2 concentration (µmol mol–1) and internal CO2 concentration (µmol mol–1) were determined with a portable photosynthesis system (LI-6800, Li-Cor Biosciences). The shoot trimmings were collected three times (DOY 164, 191 and 215), weighed to determine the fresh weight (FW, g per vine) and then combined with the rest of canopy recovered at the time of excavation. Vine fruitfulness was determined before crop thinning and expressed as the number of bunches per shoot.
At harvest (DOY 257), the grape yield (kg m–2) and the leaf : fruit ratio (light-exposed leaf area per kg of fruit) were determined per vine. The grapes were harvested and each vine was excavated separately and split into parts, including the roots, the trunk (including the cane), the canopy (including trimmings collected during the season) and the fruits. The grape bunches were pressed manually to separate the must from the pomace. The five plant parts (roots, trunk, canopy, pomace and must) were weighed to determine FW. Must aliquots were taken for chemical (100 g) and stable isotope analysis (25 g). The plant parts were dried in a 60°C oven until a constant weight, excluding the must, which was freeze-dried, for determination of the DW and were then powdered (<1300 µm).
Stable isotope analysis
The stable C and N isotope compositions of plant parts were determined by elemental analysis and isotope ratio MS with a Carlo Erba 1108 elemental analyser (Fisons Instruments) connected via a Conflo III interface to a Delta V Plus isotope ratio mass spectrometer (Thermo Fisher Scientific). The stable isotope compositions are reported in the δ notation (i.e. δ13C and δ15N values, in variations relative to international measurement standards) (Coplen 2011):

where R is the molar ratio of the heaviest (iE) to the lightest (jE) most abundant isotopes of chemical element E (e.g. 13C : 12C, 15N : 14N). The stable isotope standard for C is Vienna Pee Dee Belemnite limestone, and the standard for N is atmospheric molecular N (Coplen 2011). All isotopic analyses were performed in duplicate. The δ values are reported in milliurey (mUr) rather than ‰, in conformity with the International System of Units and according to the guidelines and recommendations of the International Union of Pure and Applied Chemistry (Coplen 2011; Brand 2011).
For calibration and normalisation of the measured isotopic ratios to the international scales (LSVEC lithium carbonate scale for δ13C, atmospheric molecular N scale for δ15N), a three- to four-point calibration was used with international reference materials and six in-house urea standards (UNIL-Urea 1 to 6) at different 13C and 15N natural abundances and different 15N enrichments (described in Spangenberg and Zufferey 2019). The δ13C and δ15N values of the in-house standards with natural 13C and 15N abundances were determined and normalised with the reference materials for glycines USGS64 (δ13C = −40.81 mUr, δ15n = 1.76 mUr), USGS65 (δ13C = −20.29 mUr, δ15n = 20.68 mUr) and USGS66 (δ13C = −0.67 mUr, δ15n = 40.83 mUr) as described by Schimmelmann et al. (2016). The 15N-enriched standards were normalised with the reference materials USGS40 (δ15n = –4.5 mUr), USGS41 (δ15n = 47.6 mUr), USGS65, USGS66, IAEA 600 (δ15n = 1.02 mUr), IAEA 310A (δ15n = 47 mUr) and IAEA 310B (δ15n = 245 mUr). For natural abundances, the repeatability and intermediate precision were better than 0.1 mUr (1 s.d.) for both δ13C and δ15N. For the 15N-enriched samples, the reproducibility of the δ15N values was 2 mUr. The total organic C (TOC) and TN concentrations (in wt %) were determined from the peak areas of the major isotopes with the calibrations for δ13C and δ15N. The repeatability was better than 0.2wt % for the TOC and TN contents.
Fruit composition
The fresh must aliquot for chemical analysis was centrifuged (2200g), the pH was measured and the content of total soluble solids (Brix), titratable acidity (expressed as g L–1 tartaric acid), and tartaric and malic acid contents (g L–1) were determined by an infrared spectrophotometer (WineScan, FOSS NIR Systems).
Free amino acids were quantified (after 1 : 100 dilution of the aliquot) by ultrahigh-performance liquid chromatography–MS in an Infinity 1290 UPLC system connected to an Agilent 6460-C Triple Quadrupole LC-MS with electrospray positive ionisation (ESI+) (Agilent Technologies). Chromatographic separation was performed on an Intrada AA column (50 × 3 mm, Imtakt) via the TI737E method detailed in the manufacturer’s instructions. Detection was performed by multiple reaction monitoring. External calibration was performed using standards for each amino acid separately according to their abundance, either in the range of 1.5–15.0 µmol L−1 for amino acids below 3% abundance or in the range of 15.0–150.0 µmol L−1 for those above 3% abundance. Standards were prepared by dissolving amino acids in acidified water (0.2 M HCl). The repeatability of the values was better than 5% and 10% for low and high abundance respectively. The amino acid concentrations were reported in mg N L–1. Ammonium was quantified with an enzymatic test kit (Boehringer Mannheim GmbH). The total FAN concentration was determined via the o-phthaldialdehyde (OPA) method using the Primary Amino Nitrogen kit (Bio Systems). Total YAN was computed by summing the NH4+ content (expressed in mg N L–1) and primary FAN (excluding the secondary amino acids proline and hydroxyproline) (Bell and Henschke 2005).
Data treatment and statistical analysis
The N quantity (NQ, in g) in each organ was calculated as:

The abundance of 15N (A%), which was the proportion of heavy isotopes per 100 atoms, was calculated as follows (Deléens et al. 1994):

The relative specific abundance (RSA, in %), which was the proportion of newly incorporated N atoms relative to total N atoms, was calculated as follows (Deléens et al. 1994):

In our case, A%nutrient = 10. The RSA represents the organ sink strength, which is independent of the organ size (Deléens et al. 1997):
The new N pool (NNP, in g) for each organ was calculated as follows:

Thus, the percent proportion (%P) of new N in an organ, also called partitioning, was calculated as:

The results are presented as the average ± s.d. The statistical analysis was performed with XLSTAT ver. 2018.1.50011 (Addinsoft). The significance of the differences between treatments was evaluated with ANOVA (P < 0.05) and the Newman–Keuls post hoc test. Principal component analysis was used to evaluate the FAN composition.
