Energy-crises in well-aerated and anoxic tissue: does tolerance require the same specific proteins and energy-efficient transport?
Hank Greenway A C and William Armstrong A BA School of Plant Biology, Faculty of Science, the University of Western Australia, Crawley, WA 6009, Australia.
B Biological Science, School of Environmental Sciences, Faculty of Science and Engineering, University of Hull, Kingston upon Hull, HU6 7RX, UK.
C Corresponding author. Email: hank.greenway@uwa.edu.au
Functional Plant Biology 45(9) 877-894 https://doi.org/10.1071/FP17250
Submitted: 31 August 2017 Accepted: 20 February 2018 Published: 12 April 2018
Abstract
Many of the profound changes in metabolism that are caused by O2 deficiency also occur in well-aerated tissues when oxidative phosphorylation is partially or wholly inhibited. For these well-aerated tissues, reduction in energy formation occurs during exposure to inhibitors of oxidative phosphorylation, cold/chilling and wounding, so we prefer the term ‘energy crisis’ metabolism over ‘anaerobic’ metabolism. In this review, we note that the overwhelming body of data on energy crises has been obtained by exposure to hypoxia-anoxia, which we will indicate when discussing the particular experiments. We suggest that even transient survival of an energy crisis requires a network of changes common to a large number of conditions, ranging from changes in development to various adverse conditions such as high salinity, drought and nutrient deficiency, all of which reduce growth. During an energy crisis this general network needs to be complemented by energy specific proteins, including the so called ‘anaerobic proteins’ and the group of ERFVII transcription factors, which induces the synthesis of these proteins. Crucially, the difference between anoxia-intolerant and -tolerant tissues in the event of a severe energy crisis would mainly depend on changes in some ‘key’ energy crisis proteins: we suggest these proteins would include phytoglobin, the V-H+PPiase and pyruvate decarboxylase. A second characteristic of a high tolerance to an energy crisis is engagement of energy efficient transport. This feature includes a sharp reduction in rates of solute transport and use of energy-efficient modifications of transport systems by primary H+ transport and secondary H+-solute transport systems. Here we also discuss the best choice of species to study an energy crisis. Further, we consider confounding of the acclimative response by responses to injury, be it due to the use of tissues intolerant to an energy crisis, or to faulty techniques.
Additional keywords: ATP, hypoxia, inhibitors of oxidative phosphorylation, pHcyt, ‘anaerobic’ enzymes, key proteins.
This review is arranged in two main parts, the first dealing with physiological aspects of ‘anaerobic metabolism’ induced in well-aerated tissues, the second with genetical aspects covering severe hypoxia-anoxia and the possible reasons for the multitude of changes in transcripts and enzymes in molecular studies. Supplementary material provided includes a table of useful definitions.
Physiology: induction of ‘anaerobic metabolism’ in well-aerated tissues
The main themes of this part of the review are: Energy crisis metabolism in well-aerated tissues, pHcyt during an ‘energy crisis’, Reductions in energy charge and ATP concentrations, and Reductions in trans-membrane transport which embraces the use of energy efficient transporters and the calamitous effect of sugar starvation during an energy crisis.
Preamble
This section presents evidence that so called ‘anaerobic metabolism’ can also be induced in well-aerated tissues by inhibitors of oxidative phosphorylation, cold temperatures, Pi deficiency and wounding. All of these treatments do, or are likely to, reduce energy production. The evidence for development of energy deficient metabolism in well-aerated tissues is still not by any means as comprehensive as for O2 deficiency. Once confirmed, the appropriate term for this syndrome would be ‘energy-deficient metabolism’ or ‘energy-crisis metabolism’ rather than ‘anaerobic metabolism’. Note also that O2 at 21 kPa in the boundary layer around a tissue/organ does not guarantee adequate O2 for all cells within. There may be high resistances in any internal supply pathway leading to steep concentration gradients in O2 and limited O2 availability both along and internal to these resistances. A gradient does not increase availability but is a reflection of both resistance and usage and with O2 availability reducing progressively down the gradient (Armstrong et al. 2009; Armstrong and Armstrong 2014).
The shift to an energy deficient metabolism probably originates with a decrease in ATP concentrations, with increases in Ca2+ levels in the cytoplasm and/or decreases in pHcyt, changing among others activities of many enzymes. Whether these three key factors always interact and/or act in concert is not certain. Major components of energy crisis metabolism are less negative trans-membrane potentials, with decreases in rates of solute transport, reduced permeability of the plasma membrane and tonoplast, and engagement of energy-efficient transport systems across membranes. We confine ourselves mainly to energy crises caused by inhibition of oxidative phosphorylation. We do comment briefly on carbohydrate starvation during an energy crisis and suggest some characters would be relevant to the two types of energy crises, for example engagement of energy saving devices would be even more important during carbohydrate starvation during an energy crisis than when the carbohydrate supply was ample.
This review is mainly confined to metabolism in the cytoplasm and to solute transport during an energy crisis. For other important aspects such as response to anoxia by mitochondria, return to air and H2O2 formation see a recent review by Shingaki-Wells et al. (2014).
In a separate paper (W. Armstrong, T. D. Colmer, P. M. Beckett and H. Greenway, unpubl. data) we will discuss formation of anoxic cores and the possible importance of the phytoglobin-nitric oxide cycle in ATP formation during an energy crisis. This cycle may occur in shells surrounding anoxic cores, but also in well-aerated tissues when oxidative phosphorylation is inhibited. Further aspects will be NO and ethylene as possible messengers between different zones, or plant organs.
Energy crisis metabolism in well-aerated tissues
In 1992, Dennis and co-workers suggested that the term ‘anaerobic response’ element involved in transcription of several anaerobic genes was a misnomer and was best replaced by: ‘energy deficiency regulatory element’. The hypoxic response element (HRE; Gibbs et al. 2015) is presumably the anaerobic response element discussed by Dennis et al. and still does not cover energy crisis in fully aerated tissues. By extension, we suggest the term ‘energy-crisis metabolism’ may be better than the usually applied ‘anaerobic metabolism’. Dennis et al. (1992) reached their conclusion mainly because transcripts of the ‘anaerobic’ enzyme, alcohol dehydrogenase (ADH), could be induced, not only by O2 deficiency, but also by a range of adverse conditions in well-aerated tissues; listed were cold temperature (<10°C) in tropical species, wilting, exposure to ABA, 2,4-D and wounding (Dennis et al. 1992). Based on this evidence for ADH, they suggested that a single promoter element associated with anaerobic genes, initiated their transcription when mitochondrial function was reduced/inhibited.
Nie and Hill (1997) also concluded that the induction of mRNA of the ‘anaerobic’ protein phytoglobin is ‘not determined by oxygen availability, but rather by ATP, or some consequence of ATP action’, with Pgb protein (Phytoglobin, synonymous with non-symbiotic haemoglobin) increasing at lower ATP. They based this crucial suggestion on their finding that mRNA of phytoglobin (Pgb) in barley aleurone tissue is induced in air by inhibitors of oxidative phosphorylation such as CO, antimycin A, cyanide, and oligomycin, usually to the same degree as exposure to low O2 imposed by N2 flushing (Nie and Hill 1997). Also, a combination of N2 and antimycin did not increase level of Pgb transcripts above the level attained by each of these factors applied separately. Using CO, it was also shown that there was induction in air of ADH and LDH transcripts, ADH is one of the ‘anaerobic’ proteins listed by Sachs et al. (1980). Furthermore, there was induction of Pgb mRNA when ATP formation by oxidative phosphorylation was inhibited by exposure to the uncoupler 2,4 DNP (Nie and Hill 1997). This uncoupler increases O2 uptake, which is in contrast with the metabolic inhibitors, further emphasising that tissue concentrations or uptake of O2 are not directly involved in inducing these transcripts. The caveat for the uncoupler is that the high O2 consumption, resulting from this uncoupler, may have led to development of anoxic cores (Armstrong and Beckett 2011a), so in this case O2 concentrations in the tissue should be measured to ensure such anoxic cores had not developed. As early as 1953 Beevers showed that ‘anaerobic’ metabolism, as evidenced by acetaldehyde and ethanol formation, became engaged during exposure to 2-4 dinitrophenol in fully aerated tissues to the same extent as in anoxia (Beevers 1953). Consistently, Felle (2005) referred to effects of inhibitors on well-aerated tissues as ‘chemical anoxia’; and Kato-Noguchi (2000) concluded that O2 itself was not involved directly in accumulation of ethanol, which occurred both during anoxia and in well-aerated tissues in which the ETC was inhibited.
As well, features of ‘anaerobic metabolism’ in air were found for leaves of red pine and paper bark: treatments were exposure to SO2, freezing, water deficits and ozone (Kimmerer and Kozlowski 1982). The relevance of ethanolic fermentation in air was also emphasised in relation to disease resistance and stress (Tadege et al. 1999).
The similar response, irrespective of the cause of the energy crisis, is also shown by a typical decrease in pHcyt occurring in cells not only rapidly after the start of anoxia, but also after addition of several inhibitors of oxidative phosphorylation during aeration (reviewed by Felle 2005), as well as, albeit more slowly, during exposure to 0°C of cultured mung bean cells (Yoshida 1994). So, a substantial case that interference with ATP synthesis in the ETC chain of mitochondria invokes ‘energy crisis metabolism’ has been made above; most of the tissues used in this work in well-aerated tissues had no reputation of high anoxia tolerance, so it would be advantageous to extend this work using extremely energy crisis-tolerant plant tissues (see ‘Species and techniques’ in the second part of this review).
Apart from inhibitors, ‘anaerobic’ proteins are also induced in well aerated tissues by low temperature, low Pi and wounding, which will now be discussed.
Low temperature: further support for induction of ‘anaerobic’ metabolism in aerobic conditions, comes from exposure of plants to temperatures between 10°C and somewhat above 0°C, particularly in tropical species. The V-H+-PPiase transcripts, proteins and enzyme activity at the tonoplast of rice shoots (cultivar IR 36; 3–10 days after imbibition) were induced by chilling for 2–6 days at 10°C, though less pronounced than during anoxia (Carystinos et al. 1995). In contrast, roots of 5 day old maize had a high constitutive level of the V-H+-PPiase and showed little increase upon exposure to 10°C (Carystinos et al. 1995). In another study with roots and shoots of a japonica rice variety and roots of maize, there were increases of 15-fold in ADH transcripts, and of 8-fold in ADH proteins after exposure for 24 h to 10°C (Christie et al. 1991). Both Christie et al. (1991) and Carystinos et al. (1995) emphasised the responses of ADH were similar to those during anaerobiosis, in both cases presumably related to energy deficits. They also emphasised there was only partial overlap between cold and anaerobiosis, with differences in the degree and time of change in ADH (Christie et al. 1991; Carystinos et al. 1995) and more in general in pattern of proteins changing (Christie et al. 1991). These data complement previous data on tropical and subtropical species, which repeatedly have been shown to produce ethanol and acetaldehyde upon chilling (Lyons 1973). By far the most perceptive insight comes from work by Lyons and Raison (1970), who showed that chilling of isolated mitochondria of tropical species below 9−12°C resulted in a steep break in the Arrhenius plot, i.e. the Q10 of O2 consumption (where Q10 = (O2 uptake at t + 10)/(O2 uptake at t)) increased from 1.3–1.6 to between 2.2 and 6.3 (Lyons and Raison 1970). This 1.5–4-fold increase of the Q10 with drop in temperature in the tropical species, compared with a Q10 of 1.75 over the full range of 25 to 1.5°C for mitochondria of chilling tolerant species. These authors speculated from observed changes under phase contrast microscopy that the mitochondrial membrane altered its structure, so reducing the uptake of substrates into the mitochondria, hence slowing oxidative phosphorylation more than rates of glycolysis; the glycolytic enzymes being located in the cytosol would retain the usual Q10 of 1.3–1.8.
The possible similar mechanism of acclimative response to O2 deficiency and to cold temperatures is also strengthened by the finding that cucumber seedlings had 70% survival after a 72 h exposure to 2°C, provided they had been pre-treated at 25°C for 12 h at 2 kPa O2. This survival compared with 5% for seedlings not pre-treated at 2 kPa O2 (Frenkel and Erez 1996). We speculate that the O2 deficiency exposure at 25°C induced energy crisis proteins – enzymes that are also important in tolerance to cold temperature. This would be an alternative to the suggestion by Frenkel and Erez (1996) that the ethanol produced during O2 deficiency promotes fluidity of membranes. However lipophilic compounds will reduce ATP concentration, so would lead to an energy crisis (see Supplement 1, available as Supplementary Material to this paper).
Pi deficiency: another example of induction in aerated tissues of several enzymes involved in response to anoxia has been established during Pi deficiency. During Pi deficiency, there are increases in a suite of PPi-dependent enzymes including the V-H+-PPiase, sucrose synthase, PFK-PPi and PPDK (Plaxton and Podesta 2006). In Brassica napus L. suspension cultures, Pi deficient cultures exceeded Pi sufficient cultures by 3-fold for the ratio V-H+-PPiase/V-H+-ATPase measured as maximum catalytic activity and by 2.5-fold for the V-H+-PPiase/V-H+-ATPase protein (Palma et al. 2000). Importantly, Pi starvation in Brassica nigra L. suspension cells, also led to 4-fold decreases in ATP and 10-fold decreases in ADP concentrations (Duff et al. 1989). Thus, several of the metabolic changes during Pi deficiency in air are similar to those occurring under anoxia (Plaxton and Tran 2011; Atwell et al. 2015). The presently known difference between plant tissues during Pi deficiency and anoxia is that NADH produced during catabolism is reconverted to NAD+ by the alternative oxidase during Pi deficiency (Plaxton and Tran 2011) and mainly via ethanol formation in anoxia (Gibbs and Greenway 2003) and possibly during anoxia also by the Pgb-NO cycle (Igamberdiev and Hill 2009; Igamberdiev et al. 2011).
