Negative short-term salt effects on the soybean–Bradyrhizobium japonicum interaction and partial reversion by calcium addition
Nacira Muñoz A B , Marianela Rodriguez A , German Robert A B and Ramiro Lascano A B CA Instituto de Fisiología y Recursos Genéticos Vegetales, Centro de Investigaciones Agropecuarias-INTA, Camino a 60 Cuadras Km 5 y ½, Córdoba Argentina.
B Cátedra de Fisiología Vegetal, Facultad de Ciencias Exactas Físicas y Naturales, Universidad Nacional de Córdoba, Av. Vélez Sarsfield 299, Córdoba Argentina.
C Corresponding author. Email: hrlascano@correo.inta.gov.ar
Functional Plant Biology 41(1) 96-105 https://doi.org/10.1071/FP13085
Submitted: 5 September 2012 Accepted: 19 July 2013 Published: 5 September 2013
Abstract
The short-term (2 h) effects of salt stress (50 and 150 mM NaCl) on early events of soybean– Bradyrhizobium japonicum (rhizobia) interaction were analysed, determining the following parameters in root hair with or without calcium addition: deformation, apoplastic superoxide radical production (O2⚫–), root hair death and sodium/potassium ion content. We also analysed whether this short-term salt stress influenced later formation of crown and noncrown nodules, determining the number and weight of nodules. The negative effect of salt stress on these characters was attenuated by the addition of 5 mM CaCl2. We also analysed the expression of pathogenesis-related proteins (PRP) genes PR-1, PR-2, PR-3, and four isoforms of PR-5. The expression of PR-2 increased under saline conditions and decreased in osmotic treatment and saline treatment supplemented with calcium in the presence of the symbiont. The changes in PR-2 expression levels, together with the death of root hairs provide a possible mechanism for the inhibition of infection by the symbiont under salinity, and suggests a possible overlap with responses to plant pathogens.
Additional keywords: ionic homeostasis, nodulation, pathogenic-related proteins, symbiotic interaction.
Introduction
Salinity involves two stress components for plants; the osmotic stress given by the reduction of water availability, and the ionic stress that is related with the ionic homeostasis alteration. Salinity affects several physiological and biochemical processes associated with plant growth and development (Zhu 2001). The negative effects of salt stress are in part a consequence of the oxidative damage induced by the enhanced production of reactive oxygen species (ROS) (Apel and Hirt 2004).
The dual role of ROS, as toxic and signal molecules, is determined by the rates and subcellular location of ROS generation and degradation (Mittler et al. 2004). The ROS production : degradation ratio is a key determinant of the function and viability of the cell (Fedoroff 2006). The NADPH oxidase complex is a major ROS source in the apoplast. Plant NADPH oxidase proteins have a large hydrophilic domain with two calcium-binding EF hand motifs in the N-terminal region and the activity of NADPH complex is directly regulated by Ca2+ (Sagi and Flhur 2001).
Soybean (Glycine max L.) has been classified as a salinity-susceptible crop, with field performance being affected when salinity exceeds 5 ds m–1 (~50 mM NaCl) (Ashraf 1994). The infection and nodulation process by Bradyrhizobium japonicum is also severely affected under salinity. The number and weight of nodules in salinised plants is reduced by 50% at concentrations of 26.6 mM NaCl (Singleton and Bohlool 1984; Delgado et al. 1994; Elsheikh and Wood 1995). Nodulation is a morphogenetic process that occurs during the plant development and salt tolerance responses in soybean is highly dependent on the developmental stage (Shao et al. 1986, 1993). How these processes are jointly affected by salt stress is still only partially understood.
The calcium addition to saline soils is a common agricultural practice that attenuates the negative effects of salt. Calcium has a key role in ionic homeostasis under salt stress (Demidchik and Maathuis 2007). Ionic homeostasis is necessary to maintain low concentrations of toxic ions and high concentrations of essential ions within the cell, and is regulated by ion fluxes. Particularly important is the sodium (Na+) : potassium (K+) ratio, which is a main determinant of tolerance response to salt stress (Munns and Tester 2008). However, the effects of calcium on the legume rhizobia symbiotic interaction have not been investigated. In this work we analysed the root hair ionic homeostasis during soybean–B. japonicum interaction with or without calcium addition.
We have characterised the apoplastic and intracellular ROS changes that occur during the root hair deformation in soybean–B. japonicum symbiotic interactions under control, osmotic and salt stress condition. These results showed a correlation between aposplatic ROS production and root hair curling, and strong negative effects of salt stress, inhibiting both processes and inducing a sustained intracellular ROS production and root hair death (Muñoz et al. 2012). The sustained production of ROS in inoculated soybean root hairs subject to saline treatments, is similar to the response observed in root hairs of Phaseolus vulgaris L. elicited with chitosan (a fungal elicitor) (Cárdenas et al. 2008; Muñoz et al. 2012). These results led us to hypothesise that under salt stress, rhizobia could be sense as a pathogen, triggering a pathogen defence response in legume plants. Likewise, in soybean, it has been noted that an incorrect recognition of the symbiont induce the synthesis of pathogenesis-related (PR) proteins (López-Baena et al. 2009). Further, saline treatments also induce PR protein in soybean (Onishi et al. 2006; Tachi et al. 2009). However, whether this induction affects the symbiotic interaction in soybean under salinity remains unexplored. In this work we analysed the changes in expression of different PR, which have been studied in soybean under salinity and symbiotic interactions, like markers of plant–pathogen responses (van Loon et al. 2006) under the short-term salt stress treatments.