Results
Vegetative growth and fruit development
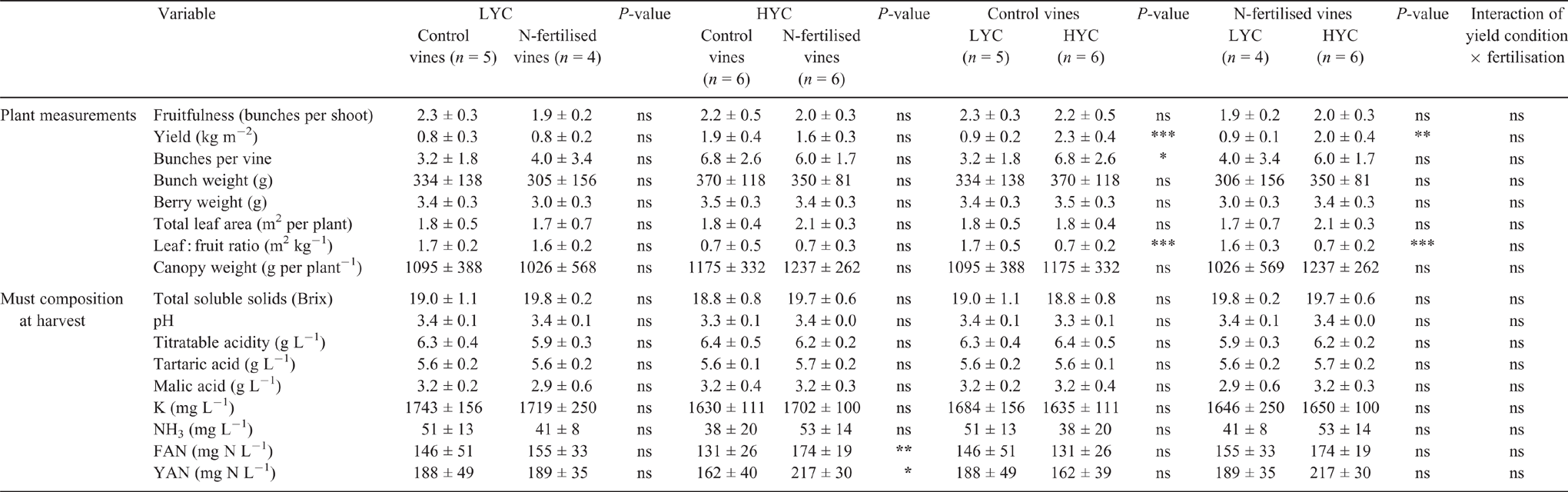
From bud burst to harvest, the canopy reached 1145 ± 360 g per plant on average. A large yield gradient was obtained, spanning from a minimum of 0.5 to a maximum 2.5 kg m–2 (Table 1). Consequently, the leaf : fruit ratio varied from a minimum of 0.5 to a maximum of 2.4 m2 kg−1, depending on the crop load. The vigour was assessed by the canopy weight and was heterogeneous. The bunch and berry weights were correlated with vigour (r = 0.71, P = 0.015 and r = 0.70, P = 0.017 respectively). However, vigour was correlated with neither crop load nor fertilisation. Indeed, crop load was manually controlled by bunch thinning and urea was applied late in the season when the canopy was already developed.
No significant change arising from N fertilisation or crop load treatments was observed in terms of photosynthetic activity and gas exchange per unit of leaf area (Table S1, available as Supplementary Material to this paper). The average chlorophyll index was homogenous and independent of fertilisation and crop load (489 ± 22 at veraison). The leaf nutrient content was constant (averages: 2.15% DW TN, 0.2% DW P, 2.9% DW Ca and 0.2% DW Mg) and not restrictive for vine development, according to the thresholds defined for Chasselas (Sinaj and Richner 2017). In contrast, the leaf K concentration was strongly related to the bunch number (r = −0.91, P = 0.013). Leaf K was not restrictive under LYC (1.7% DW K for 2.2 ± 0.4 bunches per vine) and was lower and restrictive under high-yielding conditions (HYC, 1.2% DW K for 8.1 ± 1.5 bunches vine−1). Leaf K was also positively correlated with bunch weight (r = 0.90, P = 0.015) and canopy weight (r = 0.89, P = 0.018) (data not shown).
Dry weight, total organic C, δ13C and C : N ratio
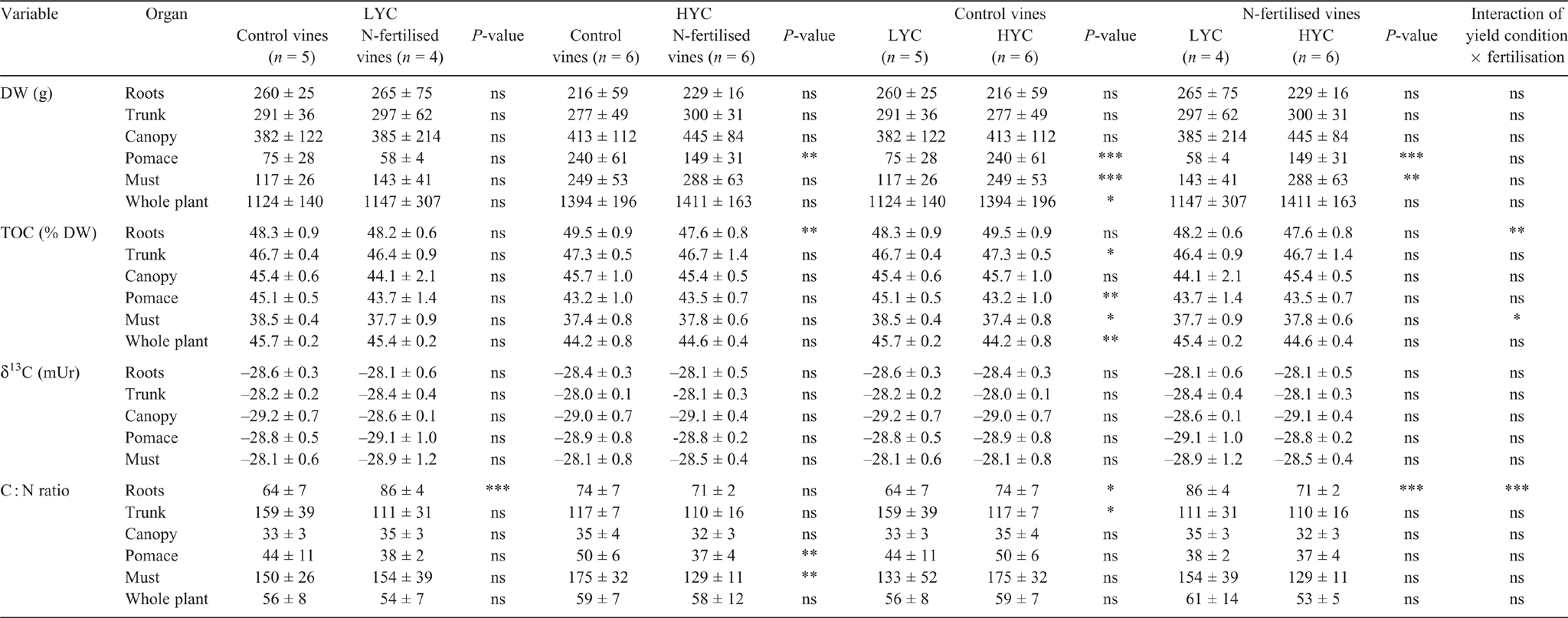
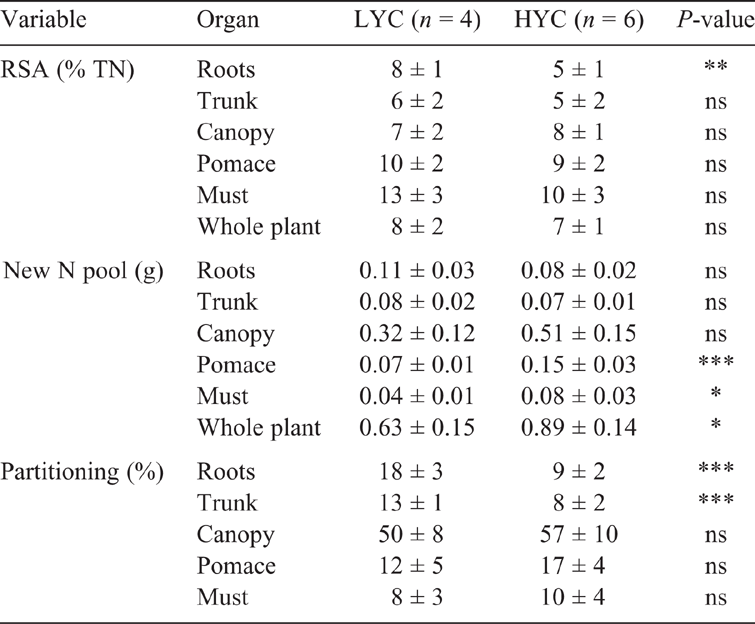
The DW, TOC, TN and C and N isotope compositions of each plant part are statistically compared and presented in Table S2. The results are similar to the ones presented in others studies (Zapata et al. 2004; Schreiner 2016). For the control vines, the whole biomass was significantly higher under HYC than under LYC (Table 2). The largest difference was observed in the pomace and must DWs. Under HYC, the root DW was 27% lower and canopy DW was 8% higher than those under LYC; these differences were not significant because of vine-to-vine variability. Similar trends were observed in the N-fertilised vines. The whole-plant TOC was significantly lower