Potential for atmospheric acid processing of mineral dust to supply bioavailable trace metals to the oceans
Anthony Stockdale A * and Michael D. Krom B C
A * and Michael D. Krom B C
A
B
C
Abstract
Mineral dust is an important external source of trace metals to the offshore ocean. Dust exposure to acids is a significant driver of the release of dissolved trace elements. This study provides an analysis of mineral dust interaction with acid, as a proxy for atmospheric processes. An insight is given into the processes that may occur in the atmosphere where desert dust may add nutrient or toxic metals to oceans.
Trace metal concentrations in oceans are influenced by several factors including biogeochemical cycling effects on distributions, concentrations and speciation. The major input of trace metals (and P) to the surface waters of the offshore ocean is mineral dust, predominantly from desert regions. This dust can be subject to acid processing in the atmosphere due to the presence of anthropogenic acidic gases (oxides of nitrogen and sulfur), potentially making trace metals more bioavailable when dust is deposited in the oceans. Here we present a study on the release of trace metals from a desert dust when exposed to a series of acid addition treatments. Al, Fe, Co, Cu, Zn and Pb are preferentially leached from the dust only when the calcite phase has been exhausted and the pH is no longer buffered at circumneutral values. Further acid additions quickly released the majority of leachable trace metals, although lower concentrations of most metals continue to be leached with further acid addition cycles. This contrasts with the behaviour of Ca and P, where in prior work it had been shown that dissolution mirrors closely the addition of protons to mineral surfaces demonstrating the related but contrasting processes for trace element dissolution.
Keywords: atmospheric processing, bioavailability, biogeochemistry, desert dust, metal leaching, nutrients, ocean chemistry, trace metals.
Nitrogen, iron and (to a more limited extent) phosphorus have been identified as limiting nutrients in oceans (Moore et al. 2013; Mahowald et al. 2018). Other biologically important trace metals do not limit productivity to the same extent as these elements. However, they can affect microbial community composition through their role as enzyme co-factors (Boyd et al. 2017). Several trace metals are essential nutrients for biological functions and the concentration and chemical speciation of these metals may directly influence the distribution of phytoplankton species in ocean and neritic environments (Bruland 1989; Morel et al. 2003; Ellwood 2004). These metals include Mn, Fe, Co, Ni, Cu and Zn. Other metals, such as Cd, Pb or Hg, or nutrient metals at elevated concentrations, may exert toxic effects (Jordi et al. 2012; Xu and Morel 2013). These effects and the chemical speciation of metals may also be modified by anthropogenic effects such as ocean acidification (Thangal et al. 2023; Stockdale et al. 2016a).
Trace metal concentrations in oceans are influenced by a number of factors. Biogeochemical cycling affects distributions, concentrations and speciation, and inputs to the oceans may come from rivers along ocean boundaries, hydrothermal circulation at mid-ocean ridges and by wind-blown dust from arid and semi-arid regions of the continents, which are particularly important away from land masses in the open ocean (Bruland and Lohan 2003). Several studies have also identified that combustion particles, such as fly ash from coal or biomass-burning power plants, are potential sources of atmospheric iron and that this can be much more soluble than mineral dust (e.g. Baldo et al. 2022).
The principal acid species in the atmosphere are oxides of nitrogen and sulfur. Although some natural processes can be a source of these acid gases (Liss et al. 1997), at present, the main source of such gases, particularly in the northern hemisphere, is anthropogenic (Seinfeld and Pandis 2006). Dust commonly forms cloud condensation nuclei (CCN) around which water condenses. This water absorbs hydrophilic gases such as SOx, NOx and HCl, which lowers the pH of the condensed water. Clouds characteristically form and evaporate many times (for example a CCN may be involved in 10–20 nonprecipitating cloud cycles over a 3–10-day period; Hoppel et al. 1990). During the evaporation stage, the pH of this water can decrease considerably. It is during this acidification phase that the dissolution of mineral phases such as calcite, apatite and other minerals containing trace metals occurs. When the process reverses and a new cloud is formed, the chemical speciation of the dissolved phases, including trace metals, often form more bioavailable chemical species (Shi et al. 2015). For example, iron oxides (goethite and haematite) will dissolve to form soluble Fe at low pH, which then precipitates to form ferrihydrite Shi et al. 2009), which is bioavailable (Barbeau et al. 1996). A similar sequence of acidification and subsequent neutralisation may affect other trace metals either alone or in association with other minerals such as ferrihydrite. Chemistry within and between clouds can be very different, with relatively high pH and low ionic strength (IS) in cloud droplets and low pH and high IS in wet aerosols (Seinfeld and Pandis 2006; Weber et al. 2016), having differing potential effects on nutrient release from mineral dust.