Early negative effects of salt stress in inoculated soybean root hairs could have consequences on later events, affecting the formation of crown nodules. Crown root nodules are those formed at an early stage after seed germination, and provide the highest amount of the biological fixed nitrogen to soybean plants (Zdor and Pueppke 1988). In saline soils the highest salt concentrations are in the upper strata, where the initial steps of the symbiotic interaction occur together with seed germination (Bernstein 1975) and thus, could have a strong effect on crown nodule formation and nitrogen biological fixation.
The aim of this work was to study the effects of short-term salt stress, with or without calcium addition, on root hair responses including root hair deformation, root hairs death, apoplastic superoxide production, root hair ionic homeostasis, pathogenesis-related protein expressions, and its consequences for nodulation.
Materials and methods
Bacterial strain and plant material
Soybean seeds disinfected with 5% sodium hypochlorite (V/V) for 5 min (Glycine max L. DM4800) were germinated on filter paper moistened with distilled water for 48 h in the dark. The seeds were incubated at 28 and 37°C during the first and second 24-h periods, respectively, to promote the growth of roots and root hairs. Bradyrhizobium japonicum USDA 138 was cultured in yeast extract mannitol (YEM) medium (Vincent 1970) at 28°C with constant agitation for 5 days (3 × 109 cells mL–1). The bacteria were washed and resuspended in sterile water.
Saline and osmotic treatments of root hairs
Two days after germination, seedlings were transferred to aerated tubes that contained sterile water, NaCl (50 or 150 mM), or sorbitol (100 or 300 mM). These series of hyperosmotic solutions developed equal osmotic pressures (–0.55 and –0.84 MPa respectively). The seedlings were inoculated with fresh bacterial cultures (1 mL of OD = 0.6; 3 × 109 cells mL–1). Measurements were performed after 2 h of treatment. Experiments with combinations of NaCl (50 or 150 mM) and CaCl2 5 mM were also performed to analyse the effects of added calcium.
Early effects of salt stress treatments on later stages of the interaction
To evaluate whether the combined early effects of salt and inoculation with B. japonicum on root hairs had an influence on nodule formation, treatments were performed on 48 h pre-germinated seeds for 2 h, as described in the previous section; then the seeds were washed with 5% Tween 20 for 40 s and rinsed eight times with sterile distilled water.
The treated and inoculated seeds were placed in plastic trays with Broughton and Dilworth nutrient solution (Broughton and Dilworth 1971) without nitrogen, aeration, photoperiod of 16 h light and 8 h dark, 25°C and grown for 21 days; after that period, nodules formed in the roots were observed and their number and weight were evaluated. In each treated plant, nodules on primary and secondary roots were differentiated to obtain the number and weight of crown nodules and non-crown nodules respectively.
Extraction of root hairs
Root hairs were extracted from roots subjected to different salt stress treatments and their respective osmotic controls with sorbitol. Root hairs were extracted by peeling the root zone containing young root hairs, which were immediately frozen in liquid nitrogen. Peeling was performed by making an incision with a scalpel under a magnifying glass and pulling the epidermal tissue that contains the root hairs with a fine-tipped clamp. Root hairs of ~200 roots (equivalent to 200 germinated seeds) generated sufficient material for a sample.
Na+ and K+ determination in roots hairs
Root hair samples subjected to different saline and osmotic treatments and saline treatment supplemented with calcium were weighed, immersed in a 1 : 10 (w/v) plant tissue : water, boiled for 30 min and centrifuged for 5 min at 12 000g. Sodium (Na+) and potassium (K+) content in the extracting solution were determined using a Jenway PFP flame photometer (Jenway Gransmore Green Felsted, Dunmow Essex, England). Ion concentration was calculated using a standard curve with known concentrations of sodium and potassium.
Root hair deformation
Root hair deformation was observed 2 h after inoculation. Root hairs from different treatments were stained with 1% (w/v) toluidine blue and studied under an optical microscope. Eight roots per treatment and four sections per root were viewed and counted (~600 root hairs per treatment). The percentage of deformed root hairs was calculated as a proportion of the total number of root hairs in each section.
Apoplastic superoxide radical production in root hairs
Superoxide levels were determined with nitroblue tetrazolium (NBT), which reacts with superoxide radicals to produce a blue formazan precipitate. Roots were incubated in 0.01% (w/v) in the dark for 30 min. The reaction was stopped with absolute ethanol and the blue precipitate was quantified under an optical microscope.
Root hair death: nuclear morphology, chromatin condensation and DNA fragmentation
Nuclear morphology, chromatin condensation and DNA fragmentation were evaluated using 4,6-diamino-2-phenylindole (DAPI). Roots were incubated 1 µg mL–1 of DAPI for 15 min. Nuclei of root hairs were visualised using epifluorescence microscopy (Nikon Eclipse Ti; Nikon, Tokyo, Japan) with filter UV-2E/C (Ex: 360/40 nm, DM: 400 nm, Em: 460/50 nm). Eight roots per treatment and four sections per root were observed and counted (~600 root hairs per treatment). The percentages of nuclei with altered morphology, chromatin condensation or DNA fragmentation were calculated as a proportion of the total number of root hairs in each section with unaltered morphology. Two nuclear characteristics were observed and recorded; chromatin condensation (relatively uniform nuclear envelope with bright and stippled nuclear material, without marked nucleolus) and DNA fragmentation (notably affected nuclear envelope and morphology).