The acclimative value of the PPi dependent enzymes is their use of PPi. This high energy compound is formed mostly during macromolecule synthesis, which includes ATP conversion to PPi, with a free energy of hydrolysis for ATP of 43 kJ mol–1 and that for PPi of 27 kJ mol–1 (Davies et al. 1993; pH 7.3). In rapidly growing tissues PPi is hydrolysed to 2 Pi via a cytosolic PPiase (Plaxton and Podesta 2006). However, in the absence of the PPiase, the high energy bond in PPi can be used as an energy source, quite valuable during an energy crisis. In this review we comment further only on the acclimative-adaptive value of the V-H+-PPiase, which can be attributed to its function in maintaining integrity of the trans-tonoplast H+ gradient during energy shortage (Plaxton and Tran 2011; Atwell et al. 2015). Activity of the V-H+ PPiase would further be stimulated by the increase in soluble Mg2+, common under anoxia (Igamberdiev and Kleczkowski 2011). This acclimation would greatly increase energy efficiency by ‘saving’ ATP (Plaxton and Tran 2011). Additionally, the V-H+-PPiase may result in preferential energy flow to the tonoplast, which would have high priority in view of the crucial value of maintenance of tonoplast energisation to cell survival (Carystinos et al. 1995). This hypothesis is quite relevant to cells in an energy crisis since defective cellular compartmentation of solutes would be fatal (Felle 2005; Atwell et al. 2015; also see ‘Reduction in trans-membrane transport’ later in this review). In this context it is relevant that in rice, the specific activity of a tonoplast fraction became, under anoxia, 10-fold higher for both the total catalytic activity and the total protein for the V-H+-PPiase than for the V-H+-ATPase (Carystinos et al. 1995).
We note that H+-PPiases have been reported for plasma membrane fractions of cotyledons of Ricinus communis L. (Long et al. 1995) and Pisum sativum L. (Robinson et al. 1996). There are some further examples in the review by Maeshima (2000). So, there may also be some preferential energy flow to the plasma membrane. This suggestion has the caveat that the fractionation of the microsomal extract runs the risk of contamination of the plasma membrane (PM) fraction with enzymes of the tonoplast and other organelles (Long et al. 1995; Robinson et al. 1996). Low levels of markers for other membrane fractions in the PM fraction are reassuring for a reasonable purity (Long et al. 1995; Robinson et al. 1996). Even so, Long et al. (1995) remained cautious, so did not reach a water tight conclusion. Nevertheless, the results of the experiments with cotyledons are very promising. In view of the crucial importance of trans-membrane transport during an energy crisis it will be informative to have experiments on possible H+-PPiases in the plasma membrane of tissues very tolerant to an energy crisis. If confirmed, these findings would reinforce the notion that part of the scarce energy is directed to trans-membrane transport, while ATP requiring reactions would remain reduced. Inter alia, these experiments would also provide a much needed test for the occurrence of PM-H+-PPiases, at least in some tissues or conditions.
Wounding: there is little information available for the link between wounding and energy deficits. Cutting primary seminal roots of maize into 1–2 cm segments resulted in transient, 1 h lasting, decreases of 13–35% in ATP and 50% in tissue K+ (Gronewald and Hanson 1982). As well, the PM membrane potential was depolarised from –118 mV to –87 mV (Gronewald et al.1979). A further link between wounding and an energy deficit is indicated by 1.5–2-fold increases in ADH activity after crushing leaves of lettuce and maize with forceps (Kato-Noguchi 2001). Similarly, 5–10 min mild agitation of cotton roots increased ADH activity 5-fold, which was as much as during anoxia, and these increases were attributed to mitochondrial damage (Dennis et al. 1992). The notion that oxidative phosphorylation was impeded after wounding is supported by decreases in CN sensitive respiration, concurrently with increases in CN resistant respiration (Gronewald et al. 1979).
The difference in degree of response between experiments can be expected since various wounding treatments may well differ in degree of injury. These results with wounding need confirmation since in some of the present experiments the tissues may have suffered a degree of O2 deficiency, due to destruction of porous structure. Whether this confounding occurred can be tested by O2 microelectrode profiling measurements across the tissues (Gibbs et al. 1998). Further, the sugar status of the tissues is usually unknown and could suffer from the excision from the source.
Is ethylene involved in development of energy crisis metabolism in well-aerated tissues?
Possible relevance of ethylene to an energy crisis is strongly indicated by experiments in which Rumex palustris Sm. plants were exposed to ethylene at 5 µL L–1 in air for 4 h (van Veen et al. 2013; further considered in W. Armstrong, T. D. Colmer, P. M. Beckett and H. Greenway (unpubl. data). The data by van Veen may have relevance for many adverse conditions: ethylene emanates from tissues during a range of adverse conditions, which include, wounding, high salinity and drought (Morgan and Drew 1997), all which may lead to an energy crisis.
New set point of pHcyt during an ‘energy crisis’
pHcyt: Felle (2005) hypothesised that energy deficits lead to a new quasi steady state with a lower set point of pHcyt 7.1–7.2 than in cells of well-aerated tissues. We review here detailed data obtained under anoxia of hypoxically pre-treated, 5–10 mm tips of intact maize roots. During anoxia these tips stabilised for at least 10 h at pHcyt 7.1–7.2 compared with 7.5 in the aerated tips and this pHcyt did not respond to large decreases in energy production caused by exposure to 1 mM fluoride and 50 mM mannose (Xia et al. 1995; pHext 6.2); in other words, there must have been ‘surplus’ energy available in the roots without fluoride or mannose, yet that energy was not used to restore pHcyt to the set point in air. Of particular importance is the observation that anoxic maize root tips at pHext 4.5 with a pHcyt 7.1, responded to fusicoccin – the stimulator of the PM-H+-ATPase – by an increase in the rate of the PM-H+-ATPase of as much as 4 µmol g–1 FW h–1 (Xia and Roberts 1996). Yet, this potential for H+ pumping across the PM had not been engaged, before fusicoccin was applied to restore the pHcyt to the set point of 7.5 in air. In Felle’s words, ‘it is pointless to restore the aerobic pH, since that would merely reinstate a high energy requiring regime’ (Felle 2005). During an energy crisis the new set point of pHcyt is mainly maintained by a biochemical rather than a biophysical pHstat.
The new pHcyt may last for a few hours and then decline further or last for days (Felle 2005). The transient pattern is found for wheat shoots (Menegus et al. 1991) and also for tips of hypoxically pre-treated 5 mm maize roots at pHext 4.5 (Xia and Roberts 1996) and 2 mm root tips of Triticum aestivum L. (Kulichikhin et al. 2007). In the maize root tips, there was a plateau of pHcyt near 7.1 between 2 and 4.5 h after start of anoxia; subsequently pHcyt dropped again precipitously to 6.2, whereas tips failed to resume elongation during 24 h of re-aeration (Xia and Roberts 1996). So, the changed metabolism in these cells only conferred transient survival. This precipitous decline of pHcyt has been used as support for the notion that death would be caused by acidosis of the cytoplasm (Greenway and Gibbs 2003). More likely the decline is a consequence of a deterioration of membrane integrity due to failure to renew proteins and liquid constituents of the membranes (Felle 2005). In this scenario one would expect a sudden collapse, as was the case in anoxic slices of storage root of red beet: upon return to air all cells retaining their anthocyanins, which are in the vacuole, restored their plasma membrane potential within a few minutes; furthermore, only those that had lost their anthocyanins remained at a Donnan free space potential (Zhang et al. 1992).
In contrast, pHcyt dropped by only 0.3–0.5 units in two extremely anoxia tolerant tissues, Potamogeton stems and rice coleoptiles and the pHcyt were maintained for days (Dixon et al. 2006; Kulichikhin et al. 2009; Table 1). The proposed new set point of pHcyt (Felle 2005) may thus be relevant to long-term exposure to anoxia-energy crisis. The question remaining is whether in anoxic rice coleoptiles and Potamogeton stems the rather small decreases in pHcyt of 0.3–0.5 units can trigger the profound changes in metabolism. Reassuringly, similar rather small changes in pHcyt triggered profound diurnal changes in Crassulacean acid metabolism (Hafke et al. 2001; Table 1).
|
One exception to the general pattern of a decrease in pHcyt during reduced energy production is that pHcyt was 7.4 in Potamogeton stems grown for 7 days since the start of sprouting of the turions in air and anoxia (Dixon et al. 2006). Overall, the discussed data on pHcyt show that tolerance to anoxia not only varies between tissues, but within the same tissues also depends on environmental conditions.
That pHcyt may act as a messenger to modify metabolism is supported by an interesting case of regulation of nitrate reductase. NO3– reductase (NR) activity and protein can be upregulated both during anoxia and during cellular acidification using respiratory inhibitors, weak acids, or when transferring NO3– deficient tissues to solutions containing NO3– (Kaiser et al. 1999). In all cases, the activation of NR would be via a decrease in pHcyt, but there is no chance that the activation of NR could be due to a decreased ATP concentration per se, since even in anoxic tissues these ATP levels remain well in excess of the known Km of the enzymes involved in regulation of NR (Table 2 for ATP concentrations in anoxia tolerant tissues; and Botrel and Kaiser 1997; Kaiser et al. 1999; for the Km of the regulatory enzyme of NR). Unfortunately, the pHcyt at which NR is activated is not known, so it is uncertain whether the modest decrease in pHcyt of the very anoxia tolerant species during anoxia is large enough to activate NR. If so, this would show that, at least in some cases, the anaerobic response consists simply of a decrease in pHcyt, caused by an energy crisis, and then engaging a system which exists in most plant tissues to modulate a key enzyme like NR. It should be noted some of the techniques of Brotel and Kaiser may have not been optimal (see Supplement 4, Some relevant examples of defects in techniques).

|
Reductions in energy charge and ATP concentrations
The induction of energy deficient metabolism in well-aerated tissue shows that in this environment, at least, the primary cause is likely to be a reduction in ATP concentrations (see also next section). The changes in metabolism are large, with changes in enzyme composition (using anoxia, see Sachs et al. 1996; Chang et al. 2000) and a slowing down of trans-membrane transport (Felle 2005). Before discussing these changes (in ‘Reduction in trans-membrane transport’), we will consider the changes in the adenine nucleotide pool.
Reductions in oxidative phosphorylation, e.g. by anoxia, rapidly lead to decreases in energy charge and ATP concentrations (Drew 1997). For example, transfer of rice embryos to anoxia resulted, within minutes, in a decline of the energy charge from 0.9 to 0.6 (Mocquot et al. 1981). Individual adenine nucleotides were not reported, but in view of the high initial level of the energy charge it is sure there must have been a large drop in ATP (see equation for energy charge in list of definitions). Subsequently, the energy charge restores to close to values found in tissues with high oxidative phosphorylation. Thus, energy charge may participate during the early acclimation to an energy crisis, but is unlikely to have much influence during the long-term survival of at least 5 days of anoxic rice coleoptiles (Kulichikhin et al. 2009; Greenway et al. 2012). Instead, ATP concentration may be important: in the extremely anoxia tolerant rice coleoptiles the assessed ATP levels in anoxia remained 30% lower than in air at 3 and 4 days after exposure to anoxia (Table 2; see also Ishizawa et al. 1999). So, ATP requiring reactions, including H+-ATPases, may be slowed during an energy crisis and that possibility will be discussed in the next section.
A 40% decrease in ATP (per unit fresh weight) after the start of anoxia in stems of Potamogeton and Sagatira (Ishizawa et al. 1999) is probably not representative for the cytoplasm since after transferring to anoxia there are likely to have been large increases in the FW : protein ratio, which might well explain most, if not all, of the drop in ATP level on a fresh weight basis. The only firm indication of a favourable energy status of these tissues is a slightly higher energy charge in anoxia than in air, rather than the usual lower energy charge.
Are the decreases in ATP level large enough to reduce activity of the H+-ATPases?
To further elucidate whether ATP concentrations limit the activity of the H+-ATPases, we need their Km values for ATP and the ATP concentration in the cytoplasm. The Km values of the plasma membrane and tonoplast H+-ATPases are very similar (Table 2). The Km of H+-ATPases at the tonoplast were assessed during patch clamp studies of isolated vacuoles; one of the advantages of patch clamp is that the ATP concentrations at the tonoplast membrane are precisely known. Km values were 600 and 800 µM for sugar beet and barley respectively (Hedrich et al. 1986, 1989). The assessed ATP concentrations in the cytosol of anoxic maize root and rice coleoptiles were at, or below, most of the Km values for ATP on the H+-ATPases (Table 2). So, it is possible that during an energy crisis ATP might restrict the rate of the H+-ATPases, at least partially, accounting for a reduced trans-membrane transport.
Clearly more firm evidence is required, including the Km of the plasma membrane H+-ATPases of the species concerned. Particularly, data on species with high tolerance to an energy crisis would be valuable to further test the hypothesis by Felle (2005) that, in anoxic tissues, PM-H+-ATPases do not become engaged to restore the pHcyt to the set point in air. It would also be worth checking whether the Km values for ATP of the H+-ATPases change when the tissues are exposed to an energy crisis.
The decrease in energy available for solute transport can be coped with in two ways. Reduction in solute transport (see ‘Reduction in trans-membrane transport’) and possible modification of transport proteins to achieve an energy efficient form, such as increased coupling ratios, e.g. numbers of protons pumped per ATP hydrolysed (see below ‘Energy budget during an energy crisis’).
Reduction in trans-membrane transport
One key feature of energy deficient metabolism is that H+-co-transport is drastically reduced in nearly all tissues, so that only a low rate of the PM-H+-ATPase is required (reviewed by Felle 2005; Atwell et al. 2015). The best known cases, based on K+ fluxes, are for rice coleoptiles (Colmer et al. 2001) and grapevine roots (Mancuso and Marras 2006). Recently it was shown for Arabidopsis that outward K+ rectifiers channels were downregulated within 1 h of the start of anoxia (Wang et al. 2017).