under HYC; it decreased in grapes (must and pomace) and increased in the trunk, although there was no variation in the roots and canopy. No difference was observed between LYC and HYC in the N-fertilised vines. N fertilisation only affected the pomace DW and the root TOC. The δ13C values varied insignificantly between a minimum of –29.2 mUr and a maximum of –28.0 mUr in all plant parts (organs and must) (Table 2). In the roots of the control vines, the C : N ratio was 16% higher in the vines under HYC than under LYC, whereas it was 27% lower under the urea treatment (Table 2). Under LYC, the must and the trunk were the plant parts with the highest C : N ratio at harvest. However, under HYC, the trunk had a lower C : N ratio (118 under HYC vs 159 under LYC) (Table S2). Differences in the C : N ratios in grapes for the different crop loads and fertilisation conditions were not significant because of the high vine-to-vine variability of TN and TOC.
Total N, NQ and δ15N
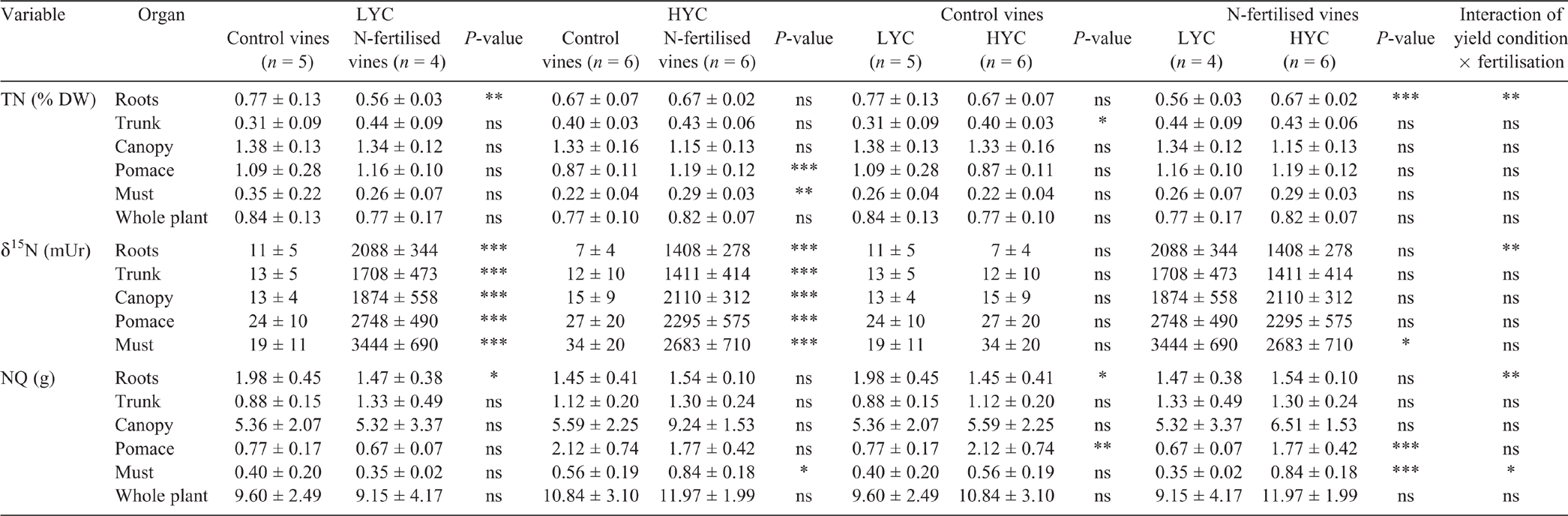
The canopy was the most concentrated plant part in terms of TN (1.4 and 1.3% DW under LYC and HYC respectively) and the must the least concentrated (0.3 and 0.2% DW under LYC and HYC respectively) (Table S2). In the control vines, only the TN in the trunk behaved differently between HYC and LYC compared with the other plant parts; the TN was 29% higher (P = 0.033) in the trunk of vines under HYC but there was no significant difference in the other plant parts (Table 3). In N-fertilised vines, only the roots had 20% more TN under HYC than under LYC (Table 3). For the vines under HYC, N fertilisation increased the TN by 34% (P = 0.003) and the NQ by 51% (P = 0.023) compared with the control vines (Table 3, Fig. 1). No significant differences were observed in the vines under LYC as a result of fertilisation. These trends mimic those observed for the YAN content. HYC increased the NQ in grapes (particularly in the pomace), independent of N fertilisation. The NQ was lower by 27% in the roots of control vines under HYC compared with those under LYC. In the control vines, the NQ increased in grapes under HYC (P = 0.006), whereas it decreased in the roots (P = 0.026). In contrast, the NQ was not depleted in the roots of the N-fertilised vines. (Table 3). In the control vines, under both yield conditions, the δ15N values increased gradually from the roots (7 ± 4 mUr) to grapes (34 ± 20 mUr). In the fertilised vines, the δ15N values were lower in the must under HYC, through the variation was insignificant in the other plant parts.