Image quantification
Apoplastic superoxide radical production determined by blue formazan staining was quantified using the image analyser program OPTIMAS 6.1 (Optimas Corporation, Bothell, WA, USA). For the quantification procedure, we selected the root zone that contained young root hairs. The total distribution of stain intensity was measured as luminance by the image processing software. This luminance was transformed into optical density (OD). OD, the final parameter that represents signal intensity, was calculated relative to the tissue area analysed.
RNA extraction
Samples subjected to different treatments were homogenised in a cold mortar with trizol (in a 1 : 10 plant material : phenol relation), mixed for 1 min and incubated at room temperature for 5 min. Then, 0.2 mL chloroform per mL of trizol was added and incubated at room temperature for 3 min. After incubation, the samples were centrifuged at 12 000g at 4°C for 15 min. The aqueous phases were transferred to clean tubes. Then, one volume of isopropanol was added and the samples were incubated at room temperature for 10 min and centrifuged at 12 000g, 4°C for 15 min. The precipitate was washed with 70% ethanol and the samples were centrifuged again at 12 000g and 4°C for 15 min. The precipitate was dried and resuspended in DEPC water and its concentration was quantified in Thermo Scientific (Waltham, MA, USA) NanoDrop 3300. Purified RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) to remove genomic DNA, according to the manufacturer’s instruction.
qRT-PCR
DNA-free RNA (1–2.5 µg) was used with oligo(dT) for first strand cDNA synthesis using the Moloney Murine Leukemia Virus for RT–PCR (Promega, Fitchburg, WI, USA), according to the manufacturer’s instruction. The lack of genomic DNA contamination was verified by qRT–PCR using primers able to amplify genomic DNA. The gene-specific primer pairs employed for the detection of transcripts of soybean were: four genes characterised under symbiotic and phatogenic interactions described by Mazarei et al. (2007), GmPR-1 (acidic PR-1 GenBank accession number BU577813, forward primer 5′-AACTATGCTCCCCCTGGCAACTATATTG-3′, reverse primer 5′-TCTGAAGTGGTAGCTTCTACATCGAAACAA-3′), GmPR-2 (basic β-1,3-endoglucanase GenBank accession number M37753, forward primer 5′-TGAAATAAGGGCCACGAGTCCAAATG-3′, reverse primer 5′-ATGGTACATGCAGACTTCAAGAATGCAGAT-3′), GmPR-3 (basic chitinase GenBank accession number AF202731, forward primer 5′-AACTACAATTACGGGCAAGCTGGCAA-3′, reverse primer 5′-TTGATGGCTTGTTTCCCTGTGCAGT-3′), GmPR-5 (thaumatin-like GenBank accession number BU765509, forward primer 5′-GCGCTTGCTCCGCTTTCAACT-3′, reverse primer 5′-CTTGGAATAGACGGTGGGCTT GC-3′), and three isoforms of soybean PR-5 characterised under salt stress, described by Tachi et al. (2009), GmOLPb (neutral PR-5 isoform GenBank accession number AB370233, forward primer 5′-ACCAATTTGGCAACCAGGAT-3′, reverse primer 5′- CATTGGTGCAGCAATACTCA-3′), GmOLPa (acidic PR-5 isoform GenBank accession number AB116251, forward primer 5′-GTACACCTCCGAACACGTTG-3′, reverse primer 5′-TGGGACACTCTCCGATGATG-3′) and GmP21e (acidic PR-5 isoform GenBank accession number AB370234, forward primer 5′-GTGCACACGTGGCATAAGGT-3′, reverse primer 5′-CACACAGCTACCGGAATTGC-3′). Gene-specific primer pairs for actin were used as an internal control forward primer 5′-AACGACCTTAATCTTCATGCTGC-3′ and reverse primer 5′-GGTAACATTGTGCTCAGTGGTGG-3′. qRT-PCR was performed in thermocycler iQ5 (BioRad, Hercules, CA, USA) at 58°C with iQ SYBR Green Supermix (BioRad), according to the manufacturer’s instruction. Relative expression levels with respect to inoculated control were calculated with the method by Livak and Schmittgen (2001).
Statistical analyses
Data were analysed using analysis of variance (ANOVA) followed by the DGC (multiple-comparison method of Di Riezo, Guzmán and Casanoves in Infostat) test. All analyses were performed using the InfoStat program (InfoStat/Profesional ver. 2007p, Grupo InfoStat, Facultad de Ciencias Agropecuarias, Universidad Nacional de Cordoba, Argentina).
Results
Root hair deformation induced by B. japonicum inoculation under saline treatments: effect of calcium addition
Root hair deformation is the first morphological response during the legume-rhizobia symbiotic interaction. The percentages of soybean root hair deformation was evaluated 2 h after inoculation with B. japonicum under saline treatments and in saline treatments supplemented with calcium (Fig. 1). Root hair deformation was not affected under 50 mM NaCl treatment. Under 150 mM NaCl, root hair deformation was significantly reduced. We noted that these responses were partially reverted with the addition of calcium.