When there is no growth, such as in excised rice coleoptiles (Huang et al. 2005a) and aged beet root slices (Zhang and Greenway 1995), the residual membrane transport during an energy deficit, can be driven in two ways: by provision of some energy for residual H+ pumping, or by previously accumulated energy coined a ‘battery’ (Greenway and Gibbs 2003; Felle 2005; Atwell et al. 2015). These alternatives can be distinguished when net K+ fluxes are known, because a K+ net influx would indicate some residual activity of the PM H+-ATPase. In contrast, the ‘battery’ would involve K+ net effluxes (Greenway and Gibbs 2003; Felle 2005). The H+, entering the cells during any co-transport, would be neutralised by organic anions rather than be excreted (Greenway and Gibbs 2003; Felle 2005). An indication of energy provision associated with residual H+ pumping was found for excised rice coleoptiles. These anoxic rice coleoptiles showed small net K+ influxes over 72 h anoxia (Huang et al. 2005a; Greenway et al. 2012) while there was substantial glucose and sucrose uptake (Zhang and Greenway 1995). In contrast, the ‘battery’ was presumably engaged in anoxia tolerant aged tissue of red beet: there was a small net rate of K+ efflux over 250 h anoxia, even in the most anoxia tolerant state achieved: being aged tissues, which were hypoxically pre-treated (HPT) and supplied with glucose (Zhang et al. 1992). The rate of K+ efflux was roughly similar to the rate of net glucose uptake (Zhang and Greenway 1995). Confirmation is required by measuring K+ and glucose fluxes in the same experiment (see Supplement 2, available as Supplementary Material to this paper). Further evidence for the action of the ‘battery’ was obtained by transferring excised, anoxic, rice coleoptiles from pH 6.5 to pH 3.5, which changed a small net K+ influx at pH 6.5 to a continuous net K + efflux, with concurrent decreases in malate and succinate, as is predicted by the ‘battery’ concept (Greenway et al. 2012). In contrast, at pH 6.5 there were even some increases in malate and succinate (Greenway et al. 2012). So, these data indicate, at least for anoxic rice coleoptiles, substantial flexibility: with either residual PM H+-ATPase activity being engaged to sustain H+-co-transport, or engagement of the ‘battery’ under more adverse conditions (Atwell et al. 2015). Eventually this ‘battery’ would, of course, be exhausted, and the substantial K+ loss may have fatal consequences (Greenway et al. 2012); as discussed by Demidchik et al. (2010) in terms of programmed cell death (see Supplement 2 for cases where the battery is predicted or alternatively, some H+-solute co-transport).
Of course, when there is rapid growth during anoxia, as for cells of Potamogeton stems (Koizumi et al. 2011) quite large K+ influxes would be predicted, but as far as we know these have not yet been measured.
Energy budget during an energy crisis
Ion transport is particularly suited for studies on energy budgets, since changes in rate of transport can be relatively easily measured, while rates of solute uptake in air are high and consume as much as 45% of the energy required for cell maintenance (Penning de Vries 1975; de Visser et al. 1992; Greenway and Gibbs 2003).
The first energy budget in anoxic plant tissues was constructed using coleoptiles of 3-day-old rice seedlings, anoxic since germination (Edwards et al. 2012) and this information was further discussed by Atwell et al. (2015), among others in relation to reductions in ion fluxes. The substantial reduction in the energy requirements for these fluxes must have contributed to survival and growth of the coleoptile over at least 5 days anoxia (Greenway et al. 2012).
Further insight was gained by exposing, acclimated excised, anoxic coleoptiles to an additional stress of 50–100 mM NaCl, which in air would greatly increase ion fluxes. This challenge could not be met during anoxia in 100 mM NaCl, when the coleoptiles died (Kurniasih et al. 2017). However, the anoxic coleoptiles survived at 50 mM NaCl for at least 90 h, allowing comparisons between ion fluxes and energy production and the construction of a preliminary model on energy efficient transport during an energy crisis. We postulated energy efficient transport would be achieved by high coupling ratios of both primary transport systems (moles H+ extruded/moles energy consumed), and secondary transport systems (moles solute transported)/moles H+ influx for symports, or antiports). In addition, membrane permeability to solutes like Na+ would be greatly reduced, thus minimising the amount of energy required for Na+ transport to the external solution or vacuole (Kurniasih et al. 2017). In general, one would expect leakage of all solutes has to be minimised and less selective transporters have to be de-activated.
Response during carbohydrate starvation
Carbohydrate starved tissues-organs have reduced time of survival (see reviews by Vartapetian et al. 2003; Gibbs and Greenway 2003). In the present review we discuss the effect of carbohydrate starvation on ion fluxes in excised, anoxic, rice coleoptiles (Huang et al. 2005a). The first distinct difference between coleoptiles, with and without exogenous glucose, was between 72 and 96 h after the start of anoxia, when coleoptiles without exogenous glucose showed net losses of K+, P and Cl–, but there was still net uptake by the coleoptiles supplied with 20 mM glucose. At this time, return to air still resulted in resumption of rapid net uptake even in the carbohydrate-starved tissues. However, in the coleoptiles without exogenous glucose, longer exposure led to substantial net efflux of ions and after return to air at 120 h after the start of anoxia, the carbohydrate starved tissues had only half to one-third of the net K+ and Cl– uptake of the coleoptiles supplied with glucose (Huang et al. 2005a). Similarly, in carbohydrate starved slices of red beetroot, the only cells which had lost their semi-permeability (see previously ‘New set point of pHcyt during an ‘energy crisis’) were detected in sugar-starved slices (Zhang et al. 1992). These results are consistent with Felle’s (2005) hypothesis that death during anoxia is caused by collapse of trans-membrane gradients. The collapse of the coleoptiles is associated with a prior long-term reduced ethanol formation, which in the coleoptiles without exogenous glucose was only one-third and one-ninth of that in 20 mM glucose as early as between 0–24 and 24–48 h after start of anoxia. This drastic reduction in inferred rates of glycolysis, and hence ATP formation, could thus be tolerated for another 48–72 h, reinforcing the notion that trans-membrane transport is preferentially allocated energy. We note that ATP concentration only declined precipitously between 72 and 96 h anoxia, from ~40 to 10 µmol g–1 FW h–1 (Huang et al. 2005a).
Conclusions
This section strongly supports the notion that ‘anaerobic metabolism’ can be induced by several adverse conditions, including O2 deficiency, but also by inhibition of oxidative phosphorylation in well-aerated tissues. This reinforces the suggestion to use terms like ‘energy-crisis’ metabolism instead of ‘anaerobic’ metabolism, so highlighting that the changes in metabolism are triggered by a decrease in ATP level and by inference in ATP formation, or one of its consequences, not by the level of O2 per se (Nie and Hill 1997). Such a revised definition would clarify understanding and improve efficiency of future investigations. However, such a change in terminology has to be based on a watertight case, since there will inevitably be confusion, particularly when comparing future and past publications. So, more detailed comparisons between responses to energy crises in anoxia and in air are a prerequisite to any change in terminology. Further elucidation of the induction of ‘energy-deficient’ metabolism in well-aerated tissues might use either one of the conditions shown to result in aspects of energy crisis metabolism in well-aerated tissues, such as wounding or cold temperatures, or a suitable inhibitor of oxidative phosphorylation, for example antimycin A, which inhibits electron flow (Stoimenova et al. 2007). In contrast to several other metabolic inhibitors, antimycin A does not prevent nitrite-driven ATP formation in anoxic mitochondria (Stoimenova et al. 2007) This ATP formation is very small relative to oxidative phosphorylation but comparable in magnitude to that achieved in glycolysis linked to ethanol formation (Stoimenova et al. 2007).
There are indications that cytoplasmic ATP concentrations, rather than energy charge, contribute to the decrease in the rate of proton extrusion by the PM H+-ATPase. Felle (2005) has presented a reasonably satisfactory scenario of how an energy crisis limits trans-membrane transport (Felle 2005) and we suggested, in addition, that the use of energy efficient transport systems contribute to a favourable energy balance. So, the energy balance, which decreases at first, is rapidly restored till energy consumption is reduced enough to match the decrease in energy production.
The second part of this paper will address some possible reasons for the large changes in transcripts and enzymes during anoxia-energy crisis, as well as some aspects of gene regulation when tissues are exposed to an energy crisis.
Genetic aspects: reasons for the multitude of changes in transcripts and enzymes in molecular studies during severe hypoxia-anoxia
This second part of the review has the following main themes: General networks, Anaerobic proteins, Key anaerobic proteins, ‘O2 sensitive’ branch of N-end rule pathway, Messengers, Choice of species, and Confounding energy crisis and injury.
Preamble
In this section we attempt to integrate physiological and molecular studies. Exposure to severe hypoxia-anoxia (energy crisis) leads to a multitude of changes in transcripts and enzymes, even in anoxia tolerant tissues. We propose that most of these changes are related to common networks, which can participate in acclimation to a wide range of conditions, which change growth and metabolism.
The terms anoxia and anaerobic are used in this section because all the data quoted were collected during hypoxia-anoxia. Of course, when the data of an energy crisis for well-aerated tissues are confirmed, and enlarged, the better term would be ‘energy crisis’.
These general networks would be complemented with changes in transcripts and enzymes specific for each particular condition, in the present case with anaerobic (energy crisis) transcripts and enzymes. Only some of these specific anaerobic enzymes would make the difference between anoxia tolerant and anoxia intolerant species. These enzymes endowing anoxia tolerance are referred to as ‘key anaerobic proteins’, a similar suggestion was made by Shingaki-Wells et al. (2014).
To detect differences between anoxia-tolerant and -intolerant tissues in the key anaerobic proteins, efficient investigation requires a judicious choice between tolerant and/or intolerant tissues, knockout lines, mutants and overexpression lines. There is also a requirement to avoid methodology proven to lead to early injury under anoxia. Otherwise, many of the changes in transcripts are likely to be associated with injury and approaching death, i.e. have no meaning for anoxia tolerance. Similar suggestions have been given for studies on plant drought resistance, to account for up and downregulation of hundreds, if not thousands, of transcripts (Blum 2011).
Networks that become engaged in response to many stresses are complemented by ‘anaerobic’ proteins
In this review we concentrate on hypoxia-anoxia responses in solution culture. These responses to anoxia-hypoxia in solution culture should be clearly distinguished from responses to flooding, including submergence of plants in soil. The latter, more complex, environments may be high in CO2 and ethylene, and include many changes in soil composition. Such complex environments were characterised by Shabala et al. (2014) as ‘of a high complex multi-factor stress type’.
Even in solution culture using anoxia tolerant rice coleoptiles, the anoxia response involves changes in thousands of transcripts and genes (Lasanthi-Kudahettige et al. 2007; Shingaki-Wells et al. 2014). The rice coleoptiles can survive anoxia for at least 5 days (Greenway et al. 2012), so there is no chance that an appreciable percentage of the changes is associated with severe injury and/or approaching death. To account for the large number of changes during hypoxia-anoxia, we propose that most of these changes are associated with networks common to most plant tissues, such networks would become engaged in many situations when growth slows down and metabolism is drastically altered (Fig. 1). For each given stress the engagement of these common networks would be complemented by transcripts and proteins specific for any particular stress, in the present case by anaerobic proteins. These anaerobic proteins will be divided into two groups depending on in which species they occur: (i) anaerobic proteins which are induced during anoxia in most plant species, especially in the roots; and (ii) key anaerobic proteins, which are only optimally expressed in anoxia tolerant tissues, such as the very anoxia tolerant rice coleoptiles, so accounting for the difference in tolerance between genotypes and tissues.
Networks common to many stresses
One good candidate for this common network is the low energy syndrome (LES) present in nearly all plants, including Arabidopsis (Tomé et al. 2014), or a similar network. LES involves thousands of changes in transcripts, translation factors and proteins and is involved in acclimation to nutrient limitation, high salt, hypoxia, low and high temperature (Tomé et al. 2014). It should be noted we think the term ‘low energy syndrome’ is unfortunate, since several of the adverse conditions, mentioned by Tomé et al. (2014), do not necessarily involve a low energy status. The LES network among others acts via metabolic reprogramming, repression of biosynthesis, energy preservation and protein breakdown. The alternative pathway (AOX) has been coined a ‘survival protein’ (Van Aken et al. 2009) and presumably belongs to or supplements the LES network. The Km of the AOX for O2 is quite high, but it may function in the well-aerated tissues, which are the main theme of the first part of this review. One main function of AOX is the oxidation of reduced nucleotides. However, in 1-day-old anoxic rice embryos there was a decrease in AOX in rice (Narsai et al. 2011), consistent with our suggestion that AOX might not be required in tissues highly tolerant to an energy crisis (W. Armstrong, T. D. Colmer, P. M. Beckett and H. Greenway, unpubl. data).
We suggest, that the N-end rule pathway (Graciet and Wellmer 2010; see shortly) is part of, or is dovetailing, the LES network. The exception is, of course, the ‘O2 sensitive’ branch of the N-end rule pathway, which will be discussed in the anaerobic protein section (‘The ‘O2 sensitive’ branch of the N-end rule pathway’ section). The N-end rule pathway has been reviewed by Graciet and Wellmer (2010), the pathway determines the type of proteins to be degraded during a large range of cellular and developmental stages. Of course, deletion of one of the principal components of these general networks would decrease acclimation to many stresses, including anoxia. For a possible example of such a failure of common networks see below ‘Hypotheses on messengers and their targets’.
Anaerobic proteins induced in most genotypes
Some of the anaerobic proteins are induced in both anoxia intolerant Arabidopsis seedlings and anoxia tolerant rice coleoptiles; one of the best known examples is ADH (see Ismond et al. 2003 for Arabidopsis; Gibbs et al. 2000 for rice). Consistently, many intolerant tissues have rates of ethanol formation as high as tolerant tissues (Drew 1997; Gibbs and Greenway 2003). Similarly, several molecular responses to low oxygen, such as induction of glycolysis and fermentation are conserved (Shingaki-Wells et al. 2014). Further, in Arabidopsis there was a 2.5-fold overexpression of ADH which did not improve anoxia tolerance of roots and shoots (Ismond et al. 2003). Consistently, it has been concluded that several molecular responses to low O2, for example overexpression of ADH by 3–4-fold in cotton roots and by 20-fold in 26-day-old rice did not improve anoxia tolerance, though in this case ethanol formation was stimulated in the overexpressed lines, particularly in cotton (Ellis et al. 2000; Rahman et al. 2001 respectively). Importantly, on a protein basis the ADH levels in Arabidopsis (Ismond et al. 2003) were similar to those found in root axes of maize (Andrews et al. 1994).