|
Foliar N assimilation, relative specific abundance and partitioning
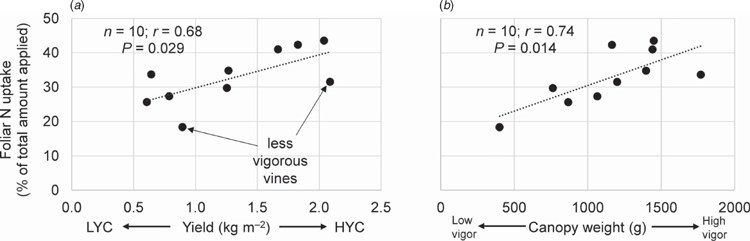
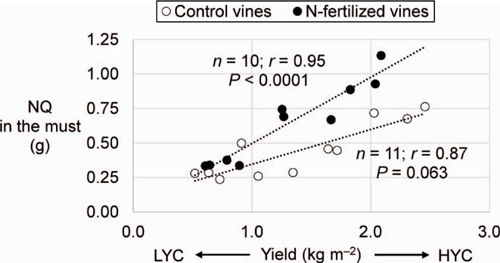
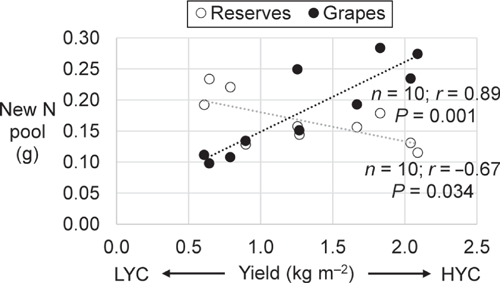
The fertiliser N uptake was 26% of the total amount applied in the vines under LYC and 37% in the vines under HYC (Fig. 2a). Indeed, the fertiliser N uptake was also a function of vine vigour (Fig. 2b). With nearly 20% of N originating from the urea application, the grapes (pomace + must) had the largest relative specific abundance (RSA) (i.e. the proportion of newly incorporated N atoms relative to total N atoms, in %) among all plant parts, regardless of the crop load (Table 4). The root RSA was 37% lower in the vines under HYC than under LYC (P = 0.009); there were no significant changes in the other plant parts. Under HYC, the new N pool was 41% higher for the whole plant (P = 0.023) and increased by 109% in grapes (pomace + must, P = 0.002), whereas it decreased by 27% in the roots (P = 0.063) and 11% in the trunk (P = 0.232) (Table 4, Fig. 3). Compared with LYC, the partitioning of new N under HYC was 50% lower in the roots and 39% lower in the trunk of the vines (both P = 0.001).
|
Fruit composition
The total soluble solids (average 19.3 ± 0.8 Brix), titratable acidity (6.2 ± 0.4 g L−1), tartaric acid (5.6 ± 0.2 g L−1), malic acid (3.1 ± 0.4 g L−1), potassium (1694 ± 148 mg L−1), ammonium (46 ± 6 mg L−1) and amino acid (151 ± 35 mg N L−1) levels and the pH (3.4 ± 0.1) remained uniform in the must despite different crop loads (Table 1). N fertilisation increased the must YAN concentration (+55 mg L−1), particularly the FAN concentration (+43 mg N L−1) in the HYC vines, although it had no effect on the vines under LYC (Table 1). The YAN concentration was correlated with plant vigour; the correlation was higher for N-fertilised vines (r = 0.82 vs r = 0.55 for the control vines; Fig. S1).
Amino acids in fruits
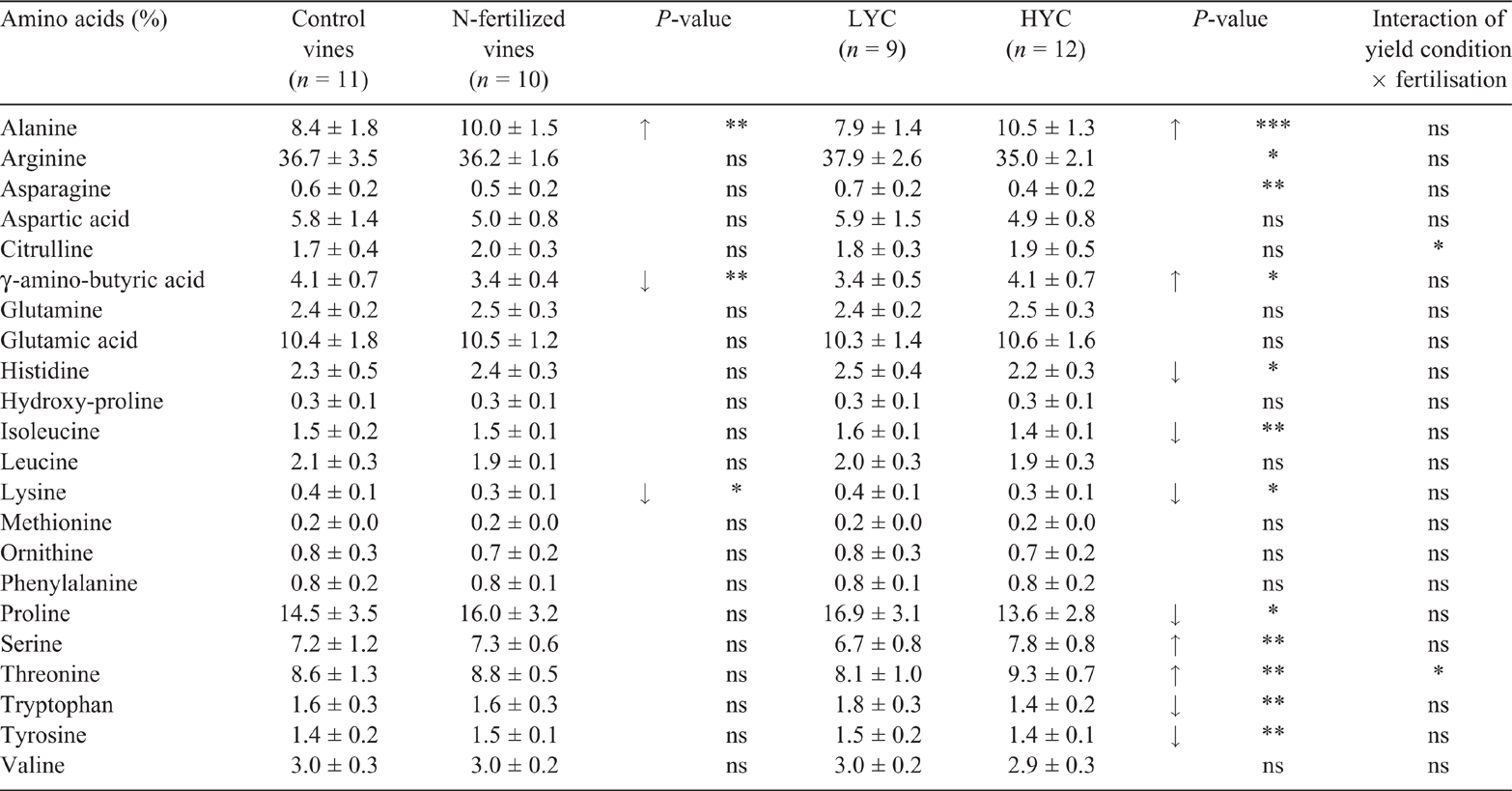
N fertilisation increased the total FAN concentration in the must (by 33%, P = 0.014) under HYC only (Table 1). The fertilised : control ratios of amino acid concentrations were calculated for each amino acid, including the nonassimilable proline and hydroxyproline (Fig. S2). The ratios under HYC were globally higher than 1.0, in contrast to the ratios under LYC (average 1.3 ± 0.2 under HYC and 1.0 ± 0.1 under LYC, P = 0.062). The differences between ratios under HYC and LYC were significant for arginine, aspartic acid, citrulline, histidine, tryptophan and tyrosine.