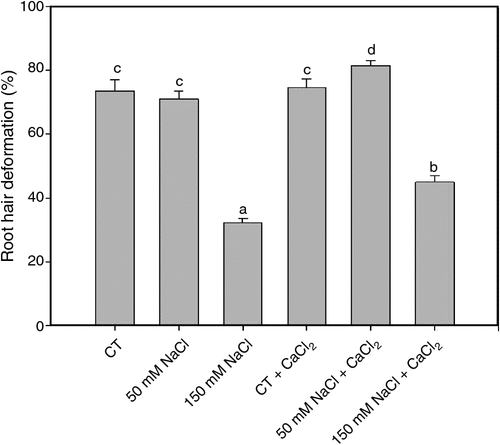
|
Apoplastic superoxide radical production in root hairs under saline treatments: effect of calcium addition
In a previous work we have demonstrated a close correlation between apoplastic superoxide production and root hair deformation. Apoplastic superoxide radical production in root hairs was determined 2 h after inoculation with B. japonicum under saline treatments and in saline treatments supplemented with calcium, (Fig. 2). The calcium addition partially reversed the inhibitory salt effect on apoplastic superoxide production, allowing a significant increase in the apoplastic superoxide radical production induced by the inoculation with B. japonicum in the 50 and 150 mM NaCl treatments.
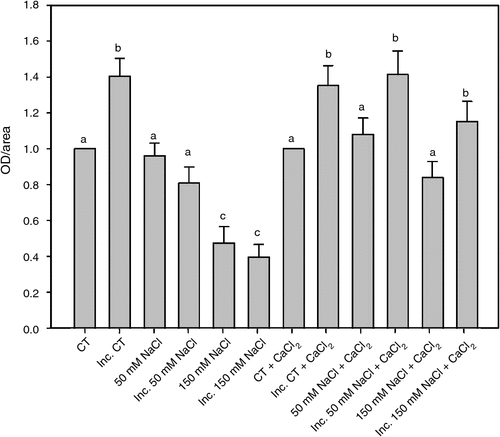
|
Na+ and K+ ion content in root hairs during the symbiotic soybean–B. japonicum interaction under salt and osmotic stress: effect of calcium addition
Salt stress usually induces changes in cellular ionic homesotasis. The content of Na+ in inoculated and non-inoculated root hairs subjected to saline treatments for 2 h increased in a dose-dependent manner compared with the control (Fig. 3a). However, K+ levels were the same in all treatments (Fig. 3b). These results show that after 2 h, the Na+ : K+ ratios increased significantly in a dose-dependent manner with respect to the control in all saline treatments, although they did not differ significantly from that of the control in osmotic treatments. The addition of calcium to saline treatments reduced the Na+ : K+ ratios with respect to the saline treatments without calcium, mainly due to a reduced influx of Na+, since no alterations in K+ content were detected (Fig. 3a, b).
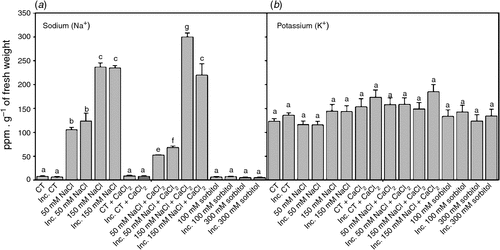
|
Root hair death under saline treatments: effect of calcium addition
Cell death is characterised by changes in the nuclear morphology. The percentages of nuclei with chromatin condensation (Fig. 4b) and DNA fragmentation (Fig. 4c) in root hairs 2 h after inoculation with B. japonicum under saline treatments and in saline treatments supplemented with calcium are shown in Fig. 4d. The nuclei of root hairs in the control treatment exhibited an orthodox conformation, with similar size and shape (Fig. 4a), and with a very low percentage of nuclei with DNA fragmentation (Fig. 4d). The inoculation of root hairs induced a slight increase of nuclei with chromatin condensation. Likewise, although the 50 mM NaCl treatment did not alter these percentages with respect to the control, 50 mM NaCl combined with B. japonicum induced root hair death, with an important increment of nuclei with DNA fragmentation and a lower increase of nuclei with chromatin condensation (Fig. 4d). These responses were similar to those in the 150 mM NaCl treatments. We noted that in saline treatments supplemented with calcium, the percentages of nuclei with DNA fragmentation were significantly reduced with respect to the unsupplemented treatments: this change was associated with a significant increase in the percentage of nuclei with chromatin condensation (Fig. 4).
