Bioindicators for monitoring atmospheric perfluorinated compounds: review of occurrences, transport, fate and analytical protocols
Nnanake-Abasi O. Offiong A * , Imeh J. Okop B * , Solomon E. Shaibu B , Edidiong S. Akwaowo D , Akwaowo I. Inyangudoh B E , Nnamso D. Ibuotenang C , Idongesit A. Victor A , George A. Robert B , Timilehin A. Adegboyega F G and Nsikak U. Benson A
A * , Imeh J. Okop B * , Solomon E. Shaibu B , Edidiong S. Akwaowo D , Akwaowo I. Inyangudoh B E , Nnamso D. Ibuotenang C , Idongesit A. Victor A , George A. Robert B , Timilehin A. Adegboyega F G and Nsikak U. Benson A
A
B
C
D
E
F
G
Handling Editor: Maya Al-Sid-Cheikh
Abstract
Perfluorinated compounds are emerging organic contaminants recently detected in various environmental matrices and remain largely unregulated. Among these matrices, air is the least studied one due to analytical challenges. This review explores emerging trends in analysing perfluorinated compounds in air with the use of bioindicators and highlights future research needs to address existing gaps in detection and monitoring.
Perfluorinated compounds (PFCs) are persistent organic pollutants with extensive industrial applications, including in firefighting foams, nonstick coatings and textiles. Their environmental contamination is widespread due to their resistance to degradation and long-range atmospheric transport, leading to their presence in various ecosystems. PFCs pose significant hazards, including bioaccumulation, endocrine disruption, hormonal imbalances and potential carcinogenic effects. Despite their ubiquity in environmental compartments, atmospheric studies remain limited due to analytical challenges. This review provides the first comprehensive analysis of biomonitoring of PFCs in the atmosphere using bioindicators. The databases consulted for the review include Web of Science, Scopus, ScienceDirect, PubMed and Google Scholar. By examining existing literature, we identify key research gaps, highlight analytical limitations and underscore the need for standardised methods to improve monitoring accuracy.
Keywords: air pollution, analytical method, atmospheric perfluorinated compounds, bioindicators, biomonitoring, emerging contaminants, PFAS, PFCs.
Introduction
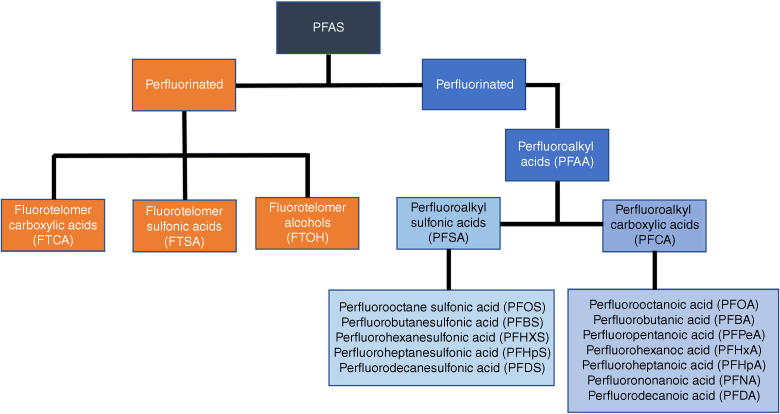
Perfluorinated compounds (PFCs) are heterogeneous groups of persistent organic pollutants with a functional group, and a carbon chain that has been fluorinated as shown in Fig. 1 (Sharifan et al. 2021). The basic family tree of PFCs (Fig. 2) consists of well-characterised legacy compounds with sulfonic, carboxylic or alcoholic functional groups (Liu B et al. 2019; Moose 2020; Evich et al. 2022). According to Liu B et al. (2019), the hydrophobic and oleophobic properties of PFCs make them extremely helpful in a variety of industries, including textiles, electronics, manufacturing, construction and automobiles. Products like textiles, paper, carpets, tiles, firefighting foam, polishes, paints, waxes, pesticides, adhesives, cosmetics and many more have all been exposed to them (Sunderland et al. 2019; DeLuca et al. 2022; Fagbayigbo et al. 2022). The main issue with perfluorinated compounds is that they are extremely stable and do not degrade through photolysis, hydrolysis or biodegradation (Lutze et al. 2018; Olatunde et al. 2020; Wang Xuelin et al. 2021). This poses a serious threat to the environment because PFCs are transported by air currents or water currents (Shoeib et al. 2006). Perfluorinated chemicals have been reported to be present in plants, soil, air, rain, animal tissues, groundwater, surface water and animal blood serum (Liu B et al. 2019; Brahana et al. 2024).
Some examples of perfluorinated compounds. (Adapted from Menger et al. 2021, with permission from Elsevier.)
The presence of PFCs in various matrices and subsequent exposures constitute both human and environmental health concerns (Zhou et al. 2021; Li Jiuyi et al. 2022). The exposure effects of PFCs on humans and other organisms have been widely studied, and many related studies are still ongoing (Liu W et al. 2018; Morales-McDevitt et al. 2021; McDonough et al. 2022; Dong et al. 2023; Zheng et al. 2023; Li and Kannan 2024; Villeneuve et al. 2024; Zhang J et al. 2024). Laboratory investigations and epidemiological studies have revealed a possible association of PFCs exposure with human thyroid dysfunction (Xie W et al. 2021). Also, PFAs have been linked with sex hormone disorders based on a study of a large cohort of males and females aged from 12 to 80 (Xie X et al. 2021). In a recent cross-sectional study involving adults, it was found that PFAs were negatively associated with gallstone disease (Shi et al. 2024). Atmospheric exposure to perfluoroalkyl and polyfluoroalkyl substances was reported to affect the growth and development of children in another study conducted in proximity of an e-waste recycling area (Hu et al. 2023). In the United States, a study found that PFAs in drinking water were associated with increased cancer incidence in the digestive, endocrine, oral cavity and respiratory systems (Li S et al. 2025). Further studies on the interactions between PFCs and antioxidant enzymes and proteins revealed possible detrimental effects on redox homeostasis in living organisms and endocrine disruption, respectively (Rajak and Ganguly 2023; Rajak et al. 2024; Cao and Ng 2025).
Despite increasing knowledge about the health risks associated with exposure to PFCs, the actual mechanisms of action and their links to specific exposure routes remain largely unknown. For instance, a study on the profiles of perfluorinated acids in liver tissues of birds was found to vary according to habitat types (Hong et al. 2022). The probability that established effects could result from synergistic interactions with other pollutants, such as microplastics, has been reported (Brahana et al. 2023; Ma et al. 2025). As a consequence of widespread concern, regulatory efforts to restrict the production and use of PFCs have been initiated in some countries, especially developing countries (Spyrakis and Dragani 2023; Figuière et al. 2025). There have also been concerted research efforts in recent times to unravel PFC sources, transport, environmental behaviour, mechanistic pathways of action, degradation, biotransformation and eventual fate.
One of the major reasons PFCs have become ubiquitous has been attributed to their ability to undergo long-range atmospheric transport (Altarawneh 2021; Owens 2021; Evich et al. 2022; Dunn et al. 2024; Young et al. 2024). It has been established that direct PFC deposition and exchange from air compartments or from particulate-bound substrates constitutes a significant contribution to surface soil and water (Wang Z et al. 2015; Shoeib et al. 2016; Chen et al. 2019; D’Ambro et al. 2021; Shimizu et al. 2021; Joudan et al. 2022; Morales-McDevitt et al. 2022; Wang F et al. 2022; Faust 2023; Feng et al. 2023; Wu Jiangyue et al. 2023; Lemay and Bourg 2025). In addition, sea spray aerosols and nucleation have been reported to contribute to the atmospheric transport and fate of PFCs (Schwidetzky et al. 2021; Medeiros et al. 2022; Sha et al. 2022). Despite the well-established relationship between atmospheric PFCs and their inputs into other environmental compartments, as well as possible human exposure and bioaccumulation, airborne levels remain highly underestimated (Lin et al. 2022a). Notably, there is limited understanding of the sources and fate of PFCs in the atmosphere (Lin et al. 2022b). Also, compared to other environmental matrices, the atmosphere has received little attention, resulting in limited data availability (Wang Xiaoping et al. 2018; Wu Jing et al. 2019). Furthermore, regional, temporal and seasonal variabilities are high in air monitoring studies (Camoiras González et al. 2021; Wang S et al. 2022). In the light of the aforementioned scenarios, special attention to atmospheric PFCs is necessary to advance our understanding and protect human and environmental health. To date, there is no review on biomonitoring of atmospheric PFCs.
For all living things, air is vital and necessary. By using plant and animal species or their fragments as ‘analytical instruments’ the practice of ‘bio-monitoring’, which intends to provide continuous, real-time analytical data and solutions, aims to avert unanticipated environmental damage as well as wasteful expenses (Gadzała-Kopciuch et al. 2004; Asif et al. 2018). This method, known as bio-indication, provides the capacity to gain a broad picture of the ecological condition based on one of its essential elements (for example, species, ecological form, population, association or community). These key components serve as biological indicators, or bioindicators, that reflect environmental conditions. Owing to their limited tolerance ranges, it is possible to identify some physical and chemical elements of the environment without the use of laboratory investigations.
Bioindicators can be categorised into qualitative and quantitative types. Qualitative bioindicators indicate the presence of a specific species in a given ecosystem. By contrast, quantitative bioindicators allow for the assessment of the ideal number or concentration of individuals of a particular species in a specific ecosystem (Gadzała-Kopciuch et al. 2004). These indicatory characteristics of living organisms are initially used in the analysis of environmental quality and the evaluation of environmental pollution. They help to gauge the rate, extent and impact of both current and future environmental changes caused by human activities. This review focuses on the use of bioindicators to detect and identify various perfluorinated compounds. Research articles reporting the use of bioindicators for monitoring of perfluorinated compounds in the air were reviewed. This was done to synthesise and elucidate the use of bioindicators in the qualitative and quantitative assessment of perfluorinated compounds in the air using databases such as Web of Science, PubMed, Google Scholar, Scopus and ScienceDirect. No limitation to time, date or year of publication was applied to the database search.
General aspects of bioindicators for air pollutants monitoring
Bioindicators are living organisms, such as plants and animals (Zaghloul et al. 2020), or functional groups (Gerlach et al. 2013), that serve as sensitive and meaningful measures of environmental conditions and changes, providing an important tool for monitoring environmental health and identifying potential threats (Stankovic and Stankovic 2013). These organisms can be used as early warning signs of environmental degradation (environmental indicators), as well as to monitor specific ecosystem stress (ecological indicators) and to assess levels of biodiversity (biodiversity indicators) at a particular location (Gerlach et al. 2013; Parmar et al. 2016; Fierro et al. 2017). Bioindicators may have multiple functions and different categories of bioindicators can be combined into a ‘bioindicator system’, which can provide a more comprehensive and holistic view of the health of an ecosystem. This system approach can enable site managers to make more informed decisions based on the combined insights provided by environmental, ecological and biodiversity indicators, which leads to more effective and sustainable management practices. The existence and distribution of bioindicator species in an ecosystem are influenced by several environmental parameters, including temperature, suspended particles, light transmission and water availability (Parmar et al. 2016). Bioindicators are a useful tool in environmental monitoring and management because they can be used to predict the natural state of an environment and infer the degree and extent of its contamination by analysing and comprehending the distribution and behaviour of relevant bioindicator species (Khatri and Tyagi 2015). Bioindicators offer numerous advantages, including the ability to identify biological impacts, monitor pollutant interactions, and detect early-stage toxicity in plants and humans. Their prevalence ensures easy availability and offers a cost-effective alternative to specialised monitoring systems. Bioindicator species are characterised by their ability to be sensitive to variations in their environment, allowing them to effectively serve as monitoring tools for the state of the air quality. This concept of bioindication applies to both biotic and abiotic responses to environmental changes, providing a broader understanding of the impacts of such changes beyond just indicating natural transformations. Thus, taxa used as bioindicators not only reflect the effects of environmental change but also demonstrate the impact of natural changes on the ecosystem, contributing to a more comprehensive understanding of the ecosystem’s overall health (Gerlach et al. 2013; Parmar et al. 2016). The use of bioindicators for monitoring pollution levels has been effectively employed to achieve broad environmental assessment objectives, including the detection of multiclass contaminants using a single species, improved sample accessibility, cost reduction in project budgets, and the simplification of laboratory analytical requirements, among other benefits.
