Forest patch modeling: using high performance computing to simulate aboveground interactions among individual trees
George E. Host A C , Harlan W. Stech B , Kathryn E. Lenz B , Kyle Roskoski A and Richard Mather BA Natural Resources Research Institute, University of Minnesota Duluth, Duluth MN, USA.
B Department of Mathematics and Statistics University of Minnesota Duluth, Duluth MN, USA.
C Corresponding author. Email: ghost@nrri.umn.edu
This paper originates from a presentation at the 5th International Workshop on Functional–Structural Plant Models, Napier, New Zealand, November 2007.
Functional Plant Biology 35(10) 976-987 https://doi.org/10.1071/FP08075
Submitted: 4 April 2008 Accepted: 24 September 2008 Published: 11 November 2008
Abstract
Functional–structural plant models (FSPMs) typically integrate suites of detailed physiological and phenological processes to simulate the growth of individual plants. Recent advances in high-performance computing have allowed FSPMs to be extended to patches of interacting trees. Here, we describe a parallel modelling strategy to run simultaneous individual tree models across an 8 × 8 patch of trees. The 64 ‘core’ trees are surrounded by multiple rings of neighbour trees to remove edge effects. A sensitivity analysis of the patch model demonstrates that computational factors such as the number of independently simulated trees (9 v. 36) or number of neighbour rings (3 v. 6) did not significantly influence model estimates of tree volume growth. Updated submodels for phenology and redistribution of overwinter carbohydrate storage allow the simulation to be more responsive to above ground competition among trees in a patch over multiple growing seasons. An 8-year patch-scale simulation of aspen clones 216 and 259 was conducted using high-resolution environmental data from the Aspen FACE Experiment, a long-term free-air carbon dioxide enrichment (FACE) study. Tree heights and volumes were comparable to 8-year growth measurements made at the Aspen FACE site.
Additional keywords: canopy light interception, computer simulation, FACE, patch-scale modeling, physiological process model, parallel processing, phenology, Populus.
Acknowledgements
We gratefully acknowledge support from the Northern Global Change Program of the USDA Forest Service, Award #05-CA-11242343–036 and the USDA Forest Service North Central Research Station Integrated Program, Agreement 03-JV-11231300–086. Computing resources were generously provided via grants from the University of Minnesota Supercomputer Institute and the University of Minnesota Duluth Visual and Digital Imaging Laboratory. We appreciate the cooperative efforts with the principals of the Aspen FACE Experiment, and in particular the support of Jaak Sober and Warren Heilmann for maintaining and sharing the long-term, high-resolution meteorological and trace gas databases for the site. Finally, this modelling effort has greatly benefited from discussions and insights from Drs Mark Kubiske, Neil Nelson, and Jud Isebrands. Additional support was provided by the University of Minnesota Duluth’s Natural Resources Research Institute and Department of Mathematics and Statistics. This is publication #489 of the Center for Water and the Environment.
Allen MT,
Prusinkiewicz P, DeJong TM
(2005) Using L-systems for modeling source-sink interactions, architecture and physiology of growing trees: the L-PEACH model. New Phytologist 166, 869–880.
| Crossref | GoogleScholarGoogle Scholar | PubMed |
and responds to direct and diffuse radiation derived from measured weather traces in conjunction with hourly temperature, humidity, and atmospheric CO2 and O3 concentrations. Photosynthetic rate, scaled by physiological leaf age, is assumed to be limited either by Rubisco-catalyzed carboxylation, (ribulose 1–5 bisphosphate carboxylase/oxygenase) or by the regeneration of RuBP controlled by electron transport rate. It is assumed that the rate of triose-phosphate utilization is not limiting based on soil quality at the FACTS-II field site (Dickson et al. 2000). The functional forms of the temperature dependencies of Rd, V
cmax and J
max are as by Harley et al. (1992). The model of detrimental effects of ozone, as described by Martin et al. (2001), interacts with the photosynthetic rate model by scaling the values of V
cmax and J
max. The water stress factor fw
(Wang et al. 2001) also scales V
cmax and J
max.
Maturation of young leaves is measured in terms of a physiological-age parameter, L age, which is the same as Leaf Plastochron Index (LPI) while a shoot continues to initiate new leaves. However, once leaf initiation ceases, each leaf’s L age continues to increase but its LPI does not. Factors based on L age, f ageV and f ageJ, scale V cmax and J max, respectively, where
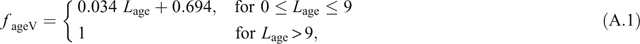
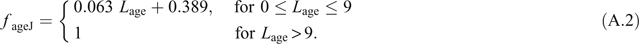
From Farquhar and von Caemmerer (1982),

where
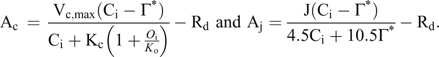
Dark respiration is computed as

and the temperature dependencies for Γ*, Ko and Kc are those published in Bernacchi et al. (2002).
The temperature dependencies of V cmax and J max are given by
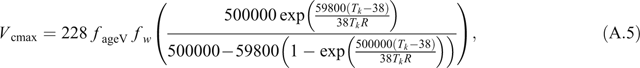
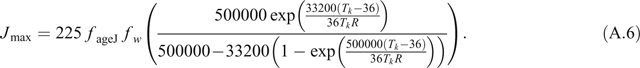
The rate of whole chain electron transport, J, is related to J max according to the equation

where the temperature dependences of ?PSII and Q2 are those published in Bernacchi et al. (2003) for growth temperature 14°C.
As by Farquhar and Sharkey (1982) and Farquhar et al. (1980),

Here,

For the experiments in this study,

though other parameterizations and variations to the stomatal conductance model (Leuning 1995; Yu et al. 2001; Wang et al. 2001) have yielded similar results.