The very high maximum catalytic activity of ADH reached under anoxia raises the question ‘to what extent this high activity is required to achieve anoxia tolerance’? The ADH-1 mutant of rice, with 20% of the maximum catalytic activity of ADH and half the rate of ethanol synthesis of the wild type, suffered fatal consequences when germinated in stagnant solution (Takahashi et al. 2014). In comparison, in maize root tips tolerance was only compromised when ADH was reduced to 5% of the wild type (Roberts et al. 1989). To what extent other putative ‘anaerobic’ enzymes can be reduced in expression without a fatal result requires further investigation.
Regarding sucrose synthase, a double mutant lacking this enzyme dies early when exposed to anoxia (Ricard et al. 1998). Similarly, participation of sucrose synthase in anaerobic catabolism of Arabidopsis was proposed by Bieniawska et al. (2007). Yet, other work with Arabidopsis shows mutants without substantial sucrose synthase, and wild types, had similar low anoxia tolerance, with all plants being dead after 24 h anoxia (Santaniello et al. 2014). It is relevant that maize roots, though only moderately tolerant to anoxia, are more tolerant than Arabidopsis. So the different conclusions by Ricard et al. (1998) and Santaniello et al. (2014) can be interpreted by assuming that deletion of sucrose synthase only matters when the tissues have at least a reasonable degree of tolerance.
We also include in this group of anaerobic proteins common to most species: the subgroup VII of ethylene responsive transcription factor (ERF VII) of the ‘O2 sensitive’ branch of the N-end rule pathway (Gibbs et al. 2011, 2015; Licausi et al. 2011). These proteins occur in both mono and dicotyledons (Nakano et al. 2006). So, though it is likely they occur in other plant species, we do not know whether either the ERF VII family and/or the ‘O2 sensitive’ branch are similar or superior in anoxia tolerant tissues than in Arabidopsis (see ‘O2 sensitive’ branch of the N-end rule pathway’ section and Supplement 3).
The key anaerobic proteins which endow anoxia tolerance
We define the key anaerobic proteins as those which make the difference between anoxia intolerant and tolerant species tissues.
These key ‘anaerobic ‘proteins’ would be expressed only in optimum amounts in tissues highly tolerant to an energy crisis, such as germinating rice seedlings. One crucial point that we make in this section is that there is now evidence that overexpressing of only one of these key anaerobic proteins, the V-H+PPiase, results in substantial improvement in performance during anaerobiosis and during cold exposure, when oxidative phosphorylation is also likely to be inhibited (see the first part of this review). Though no proof, these observations augur well for the view that only a relative small number of proteins make the difference between anoxia tolerant and intolerant species.
Importantly, the difference between anaerobic proteins and key anaerobic proteins would be in degree rather than in kind. For example, proteins such as PDC and Pgb also increase during anoxia exposure in intolerant tissues (for recent reference for Arabidopsis see Paul et al. 2016). Yet, tolerance can be still further enhanced by overexpressing the particular protein. Present evidence indicates PDC, Pgb, and the V-H+PPiase are such key anaerobic proteins. In contrast to ADH, 3–5-fold increases in expression of PDC, the entry port of pyruvate to ethanolic fermentation, substantially increased anoxia tolerance of Arabidopsis (Ismond et al. 2003). Similarly, transgenic rice with a range of PDC expressions showed an excellent correlation between maximum catalytic activity of PDC and ethanol formation of the tillers (measured after 24 h anoxia), as well as with % survival after 14 days submergence (Quimio et al. 2000). Earlier work on the rate of ethanol formation and maximum catalytic activity of PDC also led to the suggestion that anoxia intolerant wheat roots were low in PDC activity (Waters et al. 1991). Yet, there are contrary observations; in rice leaves overexpressing PDC resulted in decreased survival, possibly due to acetaldehyde toxicity (Rahman et al. 2001). Such negative results indicate that experiments involving modulation of PDC should allow for the possibility that the PDC/ADH needs to be maintained within a certain range.
Pgb levels have as far as we know, not been measured in germinating rice. However the Pgb-NO cycle in anoxic rice mitochondria produced twice as much ATP per unit protein as this cycle did in barley mitochondria (Stoimenova et al. 2007). Overexpressing the level of Pgb in Arabidopsis thaliana (L.) Heynh. led to substantial increases in anoxia tolerance, but only for anoxically shocked plants (Hunt et al. 2002). As an example, after 48 h of anoxia both shoot and root tip survival were 100% for seedlings which were pre-treated at 5% O2 for 48 h in both wild type and Pgb+, but for anoxically shocked seedlings, shoot survivals were 0 and 30% and root tip survivals were 55 and 69% for wild type and Pgb+ plants respectively (Hunt et al. 2002). In moderately anoxia-tolerant maize roots, transgenic lines with increased levels of Pgb increased ATP levels 1.6-fold, whereas deletion of the Pgb protein decreased ATP to 60% of the level in the wild type (Sowa et al. 1998).
Another key anaerobic protein would be the V-H+PPiase (Maeshima 2000; Shimaoka et al. 2004), which does occur in Arabidopsis. However, even a modest overexpression of a gene encoding the V-H+-PPiase (AVP1) in Arabidopsis significantly enhanced survival of shoots and roots after periods of anoxia of up to 24 h (Demmer 2017). Similarly, the recovery of leaf growth after transfer to air (and to a lesser extent, root extension) was up to six times faster in AVP1 overexpressers (Demmer 2017). Further, during exposure of rice seedlings for 6 days to 4°C there was higher tolerance for two lines overexpressing the OVP1 V-H+-PPiase (Zhang et al. 2011); this exposure to 4°C is likely to lead to an energy crisis (see the first part of this review). The two transgenic lines had 25 and 80% more V-H+-PPiase and 70% survival compared with 40% of the wild type (Zhang et al. 2011). Similarly, overexpressing of the V-H+-PPiase resulted in improved tolerance to drought and high salinity (Shabala et al. 2016). These data confirm that during an energy crisis overexpression of a single enzyme may lead to substantial improvement in tolerance. So, the V-H+-PPiase is likely to be one of the key anaerobic proteins, which, when fully expressed, contributes to the difference between tissues tolerant and intolerant to an energy crisis. Overall, the data obtained so far with the V-H+-PPiase show that despite the multitude of changes in transcripts and enzymes a single enzyme can substantially enhance tolerance to an energy crisis.
The ‘O2 sensitive’ branch of the N-end rule pathway
Recent works have made substantial progress with the elucidation of mechanisms of the molecular basis for formation of ‘anaerobic proteins (Gibbs et al. 2011; Licausi et al. 2011; Kosmacz et al. 2015; Paul et al. 2016). These mechanisms involve the group of ERFVII transcription factors, including RAP 2.12 and an ‘O2 dependent’ branch of the N-end rule proteolytic pathway, which is inactivated by hypoxia, thus inhibiting the breakdown of RAP 2.12 (Gibbs et al. 2011; Licausi et al. 2011; Kosmacz et al. 2015; Paul et al. 2016). This inactivation presumably leads to an increase in RAP 2.12; as well there might be de novo synthesis during hypoxia (Paul et al. 2016). The large reduction in the rate of degradation of RAP 2.12 and its transfer to the nucleus leads to formation, or maintenance, of a suite of ‘anaerobic’ proteins (Paul et al. 2016).
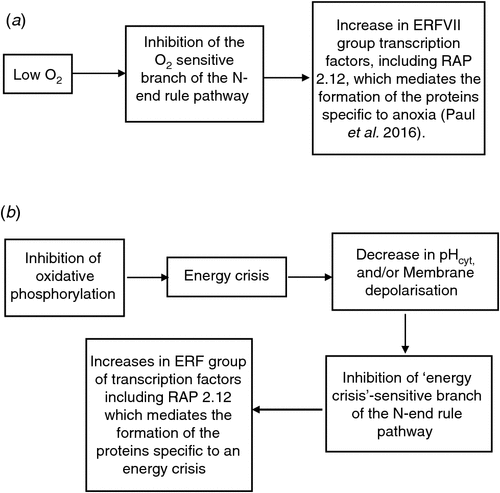
These findings provide new insight into the mechanisms of anoxia tolerance of plant tissues. Our contribution is confined to the questioning whether the pathway is regulated by O2, or by an energy crisis. The authors refer to the branch as ‘O2 sensitive’ and as an ‘O2 sensor’ (Gibbs et al. 2011; Kosmacz et al. 2015; Paul et al. 2016; Fig. 2a). Earlier, Gibbs et al. 2011, gave as an alternative to O2 sensing, that the ‘O2 sensing’ branch of the N-end rule pathway might be regulated by a consequence of decrease in energy formation, such as decrease in pHcyt, or formation of reactive O2 species (Gibbs et al. 2011; Fig. 2a). As stated in the ‘Networks’ sections above, we suggest that at least part of the induction of anaerobic metabolism is related directly to decreases in ATP concentrations. This alternative suggestion would be consistent with our notion that the branch may be an ‘energy crisis’-sensitive rather than an O2-sensitive branch of the N-end rule pathway (see Fig. 2 for the two alternatives). We suggest this terminology, since several ‘anaerobic’ enzymes also reach high levels in well-aerated tissues (see ‘Energy-crisis metabolism in well-aerated tissues’). However, as stated in the conclusions of the first part, more work is needed to establish to what extent the detail of responses to energy crisis in well-aerated tissues is similar to that in hypoxic tissues.
|
Can the O2 sensitive N-end rule pathway be inactivated in air ?
The question in the above heading is relevant to our theme, because the answer is a good test for the viability of our notion that the response to O2 deprivation is merely one example of the more general response to an energy crisis, even in well-aerated tissues. The cysteine-argine N-end rule pathway is regulated in aerated Arabidopsis and Hordeum vulgare. Inactivation occurs in response to multiple stresses including drought and exposure to 200 mM NaCl (Vicente et al. 2012). As stated by Gibbs et al. (2015) the inactivation of the ‘O2 sensitive’ branch of the N-end rule pathway in air shows that in these cases O2 is not the regulator of the inactivation-activation of the O2 sensitive’ branch of the N-end rule pathway. Gibbs et al. (2015) suggested down regulation in air may be achieved either by low NO, or by some other types of regulation, such as shielding of the N terminus of the ERF’s concerned. The principal message for our theme is that the regulation of the ‘O2 sensitive’ branch of the N-end rule pathway is very flexible. Whether this flexibility extends to our present theme, i.e. whether down regulation of the O2 sensitive’ branch of the N-end rule pathway occurs in well-aerated tissues during an energy crisis, could be tested by some of the treatments, which result in an energy crisis in air (see also ‘Energy-crisis metabolism in well-aerated tissues’).
The question of whether low NO may inactivate the pathway degrading ERF VII’s in our case is worth considering briefly.
The NO possibility tends to be unlikely in our case, since as will be argued shortly, NO production tends to increase in well-aerated tissues with inhibited phosphorylation (see ‘Energy-crisis metabolism in well-aerated tissues’); hence based on work by Vicente et al. (2012), the branch would remain active, i.e. there would be no accumulation of ‘anaerobic proteins’. First, NO is expected to increase when the ETC is inhibited (Cvetskovska and Vanlerberghe 2012), whereas in optimum conditions NO production is close to zero (Gupta and Igamberdiev 2011). Second, Vicente et al. (2012) suggested a major role for nitrate reductase (NR), during the inactivation of Cys-Arg/N branch of the N-end rule pathway. NR is a major source of NO, and NR activity is much reduced in the case of drought, allowing inactivation of the N-end rule pathway. However, as described in the ‘New set point of pHcyt during an ‘energy crisis’ section, metabolic inhibitors applied to well-aerated tissues increase nitrate reductase activity. So the present indications are that NO would not play a role in any regulation of the ‘O2 sensitive’ pathway of the N-end rule pathway in well-aerated tissues. Therefore, either Fig. 2b is not realistic, or there is another type of regulation than O2 and NO levels. One possibility may be deamination as found for the N-end rule pathway in yeasts (Graciet and Wellmer 2010). In conclusion, some of the treatments which result in ‘anaerobic’ metabolism in air (see ‘Energy-crisis metabolism in well-aerated’) would be useful to test whether case (b) in Fig. 2 is a viable proposition and the mechanism by which it is achieved. Of course, even a positive result cannot rule out that the inactivation of the ‘O2 dependent’ branch of the N-end rule pathway is triggered by two different mechanisms.
Enzymes that may not be critical for surviving an energy crisis, but which may optimise acclimation, over short or long-terms
Genes that are responsive to O2 deficits in micro-arrays cannot be expected to contribute equally to the physiology of survival and growth in tissues such as rice coleoptiles; that is changes in expression in a substantial array of genes may be needed to optimise anoxia tolerance, but only a few may endow a large measure of tolerance. For example, there are many changes during anoxia in intermediates and products linked to the TCA cycle (Shingaki-Wells et al. 2014). In our view, several of these changes might occur when the TCA cycle slows for whatever reason. Others might have a useful role during anoxia, i.e. accumulation of succinate might enlarge the capacity of the ‘battery’ (see ‘Reduction in trans-membrane transport’ and Greenway et al. 2012). The possible reactions leading to succinate have been most recently reviewed by Shingaki-Wells et al. (2014). Whether any of these changes are critical to long-term survival needs to be investigated. A relevant case may be alanine which accumulates during anoxia (Shingaki-Wells et al. 2014) to as high as 50 mM in excised rice coleoptiles (Greenway et al. 2012). However, the difficulty is that alanine-amino transferase in Arabidopsis was involved in the decrease, not in the net synthesis of alanine, presumably during re-aeration (Miyashita et al. 2007). A more straightforward case may be GABA, a metabolite considered to play a role during an energy crisis and which is formed by glutamate decarboxylase (Shingaki-Wells et al. 2014). One of the effects of the increase in GABA may be reduced K+ efflux, which was found for barley roots at 80 mM NaCl (Cuin and Shabala 2007). Several other amino acids had a similar mitigating effect, but GABA is attractive to explore for anoxia since in 3-day-old rice seedlings GABA increased after 24 h of anoxia from ~0.5 to 5.5 µmol g–1 FW in the shoots and 1.0 to 7.7 µmol g–1 FW in the roots (Aurisano et al. 1995). In view of the mitigation of K+ loss it would be of interest to investigate whether GABA also plays a role during an energy crisis in highly tolerant rice coleoptiles, which during anoxia showed some K+ net uptake at pH 6.5, but substantial K+ losses during exposure to pH 3.5 (Greenway et al. 2012) (see also ‘Energy budget during an energy crisis’).