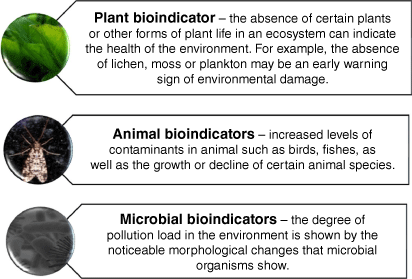
An essential part of the global efforts to prevent and lessen environmental impact is monitoring the level of pollution caused by long-distance transportation (LRT). To do this effectively across various research settings, budgets and lab configurations, bioindicators offer a valuable solution (Schick 2021). They are employed to evaluate the health of the environment and biogeographic changes that occur (Barroso et al. 2019a; Cozea et al. 2020; Mukhopadhyay et al. 2020). Additionally, they can be quickly replicated, sampled and used in locations where it’s impractical to transport and place large instruments. They can also track cumulative exposure to contaminants over time. Based on their unique responses to environmental fluctuation, various types of bioaccumulators, such as plants, animals, microorganisms and macroinvertebrates, are chosen for biomonitoring. Fig. 3 depicts bioaccumulators and their distinctive reactions to airborne contaminants.
Generally, various plant parts – including stems, bark, rings, leaves and needles – as well as lower plants like bryophytes (e.g. mosses, conifer) and lichens, are used as excellent biomonitors for assessing the level of air pollution in remote areas, which has been particularly useful in the investigation of the presence of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere (Yatim and Azman 2021). Gadzała-Kopciuch et al. (2004) conducted a detailed examination over a period of years with the goal of identifying bioindicators (both plants and animals) that accumulate harmful chemicals. The study acknowledged the complexity of assessing the toxicity of various polluting compounds, given their sheer diversity. As a result, it suggested that biomonitoring techniques should be continually refined to better predict and manage the environmental risks posed by these hazardous compounds.
Over the past few years, cases of environmental pollution and air pollution that contributed to adverse human and environmental health have been observed (Asif et al. 2018; Chiarelli et al. 2019; Hrncir et al. 2019). For example, bees have been observed as an environmental indicator of radioactive strontium-90, a byproduct of atmospheric nuclear testing (Asif et al. 2018). Also, lichens have been recognised as reliable indicators for detecting air pollution due to their sensitivity to a wide range of environmental factors. This is due to their unique physiological, morphological and anatomical characteristics (Battal et al. 2004; Asif et al. 2018; Tangahu et al. 2020; Will-Wolf et al. 2020). Yatim and Azman (2021) conducted a thorough study on the monitoring of air quality using bioindicators, which are currently being promoted and used frequently due to their benefits over other scientific methods. The benefits of utilising a bioindicator for air quality measurement include the fact that bioindicators are reasonably affordable, widely accessible and straightforward matrix for accurate atmospheric monitoring. The authors evaluated the potential of moss, specifically Leucobryum glaucum (commonly known as Holland moss), as a bioindicator for air quality monitoring across diverse settings. The research was conducted in four distinct environments: an urban area, a reserve forest, a living room and a smoking room. Moss containers fitted with grids were placed at each location and left for a 2-week period. Moss colour changes were observed and recorded weekly as a part of the physical monitoring process. Additionally, the number of grid cells showing moss growth in each container was counted to assess the moss’s survival rate. Data collection involved direct physical observation of the moss’s response to varying air quality conditions and its subsequent survival rates. The results indicated that the moss responded differently to various air quality conditions. Notably, in smoking rooms where the air quality was severely compromised, the moss changed from a fresh green to a brownish hue. This observation confirms that moss can serve as an effective bioindicator for air quality monitoring, as it adapts its appearance and growth patterns to its surrounding environment.
Numerous studies have been done on air metallic pollution using plants as bioindicators and bioaccumulators (Alatou and Sahli 2019). Scientifically, using trees as biomonitors is preferred to lichens and mosses as they reflect the heavy metals contamination of the environment for a long time (Alahabadi et al. 2017; López Berdonces et al. 2017). Compared to other plant species, trees are perennial, long-living plants with a high surface area. They show amazing flexibility and a high accumulation potential to ambient xenobiotics as a result of their huge size and prolonged persistence. Trees benefit greatly from having a vast root and shoot system that allows them to store a big amount of xenobiotics, as well as a large canopy area (Gómez et al. 2019; Wang Xingwen et al. 2022).
Moreover, these bioindicators can be used to determine if people living near these sources are being exposed to elevated levels of PFCs. Another application is using bioindicators to assess the effectiveness of pollution control measures, such as scrubbers or filters. Recent developments in this area include the use of bioindicators to monitor the effects of climate change on the levels of PFCs in the air. For instance, Godzik (2020) thoroughly investigated the impact of bioindicators in Poland due to the intensification of pollution emitted at regular intervals as a result of the impact of local emissions both in industrialisation and urbanisation.
Bargagli and Rota (2024) have established that penguins, similar to mosses and lichens, exhibit circumpolar distribution and long life spans (~20 years), as well as a strong tendency to return to their breeding sites annually. This unique combination of traits makes penguins an excellent choice for the role of bioindicators, as they can provide valuable information on long-term temporal changes in the bioavailability of contaminants across their foraging habitats, serving as an effective warning system for environmental monitoring and management efforts.
Although various studies have been carried out on the occurrence and bioaccumulation of PFCs, most of them have focused on soil and water environments. Munoz et al. (2022) investigated the bioaccumulation and trophic magnification per- and polyfluoroalkyl substances (PFAS) in an urban river food web, analysing aquatic vegetation, invertebrates, and fishes (Munoz et al. 2022). The authors concluded that differences in metabolic processes in gammarids, molluscs and insects may have been responsible for the contrast in detectable levels of perfluorooctanoic acid and fluorotelomer sulfonates in fish. In another study, the bioaccumulation of per- and polyfluoroalkyl substances by freshwater benthic macroinvertebrates was investigated (Yun et al. 2023). The authors claimed that bioaccumulation in worms was significantly higher compared to mussels and snails, whereas a rise in sediment organic carbon content led to a reduction in bioaccumulation of PFCs in benthic macroinvertebrates. There is evidence that accumulated PFCs could partially induce oxidative imbalance in aquatic organisms (Cocci et al. 2025). Variations in taxonomy and tissue type revealed higher concentrations in protein-rich tissues and in air-breathing organisms compared to those that rely on aquatic respiration (Khan et al. 2023). Possible biomagnification through the food web within marine organisms has been reported (Cheng et al. 2022). For soil-to-plant interactions, exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances have been extensively reviewed and reported (Lesmeister et al. 2021; Li Jiuyi et al. 2022).
Overview and occurrences of PFCs in air
PFCs are widely used in various industrial and consumer products, such as non-stick coatings, firefighting foams and water-resistant materials (Olsavsky et al. 2020). The presence of PFCs in the environment, particularly in the air, has raised concerns due to their potential bioaccumulation and adverse effects on human health and ecosystems. The occurrences of PFCs in air have been detected in both indoor and outdoor air, with various sources contributing to their presence (Yao et al. 2018; Liu B et al. 2019). In urban areas, PFCs are primarily emitted from manufacturing facilities, industrial processes and the use of consumer products containing these compounds (Li N et al. 2021; Wang L et al. 2024). Studies have reported the presence of PFCs in air samples collected near industrial sites, indicating that they can be released into the atmosphere during production and processing (Wang L et al. 2024). Additionally, indoor air has been identified as an important exposure pathway for humans, with elevated concentrations of certain PFCs, such as fluorotelomer alcohols (FTOHs), perfluorooctane sulfonamides (FOSAs) and perfluorooctane sulfonamidoethanols (FOSEs), detected in residential and office environments (Lange 2018; Maung et al. 2022; Shen et al. 2023). This highlights the significance of indoor air quality in relation to human exposure to PFCs.
PFCs have also been found in ambient outdoor air (Camoiras González et al. 2021), indicating their potential for long-range atmospheric transport and widespread environmental contamination (Fu et al. 2021). As a result, PFCs have been detected in remote regions, such as the Arctic, raising concerns about their global distribution and persistence in the environment (Su et al. 2016).
As shown in Table 1, there is a significant difference in the concentrations of perfluorinated compounds across various countries in the world. Approximately 3570 pg m−3 of FTOH was reported in Finland, whereas in South Africa, a lower concentration of PFOS (1.6 pg m−3) was recorded. FTOHs are one of the most frequently measured and detected PFCs, suggesting it might be more prevalent in the environment or more commonly targeted in studies. The varying levels of PFCs likely reflect differences in industrial activities, consumer product usage and environmental factors.
| Country | Target PFC | Level (pg m−3) | References | |
|---|---|---|---|---|
| Japan | FTOH | 204.0 | Li Jun et al. (2011) | |
| Finland | FTOH | 3570 | Winkens et al. (2017) | |
| China | FTOH | 230.0 | Lai et al. (2016) | |
| China | PFOA | 5.4 | Liu B et al. (2015) | |
| Czechia | PFASsA | 0.52 | Paragot et al. (2020) | |
| Canada | FTOH | 78.5 | Ahrens et al. (2012) | |
| Switzerland | FTOH | 868 | Müller et al. (2012) | |
| Germany | FTOH | 37 | Wang Xiaoping et al. (2014) | |
| South Africa | PFOS | 1.6 | Hanssen et al. (2010) | |
| South Africa | PFASsA | 289 | Groffen et al. (2018) |
The target analytes in a particular investigation by Liu B et al. (2015) on the perfluorinated chemicals in the atmosphere over Asia included fluorotelomer alcohols (FTOHs), olefins (FTOs), acrylates (FTAs), sulfonamides and sulfonamidoethanols. In their study, Chen et al. (2018) found that higher population centres in Toronto and Ontario had substantially higher levels of FTOHs in the air. By contrast, perfuorooctane sulfonate (PFOS) was the most abundant PFC, followed by perfuorooctanoic acid (PFOA), perfuoropentanoic acid (PFPeA) and perfuoroheptanoic acid (PFHpA), according to studies on the spatial distribution of perfluorinated compounds in the air near the Pearl River Delta in China (Liu B et al. 2019). PFPeA, PFOS, PFOA and PFHpA made up respectively 26, 22, 21 and 19% of PFCs in the study location (Liu B et al. 2019).
Generally, PFOA and PFOS are the PFCs that have received the most attention despite the fact that there are numerous other chemicals that fall under the same general category (Zhang T et al. 2010; Li Jun et al. 2011; Fraser et al. 2013; DeLuca et al. 2022). Consequently, the concentrations of PFOA and PFOS in the atmosphere (both indoors and outdoors) have been monitored due to extensive research on their toxicity, considering the possible hazards to human health (Shan et al. 2021; Wang P et al. 2021). Following the measured environmental concentrations in a certain study in China, the lifetime risk indices of PFOS and PFOA concentrations were substantially less than unity, implying a decreased non-oncogenic danger to people (Liu Shuyu et al. 2019). Similarly, according to another study conducted in a different location in China, it was found that the calculated hazard quotient (non-cancer risk) through breathing was less than one, depending on the concentrations of PFOS and PFOA (Guo et al. 2018; Harrad et al. 2019; Rogers et al. 2021), indicating that perfluorinated compound concentrations may not immediately pose a threat to the locals.
Although it has been established that PFCs are hazardous to animals according to the reports, studies of PFCs’ effects on human health are conflicting. It has been reported that the short half-life of PFCs in animals – ~17–19 days in mice – compared to humans – who have a significantly longer half-life of 3–5 years – makes it challenging to translate studies on the health effects of PFCs on animals to humans (Lindstrom et al. 2011). As a result, there is not enough information to draw conclusions about the relationship between PFC exposure and potential adverse effects in humans. Furthermore, although PFASs and FTOHs were found in the particle phase, Shoeib et al. (2006) concluded that it is not understood what governs this partitioning and how this may vary with particle type, temperature and the physical–chemical properties of these chemicals.