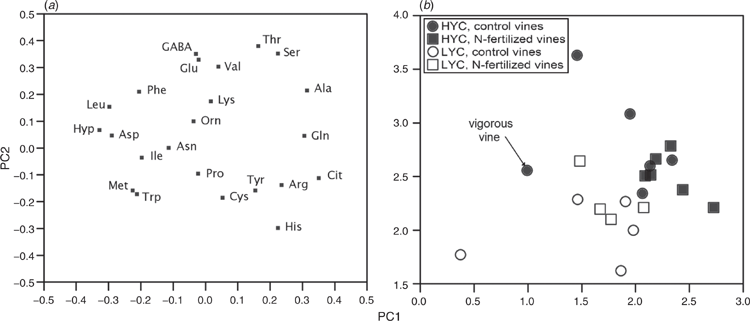
N fertilisation had a small effect on the FAN profile, with an increase in the relative abundances of alanine and a decrease in the γ-amino-butyric acid and lysine contents (Table 5). The fruit load affected the must FAN profile without any impact on the total FAN concentration (Tables 1 and 5). The alanine, γ-amino-butyric acid, serine and threonine proportions were higher under HYC than under LYC, whereas the histidine, isoleucine, lysine, proline, tryptophan and tyrosine proportions were lower. Principal component analysis was used to assess the impact of fruit load and fertilisation on the FAN profiles better (Fig. 4). The principal component analysis of the relative amino acid abundance allowed a clear discrimination of the vines under LYC from the vines under HYC, independent of the fertilisation treatment (Fig. 4b).
Discussion
Relationship between vigour and plant N nutrition
Differences were observed between vines in terms of canopy weight, leaf area and bunch weight. This natural heterogeneity was independent of both experimental treatments for crop load (P = 0.402) and urea supply (P = 0.970) and did not affect the interpretations of the trial. Canopy weight was positively correlated with the fertiliser N uptake, N concentration and N quantity in the whole plant: more vigorous plants had higher YAN in grape must (P = 0.077 in the control treatment and P = 0.004 in the urea treatment, Fig. S1). This positive impact of N nutrition on plant growth and overall development has already been demonstrated by other researchers (Holzapfel and Treeby 2007; Gatti et al. 2018).
No impact of crop load on fruit N concentration or maturation
In 2017, the optimal climatic conditions (i.e. no water restriction, suitable temperature and sufficient luminosity) were conducive to proper fruit maturation in all treatments, as explained by Mawdsley et al. (2018). The δ13C values indicate that the vines had sufficient water supply (Van Leeuwen et al. 2009). Despite the important variation of crop load between LYC and HYC (+155% in the control treatment; +117% in the urea treatment), the must TN content remained constant in both the control and urea treatments (Fig. S3). Despite the large crop load variation, all the vines reached full grape maturity in the same period and there was no differences in terms of total soluble solids, acidity, pH and YAN concentrations between the treatments, as shown in other studies (Keller et al. 2005; Wang et al. 2018), although the average leaf : fruit ratio in the HYC treatment was as low as 0.7 m2 kg–1. Unlike canopy oversizing, which induces a drop in terms of YAN concentration in the must (Spring et al. 2012), increasing the crop load did not affect YAN concentration. This result confirms the findings of Verdenal et al. (2016). Additionally, the must K concentration remained unchanged despite leaf K deficiency under HYC. Grapevines appeared to adapt their metabolism through the modulation of combined morphological and physiological mechanisms, as explained hereafter.
Limitation of root growth and smaller N reserves under HYC
The root DW was 17% and 14% lower under HYC than under LYC in the control and urea treatments respectively. This confirms the results from other research (Howell 2001; Morinaga et al. 2003). Morinaga et al. (2003) observed that under HYC, the growth of fine roots and lateral shoots is reduced, though the fine root respiration rate is higher. More C and N were mobilised from the trunk and root reserves under HYC to supply the maturing fruits (Howell 2001). C and N accumulation in the grapes appeared as a priority objective over root development and reserve refilling. Therefore, the TOC and TN contents increased in the fruits almost proportionally to the crop load, whereas root growth was consequently limited, along with the C and N storage capacity (Fig. S4).
In addition to limited root growth, the root N reserves were more solicited under HYC than under LYC: the NQ was 27% lower in the control treatment. Several studies mentioned that root N reserve accumulation is restricted by the presence of fruit before and after veraison (Rodriguez-Lovelle and Gaudillere 2002; Rossouw et al. 2017). This result suggests that several years of overproduction could potentially induce an important reduction in N reserves, which may affect vigour, bud fruitfulness and even the plant’s lifespan.
Similar photosynthetic activity and higher leaf N assimilation under HYC
The photosynthetic activity was influenced by neither crop load nor urea application. This result confirms the findings from Dayer et al. (2016), which showed no impact of crop load on CO2 assimilation. The fertiliser N uptake was, on average, 42% higher under HYC than under LYC. RSA (a measure of N sink strength, independent of organ size) was the highest in grapes. When the crop load was greatly increased, the fruit N demand increased, consequently inducing modifications in N partitioning. These results confirm the findings from Verdenal et al. (2016), which suggested that increasing foliar N assimilation is a plant reaction to crop load variations to maintain fruit N concentrations. Foliar N assimilation was a function of both plant vigour and crop load.
Additionally, a higher crop load might also have stimulated soil N uptake in contrast to root growth. Stander et al. (2017) mentioned a similar correlation between both crop load and root sink activities in mandarin (Citrus reticulata Blanco) trees. This observation may explain why after N-labelling, the TN content was significantly higher in roots under HYC (+20%), whereas the RSA of new N was lower. This result suggests the possible presence of a nonlabelled N source, which can only be root N uptake from the soil.
Effect of crop load on the FAN profile
Despite a uniform overall concentration, the must FAN profile varied significantly in relation to the crop load, although the impact of urea supply was negligible. The primary : secondary amino acid ratio reflects the nutritional value of the must to yeasts, with the secondary amino acids being the nonassimilable proline and hydroxyproline (Bell and Henschke 2005). The index, which includes all amino acids, was significantly higher under HYC than under LYC (7.5 ± 1.7 and 6.0 ± 1.1 respectively, P = 0.029), indicating a higher nutritional value.
Several experiments have already demonstrated the impacts of bunch thinning: on N distribution in the grapevine (Zufferey et al. 2015; Rossouw et al. 2017), on global grapevine development and grape maturation (Keller et al. 2005), on respiration and growth rates (Morinaga et al. 2003), on the must composition on the must aroma profiles (Wang et al. 2018) and on the volatile and phenol composition of musts (Kok 2011; Rutan et al. 2018). The variable impact of crop thinning on volatile compounds and aroma development is mainly dependent on genotype and timing (Do et al. 2010; Alem et al. 2019) and could be either positive or negative. In fact, any parameters and/or practices affecting vine balance (climate conditions, plant vigour, canopy management, crop load, etc.) might affect aroma development. Consequently, an integrative point of view would be required to control and anticipate the development of the grapes’ flavour-active compounds. Further research is still required to understand the mechanisms that balance the formation of secondary metabolite in grape in relation to FAN profiles.