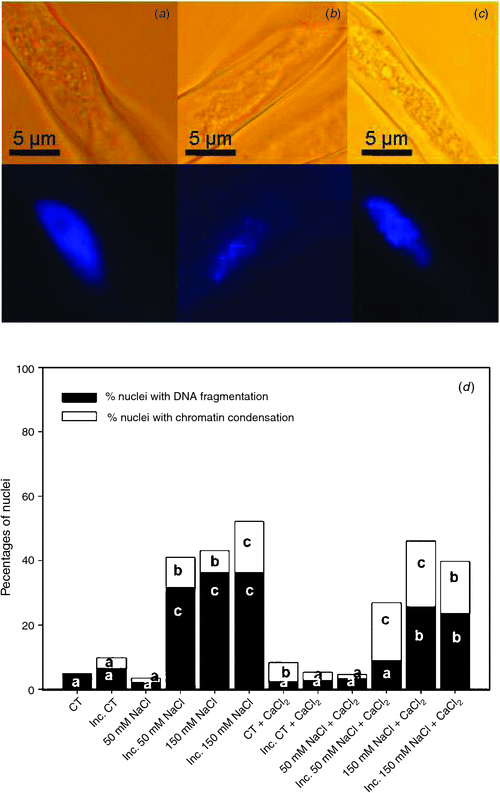
|
Expression of pathogen-related proteins
The expression levels for PR-1, PR-2 and PR-3 are shown in Fig. 5. The transcripts levels of PR-1 (Fig. 5a) increased in all conditions of saline and osmotic stress and salt stress supplemented with calcium. The expression of PR-3 (Fig. 5c) increased in 150 mM NaCl, 100 mM sorbitol and in both saline treatments supplemented with calcium in the presence of the symbiont. We noted that the expression levels increased for PR-2 (Fig. 5b) only in saline treatment and that this increase was reversed by the addition of calcium. We were not able to detect PR-5 BU765509 expression in root hairs, even though that expression was detected in other cell types of the root (data not shown). Due to the inability to detect expression of this PR-5 isoform in root hairs, we evaluated the expression of three other PR-5 isoforms (GmOLPa, GmOLPb and P21e) (Fig. 5d–f) that have been characterised in soybean under salt stress conditions (Tachi et al. 2009). The three isoforms had expression in root hair. The levels of transcripts for GmOLPa (Fig. 5d) increased in all treatments of saline and osmotic stress and salt stress supplemented with calcium. Likewise, the levels of transcripts for GmOLPb and P21e decreased or remained unaltered (Fig. 5e, f).
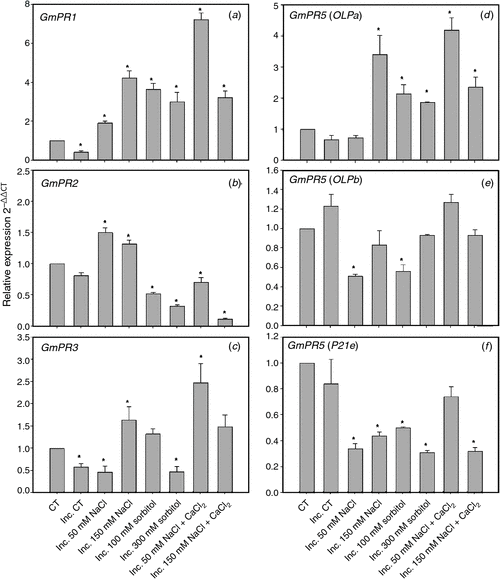
|
Effects of short-term salt stress on nodulation
The number of crown nodules in roots transiently subjected to saline stress for 2 h during the early events of symbiotic interaction significantly decreased in a dose-dependent manner with respect to the control, whereas the number of nodules in osmotic treatments showed no significant differences (Fig. 6a).
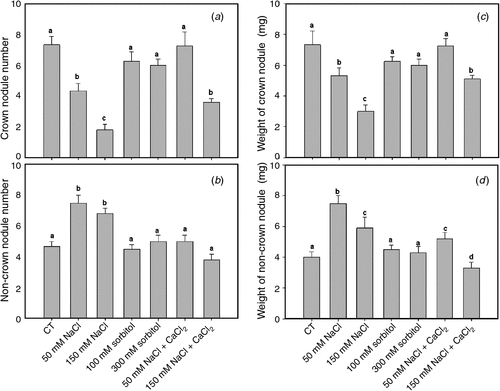
|
Non-crown nodules increased significantly in plants treated with 50 mM and 150 mM NaCl for 2 h with respect to the control (Fig. 6b). In contrast, osmotic treatments showed no significant differences in non-crown nodule number with respect to the control (Fig. 6b).
Crown nodule number in control condition supplemented with calcium for 2 h did not vary significantly with respect to the non-supplemented control; however, under saline treatments the addition of calcium partly reversed the negative effect of salt on the number of crown nodules (Fig. 6a). Further, the number of non-crown nodules in calcium-supplemented saline treatments was lower than in the non-supplemented ones (Fig. 6b).
The average weight per nodule for all treatments was also discriminated between crown and non-crown nodules (Fig. 6c, d). A positive correlation between weight and number was observed. The decrease in crown nodule number under saline treatments was correlated with smaller nodules than normal (Fig. 6c). We noted that under saline calcium-supplemented treatments, the number and weight of crown nodules increased significantly with respect to the unsupplemented saline treatment, without showing significant differences from the controls (Fig. 6c). Crown and non-crown nodules were inversely related in terms of number and weight; indeed, when number and weight of nodules decreased in the crown root, they increased in other parts of the roots (Fig. 6c, d).
Discussion
The negative effect of short-term saline treatments on crown nodule formation was dose-dependent and was produced by the ionic component of salt stress, since the number of nodules remained unaltered in the osmotic controls of these treatments. This result suggests that the ionic homeostasis of root hairs during the early events of the symbiotic interaction affects the number of nodules that later develop. The lack of infection in saline treatments is not due to a negative effect on rhizobia survival (Muñoz et al. 2012). The exogenous application of calcium to attenuate the negative effects of salt is a common agricultural practice (Rengel 1992; Shabala et al. 2006). The root hair deformation inhibition, particularly under 150 mM NaCl, was reverted with the addition of calcium. Root hair deformation is an early step that greatly improves the efficiency of Rhizobium infection. Likewise, root hair deformation occurs only in actively growing root hairs and is supported by the machinery that sustains the polarised growth of root hair. We have previously reported the importance of localised apoplastic superoxide radical production supporting the deformation that is affected under saline treatments (Muñoz et al. 2012); and in this work we show that the addition of calcium to 150 mM saline treatments preserved the apoplastic superoxide production, a condition necessary for the deformation and subsequent infection during the symbiotic interaction.