Fate and transport
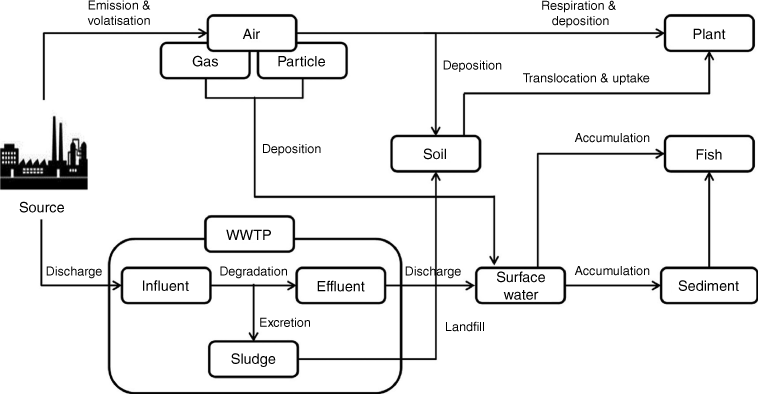
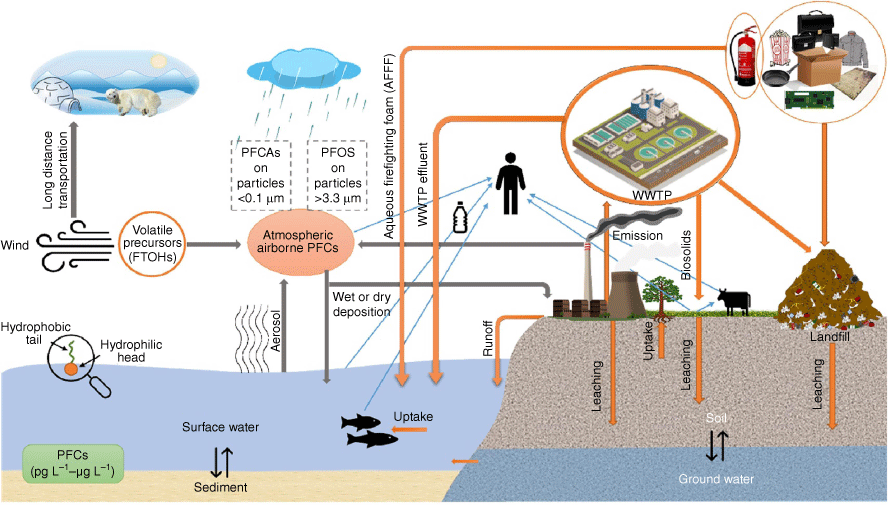
The fate and transport of PFCs in the air are determined by a number of processes and mechanisms that affect their distribution, persistence and impact on the environment and human health (Fig. 4) (Zhuo et al. 2012; Seo et al. 2019; Sungur 2022). PFCs are well known for being stable and resistant to degradation, which helps explain how they are able to travel great distances and last in the atmosphere (Ahrens 2011; Zhuo et al. 2012). These compounds have a fluorinated backbone that is hydrophobic and oleophobic–lipophobic, with a hydrophilic terminal functional group (water-loving). As a result, PFCs such as PFOS and PFOA compounds tend to partition at interfaces, like those between water and air, with the terminal functional group staying in water and the fluorinated backbone remaining in air (Owens 2021). When these compounds are released into the environment, whether intentionally or not, there are still significant gaps in our understanding of their ultimate fate and transport (Guelfo et al. 2021). This is especially true for less-studied compounds (like the majority of polyfluorinated compounds) and, to a lesser extent, for more extensively studied compounds like perfluoroalkyl acids (PFAAs), which are difficult to predict using current models.
Typical transport pathways of perfluorinated compounds (PFCs). WWTP, wastewater treatment plant. (Adapted from Seo et al. 2019 with permission from Elsevier.)
Sorption and bioaccumulation
Bioaccumulation and sorption are two critical processes that influence the fate and transport of PFCs in the environment, particularly in the air. The potential harm that PFCs impose on ecosystems varies from direct effects to their ability to sorb and bioaccumulate in the food web. These two processes play significant roles in determining how PFCs distribute, persist and affect ecological and human health systems. PFCs can be released into the environment through various means, including air emissions, wastewater discharges and the degradation of consumer products (Yao et al. 2018). When PFCs are released into the atmosphere, they can undergo bioaccumulation into the surrounding biota (Bossi et al. 2016; Zhong et al. 2019; Mao et al. 2023). The global distribution of PFCs in environmental compartments has caused wide-spread concern and highlighted the critical role of the atmosphere in their environmental fate (Li X et al. 2024).
The sorption mechanism refers to the way PFCs interact with different surfaces, such as soil, water and biota. According to Zhang D et al. (2016), physical sorption is currently the main method available for removing these organic pollutants from environmental matrices. However, PFCs can sorb to different surfaces in the environment and in living organisms through several mechanisms including hydrophobic interactions, electrostatic interactions and hydrogen bonding. These interactions are important in determining how PFCs accumulate and are transported through the environment and living organisms. Such interactions can also affect the toxicity of PFCs and their ability to be removed from the environment.
Hydrophobic interactions occur when molecules, such as PFCs, stick to other molecules due to their lack of attraction to water (Li M et al. 2021). PFCs are generally hydrophobic, and so, they tend to stick to organic matter, such as soil and plant roots making them more likely to be taken up by plants and other organisms, and difficult to remove from the environment through water treatment processes. Electrostatic interactions occur when molecules, such as PFCs, are attracted to other molecules because of their charge (Li M et al. 2019). PFCs can carry either a positive or negative charge, and this can influence their interactions with other molecules. For example, negatively charged PFCs can sorb to positively charged surfaces. Hydrogen bonding occurs when hydrogen atoms on one molecule are attracted to other atoms, such as oxygen, nitrogen or fluorine atoms on another molecule. PFCs can form hydrogen bonds with the surfaces.
The sorption mechanism of PFCs in air is similar to those in other environments (Jian et al. 2017; Liu G et al. 2021), but there are some unique characteristics of air that affect how PFCs interact with it. Air has a much lower density than water or soil, so PFC molecules in the air are more likely to interact with each other through Van der Waals forces (Jian et al. 2017). These are weak, short-range forces that occur between atoms and molecules of a substance. In addition, atmospheric conditions such as temperature, humidity and sunlight can affect the sorption of PFCs in air (Zhang D et al. 2016). Depending on the phases involved, sorption of PFCs has been demonstrated to depend on the chain length of the compounds and available functional groups as reported for sediments (Higgins and Luthy 2006).
The bioaccumulation mechanism of PFCs in the atmosphere involves the uptake of PFCs by plants, animals and other organisms through ingestion, absorption or inhalation through multiple routes as shown in Fig. 5 (Liu Z et al. 2017; Savoca and Pace 2021; Khurana et al. 2022). Once PFCs are absorbed by an organism, they can be stored in fatty tissues and biomagnified as they move up the food chain. This process can result in the accumulation of PFCs in the tissues of top predators, such as fish and birds (Giesy and Kannan 2001). Long-term bioaccumulation of PFCs in the environment may pose a risk to human health and the environment (Sturm and Ahrens 2010; Domingo and Nadal 2017; Brown et al. 2020; Teunen et al. 2021; Xing et al. 2023). However, there are limited studies on their bioaccumulation mechanisms, especially for short-chain PFCs (Zhong et al. 2019). Bioaccumulation by plants is also dependent on the chain length and can occur through the atmosphere from foliage (Yao et al. 2022). If there is atmospheric deposition on soil, uptake into plants is also possible through the roots (Zhang L et al. 2019). Once inside the plant, PFCs can accumulate in various parts, including leaves and stems (Felizeter et al. 2014). Although the release of PFCs back into the atmosphere by plants is not well documented, studies on air–vegetation exchange of organic pollutants suggest this mechanism is possible (Barber et al. 2004).
Schematic diagram of bioaccumulation mechanism of PFCs. FTOHs, fluorotelomer alcohols; PFCs, perfluorinated compounds; PFCA, perfluoroalkyl carboxylic acids; PFOS, perfluorooctane sulfonate; WWTP, wastewater treatment plant; ↓↑, partitioning. (Adapted from Khurana et al. 2022, with permission from Elsevier.)
Pathways for fate and transport of PFCs in air
PFCs can be transported through the atmosphere to other environmental matrices in three ways by advection, diffusion and deposition (Lai et al. 2016). The environmental impact of PFCs as well as strategies to mitigate their presence in the environment can be better predicted by understanding the pathways and mechanisms involved in the fate and transport of PFCs in the air. Advection refers to the movement of PFCs by wind. This can happen over short distances, such as within a city, or over long distances, such as between continents. PFCs can be carried by wind currents and dispersed across large areas, affecting regions far from the original source of contamination (Kwok et al. 2013). The random movement of PFCs in air, which can occur due to temperature differences or turbulence is termed diffusion. PFCs can spread out in the atmosphere, leading to a more even distribution over time, but also potentially increasing the area affected by contamination (Webster and Ellis 2010). Particulate matter is an important carrier during atmospheric transport (Ahrens et al. 2011; Yao et al. 2017).
Deposition involves the settling of PFCs from the air onto surfaces such as soil, water bodies and vegetation. This can occur through processes such as dry deposition, where particles settle out of the air, and wet deposition, where particles are washed out of the air by precipitation (Fang et al. 2018). Deposition can lead to contamination of terrestrial and aquatic ecosystems and is a critical pathway for the transfer of PFCs from the atmosphere to other environmental compartments. According to Said and El Zokm (2024), the primary fate of PFCs in the marine environment is through atmospheric deposition at the air–sea interface. Diffuse vapour exchange, aerosol-vapour partitioning, precipitation scavenging of vapours and particle-sorbed chemicals, and dry particle deposition are the processes that contribute to the exchange of PFCs between the air and the sea.
Degradation mechanism of PFCs in air
Lai et al. (2016) hypothesised that the interaction with OH radicals may be crucial to the atmospheric degradation of PFAS during long-range transport, particularly for short-lived species, based on their investigation of neutral polyfluoroalkyl compounds in the atmosphere over the northern South China Sea.
Owing to the unique chemical structure of PFCs, which have both hydrophilic and hydrophobic components, their sorption mechanisms can be quite complex and dependent on the properties of the adsorbents (Deng et al. 2012). This means that the behaviour of PFCs on different surfaces can vary greatly, which has implications for their environmental fate and transport. For example, PFCs may exhibit stronger interactions with certain types of chemical constituents or molecules compared to others, affecting their mobility and persistence in the environment. Understanding these sorption mechanisms is crucial for accurately predicting the behaviour of PFCs in natural systems and developing effective remediation strategies.
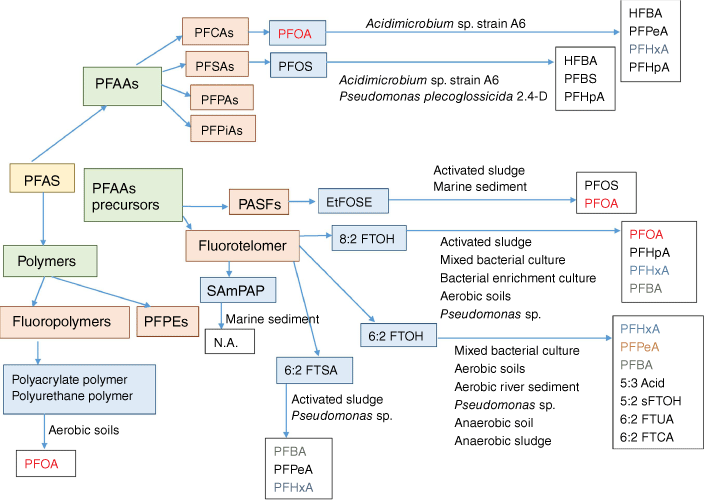
Recent studies reported that polyfluoroalkyl compounds are more vulnerable to microbial attack than perfluoroalkyl compounds, and a growing proportion of PFAS species are biodegradable (Grgas et al. 2023). Bacteria, fungi and enzymes show great potential for biodegradation of PFCs (Fig. 6). Remediation and treatment of PFASs, as well as effective decomposition of PFCs such as PFOA, is feasible using enzyme-catalysed oxidative humification reaction (ECOHR) (Colosi et al. 2009; Grgas et al. 2023). ECOHR reactions are commonly present in the soil system and characterised by a series of oxidative reactions during the humification process (Weber and Huang 2003). Laccase and peroxidase enzymes have demonstrated potential for directly degrading PFCs, but their effectiveness is not yet fully established and requires further investigation (Harris et al. 2025). Under air and water circulation system, a hybrid dielectric barrier discharge plasma and electrooxidation process, utilising multiple reactive species (˙OH, O2˙−, NO2˙−, 1O2 and ONOOH), achieved up to 90% degradation efficiency for PFOA remediation (Ajam et al. 2025).