Paytan et al. (2009) measured the release of metals from an unaltered desert dust added to filtered seawater. They observed the mass of metal release in the sequence Al > Zn > Fe > Ni, Cu, Pb ≫ Cd > Co. Their results demonstrated that colloidal and dust-derived metal in the surface ocean, and the higher solubility of acid reactive minerals, may result in higher absolute concentrations that can additionally have secondary effects on binding of other metals. Acid processing of mineral dust has been shown to have the potential to enhance the release of iron (Meskhidze et al. 2005) and phosphorus (Stockdale et al. 2016b; Herbert et al. 2018) to global oceans. It has also been shown that these processes are of increased importance in areas of the global ocean affected by atmospheric pollution of acid gases (Meskhidze et al. 2005).
Here we present a study on the release of trace metals from a desert dust when exposed to a series of acid addition treatments designed to mimic the processes of cloud formation and evaporation in the presence of acid gases. This is an extension of previous work, focussing on the potential for atmospheric acid processing of mineral dust to release bioavailable phosphorus to global oceans (Stockdale et al. 2016b). As acid addition is performed in a sequential manner, this allows only limited acid reaction with the dust at each step. This results in incremental changes to the dust mineralogy and chemistry, which in turn yields insights into the processes that may occur during atmospheric acid processing and how it may effect metal supply by this mechanism.
Experiments were carried out with an unaltered dust sample that had been deposited on a clean, flat surface during a dust storm in Rosh Pina, Israel (collected in May 2012). Based on back-trajectory data from the HYSPLIT model (https://www.ready.noaa.gov/HYSPLIT.php), the origins of this dust were the deserts of Saudi Arabia, Jordan and Iraq. Additional dust chemical and physical characteristics, including Brunauer–Emmett–Teller (BET) surface area, phosphorus speciation and mineralogical composition with X-ray diffraction (XRD) and X-ray fluorescence (XRF), are reported in Stockdale et al. (2016b).
A dust sample of 39.5 mg was wetted with 0.7 mL of Milli-Q water in a microcentrifuge tube, followed by addition of 0.7 mL of pH-1.3 HCl. This results in a solid-to-liquid ratio of >28 g L−1. The resulting slurry was placed in an end-over-end shaker for 2 min before centrifugation at 16,100g and at room temperature (20°C) for 5 min. The supernatant was extracted for analysis after which acid addition, shaking and centrifugation cycles were repeated for a further 19 acid additions. Supernatants were passed through a 0.45-μm syringe filter (13-mm Whatman Puradisc polyethersulfone), and dissolved trace elements were measured by inductively coupled plasma–mass spectrometry (ICP-MS) after dilution and acidification on a Thermo Scientific iCAP 7400 Radial ICP-MS. The limits of detection (based on three sigma for the standard deviation of blank samples) were 115, 291, 1.7, 247, 4.9, 1.9, 22.2, 0.56, 0.26 and 0.16 nmol L−1 for Ca, Al, Mn, Fe, Ni, Cu, Zn, Co, Cd and Pb respectively. Phosphorus measurements utilised the molybdate blue reaction method in conjunction with a Seal Analytical AA3 auto analyser, as previously reported (Stockdale et al. 2016b).