It has been suggested that some of the positive effects of calcium would be related to the balance in the opening and closing of non-selective cation channels (NSCC), since they involve not only the entry of sodium, but also the exit of potassium, and thereby contribute to the ionic homeostasis (Demidchik et al. 2007). Accordingly, the addition of CaCl2 had a positive effect prevented the entry of Na+ in saline treatments combined with the symbiont, possibly by regulating NSCC, such as closing CCNS-IV (voltage independent), which are the main gateways of sodium into the cell. This effect of calcium on NSCC may be an important component in the regulation of ion homeostasis that helped to reverse the infection and nodule formation in short-term saline treatments.
Furthermore, the analysis of sodium and potassium ion content in our system revealed that the Na+ contents were increased in a dose-dependent manner during saline treatments and independently of the symbiont presence, without outflow of potassium. Likewise, these contents did not change in osmotic treatments. This result is particularly important because the loss of potassium has also been studied as another negative response of the ionic effect of salt, which contributes to the imbalance of Na+ and K+, resulting in subsequent loss of ionic homeostasis and cellular death (Shabala and Cuin 2008). We have previously reported that treatments with hyperosmotic solutions of sorbitol (that developed equal osmotic pressures respect to saline treatments used in this study) did not induce cell death in root hairs (Muñoz et al. 2012), suggesting that the loss of viability could be due to the ionic component of the salt, in particular by sodium entry.
Death of soybean root hairs was detected by both high NaCl concentration (150 mM) and moderate NaCl (50 mM) combined with B. japonicum 2 h post inoculation. These cell death events were accompanied by differentials alterations in the generation of apoplastic superoxide radical and root hair deformation: absence of changes under 50 mM NaCl and a reduction under 150 mM NaCl. These results suggest that in both treatments could result in a differential induction of root hairs death involved in the subsequent decline of nodule formation. In this work we evaluated in detail the root hair death induced by saline treatments and in saline treatments supplemented with calcium. The progression of chromatin condensation can be classified into three stages during animal cell apoptosis: stage I, or ring condensation, stage II, or necklace condensation, and stage III, or nuclear collapse/disassembly (Toné et al. 2007). These stages are less clearly defined in plant cells, but the process of chromatin condensation progresses essentially in the same way and culminates in the formation of discrete domains of condensed and finally fragmented chromatin (Domínguez and Cejudo 2006; Yamada et al. 2006). The differences observed in the nucleus morphology, particularly associated with the increase of nuclei with chromatin condensation and the decrease of nuclei with DNA fragmentation in saline treatments supplemented with calcium, with respect to the unsupplemented, indicate that the addition of calcium to saline treatments inhibited or at least delayed the cell death process. This inhibition or delay in the death progress, given by calcium addition, could help to sustain the rhizobia infection and subsequent nodule formation.
We have also demonstrated a synergetic effect of salt stress and inoculation, in the induction of root hair cell death (Muñoz et al. 2012). These results led us to hypothesise that under salt stress, the symbiont could be recognised by the plant as a pathogen, and the response shifted to a plant–pathogen like response. We evaluated the expression of four PR characterised in symbiotic and pathogenic interactions of soybean (Mazarei et al. 2007; López-Baena et al. 2009) and three PR-5 isoforms of soybean characterised in saline treatments (Tachi et al. 2009). In root hairs, we were not able to detect soybean PR-5 (BU765509) expression, even though its expression is detected in the remaining root, this result suggests that this PR-5 isoform may not have expression in root hairs. Likewise, of the other three isoforms of PR-5 evaluated, two showed no change in the applied treatments or decreased expression and, only one (GmOLPa) was increased in both saline treatments as in the calcium-supplemented treatments and even in the osmotic control with sorbitol. This result indicates that in root hairs, the transcript levels of these PR5 isoforms increases in response to any osmotic pressure change in the medium. Similarly, the levels of transcripts for PR-1 and PR-3 were increased in all treatments of saline and osmotic stress and salt stress supplemented with calcium.
We also noted that the enhanced PR-2 expression under saline treatments was reversed by calcium addition, and the induction of PR-2 expression was not observed in the osmotic controls. These results, together with the reversion of sodium content, delayed root hair death and nodule formation in calcium-supplemented saline treatments and the absence of negative responses in the osmotic controls, suggest that PR-2 may be involved in the inhibition of infection and nodule formation by NaCl treatments. PR-2, a β-1,3-endoglucanase has a key role in the defence responses against several plant pathogens; by promoting the release cell wall-derived material that can act as defence elicitors (Leubner-Metzger and Meins 1999). During nodulation, the colonisation of the host plant by symbiotic rhizobia does not elicit plant defence reactions induced by pathogens, although at some stages the symbiotic infection resembles a pathogenic interaction. However, under certain circumstances, various defence reactions might take place in legume–rhizobia interactions, which can provoke the abortion of the infection in necrotic cells, concomitant with an accumulation of phenolic compounds and PR proteins (Vasse et al. 1993; Mithöfer 2002). Likewise, it was suggested that the plant controls the extent of nodule number by a systemic mechanism similar to innate immunity (Zamioudis and Pieterse 2012).