Types of microbes involved in biotransformation, biodegradation and defluorination of selected PFCs. (Adapted and reused under Open Access licence from Grgas et al. 2023.) Abbreviations: perfluoroalkyl carboxylic acid (PFCA), perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluoroalkyl sulfonic acids (PFSAs), perfluorophosphonates (PFPAs), perfluorophosphinates (PFPiAs), perfluoroalkyl acids (PFAAs), polyfluoroalkyl substance (PFAS), polyfluoroalkyl siloxane fluids (PASFs), N-ethyl perfluorooctane sulfonamidoethanol (EtFOSE), fluorotelomer alcohol (FTOH), EtFOSE-based phosphate diester (SAmPAP), perfluoropolyethers (PFPEs), heptafluorobutyric acid (HFBA), perfluorobutane sulfonate (PFBS), perfluoroheptanoic acid (PFHpA), perfluorohexanoic acid (PFHxA), perfluoropentanoic acid (PFPeA), secondary fluorotelomer alcohol (sFTOH), fluorotelomer saturated and unsaturated carboxylic acids (FTCA and FTUA) and no activity (N.A.)
Analytical protocols and challenges for biomonitoring of PFCs in air
Monitoring of PFCs in the air usually involves two conventional sampling methods: active and passive. Monitoring using bioindicators falls under the passive sampling method as it involves an indirect way of measuring contaminant concentrations in the biological sample and then ascribing the source to the atmosphere or equating the concentrations of both matrices. The fundamental challenge here is the assumption that determined concentration in the biological system only came from the atmosphere. For this reason, a preliminary analysis is usually recommended to determine three fundamental factors: (i) availability of the biological species at the site of interest; (ii) convenience of sampling and sample preparation; and (iii) ability of the biological species to act as a bioindicator given that different contaminants have different bioavailability for different species.
Numerous reports from various regions of the world have documented the presence of PFCs in various biological systems and species (Giesy and Kannan 2001; Kannan et al. 2002; Sinclair et al. 2006; Meyer et al. 2009; Zhang H et al. 2015; Gómez-Ramírez et al. 2017; Jouanneau et al. 2020; Kotthoff et al. 2020; Savoca et al. 2021; Padilha et al. 2022; Gonkowski et al. 2023; Mo et al. 2024; Wells et al. 2024). Most of the reports focus on uptake, soil–plant interactions, bioaccumulation, biotransformation, exposure scenarios or general contamination levels, amongst others. The main choice of bioindicator for PFCs monitoring and the correlation with matrix contamination substantially focuses on aqueous media. There are only a handful of studies with focus on the use of plants as bioindicators for atmospheric or airborne PFCs. This disparity may be attributed to the inability to solely track accumulation in plant species from the atmosphere. In a recent study, it was demonstrated that atmospheric particulates represent a source of C8–C12 perfluoroalkyl carboxylates and 10:2 fluorotelomer alcohol in tree bark (Zhao et al. 2021). In another study, it was established that foliar uptake outweighs root uptake for 8:2 fluorotelomer alcohol in ryegrass (Lolium perenne L.) (Yao et al. 2022). These studies confirm the possibility of air and atmospheric sources of PFCs contamination of certain plants and the possibility of suitable bioindicators to correlate possible air and atmospheric concentrations in circumstances where direct measurement by active sampling is not possible. It should be noted that using common types of passive air samplers (PAS) such as polyurethane foam (PUF) as sorbents that are mainly used for nonpolar semivolatile organic compounds are not well validated for polar compounds such as per- and polyfluorinated compounds (Karásková et al. 2018). Therefore, to achieve geographical coverage of monitoring, especially in remote areas, biomonitoring using leaves and tree barks have been recommended for monitoring the contamination status of PFCs in the atmosphere (Wang Q et al. 2020).
Like for many organic pollutants, the method of analysis for biomonitoring perfluorinated compounds in the air involves sample collection, followed by extraction, clean-up and quantification using techniques such as gas or liquid chromatography coupled with mass spectrometry. Quality control measures, such as the use of internal standards and calibration curves, are also implemented to ensure accurate quantification of perfluorinated compounds in the air samples (Liu X et al. 2014; Barroso et al. 2018; Numata et al. 2018). As reported by Houde et al. (2006), there are significant variations in inter-laboratory analytical results and poor reproducibility in biomonitoring studies of PFCs. One of the major factors for this observation, according to the authors, is that many biomonitoring data are derived from small number of laboratory studies. The authors recommended the development of certified reference materials for different matrices. This implies that method validation is very critical at this stage of ongoing research in biomonitoring of atmospheric PFCs. Barroso et al. (2018) developed an analytical method for biomonitoring of atmospheric PFCs and some other emerging organic contaminants in leaves of four tree species (Citrus aurantium, Celtis australis, Platanus hispanica and Jacaranda mimosifolia). The authors described a method involving sonication-assisted extraction, clean-up using dispersive solid-phase extraction and quantification by liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Barroso et al. 2018). Similar methods have been used by other researchers, withb some modifications in sample preparation (Table 2). Although Soxhlet extraction and other conventional techniques have been reported, most methods utilise sonication for extraction, followed by high-performance or ultra-high-performance liquid chromatography (HPLC or UHPLC) (Table 2). Leaves are the most commonly used part of trees, with pine trees being the most frequently used bioindicator.
| Location | Bioindicator used | Target analyte | Method | Levels detected | References | |
|---|---|---|---|---|---|---|
| Spain | Citris aurantium (leaves) | Multiple PFCs (6 compounds) | Sonication followed by SPE and LC-MS | 0.09–25.8 ng g–1 dry matter | Barroso et al. (2019b) | |
| Slovakia | Pinus mugo (pine needles) | Multiple PFASs (16 compounds) | Lyophilisation, extraction using automatic extractor followed by HPLC-MS | BDL–1.6 ng g–1 dry weight | Chropeňová et al. (2016) | |
| Norway | Pinus sylvestris (pine needles) | Multiple PFASs (16 compounds) | Lyophilisation, extraction using automatic extractor followed by HPLC-MS | BDL–1.9 ng g–1 dry weight | Chropeňová et al. (2016) | |
| China | Cinnamomum camphora (tree bark) | Multiple PFASs (30 compounds) | Soxhlet extraction followed by UHPLC-MS/MS | BDL–688 ng g–1 | Zhao et al. (2021) | |
| China | Pinus tabuliformis (pine needle) | 27 neutral and ionic PFASs | Sonication followed by SPE and UHPLC-MS/MS | Average of 1.44 ng g–1 dry weight (neutral) and 0.983 ng g–1 dry weight (ionic) | Wang Q et al. (2021) | |
| Spain | Leaves of Citrus aurantium, Celtis australis, Platanus hispanica, Jacaranda mimosifolia | Multiple PFCs (6 compounds) | Sonication followed by SPE and LC-MS/MS | MDL–2.20 ng g–1 dry weight | Barroso et al. (2018) | |
| South Korea | Setaria viridis (leaves) | PFASs (19 compounds) | Solvent extraction by shaking, centrifugation followed by SPE and LC-MS/MS | 0.07–1.90 ng ng–1 | Seo et al. (2019) |
PFASs, per- and polyfluoroalkyl substances; SPE, solid-phase extraction; HPLC-MS, high-performance liquid chromatography–mass spectrometry; LC-MS/MS, liquid chromatography–tandem mass spectrometry; UHPLC-MS/MS, ultra-high-performance liquid chromatography–tandem mass spectrometry; BDL, below detection limit; MDL, method detection limit.
Multiple PFCs are commonly monitored across various regions (Table 2), with levels often correlating with the degree of industrialisation and population density. Higher populations and industrial activities are associated with elevated PFC levels, likely due to increased usage or industrial emissions. Although certain PFCs may be frequently detected in specific regions, their concentrations are generally in the nanograms per gram range, and in many cases, they fall below the established detection limits (BDL) or method detection limits (MDL). Although Giesy and Kannan (2001) concluded that current PFC levels in biota are below thresholds that would cause adverse effects in laboratory animals, and potentially humans, the potential for bioaccumulation and biomagnification through the food chain underscores the importance of ongoing monitoring to ensure safer and cleaner air.
Recent advances in analytical methods for monitoring PFCs, although not specifically focused on biological samples, they may be useful for integrated biomonitoring protocols. For instance, advances in high-resolution mass spectrometry (HRMS) enhance confidence in determining elemental compositions of unknown compounds in a sample, facilitating feature identification through suspect screening and non-targeted analysis (Getzinger et al. 2021; Wallace et al. 2024). It has been reported that studies employing non-targeted analysis for air monitoring are limited (Wallace et al. 2024). A study in Ireland successfully quantified atmospheric PFCs in particulate matter (PM2.5) using a novel solid-phase extraction (SPE) method coupled with liquid chromatography–high-resolution mass spectrometry (LC-HRMS) (Kourtchev et al. 2022). A slightly different method was applied for ambient particulate matter (PM10) in Italy, recording limits of quantification in the femtograms per square metre range (Moretti et al. 2024). With the ongoing identification of new PFCs and their degradation products, inter-method differences, variability in temporal trends and the utilisation of non-conventional samples, the development of novel analytical methods remains a continuous and essential task for environmental analysts (Nakayama et al. 2019; Yamazaki et al. 2021; Saini et al. 2023; Timshina et al. 2023; Dixit et al. 2024). To minimise high matrix background and prevent contamination from commonly used multistep sample pretreatment procedures, a single-step method using selective pressurised liquid extraction (SPLE) has been developed for the analysis of PFCs in atmospheric particulate matter (Tang et al. 2024). The method, which employed LC-MS/MS for quantification, achieved method detection limits (MDLs) between 0.006 and 0.48 ng g–1. Despite this advancement, for post-degradation analysis, a systematic evaluation of matrix effects (such as ion pairing, micelle formation and complexation) using a recovery matrix for method validation is strongly recommended (Navarathna et al. 2025). In silico methods are now commonly applied to enhance understanding and mechanism, especially in biological systems, and to build transformation products libraries (Liu Sheng et al. 2024).
Future perspective and outlook
Biomonitoring for atmospheric PFCs is an ongoing area of research. Recent studies have confirmed the possibility of monitoring atmospheric PFCs using bioindicators. However, these reports are few and are limited to developed regions of the world. There are also challenges related to the reproducibility of analytical results and the need for certified reference materials for method development. Additionally, exploring a broader range of bioindicators, including plants, animals and microorganisms across various ecosystems, will enhance the robustness of biomonitoring efforts. Given the variability in the levels of compounds detected in different species, there is need for identification and method optimisation for air-derived hyperaccumulators of PFCs suitable for monitoring studies. Integrating these results with remote sensing data and atmospheric modelling could offer insights into the sources, transport and deposition patterns of PFCs on a regional and global scale.
Furthermore, studies that focus on long-term monitoring of spatio-temporal trends will be useful in understanding the long-term fate of atmospheric PFCs. Given the persistence and potential bioaccumulation of PFCs, long-term monitoring programs are critical. These programs should also consider the potential impacts of climate change on the atmospheric distribution and deposition of PFCs, as changes in temperature, precipitation patterns and extreme weather events could influence the fate and transport of these compounds.
Finally, it is possible to explore the potential of some bioindicators for phytoremediation of atmospheric PFCs in situations where biotransformation could lead to safer metabolites.
Conclusion
Bioindicators offer a promising approach to monitor PFCs in the atmosphere, especially in areas where traditional monitoring methods are impractical. The variability in PFC concentrations across different regions underscores the importance of localised monitoring using bioindicators tailored to specific environments. As research advances, the development of standardised analytical protocols and certified reference materials will be crucial in enhancing the accuracy and comparability of biomonitoring studies. Continued investigation into the bioaccumulation mechanisms and long-term effects of PFCs will further inform environmental management strategies and public health policies.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Declaration of funding
We acknowledge funding support from the Tertiary Education Trust Fund (TETFund) through the Institutional Based Research Fund awarded to Dr Imeh J. Okop.