Transport proteins
In contrast to the proteins participating in intermediary metabolism, transport proteins are seldom increased in amounts or composition as shown in an excellent review by Shabala et al. (2016). These authors found no substantial evidence for changes in most of the ‘transport proteins’. The exception being the convincing changes in the V-H+PPiase in germinating rice seedlings (Carystinos et al. 1995). The same paper showed a 2-fold increase in the maximum catalytic activity of the V-H+ATPase over 2 days anoxia; even so the protein level and maximum catalytic activity of the V-H+ATPase remained 10-fold lower than that of the V-H+PPiase (Q. Liu and B. J. Atwell, unpubl. data; Carystinos et al. 1995). Presumably, most of the other transport proteins were acclimated via regulation of existing channels and carriers. For example rapid changes in the PM-H+-ATPase are attributed to changes in membrane potential, there is no evidence for changes in PM-H+-ATPase transcripts and proteins (Shabala et al. 2016). Such very rapid changes can be elicited over a few seconds to minutes, as is observed for the PM- H+-ATPase (Shabala et al. 2016). Clearly, whether this general view holds, or whether there remain unknown changes in transport proteins, still needs to be further tested. At present, we suggest that the many fold changes in enzymes of intermediary metabolism can be attributed to the large changes in metabolism, which may occur in all conditions when environmental conditions change. Regarding transport proteins, plant tissues are always challenged by large changes in external ion concentration and other events requiring changes in transport. This general occurrence may therefore account for the predominance of regulation of existing proteins rather than induction of special transporters to cope with an energy crisis.
Summing up, we suggest that most plant species can acclimate to an energy crisis which is transient and so can survive an energy crisis for hours and sometimes as long as a day. A few very anoxia tolerant species tissues are distinguished by strong expression of some key ‘anaerobic’ proteins. The above information gives clues as to which plant’s tissues and mutants are most likely to be useful to elucidate the characteristics that lead to the vast difference between tissues in tolerance to an energy crisis.
Aquaporins
Aquaporins close under anoxia (Maurel et al. 2009) and at reduced pH (Frick et al. 2013). However, we could not find a rationale for this closure in terms of energy saving, an issue that deserves further investigation.
Hypotheses on messengers and their targets
After discussing the various components of anaerobic metabolism, we will now suggest which messengers may mediate the change from aerobic (energy plenty) to anaerobic (energy crisis) metabolism (Fig. 1).
Ca2+cyt: increases in Ca2+cyt at the onset of anoxia are well established and change activities of many enzymes (for an excellent review see Sachs et al. 1996). However, such increases in Ca2+cyt occur during several conditions apart from an energy crisis (Hepler and Wayne 1985) so Ca is not specific for anaerobic metabolism. Instead, we suggest the increase in Ca2+cyt is a messenger to engage networks common to plant tissues under several adverse conditions (Fig. 1). Ca2+ as a messenger was also involved in activation of starch hydrolysis in 4-day-old rice (Ho et al. 2017). Both changes in Ca2+ level and activation of amylases are involved in rice under multiple abiotic stresses (Ho et al. 2017). So, we suggest that the response is not specific to anoxia but part of a common network. This suggestion in no way implies that the mechanisms of such common networks are not interesting, but we have concentrated on specific anaerobic proteins and their distinction from proteins of common networks (Fig. 1).
Though not specific for anaerobiosis, the common networks would remain important for survival during anoxia. Support for this notion was found when increase in Ca2+cyt was prevented using ruthenium red: during flooding of maize, survival of the maize root tips was as short as 2 h compared with 72 h for tips with increased Ca2+cyt (Sachs et al. 1996). This assertion assumes, as indicated in Fig. 1, that high Ca2+cyt is a messenger that triggers engagement of common networks, rather than of only specific anaerobic proteins.
pHcyt: pHcyt is often considered as a messenger in several conditions resulting, among others, in activation of genes, protein kinases and changes in membrane transport (Felle 2001). Felle in this case prefers the term ‘cellular messenger’. pHcyt was ingeniously manipulated by pHext changes and applications of butyric acid (a weak acid) and methyl amine (a weak base) (Fox et al. 1995). Such treatments showed no artefacts during 3 h exposure to pHext between 4 and 10, giving sufficient time to evaluate the possible role of pHcyt as a messenger. Among others, it was demonstrated that a change in pHcyt in air did not trigger ethanol formation, i.e. as stated by Fox et al. (1995), the switch to ethanol formation requires another factor in addition to a decrease in pHcyt.
Changes in adenylate nucleotide pool: we suggest decreases in ATP, ATP/ADP, and/or energy charge might also directly change metabolism (Fig. 1). A case for ATP concentrations affecting the rate of ATP-H+ases was made in the in the first part of this review, viz. ‘Are the decreases in ATP level large enough to reduce activity of the H+-ATPases?’.
Inhibition of starch hydrolysis
Rice was the only cereal able to develop and activate starch hydrolysis during anoxia (Perata et al. 1992). Even several rice varieties are ‘sensitive’ to flooding in the germination stage (Ho et al. 2017), sensitivity due to insufficient starch hydrolysis and failure to emerge in flooded fields. The study by Ho et al. (2017) also highlights the importance of making comparisons between treatments at the same stage of development (as advocated by Blum 2011). Thus the ability to activate starch hydrolysis during anoxia is only well developed in some rice varieties. Detail on these varietal differences was obtained in a study with varieties exhibiting different abilities to develop a coleoptile during anoxia (Huang et al. 2003). In an indica variety (IR 22) that developed very slowly during anoxia, glucose in the endosperm was lower in anoxic intolerant than tolerant varieties. For example, at 3 days after the start of anoxia at germination, the endosperm of the most tolerant variety was 4 times higher in sugar than in the intolerant variety (Huang et al. 2003; Japonica and Indica respectively). No other major process seemed to be defective in the intolerant cultivar; transport from endosperm to embryo was unimpaired as shown by the high ratios of sugar in the embryo/endosperm. Further tests with excised coleoptile tips supplied with glucose showed no cultivar differences in the rate of ethanol fermentation and anoxia tolerance as measured by rapid resumption of rates of net K+ and P uptakes (Huang et al. 2003). Thus, in general, the main puzzle is why the common network of the sugar cascade often does not become engaged under anoxia and not fully activated in the intolerant rice cultivars. For the wheat, the delay in development due to the inability to activate the sugar cascade may be of advantage by delaying germination till more favourable conditions arise (Perata et al. 1992). We cannot suggest any acclimative advantage for the intermediate response of IR 22. In conclusion, the response of imbibed seeds to anoxia is an interesting phenomenon which could only be briefly considered in this review and deserves further consideration.
Choice of species for investigation
Here we compare the merits of Arabidopsis and rice coleoptiles, the most frequent objects of investigation.
Arabidopsis is a species that is very intolerant to anoxia, and has been successfully used to elucidate many aspects of response of tissues to an anoxia-energy crisis (see ‘Networks’ sections above). As is well known, the advantage of Arabidopsis is its small and sequenced genome and the ease to get mutants; its main drawback is that so far none of the known genotypes mutants has a really high tolerance to an anoxia- energy crisis.
One attractive possibility is to do similar molecular studies with rice. Rice is diploid, has a sequenced genome (Goff et al. 2002) and has many useful mutants (Hirochika et al. 2004). Further, several genotypes have a very high tolerance to anoxia, so far established for the coleoptiles during germination; the high tolerance excludes the confounding of results relevant to an energy crisis with changes due to injury and approaching death (see ‘Deficient experimental techniques’ below). The germination phase has also the advantage of the ease of imposing anoxia on a simple system: consisting only of seeds and coleoptiles. There is an extensive literature on the response to anoxia of these germinating rice seeds (recently reviewed by Atwell et al. 2015). The suggestion to further employ rice seems to contradict the view expressed by Shabala et al. (2014), that there has probably been too much emphasis on rice. However, that contradiction is only apparent when considering that these authors were concerned with the multifactor stress of flooding, whereas the present review is focussed on an energy crisis of tissues in solution culture. Hence, our emphasis on germinating rice seedlings was deliberate as the most straight forward route to elucidate anoxia tolerance. To elaborate somewhat, we caution against failure to clearly distinguish the phenomenon of germinating rice under an energy crisis and the tolerance to complete submergence of whole rice plants, in the latter case anoxia tolerance has a subsidiary role to elongation (Colmer et al. 2014). Whether rice leaves and/or roots ever have a high tolerance to anoxia-energy crisis is still doubtful (roots reviewed by Gibbs and Greenway 2003). Studies on rice mutants have been quite rewarding to elucidate responses of roots differing in suberin during flooding (Shiono et al. 2014). Regarding the use of rice mutants to study metabolism during an energy crisis, the only studies we know are with young seedlings, including mutants lacking ADH-1 (see above: ‘Anaerobic proteins induced in most genotypes’ ).
Summing up, if mutant lines for specific genes involved in an energy crisis could be found in rice, the great advantage would be that tolerant and intolerant tissues from the same species can be compared, rather than, for example, Arabidopsis with rice coleoptiles.
Confounding responses to an energy-crisis-anoxia-tolerance with responses to injury
The possible confounding of features of energy crisis tolerance with responses due to injury needs some further emphasis. Felle (2005), considering data obtained with anoxia-intolerant species, comments on the difficulty of distinguishing the period when there is a new set point for pHcyt and the subsequent period of fatal decline in pHcyt. Another example is for hypoxically pre-treated, 4.75-day-old maize roots (Carystinos et al. 1995): after a further 2 days anoxia, both the V-H+ATPase and the V-H+PPiase of the 5 mm root tips declined by 70 and 50% respectively (basis, protein of isolated microsomes). These authors suggested the likely cause for this decline in the maize root tissues was tissue damage. In contrast rice seedlings increased in both these translocases over 6 days anoxia, i.e. the patterns indicated injury in maize and acclimation in rice respectively. There is no problem of confounding changes due to acclimation with changes due to injury when using anoxia tolerant species such as red beet storage root tissue and tolerant genotypes of rice coleoptiles. Indeed these tissues need special adverse conditions, such as those leading to carbohydrate starvation; or high temperatures, to trigger a fatal decline.
Deficient experimental methodology
It is important to establish that growth resumes after re-aeration of tissues that have been exposed to an anaerobiosis energy crisis, to demonstrate there has been no permanent injury (Thomas et al. 1973). We would like to sharpen the criterion by Thomas et al. (1973) by adding ‘with a time course over the first hours after return to air’, otherwise quite serious injury might have been repaired. Unfortunately, even in recent papers there are either no recovery tests, or more frequently, the first data after transfer to aeration are obtained a few days after re-aeration, e.g. 7 days after a prior 7 h of anoxia (Paul et al. 2016) and 3 days after 12 h hypoxia (Gibbs et al. 2011). Further, the survival criteria are often photographs and visual scores on chlorosis, e.g. taken at 3 days after return from anoxia to air (Gibbs et al. 2011). None of these tests excludes serious injury during exposure to anoxia, which may have been repaired during the long period of re-aeration. Studies need time-courses of observations of sensitive indicators of cell performance starting during the first hours after re-aeration. Suitable ones include plasma membrane potential, resumption of extension growth and of energy dependent uptake using Cl– at an external concentration requiring energy dependent transport (e.g. 0.05–0.1 mM Cl–). Only when such tests show that over the period of observation there was no serious injury does it become worthwhile to do metabolic and molecular studies on anoxia tolerance. For studies on anoxia tolerance several techniques leading to injury were listed by Gibbs and Greenway (2003) and a few further comments are provided in Supplement 4.
Another serious deficiency in methodology is the failure to acclimate the tissues before transfer to anoxia; then, as demonstrated first by Saglio et al. (1988) with 5 mm tips of roots of intact maize plants, anoxic shock results in early death and/or injury. Hypoxic pretreatment is now used by many workers, but unfortunately the pre-treatment with 50 µM O2 (suitable for maize roots) has been applied to other species. This adoption may be unwarranted, since endogenous O2 concentrations depend on several factors (W. Armstrong, T. D. Colmer, P. M. Beckett and H. Greenway, unpubl. data). A simple O2 response curve of tissue respiration with a Clark electrode and cuvette can establish a likely suitable O2 concentration for hypoxic pretreatment (see Supplement 5). A second confounding factor may be deficiency of carbohydrates by limiting substrates for glycolysis. This phenomenon has been known since the 1960s (Gibbs and Greenway 2003; Vartapetian et al. 2003). In this review we have mainly considered some experiments with rice seedlings, or coleoptiles (see earlier ‘Energy budget during an energy crisis’). Sucrose addition also increased anoxia tolerance of Arabidopsis (Paul et al. 2016). So, unless the aim is to study the consequences of the interaction between hypoxia and sugar starvation, the standard procedure should be to supply sugars. Yet another pitfall is not to cater for different pattern of development during comparisons of genotypes. For example, during studies on drought resistance, samplings of different genotypes, or other treatments, are all taken on the same day, rather than taking tissues of the same level of plant water status. As another example, relative expression of CBL4, ClPK 14-15 and RAMY 3D (the start of the sugar cascade) in coleoptiles was, unexpectedly, much larger in anoxic than aerated 4-day-old rice seedlings (Ho et al. 2017). However, this difference is presumably due to early senescence of the aerated coleoptiles as indicated by a 9-fold decrease in ATP concentration between the first and third day after the start of germination in air, whereas the ATP decrease in anoxia was only 0.3-fold (Ishizawa et al. 1999), with both treatments having around the same ATP concentration on day 1. So, the better comparison would have been between coleoptiles of aerated seedlings on day 1 and anoxic seedlings at day 3.
Conclusions
The data indicating that ‘anaerobic’ metabolism can occur in well-aerated tissues, when oxidative phosphorylation is inhibited have been discussed in the conclusions to the first part of this review. We have suggested these changes in metabolism are complemented by changes in ion transport such as more energy efficient coupling ratios and reductions in membrane permeabilities to solutes (see ‘Reductions in energy charge and ATP and Reductions in trans-membrane transport’ in the first part of this review). The special case of O2 deficiency and anoxia remains worth detailed consideration, partly because this case has been intensively investigated and partly because of its relevance to flooding tolerance of plants.