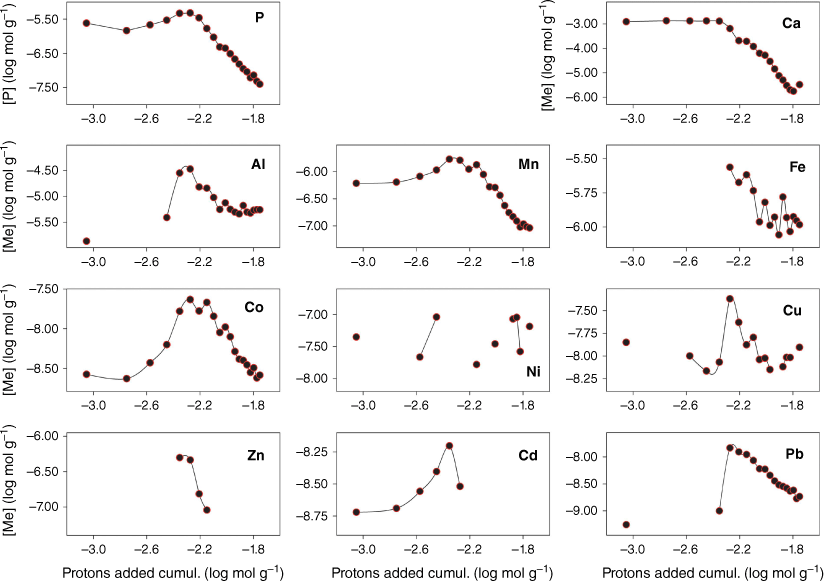
Metals are present in mineral dust in several different forms, with a range of reactive responses to the presence of acid. This can include rapid dissolution (for example calcite will continue to neutralise acid until fully dissolved; Stockdale et al. 2016b), surface desorption or partial reaction for more recalcitrant minerals. Metal solubility in atmospheric aerosols has been shown to have significant variations, with mean values of <10% for Al and Fe, and 19–69% for Mn, Co, Cu, Zn, Cd and Pb (Mahowald et al. 2018). Typically these latter elements are present in mineral dust at concentrations two to four orders of magnitude lower than Al or Fe (literature data in Table 1; Tomadin et al. 1984). The passive leaching process used in our experimental procedure ensures that the pH at the mineral surface never exceeds 1.3. Therefore, trace metal leaching would not be expected to reach values observed for sequential extraction procedures, which include peroxide and aqua regia digestion steps. Our results (Table 1) are consistent with previous observations, with low fractional leaching of Fe and Al, and other trace metals at the lower end of expectations based on concentrations and solubility in the literature (for example in Suska-Malawska et al. 2019, where 50–98% of total trace metals (except Mn) leaches only into the aqua regia extraction fraction). Ca concentrations are consistent with the high Ca content observed in the dust as calcite and to a lesser extent apatite (~12% CaO mass by XRF and 12.6% Ca mass by leaching and ICPMS; Stockdale et al. 2016b), with a total of 11.1% Ca by mass leached during the experiment.
| Element | Experimental data, Rosh Pina dust (μmol g−1) | Literature data, total metal (μmol g−1) | |
|---|---|---|---|
| P | 51.0 | ||
| Al | 59.7 | 3044 | |
| Ca | 2787 | 619 | |
| Mn | 4.34 | 13.1 | |
| Fe | 7.76 | 1009 | |
| Co | 0.06 | 0.20 | |
| Ni | 0.17 | 13.1 | |
| Cu | 0.07 | 1.0 | |
| Zn | 0.43 | ||
| Cd | 0.01 | ||
| Pb | 0.03 |
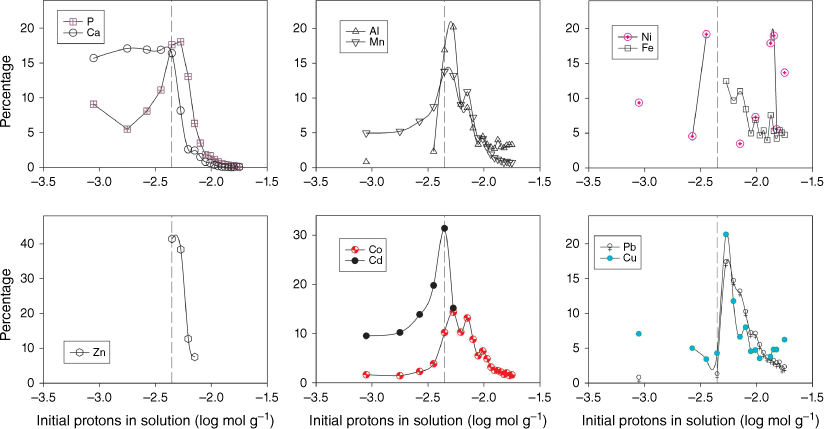
Fig. 1 shows the eluate concentration of P, Ca and trace metals during the course of the 20 stepwise acid additions. P and Ca, and to a lesser extent Mn and Cd, are mobilised into the aqueous phase early in the experiment. During this phase, neutralisation of the acid by calcite dissolution is seen (Stockdale et al. 2016b) and elevated Mn may be a result of manganese oxide dissolution, which may also be a source of co-precipitated or adsorbed Cd. Fig. 2 replots these data in terms of the percentage of total metal (and P) released at each acid addition (based on the total from across the 20 stepwise additions). This elucidates the underlying process that drives the changes in concentrations of individual metals. Al, Fe, Co, Cu, Zn and Pb are preferentially leached from the dust only when the calcite phase has been exhausted and the pH is no longer buffered at circumneutral values. The majority of metal is leached within 4–5 additions after calcite is exhausted, however, leaching of metals continues throughout the experiment in contrast to P and Ca leaching, which stops as minerals containing these elements are completely dissolved. Results suggest that Co leaching may be related to manganese oxide dissolution, as there is a close correlation between the two metal concentrations after the point of calcite removal (r2 = 0.92).