Author contributions
N.-A. O. Offiong and I. J. Okop were responsible for conceptualisation, funding, methodology, investigation, formal analysis, data visualisation, writing of the original draft, and the review, editing, revision and finalisation of the manuscript. S. E. Shaibu, E. S. Akwaowo, I. A. Victor, A. I. Inyangudoh, N. D. Ibuotenang, G. A. Robert, T. A. Adegboyega and N. U. Benson contributed the methodology, investigation, formal analysis, data visualisation, and review and editing of the written mataerial.
References
Ahrens L (2011) Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. Journal of Environmental Monitoring 13(1), 20-31.
| Crossref | Google Scholar | PubMed |
Ahrens L, Shoeib M, Del Vento S, et al. (2011) Polyfluoroalkyl compounds in the Canadian Arctic atmosphere. Environmental Chemistry 8(4), 399-406.
| Crossref | Google Scholar |
Ahrens L, Harner T, Shoeib M, et al. (2012) Improved characterization of gas–particle partitioning for per- and polyfluoroalkyl substances in the atmosphere using annular diffusion denuder samplers. Environmental Science & Technology 46(13), 7199-7206.
| Crossref | Google Scholar | PubMed |
Ajam F, Khourshidi A, Rabieian M, Taghavijeloudar M (2025) Per- and polyfluoroalkyl degradation in a hybrid dielectric barrier discharge plasma and electrooxidation system through involving more reactive species by air and water circulation. Journal of Hazardous Materials 488, 137287.
| Crossref | Google Scholar | PubMed |
Alahabadi A, Ehrampoush MH, Miri M, et al. (2017) A comparative study on capability of different tree species in accumulating heavy metals from soil and ambient air. Chemosphere 172, 459-467.
| Crossref | Google Scholar | PubMed |
Alatou H, Sahli L (2019) Using tree leaves and barks collected from contaminated and uncontaminated areas as indicators of air metallic pollution. International Journal of Phytoremediation 21(10), 985-997.
| Crossref | Google Scholar | PubMed |
Altarawneh M (2021) A closer look into the contribution of atmospheric gas-phase pathways in the formation of perfluorocarboxylic acids. Atmospheric Pollution Research 12(12), 101255.
| Crossref | Google Scholar |
Asif N, Malik M, Chaudhry FN (2018) A review of on environmental pollution bioindicators. Pollution 4(1), 111-118.
| Crossref | Google Scholar |
Barber JL, Thomas GO, Kerstiens G, Jones KC (2004) Current issues and uncertainties in the measurement and modelling of air–vegetation exchange and within-plant processing of POPs. Environmental Pollution 128(1–2), 99-138.
| Crossref | Google Scholar | PubMed |
Bargagli R, Rota E (2024) Environmental contamination and climate change in Antarctic ecosystems: an updated overview. Environmental Science: Advances 3(4), 543-560.
| Crossref | Google Scholar |
Barroso PJ, Martín J, Santos JL, et al. (2018) Analytical method for the evaluation of the outdoor air contamination by emerging pollutants using tree leaves as bioindicators. Analytical and Bioanalytical Chemistry 410(2), 417-428.
| Crossref | Google Scholar | PubMed |
Barroso PJ, Santos JL, Martín J, et al. (2019a) Emerging contaminants in the atmosphere: analysis, occurrence and future challenges. Critical Reviews in Environmental Science and Technology 49(2), 104-171.
| Crossref | Google Scholar |
Barroso PJ, Martín J, Santos JL, et al. (2019b) Evaluation of the airborne pollution by emerging contaminants using bitter orange (Citrus aurantium) tree leaves as biosamplers. Science of The Total Environment 677, 484-492.
| Crossref | Google Scholar | PubMed |
Battal P, Aslan A, Turker M, et al. (2004) Effect of the air pollutant sulfur dioxide on phytohormone levels in some lichens. Fresenius Environmental Bulletin 13(5), 436-440.
| Google Scholar |
Bossi R, Vorkamp K, Skov H (2016) Concentrations of organochlorine pesticides, polybrominated diphenyl ethers and perfluorinated compounds in the atmosphere of North Greenland. Environmental Pollution 217, 4-10.
| Crossref | Google Scholar | PubMed |
Brahana PJ, Al Harraq A, Saab LE, et al. (2023) Uptake and release of perfluoroalkyl carboxylic acids (PFCAs) from macro and microplastics. Environmental Science: Processes & Impacts 25(9), 1519-1531.
| Crossref | Google Scholar | PubMed |
Brahana PJ, Patel R, Bharti B (2024) Surface science view of perfluoroalkyl acids (PFAAs) in the environment. ACS Environmental Au 4(4), 173-185.
| Crossref | Google Scholar | PubMed |
Brown JB, Conder JM, Arblaster JA, Higgins CP (2020) Assessing human health risks from per- and polyfluoroalkyl substance (PFAS)-impacted vegetable consumption: a tiered modeling approach. Environmental Science & Technology 54(23), 15202-15214.
| Crossref | Google Scholar | PubMed |
Camoiras González P, Sadia M, Baabish A, et al. (2021) Air monitoring with passive samplers for perfluoroalkane substances in developing countries (2017–2019). Chemosphere 282, 131069.
| Crossref | Google Scholar | PubMed |
Cao Y, Ng CA (2025) High-throughput screening of protein interactions with per- and polyfluoroalkyl substances (PFAS) used in photolithography. Journal of Hazardous Materials 487, 137235.
| Crossref | Google Scholar | PubMed |
Chen H, Yao Y, Zhao Z, et al. (2018) Multimedia distribution and transfer of per- and polyfluoroalkyl substances (PFASs) surrounding two fluorochemical manufacturing facilities in Fuxin, China. Environmental Science & Technology 52(15), 8263-8271.
| Crossref | Google Scholar | PubMed |
Chen H, Zhang L, Li M, et al. (2019) Per- and polyfluoroalkyl substances (PFASs) in precipitation from mainland China: contributions of unknown precursors and short-chain (C2 C3) perfluoroalkyl carboxylic acids. Water Research 153, 169-177.
| Crossref | Google Scholar | PubMed |
Cheng H, Lv C, Li J, et al. (2022) Bioaccumulation and biomagnification of emerging poly- and perfluoroalkyl substances in marine organisms. Science of The Total Environment 851, 158117.
| Crossref | Google Scholar | PubMed |
Chiarelli R, Martino C, Roccheri MC (2019) Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress & Chaperones 24(4), 675-687.
| Crossref | Google Scholar | PubMed |
Chropeňová M, Karásková P, Kallenborn R, et al. (2016) Pine needles for the screening of perfluorinated alkylated substances (PFASs) along ski tracks. Environmental Science & Technology 50(17), 9487-9496.
| Crossref | Google Scholar | PubMed |
Cocci P, Stecconi T, Minicucci M, et al. (2025) Levels and oxidative toxicity of microplastics and perfluoroalkyl substances (PFASs) in different tissues of sea cucumber (Holothuria tubulosa). Science of The Total Environment 962, 178472.
| Crossref | Google Scholar | PubMed |
Colosi LM, Pinto RA, Huang Q, Weber WJ Jr (2009) Peroxidase‐mediated degradation of perfluorooctanoic acid. Environmental Toxicology and Chemistry 28(2), 264-271.
| Crossref | Google Scholar | PubMed |
Cozea A, Manea G-C, Bucur E, et al. (2020) Sensitive bioindicator plants studies, under the environmental conditions of climate change impact. Proceedings of the International Conference on Business Excellence 14(1), 50-58.
| Crossref | Google Scholar |
D’Ambro EL, Pye H, Bash JO, et al. (2021) Characterizing the air emissions, transport, and deposition of per- and polyfluoroalkyl substances from a fluoropolymer manufacturing facility. Environmental Science & Technology 55(2), 862-870.
| Crossref | Google Scholar | PubMed |
DeLuca NM, Minucci JM, Mullikin A, et al. (2022) Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: a systematic review. Environment International 162(December 2020), 107149.
| Crossref | Google Scholar | PubMed |
Deng S, Zhang Q, Nie Y, et al. (2012) Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environmental Pollution 168, 138-144.
| Crossref | Google Scholar | PubMed |
Dixit F, Antell EH, Faber KA, et al. (2024) Closing PFAS analytical gaps: Inter-method evaluation of total organofluorine techniques for AFFF-impacted water. Journal of Hazardous Materials Letters 5, 100122.
| Crossref | Google Scholar |
Domingo JL, Nadal M (2017) Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. Journal of Agricultural and Food Chemistry 65(3), 533-543.
| Crossref | Google Scholar | PubMed |
Dong F, Pan Y, Zhang J, et al. (2023) Comprehensive assessment of exposure pathways for perfluoroalkyl ether carboxylic acids (PFECAs) in residents near a fluorochemical industrial park: the unanticipated role of cereal consumption. Environmental Science & Technology 57(48), 19442-19452.
| Crossref | Google Scholar | PubMed |
Dunn M, Vojta S, Soltwedel T, et al. (2024) Passive sampler derived profiles and mass flows of perfluorinated alkyl substances (PFASs) across the Fram Strait in the North Atlantic. Environmental Science & Technology Letters 11(2), 166-171.
| Crossref | Google Scholar | PubMed |
Evich MG, Davis M, McCord JP, et al. (2022) Per- and polyfluoroalkyl substances in the environment. Science 375(6580), eabg9065.
| Crossref | Google Scholar | PubMed |
Fagbayigbo BO, Opeolu BO, Fatoki OS, et al. (2022) Sorption and partitioning of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) onto sediments of Diep and Plankenburg River systems Western Cape, South Africa. Environmental Technology and Innovation 25, 102110.
| Crossref | Google Scholar |
Fang X, Wang Q, Zhao Z, et al. (2018) Distribution and dry deposition of alternative and legacy perfluoroalkyl and polyfluoroalkyl substances in the air above the Bohai and Yellow Seas, China. Atmospheric Environment 192, 128-135.
| Crossref | Google Scholar |
Faust JA (2023) PFAS on atmospheric aerosol particles: a review. Environmental Science: Processes & Impacts 25(2), 133-150.
| Crossref | Google Scholar | PubMed |
Felizeter S, McLachlan MS, De Voogt P (2014) Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops. Journal of Agricultural and Food Chemistry 62(15), 3334-3342.
| Crossref | Google Scholar | PubMed |
Feng X, Yi S, Shan G, et al. (2023) Occurrence of perfluoroalkyl substances in the environment compartments near a mega fluorochemical industry: implication of specific behaviors and emission estimation. Journal of Hazardous Materials 445, 130473.
| Crossref | Google Scholar | PubMed |
Fierro P, Valdovinos C, Vargas-Chacoff L, et al. (2017) Macroinvertebrates and fishes as bioindicators of stream water pollution. In ‘Water Quality’. (Ed. H Tutu) pp. 23–38. (IntechOpen) doi:10.5772/65084
Figuière R, Miaz LT, Savvidou E, et al. (2025) An overview of potential alternatives for the multiple uses of per- and polyfluoroalkyl substances. Environmental Science & Technology 59, 2031-2042.
| Crossref | Google Scholar |
Fraser AJ, Webster TF, Watkins DJ, et al. (2013) Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environment International 60(1), 128-136.
| Crossref | Google Scholar | PubMed |
Fu J, Fu K, Chen Y, et al. (2021) Long-range transport, trophic transfer, and ecological risks of organophosphate esters in remote areas. Environmental Science & Technology 55(15), 10192-10209.
| Crossref | Google Scholar | PubMed |
Gadzała-Kopciuch R, Berecka B, Bartoszewicz J, et al. (2004) Some considerations about bioindicators in environmental monitoring. Polish Journal of Environmental Studies 13(5), 453-462.
| Google Scholar |
Gerlach J, Samways M, Pryke J (2013) Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. Journal of Insect Conservation 17(4), 831-850.
| Crossref | Google Scholar |
Getzinger GJ, Higgins CP, Ferguson PL (2021) Structure database and in silico spectral library for comprehensive suspect screening of per- and polyfluoroalkyl substances (PFASs) in environmental media by high-resolution mass spectrometry. Analytical Chemistry 93(5), 2820-2827.
| Crossref | Google Scholar | PubMed |
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environmental Science & Technology 35(7), 1339-1342.
| Crossref | Google Scholar | PubMed |
Godzik B (2020) Use of bioindication methods in national, regional and local monitoring in Poland—changes in the air pollution level over several decades. Atmosphere 11(2), 143.