The frequently observed numerous changes in transcripts and enzyme levels during anoxia (see ‘Networks’ sections) can partly be explained by injury and approaching death associated with use of anoxia intolerant species and/or faulty techniques. However, even in anoxia tolerant germinating rice seedlings investigated in experiments with satisfactory techniques, there are still multitudes of changes in levels of transcripts and proteins (Huang et al. 2005b; Lasanthi-Kudahettige et al. 2007; Shingaki-Wells et al. 2014). We propose that these are mainly related to the large change in metabolism changing from a condition providing plenty of energy to an acute energy crisis. Many of these changes may be related to the LES network common to a range of adverse conditions where metabolism is drastically altered. The N-end rule pathway (excluding the ‘O2 sensitive’ branch) would also be relevant to many conditions when metabolism changes. These general networks would need to be complemented by a much smaller array of enzymes associated specifically with an energy crisis, caused by inhibition of oxidative phosphorylation. Of these key energy crisis proteins several (ADH among others) occur in anoxia intolerant and tolerant tissues. Another important aspect relates to the de novo synthesis of the energy crisis enzymes involving ERF transcription factors and the ‘O2 sensitive’ branch of the N-end rule pathway. We suggest there may be another group of anaerobic proteins, which we call ‘key anoxic proteins’, which would occur in sufficient amounts only in tolerant tissues so that these proteins would contribute to the difference in tolerance between species tissues. Presently it seems the energy crisis enzymes occurring in both tolerant and intolerant tissues are mainly those involved in glycolysis. The key energy crisis proteins expressed specifically in tolerant tissue include Pgb, the V-H+PPiase and PDC. In contrast, changes in proteins of carriers and channels for ion transport have so far not been found, excluding the well-known case of the V-H+PPiase. Instead, regulation of existing proteins of ion transporters seems to be the norm. In view of the crucial importance of maintaining trans-membrane gradients we suggest it would be of value to research possible differences in regulation of ion transport between anoxia tolerant and intolerant tissues.
Carbohydrate starvation leads to irreversible injury of rice coleoptiles, even so this injury occurred only as late as 50 h after ethanolic fermentation had already been drastically curtailed. Clearly at least all energy saving devices discussed are particularly relevant to cases where an energy crisis is due to carbohydrate starvation.
Finally, we suggest there are two main routes for gene manipulation to further the elucidation of mechanisms of tolerance to an energy crisis: (1) comparisons between very tolerant and intolerant species, and (2) manipulations within a particular species. For comparisons with tolerant tissues like germinating rice, one choice would be Arabidopsis, with the vast information and its huge genetic variation. However, it may be more useful in the future to concentrate on rice, since its genome yields ample genetic variation and can be relatively easily manipulated. The great advantage would be that the anoxia intolerant and tolerant genotypes would be rather similar, except in respect to their difference in tolerance to an energy crisis.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Steve Tyerman for many incisive criticisms, mainly on the transport sections, as well as encouragement for completing the review; Tim Colmer for excellent suggestions and comments during the last stage of drafting the paper; Rana Munns for much encouragement and helpful comments throughout the drafting of this review; Sergey Shabala for guiding us to several relevant papers on solute transport; Brian Atwell for helpful comments on an early draft; and the anonymous referee for greatly helping us to get the molecular biology aspects in reasonable shape.
References
Andrews DL, Drew MC, Johnson JR, Cobb BG (1994) The response of maize seedlings of different ages to hypoxic and anoxic stress. Changes in induction of Adh1mRNA, ADH activity, and survival of anoxia. Plant Physiology 105, 53–60.| The response of maize seedlings of different ages to hypoxic and anoxic stress. Changes in induction of Adh1mRNA, ADH activity, and survival of anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXjtF2msLo%3D&md5=97bc2bf650ff4caac6298b4ca313a6d1CAS |
Armstrong W, Armstrong J (2014) Plant internal oxygen transport (diffusion and convection) and measuring and modelling oxygen gradients. Plant Cell Monographs 21, 267–298.
Armstrong W, Beckett PM (2011a) Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytologist 190, 431–441.
| Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1Gktbw%3D&md5=58920a23bc54f35d9f120163fe2e17e0CAS |
Armstrong W, Beckett PM (2011b) The respiratory down-regulation debate. New Phytologist 190, 276–278.
| The respiratory down-regulation debate.Crossref | GoogleScholarGoogle Scholar |
Armstrong W, Webb T, Darwent M, Beckett PM (2009) Measuring and interpreting respiratory critical oxygen pressures in roots. Annals of Botany 103, 281–293.
| Measuring and interpreting respiratory critical oxygen pressures in roots.Crossref | GoogleScholarGoogle Scholar |
Atwell BJ, Greenway H, Colmer TD (2015) Efficient use of energy in anoxia-tolerant plants with focus on germinating rice seedlings. New Phytologist 206, 36–56.
| Efficient use of energy in anoxia-tolerant plants with focus on germinating rice seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXjtF2ru70%3D&md5=50568154a0acea9c816a381d9784cf46CAS |
Aurisano N, Bertani A, Reggiani R (1995) Anaerobic accumulation of 4-aminobutarate in rice seedlings: causes and significance. Phytochemistry 38, 1147–1150.
| Anaerobic accumulation of 4-aminobutarate in rice seedlings: causes and significance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlsVGqsbo%3D&md5=1c31955eb8bc7122781e2e379674547bCAS |
Beevers H (1953) 2,4-dinitrophenol and plant respiration. American Journal of Botany 40, 91–96.
| 2,4-dinitrophenol and plant respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG3sXisF2itw%3D%3D&md5=657fcaf2a2c1445d843229b875684c8cCAS |
Bieniawska Z, Barrett PP, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. The Plant Journal 49, 810–828.
| Analysis of the sucrose synthase gene family in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjs1WrtLw%3D&md5=e129bfbba04ed71c453fb95b22ddf1cdCAS |
Blum A (2011) Drought resistance – is it really a complex trait? Functional Plant Biology 38, 753–757.
| Drought resistance – is it really a complex trait?Crossref | GoogleScholarGoogle Scholar |
Botrel A, Kaiser WM (1997) Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta 201, 496–501.
| Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtVehsrY%3D&md5=3d17694a0a0fec9494c073401592ad2cCAS |
Carystinos GD, Macdonald HR, Monroy AF, Dhindsa RS, Poole RJ (1995) Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiology 108, 641–649.
| Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtlaqsrk%3D&md5=466d3ef9deb22611d8005169f8b48013CAS |
Chang WWP, Huang L, Shin M, Webster C, Burlingname ALR, Roberts JKM (2000) Patterns of protein synthesis and tolerance of anoxia in root tips of Zea mays seedling acclimated to low oxygen concentrations and identification of proteins by mass spectrometry. Plant Physiology 122, 295–317.
| Patterns of protein synthesis and tolerance of anoxia in root tips of Zea mays seedling acclimated to low oxygen concentrations and identification of proteins by mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktFCjt7o%3D&md5=7798d1cc91e6c947c782b83fe35a74bbCAS |
Christie PJ, Hahn M, Walbot BM (1991) Low-temperature accumulation of alcohol dehydrogenase-1 mRNA and protein activity in maize and rice seedlings. Plant Physiology 95, 699–706.
| Low-temperature accumulation of alcohol dehydrogenase-1 mRNA and protein activity in maize and rice seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitVelsbY%3D&md5=a9e7f778375eda217104dd17f2008f8aCAS |
Colmer TD, Huang S, Greenway H (2001) Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia. Journal of Experimental Botany 52, 1507–1517.
| Evidence for down-regulation of ethanolic fermentation and K+ effluxes in the coleoptile of rice seedlings during prolonged anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlvVCmtbk%3D&md5=1cfe9f21e7558d11eb1a7ed12790abd4CAS |
Colmer TD, Armstrong W, Greenway H, Ismail AM, Kirk GJD, Atwell BJ (2014) Physiological mechanisms in flooding tolerance of rice: transient complete submergence and prolonged standing water. Progress in Botany 75, 255–307.
| Physiological mechanisms in flooding tolerance of rice: transient complete submergence and prolonged standing water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXotlOms7g%3D&md5=034c2f017f9f49b793db299b34f76cb4CAS |
Cuin TA, Shabala S (2007) Amino acids regulate salinity induced potassium efflux in barley root epidermis. Planta 225, 753–761.
| Amino acids regulate salinity induced potassium efflux in barley root epidermis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1yht7s%3D&md5=6c3876251f1e5851ae25e62de292d06eCAS |
Cvetskovska M, Vanlerberghe GC (2012) Alternative oxidase modulates leaf mitochondrial concentration of superoxide and nitric oxide. New Phytologist 195, 32–39.
Davies JM, Poole RJ, Sanders D (1993) The computed free energy change of hydrolysis of inorganic pyrophosphate and ATP: apparent significance for inorganic-pyrophosphate-driven reactions of intermediary metabolism. Biochimica et Biophysica Acta 1141, 29–36.
| The computed free energy change of hydrolysis of inorganic pyrophosphate and ATP: apparent significance for inorganic-pyrophosphate-driven reactions of intermediary metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhvVKgsr0%3D&md5=6299aeccd3bf4258a1a81156173c4d60CAS |
de Visser R, Spitters CJT, Bouma TJ (1992) Energy cost of protein turnover: theoretical calculation and experimental estimation from regression of respiration on protein concentration of full-grown leaves. In ‘Molecular and biochemical and physiological aspects of plant respiration’. (Eds H Lambers, LWH van der Plas) pp. 493–508. (SPB Academic Publishing: the Hague, The Netherlands)
Demidchik V, Cuin TA, Svistunenko D, Smith SL, Millar AJ, Shabala S, Yurin Y (2010) Arabidopsis root K+ efflux conductance activated by hydroxyl radicles: single channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479.
| Arabidopsis root K+ efflux conductance activated by hydroxyl radicles: single channel properties, genetic basis and involvement in stress-induced cell death.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1KmtL0%3D&md5=61b426f853897fdb74cb55d3ea12ffa5CAS |
Demmer NK (2017) A reverse genetic approach to low oxygen tolerance in Arabidopsis. Masters thesis. Macquarie University, Sydney, Australia.
Dennis ES, Olive M, Dolferus R, Millar A, Peacock WJ, Setter TL (1992) Biochemistry and molecular biology of the anaerobic response. In ‘Inducible plant proteins: their biochemistry and molecular biology. Society for Experimental Biology Seminar Series 49’. (Ed. JL Wray) pp. 231–245. (Cambridge University Press: Cambridge, UK)
Dixon MH, Hill SA, Jackson MB, Ratcliffe RG, Sweetlove LJ (2006) Physiological and metabolic adaptations of Potamogeton pectinatus L. tubers support rapid elongation of stem tissue in the absence of oxygen. Plant & Cell Physiology 47, 128–140.
| Physiological and metabolic adaptations of Potamogeton pectinatus L. tubers support rapid elongation of stem tissue in the absence of oxygen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht12ksb0%3D&md5=dbbafc60c3969eb1e0547b67cffc1567CAS |
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48, 223–250.
| Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjs1ensLc%3D&md5=8d3ab7e73edb9d21695fde1ffee62164CAS |
Duff SMG, Moorhead GBG, Lefebre DD, Plaxton WC (1989) Phosphate starvation inducible ‘bypasses’ of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiology 90, 1275–1278.
| Phosphate starvation inducible ‘bypasses’ of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXlslWhs7w%3D&md5=32a28baea9bcf1dc6b5236bb49476a66CAS |
Edwards JM, Roberts TH, Atwell BJ (2012) Quantifying ATP turnover in anoxic rice coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis. Journal of Experimental Botany 63, 4389–4402.
| Quantifying ATP turnover in anoxic rice coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1egtbfF&md5=3f07fc5178716e1578156bccea9f602bCAS |
Ellis MH, Millar AA, Llewellyn DJ, Peacock WJ, Dennis ES (2000) Transgenic cotton (Gossypium hirsutum) over-expressing alcohol dehydrogenase shows increased ethanol fermentation but no increase in tolerance to oxygen deficiency. Australian Journal of Plant Physiology 27, 1041–1050.
Felle HH (2001) pH: signal and messenger in plant cells. Plant Biology 3, 577–591.
| pH: signal and messenger in plant cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVyktA%3D%3D&md5=24f1d4e6fe3c29039a63840392d97b5fCAS |
Felle HH (2005) pH regulation in anoxic plants. Annals of Botany 96, 519–532.
| pH regulation in anoxic plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGitLnK&md5=76452e1ae996ef6417c4f3d487525ad4CAS |
Fox GG, Maccallan NR, Ratcliffe RG (1995) Manipulating cytoplasmic pH under anoxia: a critical test of the role of pH in the switch from aerobic to anaerobic metabolism. Planta 195, 324–330.
| Manipulating cytoplasmic pH under anoxia: a critical test of the role of pH in the switch from aerobic to anaerobic metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXjt1ers7k%3D&md5=cd0ce8d3f91b22009171c1fb25094b21CAS |
Frenkel C, Erez A (1996) Induction of chilling tolerance in cucumber (Cucumis sativus) seedlings by endogenous and applied ethanol. Physiologia Plantarum 96, 593–600.
| Induction of chilling tolerance in cucumber (Cucumis sativus) seedlings by endogenous and applied ethanol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xisleqsb8%3D&md5=aa7b5f3b9867816e493a4d153c0cf106CAS |
Frick A, Jarva M, Tömroth-Horsefield S (2013) Structural basis for pH gating of plant aquaporins. FEBS Letters 587, 989–993.
| Structural basis for pH gating of plant aquaporins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvVOltrc%3D&md5=093249041ac2fd08e8fd836814466743CAS |
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I Growth, survival and anaerobic catabolism. Functional Plant Biology 30, 1–47.
| Mechanisms of anoxia tolerance in plants. I Growth, survival and anaerobic catabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitVCjtLc%3D&md5=570511dfec73ed1b3d9d0443db4b346dCAS |
Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H (1998) Response to oxygen deficiency in primary roots of maize. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem. Australian Journal of Plant Physiology 25, 745–758.
| Response to oxygen deficiency in primary roots of maize. I. Development of oxygen deficiency in the stele reduces radial solute transport to the xylem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXnsFCqtb8%3D&md5=37ceacecd41dc599a85774511c5a2f9eCAS |
Gibbs J, Morrell S, Valdez A, Setter TL, Greenway H (2000) Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. Journal of Experimental Botany 51, 785–796.
| Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtF2jsLo%3D&md5=b702d1ba3c6e91a2d0692c435559ce83CAS |
Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, Holdsworth MJ (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway. Nature 479, 415–418.
| Homeostatic response to hypoxia is regulated by the N-end rule pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGks7nP&md5=51168b0e4aa8f5b38b129691ff62c6c6CAS |
Gibbs DJ, Conde JV, Berckhan S, Prahad G, Mendiondo GM, Holdsworth MJ (2015) Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress response in plants. Plant Physiology 169, 23–31.
| Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress response in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xkslyhtw%3D%3D&md5=62cf611507e90acd52683e0715de38f7CAS |
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296, 92–100.
| A draft sequence of the rice genome (Oryza sativa L. ssp. japonica).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XivVSqtrw%3D&md5=a0eadafa4c4c0ed09484419680a4a0f6CAS |
Graciet E, Wellmer F (2010) The plant N-end rule pathway: structure and functions. Trends in Plant Science 15, 447–453.
| The plant N-end rule pathway: structure and functions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvV2isbw%3D&md5=fba8fbf81fcaa0bd66c49bce6336d13dCAS |
Greenway H, Gibbs J (2003) Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to energy consuming processes. Functional Plant Biology 30, 999–1036.
| Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to energy consuming processes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1Cgtbs%3D&md5=0a842b6f7567d0e49c88151c82f26900CAS |
Greenway H, Kulichikhin KY, Cawthray GR, Colmer TD (2012) pH regulation in anoxic rice coleoptiles at pH 3.5: biochemical pH stat and net H+ influx in the presence and absence of NO3 –. Journal of Experimental Botany 63, 1969–1983.
| pH regulation in anoxic rice coleoptiles at pH 3.5: biochemical pH stat and net H+ influx in the presence and absence of NO3 –.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjslehtrc%3D&md5=59b099306046fcc335c421f5ce3f8077CAS |
Gronewald JW, Hanson JB (1982) Adenine nucleotide content of corn roots as affected by injury and subsequent washing. Plant Physiology 69, 1252–1256.
| Adenine nucleotide content of corn roots as affected by injury and subsequent washing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XkvV2jtbY%3D&md5=a493c8efb5d8dbb1d3d542d7808cb86cCAS |
Gronewald JW, Cheeseman HM, Hanson JB (1979) Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiology 63, 255–259.
| Comparison of the responses of corn root tissue to fusicoccin and washing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhsVylurs%3D&md5=eca4409886d766d4662bc6bfc0711240CAS |
Gupta KJ, Igamberdiev AU (2011) . Mitochondrion 11, 537–543.
| .Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXosVSnt7g%3D&md5=c54b86573662adeddccc65f30ae43fe0CAS |
Hafke JB, Neff R, Huett MT, Luettge U, Thiel G (2001) Day-to-night variations of cytoplasmic pH in a crassulacean acid metabolism plant. Protoplasma 216, 164–170.
| Day-to-night variations of cytoplasmic pH in a crassulacean acid metabolism plant.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnos1Oktg%3D%3D&md5=c5abe051017c64624f6b991509f93826CAS |
Hedrich R, Fluegge UI, Fernandez JM (1986) Patch-clamp studies of ion transport in isolated plant vacuoles FEBS Letters 204, 228–232.
| Patch-clamp studies of ion transport in isolated plant vacuolesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xls1yqu70%3D&md5=0ef5407e5728bea7b8af9e4c28671fe9CAS |
Hedrich R, Kurdjian A, Guern J, Fluegge UI (1989) Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysomal compartment. EMBO Journal 8, 2835–2841.
Hepler PK, Wayne RO (1985) Calcium and plant development. Annual Review of Plant Physiology 36, 397–439.
| Calcium and plant development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXktlCrtrs%3D&md5=668ab1ccce279f609f7a03e82559616cCAS |
Hirochika H, Guiderdoni E, An G, Hsing YI, Eun MY, Han CD, Upadhyaya N, Ramachandran S, Zhang Q, Pereira A, Sundaresan V, Leung H (2004) Rice mutant resources for gene discovery. Plant Molecular Biology 54, 325–334.
| Rice mutant resources for gene discovery.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmtVOit7c%3D&md5=06ad0b535bbd66d43d090b90b4c111cfCAS |
Ho VT, Tran AN, Cardarelly F, Perata P, Pucciariello C (2017) A calcineurin B-like protein participates in low oxygen signalling in rice. Functional Plant Biology 44, 917–928.
| A calcineurin B-like protein participates in low oxygen signalling in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhtlSitLjN&md5=8a3fde5c16a312497878779d18f2e87fCAS |
Huang S, Greenway H, Colmer TD (2003) Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-‘intolerant’, but not of a -‘tolerant’, genotype. Journal of Experimental Botany 54, 2363–2373.
| Anoxia tolerance in rice seedlings: exogenous glucose improves growth of an anoxia-‘intolerant’, but not of a -‘tolerant’, genotype.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXns1Cnsb8%3D&md5=c586081cb26df5e3a20a4b42ce7aca74CAS |
Huang S, Ishizawa K, Greenway H, Colmer TD (2005a) Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rates of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia. Journal of Experimental Botany 56, 2453–2463.
| Manipulation of ethanol production in anoxic rice coleoptiles by exogenous glucose determines rates of ion fluxes and provides estimates of energy requirements for cell maintenance during anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVahtLjP&md5=83f22931086d30f82165c1d4773428fcCAS |
Huang S, Greenway H, Colmer TD, Millar AH (2005b) Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth, and energy utilisation. Annals of Botany 96, 703–715.
| Protein synthesis by rice coleoptiles during prolonged anoxia: implications for glycolysis, growth, and energy utilisation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGitLbL&md5=defffdb3b3b6207badabe61990c270e4CAS |
Hunt PW, Klok EJ, Trevaskis B, Watts RA, Ellis MH, Peacock WJ, Dennis EJ (2002) Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America 99, 17197–17202.
| Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvV2qtA%3D%3D&md5=a33c34d0d34bfa626e5eeef6344a4899CAS |
Igamberdiev AU, Hill RD (2009) Plant mitochondrial function during plant anaerobiosis. Annals of Botany 103, 259–268.
| Plant mitochondrial function during plant anaerobiosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjsFCkur8%3D&md5=d7900b97e73bca49ba8afab39e0c8bf3CAS |
Igamberdiev AU, Kleczkowski LA (2011) Magnesium and cell energetics in plants under anoxia The Biochemical Journal 437, 373–379.
| Magnesium and cell energetics in plants under anoxiaCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVeksbk%3D&md5=78538d434e68944568df7a98060e484bCAS |
Igamberdiev AU, Bykova NV, Hill RD (2011) Structural and functional properties of class 1 plant hemoglobins. IUBMB Life 63, 146–152.
| Structural and functional properties of class 1 plant hemoglobins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVWitL0%3D&md5=dd16087ea77fabeb65399dcb0e4d6aa0CAS |
Ishizawa K, Murakami S, Kawakani Y, Kuzumochi H (1999) Growth and energy status of arrowhead tubers, pondweed turions and rice seedlings under anoxic condition. Plant, Cell & Environment 22, 505–514.
| Growth and energy status of arrowhead tubers, pondweed turions and rice seedlings under anoxic condition.Crossref | GoogleScholarGoogle Scholar |
Ismond KP, Dolferus R, De Pauw M, Dennis ES (2003) Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiology 132, 1292–1302.
| Enhanced low oxygen survival in Arabidopsis through increased metabolic flux in the fermentative pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsFGhtLk%3D&md5=7208ed40db56425fc965a851b89919d8CAS |
Kaiser WWM, Weiner H, Huber SC (1999) Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiologia Plantarum 105, 384–389.
| Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity.Crossref | GoogleScholarGoogle Scholar |
Kato-Noguchi H (2000) Evaluation of the importance of lactate for the activation of ethanol formation in lettuce roots in anoxia. Physiologia Plantarum 109, 28–33.
| Evaluation of the importance of lactate for the activation of ethanol formation in lettuce roots in anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjs1Ggs7k%3D&md5=2e9a58a6f236634c00309720c51ac60eCAS |
Kato-Noguchi H (2001) Wounding stress induces alcohol dehydrogenase in maize and lettuce seedlings. Plant Growth Regulation 35, 285–288.
| Wounding stress induces alcohol dehydrogenase in maize and lettuce seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XisVyqtr8%3D&md5=407b9292ab5aff539fd4a8b753bc2322CAS |
Kimmerer TW, Kozlowski TT (1982) Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress. Plant Physiology 69, 840–847.
| Ethylene, ethane, acetaldehyde, and ethanol production by plants under stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xhslyjt78%3D&md5=afa5b85b317fee86b8af3b6eb6df1d68CAS |
Koizumi Y, Hara Y, Yasaki Y, Sakano S, Isazawa K (2011) Involvement of plasma membrane H+-ATPase in elongation of stems in pond weed (Potamogeton distinctus) turions. New Phytologist 190, 421–430.
| Involvement of plasma membrane H+-ATPase in elongation of stems in pond weed (Potamogeton distinctus) turions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1Gktb8%3D&md5=7fe2facca7b2687fc5ca6f330ac386d0CAS |
Kosmacz N, Parlanti S, Schwarzlander M, Kragler F, Licausi F, van Dongen JT (2015) The stability and nuclear localisation of the transcription factor RAP 2.12 are dynamically related by oxygen concentration. Plant, Cell & Environment 38, 1094–1103.
| The stability and nuclear localisation of the transcription factor RAP 2.12 are dynamically related by oxygen concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXotVSmtrw%3D&md5=e5ff2e2f90fa4b2cf11125737052bd6fCAS |
Kulichikhin KY, Aitio O, Chirkova TV, Fagerstedt KV (2007) Effect of O2 concentration on intra cellular pH, glucose6P and NTP content in rice (Oryza sativa) and wheat (Triticum aestivum) root tips: in vivo 31-P-NMR. Physiologia Plantarum 129, 507–518.
| Effect of O2 concentration on intra cellular pH, glucose6P and NTP content in rice (Oryza sativa) and wheat (Triticum aestivum) root tips: in vivo 31-P-NMR.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVykur0%3D&md5=99e473b057dafc6637acecd2e996027aCAS |
Kulichikhin KY, Greenway H, Byrne L, Colmer TD (2009) Regulation of intracellular pH during anoxia in rice coleoptiles in acidic and near neutral circumstances. Journal of Experimental Botany 60, 2119–2128.
| Regulation of intracellular pH during anoxia in rice coleoptiles in acidic and near neutral circumstances.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtFSjuro%3D&md5=eb4afcfd9f1431cfd216286b5adfe930CAS |
Kurniasih B, Greenway H, Colmer TD (2017) Energetics of acclimation to NaCl by submerged, anoxic rice seedlings. Annals of Botany 119, 129–142.
| Energetics of acclimation to NaCl by submerged, anoxic rice seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC1cXltlCqtbg%3D&md5=4a28de0b1cad8404e0770777fe0c2fbdCAS |
Lasanthi-Kudahettige R, Magneschi L, Loretti E, Gonzali L, Licausite F Novi G, Berretta O, Viluti F, Alpi A, Perata P (2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiology 144, 218–231.
| Transcript profiling of the anoxic rice coleoptile.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXls1KjtL8%3D&md5=ab70cb4c225c9bc0913a03f46338b95aCAS |
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilisation. Nature 479, 419–422.
| Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilisation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlGks73L&md5=20e41658c596e0f7aba12c5898629e03CAS |
Long AR, Lorraine E, Nelson SJ, Hall JL (1995) Localisation of membrane phosphatase activity in Ricinus communis seedlings. Journal of Plant Physiology 146, 629–638.
| Localisation of membrane phosphatase activity in Ricinus communis seedlings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXotlOgu7g%3D&md5=b8d227943a0d3ab472d7e7a512b2dbcaCAS |
Lyons JM (1973) Chilling injury in plants. Annual Review of Plant Physiology 24, 445–466.
| Chilling injury in plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3sXlt1emurg%3D&md5=e445bc3c067c4bf3a11ce1d7286032c2CAS |
Lyons JM, Raison JK (1970) Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiology 45, 386–389.
| Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXks1amtL4%3D&md5=91523fc0030c6e1d41e7e48d63ce8a18CAS |
Maeshima M (2000) Vacuolar pyrophosphatases. Biochimica et Biophysica Acta 1465, 37–51.
| Vacuolar pyrophosphatases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXit1Wgtr4%3D&md5=38f36deb5ccb723abf471e76e401981aCAS |
Mancuso S, Marras AM (2006) Adaptive responses of Vitis root to anoxia. Plant & Cell Physiology 47, 401–409.
| Adaptive responses of Vitis root to anoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsVCltbw%3D&md5=bfa69a4ee5afdd4b9dea368ad134c306CAS |
Maurel C, Santoni V, Doang-Trung L, Wudic MM, Verdoucq L (2009) The cellular dynamic of plant aquaporin expression and function. Current Opinion in Plant Biology 12, 690–698.
| The cellular dynamic of plant aquaporin expression and function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2murnM&md5=25d0f19c5f54c4c186b9f8f09a866993CAS |
Menegus F, Cattaruzza L, Mattana M, Beffagna N, Ragg E (1991) Response to anoxia in rice and wheat seedlings. Changes in the pH of intracellular compartments, glucose-6-phosphate level and metabolic rate. Plant Physiology 95, 760–767.
| Response to anoxia in rice and wheat seedlings. Changes in the pH of intracellular compartments, glucose-6-phosphate level and metabolic rate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXitVeltrw%3D&md5=ab90046e8f750b3843fd65b8d49b49fcCAS |
Miyashita Y, Dolferus R, Ismond KP, Good AG (2007) Alanine amino transferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. The Plant Journal 49, 1108–1121.
| Alanine amino transferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXktFGitbg%3D&md5=5c7b21195f3d9d0ecefa0098b541ac10CAS |
Mocquot B, Prat C, Mouches C, Pradet AP (1981) Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiology 68, 634–640.