Phosphate and metal concentrations at each step of a sequential acid addition (0.7 mL of pH-1.3 HCl, 20 iterations) to a sample of 39.5 mg of atmospheric dust. Missing points are where sample concentrations were below the limits of detection.
Percentage of element released in each acid addition step (relative to the total across the 20 additions). Vertical lines denote where calcium carbonate (calcite) is likely to be depleted and the pH is expected to remain low. Missing points are where the sample concentration was below the limits of detection.
In a modelling study of phosphorus release from atmospheric acid processing, Herbert et al. (2018) predicted that close to dust source regions (Saharan, Arabian, Gobi, Patagonian, Kalahari, Great Basin and Great Australian deserts) there is relatively little acid processing and hence the P is not modified to a direct bioavailable form. Further away from source regions and particularly over industrialised regions such as Eastern China, atmospheric acid processing is the dominant source for bioavailable P. This process is enhanced as dust accumulates condensed acids during long-range transport and when dust loading is low in proximity to local pollution sources. These are likely to be the same conditions under which leaching of bioavailable metal is enhanced.
We report important levels of acid-mobilised trace metal in our dust sample. Should these levels occur in the atmosphere as a result of atmospheric cycling of the droplet and aerosol pH and IS described above and the formation of iron (and perhaps manganese) nanoparticle oxides, it is likely to increase the bioavailability of metals due to conversion into more available co-precipitated and surface sorbed forms. This is consistent with observations in other high trace metal concentration environments such as acid mine drainage, where secondary iron precipitates have been found to be natural scavengers of trace metals (Baleeiro et al. 2018).
The experimental acidification process used here ensures that the pH at the mineral surface, even after neutralising minerals have been exhausted, is no greater than 1.3. This may minimise any potential kinetic effects of trace element solubility. Ito and Shi (2016) showed that the solubility of atmospherically relevant Fe minerals follows pH-dependent rate constants. Further work is needed to elucidate the pH dependence and kinetics of processes that may influence trace metal release from mineral dust in the atmosphere.
The effect of atmospheric acid processing may have a significant effect on the availability of metals compared to dry deposition or wet deposition that does not undergo acid processing. Benaltabet et al. (2023) studied dust deposition events in the Red Sea and concluded that, although these events are long-term contributors to trace metals in marine environments, increases in dust loads during storms are associated with lower levels of trace metals in seawater, attributed to adsorption onto dust particles sinking in seawater. It has also been shown that these processes are of increased importance in areas of the global ocean affected by atmospheric pollution of acid gases (Meskhidze et al. 2005; Jickells et al. 2016).
We have presented data indicating that atmospheric processing of mineral dust can enhance the bioavailability of acid leach derived trace metals. Further work is required to quantify these processes for a broader range of dust sources, both mineral (desert type) dust and anthropogenically derived dust that typically are enhanced in Cu, Pb and Co. Also, to quantify these effects based on climate modelling. Nevertheless, this work gives an insight into the differing processes that may occur in the atmosphere, which may result in desert dust adding nutrient or toxic metals to the surface oceans.
Declaration of funding
This research did not receive any specific funding. However, it stems from previous work, Grant RPG 406, entitled ‘Understanding the Delivery of P Nutrient to the Oceans’, from the Leverhulme Trust.
Dedication
This manuscript is submitted in honour of the career of Ed Tipping. I (A. Stockdale) first worked with Ed on a small project in the summer of 2005 between my undergraduate and PhD studies. After my PhD, a 1-year formal collaboration spawned a large series of informal projects, some of which are still ongoing. Throughout this time it has been a privilege to work with such a great scientist, whose influence is felt far beyond the environmental chemistry community. I wish you all the best for your future endeavours, which will undoubtedly include more great science. Thanks, Ed.