| Crossref | Google Scholar |
Gómez L, Contreras A, Bolonio D, et al. (2019) Phytoremediation with trees. In ‘Molecular Physiology and Biotechnology of Trees’, 1st edn. (Ed. FM Cánovas) Advances in Botanical Research, pp. 281–321. (Elsevier Ltd) doi:10.1016/bs.abr.2018.11.010
Gómez-Ramírez P, Bustnes JO, Eulaers I, et al. (2017) Per- and polyfluoroalkyl substances in plasma and feathers of nestling birds of prey from northern Norway. Environmental Research 158, 277-285.
| Crossref | Google Scholar | PubMed |
Gonkowski S, Martín J, Kortas A, et al. (2023) Assessment of perfluoroalkyl substances concentration levels in wild bat guano samples. Scientific Reports 13(1), 22707.
| Crossref | Google Scholar | PubMed |
Grgas D, Petrina A, Štefanac T, et al. (2023) A review: per- and polyfluoroalkyl substances—biological degradation. Toxics 11(5), 446.
| Crossref | Google Scholar | PubMed |
Groffen T, Wepener V, Malherbe W, Bervoets L (2018) Distribution of perfluorinated compounds (PFASs) in the aquatic environment of the industrially polluted Vaal River, South Africa. Science of The Total Environment 627, 1334-1344.
| Crossref | Google Scholar | PubMed |
Guelfo JL, Korzeniowski S, Mills MA, et al. (2021) Environmental sources, chemistry, fate, and transport of per‐ and polyfluoroalkyl substances: state of the science, key knowledge gaps, and recommendations presented at the August 2019 SETAC Focus Topic Meeting. Environmental Toxicology and Chemistry 40(12), 3234-3260.
| Crossref | Google Scholar | PubMed |
Guo M, Lyu Y, Xu T, et al. (2018) Particle size distribution and respiratory deposition estimates of airborne perfluoroalkyl acids during the haze period in the megacity of Shanghai. Environmental Pollution 234, 9-19.
| Crossref | Google Scholar | PubMed |
Hall DM, Martins DJ (2020) Human dimensions of insect pollinator conservation. Current Opinion in Insect Science 38, 107-114.
| Crossref | Google Scholar | PubMed |
Hanssen L, Röllin H, Odland JØ, et al. (2010) Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. Journal of Environmental Monitoring 12(6), 1355-1361.
| Crossref | Google Scholar | PubMed |
Harrad S, Wemken N, Drage DS, et al. (2019) Perfluoroalkyl substances in drinking water, indoor air and dust from ireland: implications for human exposure. Environmental Science & Technology 53, 13449-13457.
| Crossref | Google Scholar | PubMed |
Harris BA, Zhou J, Clarke BO, Leung I (2025) Enzymatic degradation of PFAS: current status and ongoing challenges. ChemSusChem 18, e202401122.
| Crossref | Google Scholar | PubMed |
Higgins CP, Luthy RG (2006) Sorption of perfluorinated surfactants on sediments. Environmental Science & Technology 40(23), 7251-7256.
| Crossref | Google Scholar | PubMed |
Hong SH, Reiner JL, Jang M, et al. (2022) Levels and profiles of perfluorinated alkyl acids in liver tissues of birds with different habitat types and trophic levels from an urbanized coastal region of South Korea. Science of The Total Environment 806, 151263.
| Crossref | Google Scholar |
Houde M, Martin JW, Letcher RJ, et al. (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environmental Science & Technology 40(11), 3463-3473.
| Crossref | Google Scholar | PubMed |
Hrncir M, Maia-Silva C, Farina WM (2019) Honey bee workers generate low-frequency vibrations that are reliable indicators of their activity level. Journal of Comparative Physiology – A. Neuroethology, Sensory, Neural, and Behavioral Physiology 205(1), 79-86.
| Crossref | Google Scholar | PubMed |
Hu H, Zeng X, Zheng K, et al. (2023) Risk assessment and partitioning behavior of PFASs in environmental matrices from an e-waste recycling area. Science of The Total Environment 905, 167707.
| Crossref | Google Scholar | PubMed |
Jian JM, Guo Y, Zeng L, et al. (2017) Global distribution of perfluorochemicals (PFCs) in potential human exposure source – a review. Environment International 108(May), 51-62.
| Crossref | Google Scholar | PubMed |
Jouanneau W, Bårdsen BJ, Herzke D, et al. (2020) Spatiotemporal analysis of perfluoroalkyl substances in white-tailed eagle (Haliaeetus albicilla) nestlings from Northern Norway—a ten-year study. Environmental Science & Technology 54(8), 5011-5020.
| Crossref | Google Scholar | PubMed |
Joudan S, Orlando JJ, Tyndall GS, et al. (2022) Atmospheric fate of a new polyfluoroalkyl building block, C3 F7 OCHFCF2 SCH2 CH2 OH. Environmental Science & Technology 56(10), 6027-6035.
| Crossref | Google Scholar | PubMed |
Kannan K, Choi JW, Iseki N, et al. (2002) Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 49(3), 225-231.
| Crossref | Google Scholar | PubMed |
Karásková P, Codling G, Melymuk L, et al. (2018) A critical assessment of passive air samplers for per- and polyfluoroalkyl substances. Atmospheric Environment 185, 186-195.
| Crossref | Google Scholar |
Khan B, Burgess RM, Cantwell MG (2023) Occurrence and bioaccumulation patterns of per- and polyfluoroalkyl substances (PFAS) in the marine environment. ACS ES&T Water 3(5), 1243-1259.
| Crossref | Google Scholar | PubMed |
Khatri N, Tyagi S (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Frontiers in Life Science 8(1), 23-39.
| Crossref | Google Scholar |
Khurana P, Khurana P, Hasaneen N, et al. (2022) Co-transport of PFCs in the environment – an interactive story. Current Research in Green and Sustainable Chemistry 5, 100302.
| Crossref | Google Scholar |
Kotthoff M, Fliedner A, Rüdel H, et al. (2020) Per- and polyfluoroalkyl substances in the German environment – levels and patterns in different matrices. Science of The Total Environment 740, 140116.
| Crossref | Google Scholar | PubMed |
Kourtchev I, Hellebust S, Heffernan E, et al. (2022) A new on-line SPE LC-HRMS method for the analysis of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in PM2.5 and its application for screening atmospheric particulates from Dublin and Enniscorthy, Ireland. Science of The Total Environment 835, 155496.
| Crossref | Google Scholar | PubMed |
Kwok KY, Yamazaki E, Yamashita N, et al. (2013) Transport of Perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: implications for sources. Science of The Total Environment 447, 46-55.
| Crossref | Google Scholar | PubMed |
Lai S, Song J, Song T, et al. (2016) Neutral polyfluoroalkyl substances in the atmosphere over the northern South China Sea. Environmental Pollution 214, 449-455.
| Crossref | Google Scholar | PubMed |
Lange CC (2018) Anaerobic biotransformation of N ‐methyl perfluorobutanesulfonamido ethanol and N ‐ethyl perfluorooctanesulfonamido ethanol. Environmental Toxicology and Chemistry 37(3), 768-779.
| Crossref | Google Scholar | PubMed |
Lemay AC, Bourg IC (2025) Interactions between per- and polyfluoroalkyl substances (PFAS) at the water–air interface. Environmental Science & Technology 59, 2201-2210.
| Crossref | Google Scholar |
Lesmeister L, Lange FT, Breuer J, et al. (2021) Extending the knowledge about PFAS bioaccumulation factors for agricultural plants – a review. Science of The Total Environment 766, 142640.
| Crossref | Google Scholar | PubMed |
Li W-L, Kannan K (2024) Determination of legacy and emerging per- and polyfluoroalkyl substances (PFAS) in indoor and outdoor air. ACS ES&T Air 1(9), 1147-1155.
| Crossref | Google Scholar |
Li J, Del Vento S, Schuster J, et al. (2011) Perfluorinated compounds in the Asian atmosphere. Environmental Science & Technology 45(17), 7241-7248.
| Crossref | Google Scholar |
Li J, Sun J, Li P (2022) Exposure routes, bioaccumulation and toxic effects of per- and polyfluoroalkyl substances (PFASs) on plants: a critical review. Environment International 158, 106891.
| Crossref | Google Scholar | PubMed |
Li M, Sun F, Shang W, et al. (2019) Theoretical studies of perfluorochemicals (PFCs) adsorption mechanism on the carbonaceous surface. Chemosphere 235, 606-615.
| Crossref | Google Scholar | PubMed |
Li M, Sun F, Shang W, et al. (2021) Removal mechanisms of perfluorinated compounds (PFCs) by nanofiltration: roles of membrane-contaminant interactions. Chemical Engineering Journal 406(August 2020), 126814.
| Crossref | Google Scholar |
Li N, Ying GG, Hong H, Deng WJ (2021) Perfluoroalkyl substances in the urine and hair of preschool children, airborne particles in kindergartens, and drinking water in Hong Kong. Environmental Pollution 270, 116219.
| Crossref | Google Scholar | PubMed |
Li S, Oliva P, Zhang L, et al. (2025) Associations between per- and polyfluoroalkyl substances (PFAS) and county-level cancer incidence between 2016 and 2021 and incident cancer burden attributable to PFAS in drinking water in the United States. Journal of Exposure Science & Environmental Epidemiology [Published online 9 January 2025].
| Crossref | Google Scholar | PubMed |
Li X, Wang Y, Cui J, et al. (2024) Occurrence and fate of per- and polyfluoroalkyl substances (PFAS) in atmosphere: size-dependent gas-particle partitioning, precipitation scavenging, and amplification. Environmental Science & Technology 58(21), 9283-9291.
| Crossref | Google Scholar | PubMed |
Lin H, Taniyasu S, Yamazaki E, et al. (2022a) Fluorine mass balance analysis and per- and polyfluoroalkyl substances in the atmosphere. Journal of Hazardous Materials 435, 129025.
| Crossref | Google Scholar | PubMed |
Lin H, Taniyasu S, Yamashita N, et al. (2022b) Per- and polyfluoroalkyl substances in the atmospheric total suspended particles in Karachi, Pakistan: profiles, potential sources, and daily intake estimates. Chemosphere 288, 132432.
| Crossref | Google Scholar | PubMed |
Lindstrom AB, Strynar MJ, Libelo EL (2011) Polyfluorinated compounds: past, present, and future. Environmental Science & Technology 45(19), 7954-7961.
| Crossref | Google Scholar | PubMed |
Liu B, Zhang H, Yao D, et al. (2015) Perfluorinated compounds (PFCs) in the atmosphere of Shenzhen, China: spatial distribution, sources and health risk assessment. Chemosphere 138, 511-518.
| Crossref | Google Scholar |
Liu B, Xie L, Zhang H, et al. (2019) Spatial distribution of perfluorinated compounds in atmosphere of the Pearl River Delta, China. Archives of Environmental Contamination and Toxicology 77(2), 180-187.
| Crossref | Google Scholar |
Liu G, Stewart BA, Yuan K, et al. (2021) Comprehensive adsorption behavior and mechanism of PFOA and PFCs in various subsurface systems in China. Science of The Total Environment 794, 148463.
| Crossref | Google Scholar | PubMed |
Liu S, Yang R, Yin N, et al. (2019) Environmental and human relevant PFOS and PFOA doses alter human mesenchymal stem cell self-renewal, adipogenesis and osteogenesis. Ecotoxicology and Environmental Safety 169(November 2018), 564-572.
| Crossref | Google Scholar | PubMed |
Liu S, Dukes DA, Koelmel JP, et al. (2024) Expanding PFAS identification with transformation product libraries: nontargeted analysis reveals biotransformation products in mice. Environmental Science & Technology 59, 119-131.
| Crossref | Google Scholar |
Liu W, He W, Wu J, et al. (2018) Distribution, partitioning and inhalation exposure of perfluoroalkyl acids (PFAAs) in urban and rural air near Lake Chaohu, China. Environmental Pollution 243, 143-151.
| Crossref | Google Scholar | PubMed |
Liu X, Guo Z, Krebs KA, et al. (2014) Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere 98(November), 51-57.
| Crossref | Google Scholar | PubMed |
Liu Z, Lu Y, Shi Y, et al. (2017) Crop bioaccumulation and human exposure of perfluoroalkyl acids through multi-media transport from a mega fluorochemical industrial park, China. Environment International 106, 37-47.