Preservation of root N reserves through foliar urea supply
The uptake and the subsequent impact of foliar N fertilisation highly depended on the crop load. Fertilisation had no influence on the fruit YAN under LYC. However, the fertiliser N uptake was higher under HYC (Fig. 2); consequently, the fruit YAN was 34% higher (P = 0.021, Table 1). Under these conditions, the partitioning of new N was largely influenced by crop load: significantly smaller fractions of new N were allocated to the roots and trunk (−50% and −38%, respectively), whereas larger fractions tended to be allocated to the canopy and fruits (+14% and +35%, respectively). The positive impact of urea fertilisation on the must YAN content confirms many results from other studies (Dufourcq et al. 2009; Nisbet et al. 2014; Verdenal et al. 2015; Hannam et al. 2016). The newcontribution of this experiment is the positive correlation between NUE and the crop load.
In contrast to the control treatment, the urea supply maintained a root NQ that was unchanged despite variation in the crop load. Thus the urea supply allowed the N demand of fruits to be satisfied while preserving the root N reserves, potentially increasing plant sustainability under HYC. Reserve N refilling is essential for the following season growth (Holzapfel and Treeby 2007). The relationship between fruits and roots must be clarified to improve perennial fruit crop production, as root development and reserve capacity influence the following year’s production.
To conclude, this experiment demonstrates the high potential of crop limitation to control plant NUE and ultimately fruit N composition at harvest. The results indicate that root development and activity are both key factors for understanding the mechanisms that balance plant N nutrition. Grapevines were in a constant search for fruit nutrition balance. They actively modulated root N reserve mobilisation and fertiliser N uptake to maintain a uniform N concentration in the must, despite crop load variations. Fertiliser N uptake and assimilation were strongly stimulated under HYC in answer to the higher fruit N demand and, consequently, preserved N reserves from excessive mobilisation and downsizing. Compared with HYC, LYC did not improve the YAN concentration in the must but only affected the FAN profile, suggesting a modification of the potential aroma profile. It is therefore questionable whether the crop load limitation always has a positive impact on the grapes’ composition and ultimately on the wine quality. This study encourages further research on the potential of agricultural practices to monitor NUE, with the aim of enhancing crop quality and sustainability.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the vineyard and laboratory teams of Agroscope and Sol-Conseil for their support in this project. Special thanks are given to Philippe Duruz and Laure Passot for their valuable work in the field and to Jonas Siegrist and Sylvain Schnee for their help in the preparation of the samples. This research did not receive any specific funding.
References
Alem H, Rigou P, Schneider R, Ojeda H, Torregrosa L (2019) Impact of agronomic practices on grape aroma composition: a review. Journal of the Science of Food and Agriculture 99, 975–985.| Impact of agronomic practices on grape aroma composition: a review.Crossref | GoogleScholarGoogle Scholar | 30142253PubMed |
Bates TR, Dunst RM, Joy P (2002) Seasonal dry matter, starch, and nutrient distribution in ‘Concord’ grapevine roots. HortScience 37, 313––316.
| Seasonal dry matter, starch, and nutrient distribution in ‘Concord’ grapevine roots.Crossref | GoogleScholarGoogle Scholar |
Bell S-J, Henschke PA (2005) Implications of nitrogen nutrition for grapes, fermentation and wine. Australian Journal of Grape and Wine Research 11, 242–295.
| Implications of nitrogen nutrition for grapes, fermentation and wine.Crossref | GoogleScholarGoogle Scholar |
Boss PK, Bottcher C, Davies C (2014) Various influences of harvest date and fruit sugar content on different wine flavor and aroma compounds. American Journal of Enology and Viticulture 65, 341–353.
| Various influences of harvest date and fruit sugar content on different wine flavor and aroma compounds.Crossref | GoogleScholarGoogle Scholar |
Brand WA (2011) New reporting guidelines for stable isotopes – an announcement to isotope users. Isotopes in Environmental and Health Studies 47, 535–536.
| New reporting guidelines for stable isotopes – an announcement to isotope users.Crossref | GoogleScholarGoogle Scholar | 22166155PubMed |
Carbonneau A (1995) La surface foliaire exposée potentielle. Guide pour sa mesure. Le Progrès Agricole et Viticole 112, 204–212.
Chaumont M, Morot-Gaudry J-F, Foyer CH (1994) Seasonal and diurnal changes in photosynthesis and carbon partitioning in Vitis vinifera leaves in vines with and without fruit. Journal of Experimental Botany 45, 1235–1243.
| Seasonal and diurnal changes in photosynthesis and carbon partitioning in Vitis vinifera leaves in vines with and without fruit.Crossref | GoogleScholarGoogle Scholar |
Chaves MM (1984) Photosynthesis and assimilate partition in fruiting and non-fruiting grapevine shoots. In. ‘Advances in Photosynthesis Research: Proceedings of the VIth International Congress on Photosynthesis’, 1–6 August 1983, Brussels, Belgium. (Ed C Sybesma) Volume IV. pp. 145–148. (Springer Netherlands: Dordrecht)
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Communications in Mass Spectrometry 25, 2538–2560.
| Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results.Crossref | GoogleScholarGoogle Scholar | 21910288PubMed |
Dai ZW, Vivin P, Barrieu F, Ollat N, Delrot S (2010) Physiological and modelling approaches to understand water and carbon fluxes during grape berry growth and quality development: a review. Australian Journal of Grape and Wine Research 16, 70–85.
| Physiological and modelling approaches to understand water and carbon fluxes during grape berry growth and quality development: a review.Crossref | GoogleScholarGoogle Scholar |
Dayer S, Prieto JA, Galat E, Peña JP (2016) Leaf carbohydrate metabolism in Malbec grapevines: combined effects of regulated deficit irrigation and crop load. Australian Journal of Grape and Wine Research 22, 115–123.
| Leaf carbohydrate metabolism in Malbec grapevines: combined effects of regulated deficit irrigation and crop load.Crossref | GoogleScholarGoogle Scholar |
Deléens E, Cliquet J-B, Prioul J-L (1994) Use of 13C and 15N plant label near natural abundance for monitoring carbon and nitrogen partitioning. Australian Journal of Plant Physiology 21, 133–146.