Morgan PW, Drew MC (1997) Ethylene and plant response to stress. Physiologia Plantarum 100, 620–630.
| Ethylene and plant response to stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXks1yqsLw%3D&md5=bc148b30d9a99ef5d039c7dc9241b217CAS |
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432.
| Genome-wide analysis of the ERF gene family in Arabidopsis and rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsV2itLk%3D&md5=10a8d7bf07bbe379a42316ecfa8e992fCAS |
Narsai R, Rocha M, Geigenberger P, Whelan J, van Dongen JT (2011) Comparative analysis between plant species of transcriptional and metabolic response to hypoxia. New Phytologist 190, 472–487.
| Comparative analysis between plant species of transcriptional and metabolic response to hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmt1Gktbs%3D&md5=514ed7c571bc1c077b78d7d988ccd4a4CAS |
Nie X, Hill RD (1997) Mitochondrial respiration and hemoglobin gene expression in barley aleurone layers. Plant Physiology 114, 835–840.
| Mitochondrial respiration and hemoglobin gene expression in barley aleurone layers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkslOqs7c%3D&md5=5409623f7a9c76ea9647cd2b93214d07CAS |
Palma DA, Blumwald E, Plaxton WC (2000) Upregulation of H+-translocating pyrophosphatase by phosphate starvation of Brassica napus (rapeseed) suspension cell cultures. FEBS Letters 486, 155–158.
| Upregulation of H+-translocating pyrophosphatase by phosphate starvation of Brassica napus (rapeseed) suspension cell cultures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXoslGhs78%3D&md5=c67863a4190bf475702e2e1ff596845aCAS |
Paul MV, Lyer S, Amerhausen C, Lehmann M, van Dongen JT, Geigenberger P (2016) Oxygen sensing via the ethylene response transcription factor RAP 2.12 affects plant performance under both normoxia and hypoxia. Plant Physiology 172, 141–153.
| Oxygen sensing via the ethylene response transcription factor RAP 2.12 affects plant performance under both normoxia and hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhvFOrtbbO&md5=308ac96f146bc0fa20e3efc4a9a4800aCAS |
Penning de Vries FWT (1975) The cost of maintenance processes in plant cells. Annals of Botany 39, 77–92.
| The cost of maintenance processes in plant cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XhsVOmu74%3D&md5=5cfe64677d4b929bc274bb819296162cCAS |
Perata P, Pozuela-Romero J, Akazawa T, Yamaguchi J (1992) Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188, 611–617.
| Effect of anoxia on starch breakdown in rice and wheat seeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXhvFensw%3D%3D&md5=e2f17b436889ec3d4be00c31f1070912CAS |
Plaxton WC, Podesta FE (2006) The functional organisation and control of plant respiration. Critical Reviews in Plant Sciences 25, 159–198.
| The functional organisation and control of plant respiration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtVKqtrs%3D&md5=e2524493098e7efde366d31ba639e551CAS |
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiology 156, 1006–1015.
| Metabolic adaptations of phosphate-starved plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFWluro%3D&md5=eff040b63f05bcc7192929554a090822CAS |
Quimio CA, Torrizo LB, Setter TL, Ellis M, Grover A, Abrigo EM, Oliva NP, Ella ES, Carpena AL, Ito O, Peacock WJ, Dennis E, Dattal SK (2000) Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase. Journal of Plant Physiology 156, 516–521.
| Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXksVOntb4%3D&md5=fa69a6f29bd13fa0ef0bea7b299741fdCAS |
Rahman M, Grover A, Peacock WJ, Dennis ES (2001) Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Australian Journal of Plant Physiology 28, 1231–1241.
Ricard B, van Toya T, Chourey P, Saglio P (1998) Evidence for the critical role of sucrose synthase for anoxia tolerance of maize roots using a double mutant. Plant Physiology 116, 1323–1331.
| Evidence for the critical role of sucrose synthase for anoxia tolerance of maize roots using a double mutant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisFyqsb0%3D&md5=0bd843ea1e0ed43f59f62b50558d9ef5CAS |
Roberts JKM, Chang K, Webster C, Callis J, Walbot V (1989) Dependence of ethanolic fermentation, cytoplasmic pH regulation, and viability on the activity of alcohol dehydrogenase in hypoxic maize root tips. Plant Physiology 89, 1275–1278.
| Dependence of ethanolic fermentation, cytoplasmic pH regulation, and viability on the activity of alcohol dehydrogenase in hypoxic maize root tips.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitV2ns7Y%3D&md5=95706ac6de896e8e8bdbe6aadb786500CAS |
Robinson DC, Haschke H-P, Hinz G, Hoh B, Maeshima M, Marty M (1996) Immunological detection of tonoplast polypeptides in membrane of pea cotelydons. Planta 198, 95–103.
| Immunological detection of tonoplast polypeptides in membrane of pea cotelydons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXhtVSntbrJ&md5=8fb6bfa1b07643713f05eb1766a77b72CAS |
Sachs MM, Freeling M, Okimoto R (1980) The anaerobic proteins of maize. Cell 20, 761–767.
| The anaerobic proteins of maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXltFSltL8%3D&md5=1c0a21cecaf7adea750a54836a707900CAS |
Sachs MM, Subbaiah CS, Saab IN (1996) Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47, 1–15.
| Anaerobic gene expression and flooding tolerance in maize.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtlajtL4%3D&md5=04016488acd3c14549d3bd979b098fe4CAS |
Saglio PH, Drew MC, Pradet A (1988) Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays. Plant Physiology 86, 61–66.
| Metabolic acclimation to anoxia induced by low (2–4 kPa partial pressure) oxygen pretreatment (hypoxia) in root tips of Zea mays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXht1ymurY%3D&md5=b0931b5d6c4d9b22b6f01a079faf8bcdCAS |
Santaniello A, Loretti E, Gonazli S, Novi G, Perata P (2014) A reassessment of the role of sucrose synthase in the hypoxic sucrose-ethanol transition in Arabidopsis. Plant, Cell & Environment 37, 2294–2302.
Shabala S, Shabala L, Baralo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptative responses to oxygen deprivation and soil flooding. Plant, Cell & Environment 37, 2216–2233.
Shabala S, Bose J, Fuglsang A, Pottosin I (2016) On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. Journal of Experimental Botany 67, 1015–1031.
| On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtlWiu77K&md5=1516d9e031430b3d00acfacf39c5330fCAS |
Shimaoka T, Ohnishi M, Sazuka T, Mitsuhashi N, Hara-Nishimura I, Shimazaki K, Maeshima M, Yokota A, Tomizawa K, Mimura T (2004) Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant & Cell Physiology 45, 672–683.
| Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltlWrtbw%3D&md5=5c9e99e767d02cde508213a9e070e7d7CAS |
Shimazaki K, Konde M (1987) Plasma membrane H+-ATPase in guard cell protoplasts from Vicia faba Plant & Cell Physiology 28, 893–900.
| Plasma membrane H+-ATPase in guard cell protoplasts from Vicia faba Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXlsV2qtbg%3D&md5=fd88f9cac8222ff9d38cb67ee25a4780CAS |
Shingaki-Wells R, Millar AH, Whelan J, Narsai R (2014) What happens to plant mitochondria under low oxygen? An omics review of the responses to low oxygen and reoxygenation. Plant, Cell & Environment 37, 2260–2277.
Shiono K, Ando M, Nishiuchi S, Takahashi H, Watanabe K, Nakamura M, Matsuo Y, Yasuno N, Yamanouchi U, Fujimoto M, et al (2014) RCN/OsABCG5, an ATP‐binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). The Plant Journal 80, 40–51.
| RCN/OsABCG5, an ATP‐binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsFOnsb%2FM&md5=a6f16f819b403c244254a58513575431CAS |
Sowa AW, Duff SMG, Guy PA, Hill RD (1998) Altering haemoglobin levels changes energy status in maize cells under hypoxia. Proceedings of the National Academy of Sciences of the United States of America 95, 10317–10321.
| Altering haemoglobin levels changes energy status in maize cells under hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsFGgsbg%3D&md5=c5773e478b4d1c4e0be571928dee8e03CAS |
Spickett CM, Smirnoff N, Ratcliffe RG (1993) An in vivo nuclear magnetic resonance investigation of ion transport in maize (Zea mays) and Spartina anglica roots during exposure to high salt concentrations. Plant Physiology 102, 629–638.
| An in vivo nuclear magnetic resonance investigation of ion transport in maize (Zea mays) and Spartina anglica roots during exposure to high salt concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlvF2rsL8%3D&md5=a69447857b95e3c8caac2aa1087193ccCAS |
Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD (2007) Nitrite driven ATP synthesis in barley and rice roots mitochondria. Planta 226, 465–474.
| Nitrite driven ATP synthesis in barley and rice roots mitochondria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmt1Sru7o%3D&md5=714d4b1c59ae17164c01fcd983cda329CAS |
Sze H (1985) H+-translocating ATPases: advances using membrane vesicles. Annual Review of Plant Physiology 36, 175–208.
| H+-translocating ATPases: advances using membrane vesicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXksFSis7g%3D&md5=645c603a6820a4087716b44e208c1cd3CAS |
Tadege M, Dupuis I, Kuhlemeier C (1999) Etahanolic fermentation: new functions for an old pathway. Trends in Plant Science 4, 320–325.
| Etahanolic fermentation: new functions for an old pathway.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2sbgslKlsw%3D%3D&md5=26c3631dada85704461eeb29f56cf4fdCAS |
Takahashi H, Greenway H, Matsumura H, Tsutsumi N, Nakazono M (2014) Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Annals of Botany 113, 851–859.
| Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXkslCrt7w%3D&md5=efc1f13c2e2507d7ea084a0952be9476CAS |
Thomas M, Ranson SL, Richardson JA (1973) ‘Plant physiology.’ (5th edn) (Longman: London)
Tomé F, Nägele T, Adamo M, Garg A, Marco-Ilorca C, Nukarinen E, Pedrotti L, Peviani A, Simeunovic A, Tatkiewicz A, Tomar M, Gamm M (2014) The low energy signalling network. Frontiers in Plant Science 5, 353
Van Aken O, Giraud E, Clifton R, Whelan J (2009) Alternative oxidase: a target and regulator of stress responses. Physiologia Plantarum 137, 354–361.
| Alternative oxidase: a target and regulator of stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFCgtb7M&md5=79d8aca874b0fd43e61e13566b812908CAS |
van Veen H, Mustroph A, Barding GA, Vergeer-van Eijk M, Welschen-Evertman RAM, Pedersen O, Visser EJW, Larive CK, Pierik R, Bailey-Serres J, Voesenek LACJ, Sasidharan R (2013) Two Rumex species from contrasting hydrological niches regulate flooding tolerance through different mechanism. The Plant Cell 25, 4691–4707.
| Two Rumex species from contrasting hydrological niches regulate flooding tolerance through different mechanism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVOqsrk%3D&md5=319ca469c04e3903db7ad553b11071ecCAS |
Vartapetian BB, Andreeva TN, Generova IP, Polyakova LI, Mazlova IP, Dolchik YI, Stephanova AY (2003) Functional electron microscopy in studies on anaerobic stress. Annals of Botany 91, 155–172.
| Functional electron microscopy in studies on anaerobic stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitVCksLc%3D&md5=f391c6afc10cf52284a2d56b37f519b2CAS |
Vicente J, Mendiondo GM, Movahedi M, Peirats-Llobet M, Juan YT, Shen YY, Dambire C, Smart K, Rodriguez PL, Charng YY, Gray JE, Holdsworth MJ (2012) The Cys-Arg/N-end rule pathway is a general sensor of abiotic stress in flowering plants. Current Biology 27, 3181–3190.
Wang F, Chen Z-H, Liu X, Colmer TD, Shabala L, Salih A, Zhou M, Shabala S (2017) Revealing the role of GORK channels and NADH oxidase in acclimation to hypoxia in Arabidoposis. Journal of Experimental Botany 68, 3191–3204.
Waters I, Morrell S, Greenway H, Colmer TD (1991) Effects of anoxia on wheat seedlings. II Effects of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation and sugar levels. Journal of Experimental Botany 42, 1437–1447.
| Effects of anoxia on wheat seedlings. II Effects of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation and sugar levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XjvVWmsg%3D%3D&md5=731694ea72148562493c5d97b9c64bc7CAS |
Xia JH, Roberts JKM (1996) Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiology 111, 227–233.
| Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivFekurw%3D&md5=271a7c5443b5dcef2d022f0eb1817a2eCAS |
Xia JH, Saglio P, Roberts JKM (1995) Nucleotide levels do not critically determine survival of maize root tips acclimated to a low-oxygen environment. Plant Physiology 108, 589–595.
| Nucleotide levels do not critically determine survival of maize root tips acclimated to a low-oxygen environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtlaqsr8%3D&md5=56596fac73f3fc92daa49a6209913073CAS |
Yoshida S (1994) Low temperature-induced acidosis in cultured mung bean (Vigna radiata (L.) Wilczec cells. Plant Physiology 104, 1131–1138.
| Low temperature-induced acidosis in cultured mung bean (Vigna radiata (L.) Wilczec cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXjtF2ltL4%3D&md5=7f9739948f54f74a7432fdb7b90fb9d3CAS |
Zhang Q, Greenway H (1995) Membrane transport in anoxic rice coleoptiles and storage tissues of beet root. Australian Journal of Plant Physiology 22, 965–975.
| Membrane transport in anoxic rice coleoptiles and storage tissues of beet root.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtF2jsLs%3D&md5=212564b871fcd3bad4290ccde81351d9CAS |
Zhang Q, Lauchli A, Greenway H (1992) Effects of anoxia on solute leakage from beetroot storage tissue. Journal of Experimental Botany 43, 897–905.
| Effects of anoxia on solute leakage from beetroot storage tissue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xlt1yhtrY%3D&md5=e64662abafa440611692011e4ff1f561CAS |
Zhang J, Li J, Wang X, Chen J (2011) OVP 1, a vacuolar H+-translocase inorganic pyrophosphatase (V-PPase), overexpression improved rice cold tolerance. Plant Physiology and Biochemistry 49, 33–38.
| OVP 1, a vacuolar H+-translocase inorganic pyrophosphatase (V-PPase), overexpression improved rice cold tolerance.Crossref | GoogleScholarGoogle Scholar |