| Crossref | Google Scholar | PubMed |
López Berdonces MA, Higueras PL, Fernández-Pascual M, et al. (2017) The role of native lichens in the biomonitoring of gaseous mercury at contaminated sites. Journal of Environmental Management 186, 207-213.
| Crossref | Google Scholar | PubMed |
Lutze HV, Brekenfeld J, Naumov S, et al. (2018) Degradation of perfluorinated compounds by sulfate radicals – new mechanistic aspects and economical considerations. Water Research 129, 509-519.
| Crossref | Google Scholar | PubMed |
Ma M, Coulon F, Tang Z, et al. (2025) Unveiling the truth of interactions between microplastics and per- and polyfluoroalkyl substances (PFASs) in wastewater treatment plants: microplastics as a carrier of PFASs and beyond. Environmental Science & Technology 54, 2211-2221.
| Crossref | Google Scholar |
Mao W, Li M, Xue X, et al. (2023) Bioaccumulation and toxicity of perfluorooctanoic acid and perfluorooctane sulfonate in marine algae Chlorella sp. Science of The Total Environment 870, 161882.
| Crossref | Google Scholar | PubMed |
Maung TZ, Bishop JE, Holt E, et al. (2022) Indoor air pollution and the health of vulnerable groups: a systematic review focused on particulate matter (PM), volatile organic compounds (VOCs) and their effects on children and people with pre-existing lung disease. International Journal of Environmental Research and Public Health 19(14), 8752.
| Crossref | Google Scholar | PubMed |
McDonough CA, Li W, Bischel HN, et al. (2022) Widening the lens on PFASs: direct human exposure to perfluoroalkyl acid precursors (pre-PFAAs). Environmental Science & Technology 56(10), 6004-6013.
| Crossref | Google Scholar | PubMed |
Medeiros FS, Mota C, Chaudhuri P (2022) Perfluoropropionic acid-driven nucleation of atmospheric molecules under ambient conditions. The Journal of Physical Chemistry A 126(45), 8449-8458.
| Crossref | Google Scholar | PubMed |
Menger RF, Funk E, Henry CS, Borch T (2021) Sensors for detecting per- and polyfluoroalkyl substances (PFAS): a critical review of development challenges, current sensors, and commercialization obstacles. Chemical Engineering Journal 417, 129133.
| Crossref | Google Scholar | PubMed |
Meyer J, Jaspers VL, Eens M, de Coen W (2009) The relationship between perfluorinated chemical levels in the feathers and livers of birds from different trophic levels. Science of The Total Environment 407(22), 5894-5900.
| Crossref | Google Scholar | PubMed |
Mo L, Wan N, Zhou B, et al. (2024) Per- and polyfluoroalkyl substances in waterbird feathers around Poyang Lake, China: compound and species-specific bioaccumulation. Ecotoxicology and Environmental Safety 273, 116141.
| Crossref | Google Scholar | PubMed |
Morales-McDevitt ME, Becanova J, Blum A, et al. (2021) The air that we breathe: neutral and volatile PFAS in indoor air. Environmental Science & Technology Letters 8(10), 897-902.
| Crossref | Google Scholar | PubMed |
Morales-McDevitt ME, Dunn M, Habib A, et al. (2022) Poly- and perfluorinated alkyl substances in air and water from Dhaka, Bangladesh. Environmental Toxicology and Chemistry 41(2), 334-342.
| Crossref | Google Scholar | PubMed |
Moretti S, Castellini S, Barola C, et al. (2024) Determination of perfluorinated and polyfluorinated alkyl substances (PFASs) in PM10 samples: analytical method, seasonal trends, and implications for urban air quality in the city of Terni (Central Italy). Separations 11(2), 42.
| Crossref | Google Scholar |
Mukhopadhyay S, Dutta R, Das P (2020) A critical review on plant biomonitors for determination of polycyclic aromatic hydrocarbons (PAHs) in air through solvent extraction techniques. Chemosphere 251, 126441.
| Crossref | Google Scholar | PubMed |
Müller CE, Gerecke AC, Bogdal C, et al. (2012) Atmospheric fate of poly- and perfluorinated alkyl substances (PFASs): I. Day–night patterns of air concentrations in summer in Zurich, Switzerland. Environmental Pollution 169, 196-203.
| Crossref | Google Scholar | PubMed |
Munoz G, Mercier L, Duy SV, et al. (2022) Bioaccumulation and trophic magnification of emerging and legacy per- and polyfluoroalkyl substances (PFAS) in a St Lawrence River food web. Environmental Pollution 309, 119739.
| Crossref | Google Scholar | PubMed |
Nakayama SF, Yoshikane M, Onoda Y, et al. (2019) Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends in Analytical Chemistry 121, 115410.
| Crossref | Google Scholar |
Navarathna C, Boateng RA, Luo L (2025) Challenges in PFAS postdegradation analysis: insights from the PFAS-CTAB model system. ACS Measurement Science Au 5, 135-144.
| Crossref | Google Scholar |
Numata JKJ, Klinger BZR, Just FHP (2018) Suitability of wild boar (Sus scrofa) as a bioindicator for environmental pollution with perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). Archives of Environmental Contamination and Toxicology 25, 102110.
| Crossref | Google Scholar |
Olatunde OC, Kuvarega AT, Onwudiwe DC (2020) Photo enhanced degradation of polyfluoroalkyl and perfluoroalkyl substances. Heliyon 6(12), e05614.
| Crossref | Google Scholar | PubMed |
Olsavsky NJ, Kearns VM, Beckman CP, et al. (2020) Research and regulatory advancements on remediation and degradation of fluorinated polymer compounds. Applied Sciences 10(19), 6921.
| Crossref | Google Scholar |
Owens CV (2021) Review of source and transportation pathways of perfluorinated compounds through the air. Journal of Environmental Health 83(6), 20-27.
| Google Scholar | PubMed |
Padilha J, de Carvalho GO, Willems T, et al. (2022) Perfluoroalkylated compounds in the eggs and feathers of resident and migratory seabirds from the Antarctic Peninsula. Environmental Research 214, 114157.
| Crossref | Google Scholar | PubMed |
Papa G, Maier R, Durazzo A, et al. (2022) The honey bee Apis mellifera: an insect at the interface between human and ecosystem health. Biology 11(2), 233.
| Crossref | Google Scholar | PubMed |
Paragot N, Bečanová J, Karásková P, et al. (2020) Multi-year atmospheric concentrations of per- and polyfluoroalkyl substances (PFASs) at a background site in central Europe. Environmental Pollution 265, 114851.
| Crossref | Google Scholar | PubMed |
Parmar TK, Rawtani D, Agrawal YK (2016) Bioindicators: the natural indicator of environmental pollution. Frontiers in Life Science 9(2), 110-118.
| Crossref | Google Scholar |
Rajak P, Ganguly A (2023) The ligand-docking approach explores the binding affinity of PFOS and PFOA for major endogenous antioxidants: a potential mechanism to fuel oxidative stress. Sustainable Chemistry for the Environment 4, 100047.
| Crossref | Google Scholar |
Rajak P, Ganguly A, Dey S (2024) An in silico insight on inhibitory potential of short-chain PFHxA and PFHxS against endogenous antioxidant enzymes. Sustainable Horizons 12, 100116.
| Crossref | Google Scholar |
Rogers RD, Reh CM, Breysse P (2021) Advancing per- and polyfluoroalkyl substances (PFAS) research: an overview of ATSDR and NCEH activities and recommendations. Journal of Exposure Science & Environmental Epidemiology 31(6), 961-971.
| Crossref | Google Scholar | PubMed |
Said TO, El Zokm GM (2024) Fate, bioaccumulation, remediation, and prevention of POPs in aquatic systems regarding future orientation. In ‘Persistent Organic Pollutants in Aquatic Systems’. (Eds TO Said, GM El Zokm) Emerging Contaminants and Associated Treatment Technologies, pp. 115–148. (Springer Nature: Cham, Switzerland) doi:10.1007/978-3-031-53341-9_6
Saini A, Chinnadurai S, Schuster JK, et al. (2023) Per- and polyfluoroalkyl substances and volatile methyl siloxanes in global air: spatial and temporal trends. Environmental Pollution 323, 121291.
| Crossref | Google Scholar | PubMed |
Savoca D, Pace A (2021) Bioaccumulation, biodistribution, toxicology and biomonitoring of organofluorine compounds in aquatic organisms. International Journal of Molecular Sciences 22(12), 6276.
| Crossref | Google Scholar | PubMed |
Savoca D, Melfi R, Palumbo Piccionello A, et al. (2021) Presence and biodistribution of perfluorooctanoic acid (PFOA) in Paracentrotus lividus highlight its potential application for environmental biomonitoring. Scientific Reports 11, 118763.
| Crossref | Google Scholar |
Schwidetzky R, Sun Y, Fröhlich-Nowoisky J, et al. (2021) Ice nucleation activity of perfluorinated organic acids. The Journal of Physical Chemistry Letters 12(13), 3431-3435.
| Crossref | Google Scholar | PubMed |
Seo S-H, Son MH, Shin ES, et al. (2019) Matrix-specific distribution and compositional profiles of perfluoroalkyl substances (PFASs) in multimedia environments. Journal of Hazardous Materials 364, 19-27.
| Crossref | Google Scholar | PubMed |
Sha B, Johansson JH, Tunved P, et al. (2022) Sea spray aerosol (SSA) as a source of perfluoroalkyl acids (PFAAs) to the atmosphere: field evidence from long-term air monitoring. Environmental Science & Technology 56(1), 228-238.
| Crossref | Google Scholar | PubMed |
Shan G, Xiang Q, Feng X, et al. (2021) Occurrence and sources of per- and polyfluoroalkyl substances in the ice-melting lakes of Larsemann Hills, east Antarctica. Science of The Total Environment 781, 146747.
| Crossref | Google Scholar | PubMed |
Sharifan H, Bagheri M, Wang D, et al. (2021) Fate and transport of per- and polyfluoroalkyl substances (PFASs) in the vadose zone. Science of The Total Environment 771, 145427.
| Crossref | Google Scholar | PubMed |
Shen P, Song X, Li N, Zhao C (2023) Concentrations and distributions of fluorotelomer alcohols and perfluoroalkane sulfonamido substances in the atmosphere in the Pearl River Delta, China. Journal of Environmental Science and Health – A. Toxic/Hazardous Substances and Environmental Engineering 58(3), 183-190.
| Crossref | Google Scholar | PubMed |
Shi T, Li D, Li D, et al. (2024) Individual and joint associations of per- and polyfluoroalkyl substances (PFAS) with gallstone disease in adults: a cross-sectional study. Chemosphere 358, 142168.
| Crossref | Google Scholar | PubMed |
Shimizu MS, Mott R, Potter A, et al. (2021) Atmospheric deposition and annual flux of legacy perfluoroalkyl substances and replacement perfluoroalkyl ether carboxylic acids in Wilmington, NC, USA. Environmental Science & Technology Letters 8(5), 366-372.
| Crossref | Google Scholar |
Shoeib M, Harner T, Vlahos P (2006) Perfluorinated chemicals in the arctic atmosphere. Environmental Science & Technology 40(24), 7577-7583.
| Crossref | Google Scholar | PubMed |
Shoeib M, Schuster J, Rauert C, et al. (2016) Emission of poly and perfluoroalkyl substances, UV-filters and siloxanes to air from wastewater treatment plants. Environmental Pollution 218, 595-604.
| Crossref | Google Scholar | PubMed |
Sinclair E, Mayack DT, Roblee K, et al. (2006) Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Archives of Environmental Contamination and Toxicology 50(3), 398-410.
| Crossref | Google Scholar | PubMed |
Spyrakis F, Dragani TA (2023) The EU’s per- and polyfluoroalkyl substances (PFAS) ban: a case of policy over science. Toxics 11(9), 721.
| Crossref | Google Scholar | PubMed |
Stankovic S, Stankovic AR (2013) Bioindicators of Toxic Metals. In ‘Green Materials for Energy, Products and Depollution’. (Eds E Lichtfouse, J Schwarzbauer, D Robert) Environmental Chemistry for a Sustainable World, pp. 151–228. (Springer: Dordrecht, Netherlands) doi:10.1007/978-94-007-6836-9_5
Sturm R, Ahrens L (2010) Trends of polyfluoroalkyl compounds in marine biota and in humans. Environmental Chemistry 7(6), 457-484.