Deléens E, Morot-Gaudry J-F, Martin F, Thoereux A, Gojon A (1997) Méthodologie 15N. In ‘Assimilation de l’azote chez les plantes’. pp. 265–280. (Institut National de la Recherche Agronomique (INRA): Paris)
Do PT, Prudent M, Sulpice R, Causse M, Fernie AR (2010) The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population. Plant Physiology 154, 1128–1142.
| The influence of fruit load on the tomato pericarp metabolome in a Solanum chmielewskii introgression line population.Crossref | GoogleScholarGoogle Scholar | 20841452PubMed |
Dufourcq T, Charrier F, Poupault P, Schneider R, Gontier L, Serrano E (2009) Foliar spraying of nitrogen and sulfur at veraison: a viticultural technique to improve aromatic composition of white and rosés wines. In ‘Proceedings of the 16th International GiESCO Symposium’, Davis, USA). pp. 379–383. (UC Davis: Davis)
Gatti M, Squeri C, Garavani A, Vercesi A, Dosso P, Diti I, Poni S (2018) Effects of variable rate nitrogen application on cv. Barbera performance: vegetative growth and leaf nutritional status. American Journal of Enology and Viticulture 69, 196–209.
| Effects of variable rate nitrogen application on cv. Barbera performance: vegetative growth and leaf nutritional status.Crossref | GoogleScholarGoogle Scholar |
González-Barreiro C, Rial-Otero R, Cancho-Grande B, Simal-Gándara J (2015) Wine aroma compounds in grapes: a critical review. Critical Reviews in Food Science and Nutrition 55, 202–218.
| Wine aroma compounds in grapes: a critical review.Crossref | GoogleScholarGoogle Scholar |
Gutiérrez-Gamboa G, Romanazzi G, Garde-Cerdán T, Pérez-Álvarez EP (2019) A review of the use of biostimulants in the vineyard for improved grape and wine quality: effects on prevention of grapevine diseases. Journal of the Science of Food and Agriculture 99, 1001–1009.
| A review of the use of biostimulants in the vineyard for improved grape and wine quality: effects on prevention of grapevine diseases.Crossref | GoogleScholarGoogle Scholar | 30198154PubMed |
Hachiya T, Sakakibara H (2017) Interactions between nitrate and ammonium in their uptake, allocation, assimilation and signaling in plants. Journal of Experimental Botany 68, 2501–2512.
Hannam KD, Neilsen GH, Neilsen D, Midwood AJ, Millard P, Zhang Z, Thornton B, Steinke D (2016) Amino acid composition of grape (Vitis vinifera L.) juice in response to applications of urea to the soil or foliage. American Journal of Enology and Viticulture 67, 47–55.
| Amino acid composition of grape (Vitis vinifera L.) juice in response to applications of urea to the soil or foliage.Crossref | GoogleScholarGoogle Scholar |
Holzapfel BP, Treeby MT (2007) Effects of timing and rate of N supply on leaf nitrogen status, grape yield and juice composition from Shiraz grapevines grafted to one of three different rootstocks. Australian Journal of Grape and Wine Research 13, 14–22.
| Effects of timing and rate of N supply on leaf nitrogen status, grape yield and juice composition from Shiraz grapevines grafted to one of three different rootstocks.Crossref | GoogleScholarGoogle Scholar |
Howell GS (2001) Sustainable grape productivity and the growth–yield relationship: a review. American Journal of Enology and Viticulture 52, 165–174.
Jackson DI, Lombard PB (1993) Environmental and management practices affecting grape composition and wine quality – a review. American Journal of Enology and Viticulture 44, 409–430.
Kant S, Bi Y-M, Rothstein SJ (2011) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Journal of Experimental Botany 62, 1499–1509.
| Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency.Crossref | GoogleScholarGoogle Scholar | 20926552PubMed |
Keller M, Mills LJ, Wample RL, Spayd SE (2005) Cluster thinning effects on three deficit-irrigated Vitis vinifera cultivars. American Journal of Enology and Viticulture 56, 91–103.
Kliewer WM, Dokoozlian N (2005) Leaf area/crop weight ratios of grapevines: influence on fruit composition and wine quality. American Journal of Enology and Viticulture 56, 170–181.
Kok D (2011) Influences of pre-and post-veraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. Journal of Food Agriculture and Environment 9, 22–26.
Lancashire PD, Bleiholder H, van den Boom T, Langelüddeke P, Stauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Annals of Applied Biology 119, 561–601.
| A uniform decimal code for growth stages of crops and weeds.Crossref | GoogleScholarGoogle Scholar |
Mabrouk H, Carbonneau A (1996) Une méthode simple de détermination de la surface foliaire de la vigne (Vitis vinifera L.). Le Progrès Agricole et Viticole 113, 392–398.
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany 105, 1141–1157.
| Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture.Crossref | GoogleScholarGoogle Scholar | 20299346PubMed |
Mawdsley PFW, Dodson Peterson JC, Casassa LF (2018) Agronomical and chemical effects of the timing of cluster thinning on Pinot Noir (clone 115) grapes and wines. Fermentation 4, 60
| Agronomical and chemical effects of the timing of cluster thinning on Pinot Noir (clone 115) grapes and wines.Crossref | GoogleScholarGoogle Scholar |
Morinaga K, Imai S, Yakushiji H, Koshita Y (2003) Effects of fruit load on partitioning of 15N and 13C, respiration, and growth of grapevine roots at different fruit stages. Scientia Horticulturae 97, 239–253.
| Effects of fruit load on partitioning of 15N and 13C, respiration, and growth of grapevine roots at different fruit stages.Crossref | GoogleScholarGoogle Scholar |
Nisbet MA, Martinson TE, Mansfield AK (2014) Accumulation and Prediction of Yeast Assimilable Nitrogen in New York Winegrape Cultivars. American Journal of Enology and Viticulture 65, 325–332.
| Accumulation and Prediction of Yeast Assimilable Nitrogen in New York Winegrape Cultivars.Crossref | GoogleScholarGoogle Scholar |
Ojeda-Real LA, Lobit P, Cárdenas-Navarro R, Grageda-Cabrera O, Farías-Rodríguez R, Valencia-Cantero E, Macías-Rodríguez L (2009) Effect of nitrogen fertilization on quality markers of strawberry (Fragaria × ananassa Duch. cv. Aromas). Journal of the Science of Food and Agriculture 89, 935–939.
| Effect of nitrogen fertilization on quality markers of strawberry (Fragaria × ananassa Duch. cv. Aromas).Crossref | GoogleScholarGoogle Scholar |
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen–Geiger climate classification. Hydrology and Earth System Sciences Discussions 4, 439–473.
| Updated world map of the Köppen–Geiger climate classification.Crossref | GoogleScholarGoogle Scholar |
Petrie PR, Clingeleffer PR (2006) Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate. Australian Journal of Grape and Wine Research 12, 21–29.
| Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate.Crossref | GoogleScholarGoogle Scholar |
Porro D, Stefanini M, Dorigatti C (2010) Nitrogen foliar uptake and partitioning in Cabernet Sauvignon grapevines. Acta Horticulturae 868, 185–190.