The impossibility of re-infection in the root zone that was subjected to salt stress suggests a priming effect. In addition, the re-infection events that occurred in the non-crown root zone after removing the stress treatment strongly suggest that, given the inability of B. japonicum to infect the salt-primed area, the formation of nodules would have been induced in other parts of the root after the stress period. This redistribution of root nodules is not due to differences in root architecture, and shows how short-term and early saline stress affects the number and distribution of soybean nodules. This shift on the nodulation pattern, from crown to non-crown nodules induced by short-term salt effect, have an important negative effect on biological nitrogen fixation in soybean crops, because crown nodules provide the greater amount of fixed nitrogen to the soybean plant (Zdor and Pueppke 1988).
Finally, considering that in field conditions the crown nodules are formed after infection of root hairs during the first days after germination, the increase of soil salinity at this stage can be relevant for the subsequent formation of crown nodules, and the addition of calcium to the soil as an agricultural practice can attenuate the negative effects of salt on the infection and nodulation process. Future experiments will be conducted to study the priming effects induced by short-term salt stress exposure and the consequences in different responses that are related to the microorganism interactions in this experimental system.
Acknowledgements
This work was supported by grants from the Agencia de Promoción Científica y Tecnológica, National Microscopy Commission and the Instituto Nacional de Tecnología Agropecuaria (INTA), Argentina. RL is a fellow of CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina).
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399.| Reactive oxygen species: metabolism, oxidative stress, and signal transduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvFeisL0%3D&md5=bb62c10883f0f45b9778cbbe05a12a70CAS | 15377225PubMed |
Ashraf M (1994) Breeding for salinity tolerance in plants. Critical Reviews in Plant Sciences 13, 17–42.
Bernstein L (1975) Effects of salinity and sodicity on plant growth. Annual Review of Phytopathology 13, 295–312.
| Effects of salinity and sodicity on plant growth.Crossref | GoogleScholarGoogle Scholar |
Broughton W, Dilworth M (1971) Control of leghaemoglobin synthesis in snake beans. Biochemical journal 125, 1075–1080.
Cárdenas L, Martinez A, Sanchez F, Quinto C (2008) Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). The Plant Journal 56, 802–813.
| Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs).Crossref | GoogleScholarGoogle Scholar | 18680562PubMed |
Delgado J, Ligero F, Liuch C (1994) Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plant. Soil Biology & Biochemistry 26, 371–376.
| Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXis12is70%3D&md5=a8278e9db81641c4472249a9fe6e1557CAS |
Demidchik V, Maathuis F (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytologist 175, 387–404.
| Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFSqs7w%3D&md5=e6d9d391cb0668058f9488556bf96e8aCAS | 17635215PubMed |
Demidchik V, Shabala S, Davies J (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2 + flux and plasma membrane Ca2 + channels. The Plant Journal 49, 377–386.
| Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2 + flux and plasma membrane Ca2 + channels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXisVyisLk%3D&md5=252bc33c2375ea34e20f6298997548cdCAS | 17181775PubMed |
Domínguez F, Cejudo FJ (2006) Identification of a nuclear-localized nuclease from wheat cells undergoing programmed cell death that is able to trigger DNA fragmentation and apoptotic morphology on nuclei from human cells. Biochemical Journal 307, 529–536.
Elsheikh E, Wood M (1995) Nodulation and N2 fixation by soybean inoculated with salt-tolerant rhizobia or salt-sensitive bradyrhizobia in saline soil. Soil Biology & Biochemistry 27, 657–661.
| Nodulation and N2 fixation by soybean inoculated with salt-tolerant rhizobia or salt-sensitive bradyrhizobia in saline soil.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt12rt7s%3D&md5=cc96fa94177cd480fa161fec9b2ec205CAS |
Fedoroff N (2006) Redox regulatory mechanisms in cellular stress responses. Annals of Botany 98, 289–300.
| Redox regulatory mechanisms in cellular stress responses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1yltLc%3D&md5=a9fc96e54d0386146e5c79ef9a701fa0CAS | 16790465PubMed |
Leubner-Metzger G, Meins F (1999) Functions and regulation of plant β-1,3-glucanases (PR-2). In: ‘Pathogenesis-related proteins in plants.’ (Eds SK Datta, S Muthukrishnan) pp. 49–76. (CRC Press: Boca Raton, FL, USA)
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods 25, 402–408.
| Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFelt7s%3D&md5=cda22da73d6034ad2e4c74827f633baaCAS | 11846609PubMed |
López-Baena J, Monreal JA, Pérez-Montaño F, Guasch-Vidal B, Bellogín R, Vinardell J, Ollero F (2009) The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean. Molecular Plant-Microbe Interactions 22, 1445–1454.
| The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean.Crossref | GoogleScholarGoogle Scholar |
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ (2007) GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Molecular Plant-Microbe Interactions 20, 107–119.
| GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVyrsA%3D%3D&md5=0a56cfca1adb6122e58458a111e1bb77CAS | 17313162PubMed |
Mithöfer A (2002) Suppression of plant defence in rhizobia–legume symbiosis. Trends in Plant Science 7, 440–444.
| Suppression of plant defence in rhizobia–legume symbiosis.Crossref | GoogleScholarGoogle Scholar | 12399178PubMed |
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498.
| Reactive oxygen gene network of plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXotF2msrg%3D&md5=f784349d5767e5607415f067ce056dcbCAS | 15465684PubMed |
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681.