| Crossref | Google Scholar |
Su H, Lu Y, Wang P, et al. (2016) Perfluoroalkyl acids (PFAAs) in indoor and outdoor dusts around a mega fluorochemical industrial park in China: implications for human exposure. Environment International 94, 667-673.
| Crossref | Google Scholar | PubMed |
Sunderland EM, Hu X, Dassuncao C (2019) A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of Exposure Science & Environmental Epidemiology 29, 131-147.
| Crossref | Google Scholar | PubMed |
Sungur Ş (2022) Environmental fate and transportation of perfluorinated compounds. In ‘Emerging Contaminants in the Environment’. (Eds H Sarma, DC Dominguez, W-Y Lee) pp. 203–224. (Elsevier) doi:10.1016/B978-0-323-85160-2.00017-2
Tang H, Wang Y, Si S, et al. (2024) Quantification of perfluorinated compounds in atmospheric particulate shows potential connection with environmental event. Journal of Environmental Sciences 136, 237-247.
| Crossref | Google Scholar | PubMed |
Tangahu BV, Kartika AAG, Humaira NG (2020) The lichen type identification as a bioindicator of air quality of Sukolilo District in Surabaya, Indonesia. Technology Reports of Kansai University 62(03), 743-750.
| Google Scholar |
Teunen L, Bervoets L, Belpaire C, et al. (2021) PFAS accumulation in indigenous and translocated aquatic organisms from Belgium, with translation to human and ecological health risk. Environmental Sciences Europe 33(1), 39.
| Crossref | Google Scholar |
Timshina AS, Sobczak WJ, Griffin EK, et al. (2023) Up in the air: polyfluoroalkyl phosphate esters (PAPs) in airborne dust captured by air conditioning (AC) filters. Chemosphere 325, 138307.
| Crossref | Google Scholar | PubMed |
Villeneuve DL, Blackwell BR, Bush K, et al. (2024) Transcriptomics‐based points of departure for Daphnia magna exposed to 18 per‐ and polyfluoroalkyl substances: transcriptomic ODs for PFAS‐exposed D. magna. Environmental Toxicology and Chemistry etc.5838 [Published online early 7 March 2024].
| Crossref | Google Scholar |
Wallace MAG, Smeltz MG, Mattila JM, et al. (2024) A review of sample collection and analytical methods for detecting per- and polyfluoroalkyl substances in indoor and outdoor air. Chemosphere 358, 142129.
| Crossref | Google Scholar | PubMed |
Wang F, Wang W, Zhao D, et al. (2022) Source apportionment and wet deposition of atmospheric poly- and per-fluoroalkyl substances in a metropolitan city centre of southwest China. Atmospheric Environment 273, 118983.
| Crossref | Google Scholar |
Wang L, Chen L, Wang J, et al. (2024) Spatial distribution, compositional characteristics, and source apportionment of legacy and novel per- and polyfluoroalkyl substances in farmland soil: a nationwide study in mainland China. Journal of Hazardous Materials 470, 134238.
| Crossref | Google Scholar | PubMed |
Wang P, Zhang M, Li Q, Lu Y (2021) Atmospheric diffusion of perfluoroalkyl acids emitted from fluorochemical industry and its associated health risks. Environment International 146, 106247.
| Crossref | Google Scholar | PubMed |
Wang Q, Ruan Y, Lin H, Lam P (2020) Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment. Science of The Total Environment 737, 139804.
| Crossref | Google Scholar | PubMed |
Wang Q, Zhao Z, Ruan Y, et al. (2021) Occurrence and seasonal distribution of legacy and emerging per- and polyfluoroalkyl substances (PFASs) in different environmental compartments from areas around ski resorts in northern China. Journal of Hazardous Materials 407, 124400.
| Crossref | Google Scholar | PubMed |
Wang S, Lin X, Li Q, et al. (2022) Neutral and ionizable per- and polyfluoroalkyl substances in the urban atmosphere: occurrence, sources and transport. Science of The Total Environment 823, 153794.
| Crossref | Google Scholar | PubMed |
Wang X, Halsall C, Codling G, et al. (2014) Accumulation of perfluoroalkyl compounds in Tibetan mountain snow: temporal patterns from 1980 to 2010. Environmental Science & Technology 48(1), 173-181.
| Crossref | Google Scholar | PubMed |
Wang X, Schuster J, Jones KC, et al. (2018) Occurrence and spatial distribution of neutral perfluoroalkyl substances and cyclic volatile methylsiloxanes in the atmosphere of the Tibetan Plateau. Atmospheric Chemistry and Physics 18(12), 8745-8755.
| Crossref | Google Scholar |
Wang X, Chen Z, Wang Y, Sun W (2021) A review on degradation of perfluorinated compounds based on ultraviolet advanced oxidation. Environmental Pollution 291(August), 118014.
| Crossref | Google Scholar | PubMed |
Wang X, Sial MU, Bashir MA, et al. (2022) Pesticides xenobiotics in soil ecosystem and their remediation approaches. Sustainability 14(6), 3353.
| Crossref | Google Scholar |
Wang Z, Xie Z, Möller A, et al. (2015) Estimating dry deposition and gas/particle partition coefficients of neutral poly-/perfluoroalkyl substances in northern German coast. Environmental Pollution 202, 120-125.
| Crossref | Google Scholar | PubMed |
Weber WJ, Huang Q (2003) Inclusion of persistent organic pollutants in humification processes: direct chemical incorporation of phenanthrene via oxidative coupling. Environmental Science & Technology 37(18), 4221-4227.
| Crossref | Google Scholar | PubMed |
Webster E, Ellis DA (2010) Potential role of sea spray generation in the atmospheric transport of perfluorocarboxylic acids. Environmental Toxicology and Chemistry 29(8), 1703-1708.
| Crossref | Google Scholar | PubMed |
Wells MR, Coggan TL, Stevenson G, et al. (2024) Per- and polyfluoroalkyl substances (PFAS) in little penguins and associations with urbanisation and health parameters. Science of The Total Environment 912, 169084.
| Crossref | Google Scholar | PubMed |
Will-Wolf S, Jovan S, Amacher MC, et al. (2020) Lichen elemental indicators for air pollution in eastern United States forests; a pilot study in the upper Midwest. General Technical Report PNW-GTR-985, US Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, OR, USA.
Winkens K, Koponen J, Schuster J, et al. (2017) Perfluoroalkyl acids and their precursors in indoor air sampled in children’s bedrooms. Environmental Pollution 222, 423-432.
| Crossref | Google Scholar | PubMed |
Wu J, Jin H, Li L, et al. (2019) Atmospheric perfluoroalkyl acid occurrence and isomer profiles in Beijing, China. Environmental Pollution 255, 113129.
| Crossref | Google Scholar | PubMed |
Wu JY, Fang G, Wang X, et al. (2023) Occurrence, partitioning and transport of perfluoroalkyl acids in gas and particles from the southeast coastal and mountainous areas of China. Environmental Science and Pollution Research International 30(12), 32790-32798.
| Crossref | Google Scholar | PubMed |
Xie W, Zhong W, Appenzeller BMR, et al. (2021) Nexus between perfluoroalkyl compounds (PFCs) and human thyroid dysfunction: a systematic review evidenced from laboratory investigations and epidemiological studies. Critical Reviews in Environmental Science and Technology 51(21), 2485-2530.
| Crossref | Google Scholar |
Xie X, Weng X, Liu S, et al. (2021) Perfluoroalkyl and polyfluoroalkyl substance exposure and association with sex hormone concentrations: results from the NHANES 2015–2016. Environmental Sciences Europe 33(1), 69.
| Crossref | Google Scholar | PubMed |
Xing Y, Zhou Y, Zhang X, et al. (2023) The sources and bioaccumulation of per- and polyfluoroalkyl substances in animal-derived foods and the potential risk of dietary intake. Science of The Total Environment 905, 167313.
| Crossref | Google Scholar | PubMed |
Yamazaki E, Taniyasu S, Wang X, Yamashita N (2021) Per- and polyfluoroalkyl substances in surface water, gas and particle in open ocean and coastal environment. Chemosphere 272, 129869.
| Crossref | Google Scholar | PubMed |
Yao Y, Chang S, Zhao Y, et al. (2017) Per- and poly-fluoroalkyl substances (PFASs) in the urban, industrial, and background atmosphere of northeastern China coast around the Bohai Sea: occurrence, partitioning, and seasonal variation. Atmospheric Environment 167, 150-158.
| Crossref | Google Scholar |
Yao Y, Zhao Y, Sun H, et al. (2018) Per- and polyfluoroalkyl substances (PFASs) in indoor air and dust from homes and various microenvironments in China: implications for human exposure. Environmental Science & Technology 52(5), 3156-3166.
| Crossref | Google Scholar |
Yao Y, Lan Z, Zhu H, et al. (2022) Foliar uptake overweighs root uptake for 8:2 fluorotelomer alcohol in ryegrass (Lolium perenne L.): a closed exposure chamber study. Science of The Total Environment 829, 154660.
| Crossref | Google Scholar | PubMed |
Yatim NM, Azman NIA (2021) Moss as bio-indicator for air quality monitoring at different air quality environment. International Journal of Engineering and Advanced Technology 10(5), 43-47.
| Crossref | Google Scholar |
Young CJ, Joudan S, Tao Y, et al. (2024) High time resolution ambient observations of gas-phase perfluoroalkyl carboxylic acids: implications for atmospheric sources. Environmental Science & Technology Letters 11(12), 1348-1354.
| Crossref | Google Scholar |
Yun X, Lewis AJ, Stevens-King G, et al. (2023) Bioaccumulation of per- and polyfluoroalkyl substances by freshwater benthic macroinvertebrates: impact of species and sediment organic carbon content. Science of The Total Environment 866, 161208.
| Crossref | Google Scholar | PubMed |
Zaghloul A, Saber M, Gadow S, et al. (2020) Biological indicators for pollution detection in terrestrial and aquatic ecosystems. Bulletin of the National Research Centre 44(1), 127.
| Crossref | Google Scholar |
Zhang D, Luo Q, Gao B, et al. (2016) Sorption of perfluorooctanoic acid, perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 144, 2336-2342.
| Crossref | Google Scholar | PubMed |
Zhang H, Liu W, He X, et al. (2015) Uptake of perfluoroalkyl acids in the leaves of coniferous and deciduous broad‐leaved trees. Environmental Toxicology and Chemistry 34(7), 1499-1504.
| Crossref | Google Scholar | PubMed |
Zhang J, Hu L, Xu H (2024) Dietary exposure to per- and polyfluoroalkyl substances: potential health impacts on human liver. Science of The Total Environment 907, 167945.
| Crossref | Google Scholar | PubMed |
Zhang L, Sun H, Wang Q, et al. (2019) Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2–C8) by wheat (Triticum acstivnm L.). Science of The Total Environment 654, 19-27.
| Crossref | Google Scholar | PubMed |
Zhang T, Wu Q, Sun HW, et al. (2010) Perfluorinated compounds in whole blood samples from infants, children, and adults in China. Environmental Science & Technology 44(11), 4341-4347.
| Crossref | Google Scholar | PubMed |
Zhao N, Zhao M, Liu W, Jin H (2021) Atmospheric particulate represents a source of C8–C12 perfluoroalkyl carboxylates and 10:2 fluorotelomer alcohol in tree bark. Environmental Pollution 273, 116475.
| Crossref | Google Scholar | PubMed |
Zheng G, Eick SM, Salamova A (2023) Elevated levels of ultrashort- and short-chain perfluoroalkyl acids in US homes and people. Environmental Science & Technology 57(42), 15782-15793.
| Crossref | Google Scholar | PubMed |
Zhong W, Zhang L, Cui Y, et al. (2019) Probing mechanisms for bioaccumulation of perfluoroalkyl acids in carp (Cyprinus carpio): impacts of protein binding affinities and elimination pathways. Science of The Total Environment 647, 992-999.
| Crossref | Google Scholar | PubMed |
Zhou Y, Zhou Z, Lian Y, et al. (2021) Source, transportation, bioaccumulation, distribution and food risk assessment of perfluorinated alkyl substances in vegetables: a review. Food Chemistry 349, 129137.
| Crossref | Google Scholar | PubMed |
Zhuo Q, Deng S, Yang B, et al. (2012) Degradation of perfluorinated compounds on a boron-doped diamond electrode. Electrochimica Acta 77, 17-22.
| Crossref | Google Scholar |