Reeve AL, Skinkis PA, Vance AJ, Lee J, Tarara JM (2016) Vineyard floor management influences ‘Pinot noir’ vine growth and productivity more than cluster thinning. HortScience 51, 1233–1244.
| Vineyard floor management influences ‘Pinot noir’ vine growth and productivity more than cluster thinning.Crossref | GoogleScholarGoogle Scholar |
Rodriguez-Lovelle B, Gaudillere JP (2002) Carbon and nitrogen partitioning in either fruiting or non-fruiting grapevines: effects of nitrogen limitation before and after veraison. Australian Journal of Grape and Wine Research 8, 86–94.
| Carbon and nitrogen partitioning in either fruiting or non-fruiting grapevines: effects of nitrogen limitation before and after veraison.Crossref | GoogleScholarGoogle Scholar |
Rossouw GC, Smith JP, Barril C, Deloire A, Holzapfel BP (2017) Implications of the presence of maturing fruit on carbohydrate and nitrogen distribution in grapevines under postveraison water constraints. Journal of the American Society for Horticultural Science 142, 71–84.
| Implications of the presence of maturing fruit on carbohydrate and nitrogen distribution in grapevines under postveraison water constraints.Crossref | GoogleScholarGoogle Scholar |
Rutan TE, Herbst-Johnstone M, Kilmartin PA (2018) Effect of cluster thinning Vitis vinifera cv. Pinot Noir on wine volatile and phenolic composition. Journal of Agricultural and Food Chemistry 66, 10053–10066.
| Effect of cluster thinning Vitis vinifera cv. Pinot Noir on wine volatile and phenolic composition.Crossref | GoogleScholarGoogle Scholar | 30175910PubMed |
Schimmelmann A, Qi H, Coplen TB, Brand WA, Fong J, Meier-Augenstein W, Kemp HF, Toman B, Ackermann A, Assonov S, Aerts-Bijma AT, Brejcha R, Chikaraishi Y, Darwish T, Elsner M, Gehre M, Geilmann H, Gröning M, Hélie J-F, Herrero-Martín S, Meijer HAJ, Sauer PE, Sessions AL, Werner RA (2016) Organic reference materials for hydrogen, carbon, and nitrogen stable isotope-ratio measurements: caffeines, n-alkanes, fatty acid methyl esters, glycines, l-valines, polyethylenes, and oils. Analytical Chemistry 88, 4294–4302.
| Organic reference materials for hydrogen, carbon, and nitrogen stable isotope-ratio measurements: caffeines, n-alkanes, fatty acid methyl esters, glycines, l-valines, polyethylenes, and oils.Crossref | GoogleScholarGoogle Scholar | 26974360PubMed |
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965) Sap pressure in vascular plants. Science 148, 339–346.
| Sap pressure in vascular plants.Crossref | GoogleScholarGoogle Scholar | 17832103PubMed |
Schreiner RP (2016) Nutrient uptake and distribution in young Pinot Noir grapevines over two seasons. American Journal of Enology and Viticulture 67, 436–448.
| Nutrient uptake and distribution in young Pinot Noir grapevines over two seasons.Crossref | GoogleScholarGoogle Scholar |
Schreiner RP, Scagel CF, Lee J (2014) N, P, and K supply to Pinot Noir grapevines: impact on berry phenolics and free amino acids. American Journal of Enology and Viticulture 65, 43–49.
| N, P, and K supply to Pinot Noir grapevines: impact on berry phenolics and free amino acids.Crossref | GoogleScholarGoogle Scholar |
Sinaj S, Richner W (2017) ‘Principes de fertilisation des cultures agricoles en Suisse (PRIF 2017).’ Recherche Agronique Suisse 8 (6). Publication spéciale.
Spangenberg JE, Zufferey V (2019) Carbon isotope compositions of whole wine, wine solid residue, and wine ethanol, determined by EA/IRMS and GC/C/IRMS, can record the vine water status—a comparative reappraisal. Analytical and Bioanalytical Chemistry 411, 2031–2043.
| Carbon isotope compositions of whole wine, wine solid residue, and wine ethanol, determined by EA/IRMS and GC/C/IRMS, can record the vine water status—a comparative reappraisal.Crossref | GoogleScholarGoogle Scholar | 30714082PubMed |
Spring J-L, Zufferey V (2000) Intérêt de la détermination de l’indice chlorophyllien du feuillage en viticulture. Revue Suisse de Viticulture, d’Arboriculture et d’Horticulture 32, 323–328.
Spring J-L, Verdenal T, Zufferey V, Viret O (2012) Nitrogen dilution in excessive canopies of Chasselas and Pinot Noir cvs. Journal International des Sciences de la Vigne et du Vin 46, 233–240.
| Nitrogen dilution in excessive canopies of Chasselas and Pinot Noir cvs.Crossref | GoogleScholarGoogle Scholar |
Stander OPJ, Barry GH, Cronjé PJR (2017) Fruit-load-induced starch accumulation causes leaf chlorosis in “off” ‘Nadorcott’ mandarin trees. Scientia Horticulturae 222, 62–68.
| Fruit-load-induced starch accumulation causes leaf chlorosis in “off” ‘Nadorcott’ mandarin trees.Crossref | GoogleScholarGoogle Scholar |
Sweetman C, Sadras VO, Hancock RD, Soole KL, Ford CM (2014) Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. Journal of Experimental Botany 65, 5975–5988.
| Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit.Crossref | GoogleScholarGoogle Scholar | 25180109PubMed |
Van Leeuwen C, Tregoat O, Choné X, Bois B, Pernet D, Gaudillere JP (2009) Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? Journal International des Sciences de la Vigne et du Vin 43, 121–134.
| Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes?Crossref | GoogleScholarGoogle Scholar |
Verdenal T, Spangenberg JE, Zufferey V, Lorenzini F, Spring JL, Viret O (2015) Effect of fertilisation timing on the partitioning of foliar-applied nitrogen in Vitis vinifera cv. Chasselas: a 15N labelling approach. Australian Journal of Grape and Wine Research 21, 110–117.
| Effect of fertilisation timing on the partitioning of foliar-applied nitrogen in Vitis vinifera cv. Chasselas: a 15N labelling approach.Crossref | GoogleScholarGoogle Scholar |
Verdenal T, Spangenberg JE, Zufferey V, Lorenzini F, Dienes-Nagy A, Gindro K, Spring JL, Viret O (2016) Leaf-to-fruit ratio affects the impact of foliar-applied nitrogen on N accumulation in the grape must. Journal International des Sciences de la Vigne et du Vin 50, 23–33.
| Leaf-to-fruit ratio affects the impact of foliar-applied nitrogen on N accumulation in the grape must.Crossref | GoogleScholarGoogle Scholar |
Wang Y-T, Huang S-W, Liu R-L, Jin J-Y (2007) Effects of nitrogen application on flavor compounds of cherry tomato fruits. Journal of Plant Nutrition and Soil Science 170, 461–468.
| Effects of nitrogen application on flavor compounds of cherry tomato fruits.Crossref | GoogleScholarGoogle Scholar |
Wang Y, He Y-N, Chen W-K, He F, Chen W, Cai X-D, Duan C-Q, Wang J (2018) Effects of cluster thinning on vine photosynthesis, berry ripeness and flavonoid composition of Cabernet Sauvignon. Food Chemistry 248, 101–110.
| Effects of cluster thinning on vine photosynthesis, berry ripeness and flavonoid composition of Cabernet Sauvignon.Crossref | GoogleScholarGoogle Scholar | 29329832PubMed |
Zapata C, Deleens E, Chaillou S, Magne C (2004) Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). Plant Physiology 161, 1031–1040.
| Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.).Crossref | GoogleScholarGoogle Scholar |
Zufferey V, Murisier F, Belcher S, Lorenzini F, Vivin P, Spring JL, Viret O (2015) Nitrogen and carbohydrate reserves in the grapevine (Vitis vinifera L. ‘Chasselas’): the influence of the leaf to fruit ratio. Vitis 54, 183–188.