| Mechanisms of salinity tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntFaqtrw%3D&md5=0ce1f7aad22c73fffb329d4603f0e836CAS | 18444910PubMed |
Muñoz N, Robert G, Melchiorre M, Racca R, Lascano R (2012) Saline and osmotic stress differentially affects apoplastic and intracellular reactive oxygen species production, curling and death of root hair during Glycine max L.–Bradyrhizobium japonicum interaction. Environmental and Experimental Botany 78, 76–83.
| Saline and osmotic stress differentially affects apoplastic and intracellular reactive oxygen species production, curling and death of root hair during Glycine max L.–Bradyrhizobium japonicum interaction.Crossref | GoogleScholarGoogle Scholar |
Onishi M, Tachi H, Kojima T, Shiraiwa M, Takahara H (2006) Molecular cloning and characterization of a novel salt-inducible gene encoding an acidic isoform of PR-5 protein in soybean (Glycine max (L.) Merr.). Plant Physiology and Biochemistry 44, 574–580.
| Molecular cloning and characterization of a novel salt-inducible gene encoding an acidic isoform of PR-5 protein in soybean (Glycine max (L.) Merr.).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Cmt7vP&md5=02e942f72962752e1f8c3e2ce08caffcCAS | 17070691PubMed |
Rengel Z (1992) The role of calcium in salt toxicity. Plant, Cell & Environment 15, 625–632.
| The role of calcium in salt toxicity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmtlSjtL4%3D&md5=2dd1977b1ba9e329b4156423baec7757CAS |
Sagi M, Fluhr R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiology 126, 1281–1290.
| Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFemt7s%3D&md5=ff39ba69af81fbbff142fcd4365aba99CAS | 11457979PubMed |
Shabala S, Cuin T (2008) Potassium transport and plant salt tolerance. Physiologia Plantarum 133, 651–669.
| Potassium transport and plant salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXps1Oit70%3D&md5=b00298e506b7189fc2da0a5277d6448aCAS | 18724408PubMed |
Shabala S, Demidchik V, Shabala L, Cuin T, Smith J, Miller A, Davies J, Newman I (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiology 141, 1653–1665.
| Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XosVKitbo%3D&md5=9cf8fa017b3c4ebb820d7488c42c9af4CAS | 16798942PubMed |
Shao H, Song Z, Liu L (1986) Preliminary studies on the evaluation of salt tolerance in soybean varieties. Acta Agronomic Science 6, 30–35.
Shao H, Wan C, Chang R, Chen Y (1993) Preliminary study on the damage of plasma membrane caused by salt stress. Crops 1, 39–40.
Singleton P, Bohlool B (1984) Effect of salinity on nodule formation by soybean. Plant Physiology 74, 72–76.
| Effect of salinity on nodule formation by soybean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXot1yksw%3D%3D&md5=b5a8baa8d26d348fb8411e93da5ad066CAS | 16663389PubMed |
Tachi H, Yamada K, Kojima T, Shiraiwa M, Takahara H (2009) Molecular characterization of a novel soybean gene encoding a neutral PR-5 protein induced by high-salt stress. Plant Physiology and Biochemistry 47, 73–79.
| Molecular characterization of a novel soybean gene encoding a neutral PR-5 protein induced by high-salt stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFamtbbP&md5=536f14d1c24e636cd2042aa63b76042dCAS | 19010689PubMed |
Toné S, Sugimoto K, Tanda K, Suda T, Uehira K, Kanouchi H, Samejima K, Minatogawa Y, Earnshaw W (2007) Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis. Experimental Cell Research 313, 3635–3644.
| Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis.Crossref | GoogleScholarGoogle Scholar | 17643424PubMed |
van Loon LC, Rep M, Pieterse CMJ (2006) Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology 44, 135–162.
| Significance of inducible defense-related proteins in infected plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVylsLnK&md5=d541a733cfd42d90d8b1c378e280288eCAS | 16602946PubMed |
Vasse J, de Billy F, Truchet G (1993) Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction. The Plant Journal 4, 555–566.
| Abortion of infection during the Rhizobium meliloti-alfalfa symbiotic interaction is accompanied by a hypersensitive reaction.Crossref | GoogleScholarGoogle Scholar |
Vincent J (1970) ‘A manual for the practical study of the root-nodule bacteria.’ (Blackwell Scientific Publications: Oxford)
Yamada T, Ichimura K, Van Doorn WG (2006) DNA degradation and nuclear degeneration during programmed cell death in Antirrhinum, Argyranthemum and Petunia. Journal of Experimental Botany 57, 3543–3552.
| DNA degradation and nuclear degeneration during programmed cell death in Antirrhinum, Argyranthemum and Petunia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlCnsLvF&md5=ab16873e9bbd0f26131005d07de4d009CAS | 16957019PubMed |
Zamioudis C, Pieterse C (2012) Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions 25, 139–150.
| Modulation of host immunity by beneficial microbes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFWisbc%3D&md5=0df5f89b667b9615d22b885fe3fde06fCAS | 21995763PubMed |
Zdor R, Pueppke S (1988) Early infection and competition for nodulation of soybean by Bradyrhizobium japonicum 123 and 138. Applied and Environmental Microbiology 54, 1996–2002.
Zhu JK (2001) Plant salt tolerance. Trends in Plant Science 6, 66–71.
| Plant salt tolerance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsFyjtLs%3D&md5=36de2a21d4f2c605397e01ce5240d467CAS | 11173290PubMed |