References
Baldo C, Ito A, Krom MD, Li W, Jones T, Drake N, Ignatyev K, Davidson N, Shi Z (2022) Iron from coal combustion particles dissolves much faster than mineral dust under simulated atmospheric acidic conditions. Atmospheric Chemistry and Physics 22, 6045-6066.
| Crossref | Google Scholar |
Baleeiro A, Fiol S, Otero-Fariña A, Antelo J (2018) Surface chemistry of iron oxides formed by neutralization of acidic mine waters: removal of trace metals. Applied Geochemistry 89, 129-137.
| Crossref | Google Scholar |
Barbeau K, Moffett JW, Caron DA, Croot PL, Erdner DL (1996) Role of protozoan grazing in relieving iron limitation of phytoplankton. Nature 380, 61-64.
| Crossref | Google Scholar |
Benaltabet T, Lapid G, Torfstein A (2023) Response of dissolved trace metals to dust storms, sediment resuspension, and flash floods in oligotrophic oceans. Global Biogeochemical Cycles 37, e2023GB007858.
| Crossref | Google Scholar |
Boyd PW, Ellwood MJ, Tagliabue A, Twining BS (2017) Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nature Geoscience 10, 167-173.
| Crossref | Google Scholar |
Bruland KW (1989) Complexation of zinc by natural organic ligands in the central North Pacific. Limnology and Oceanography 34(2), 269-285.
| Crossref | Google Scholar |
Bruland KW, Lohan MC (2003) 6.02 - Controls of trace metals in seawater. In ‘Treatise on Geochemistry. Vol. 6’. (Eds HD Holland, KK Turekian) pp. 23-47. (Pergamon) 10.1016/B0-08-043751-6/06105-3
Ellwood MJ (2004) Zinc and cadmium speciation in subantarctic waters east of New Zealand. Marine Chemistry 87(1−2), 37-58.
| Crossref | Google Scholar |
Herbert RJ, Krom MD, Carslaw KS, Stockdale A, Mortimer RJG, Benning LG, Pringle K, Browse J (2018) The effect of atmospheric acid processing on the global deposition of bioavailable phosphorus from dust. Global Biogeochemical Cycles 32, 1367-1385.
| Crossref | Google Scholar |
Hoppel WA, Fitzgerald JW, Frick GM, Larson RE, Mack EJ (1990) Aerosol size distributions and optical properties found in the marine boundary layer over the Atlantic Ocean. Journal of Geophysical Research: Atmospheres 95, 3659-3686.
| Crossref | Google Scholar |
Ito A, Shi Z (2016) Delivery of anthropogenic bioavailable iron from mineral dust and combustion aerosols to the ocean. Atmospheric Chemistry and Physics 16, 85-99.
| Crossref | Google Scholar |
Jickells TD, Baker AR, Chance R (2016) Atmospheric transport of trace elements and nutrients to the oceans. Philosophical Transactions of the Royal Society of London – A. Mathematical, Physical and Engineering Sciences 374, 20150286.
| Crossref | Google Scholar | PubMed |
Jordi A, Basterretxea G, Tovar-Sánchez A, Alastuey A, Querol X (2012) Copper aerosols inhibit phytoplankton growth in the Mediterranean Sea. Proceedings of the National Academy of Sciences 109(52), 21246-21249.
| Crossref | Google Scholar | PubMed |
Liss PS, Hatton AD, Malin G, Nightingale PD, Turner SM (1997) Marine sulphur emissions. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 352, 159-169.
| Crossref | Google Scholar |
Mahowald NM, Hamilton DS, Mackey KRM, Moore JK, Baker AR, Scanza RA, Zhang Y (2018) Aerosol trace metal leaching and impacts on marine microorganisms. Nature Communications 9(1), 2614.
| Crossref | Google Scholar | PubMed |
Meskhidze N, Chameides WL, Nenes A (2005) Dust and pollution: a recipe for enhanced ocean fertilization? Journal of Geophysical Research: Atmospheres 110, D03301.
| Crossref | Google Scholar |
Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW, Galbraith ED, Geider RJ, Guieu C, Jaccard SL, Jickells TD, La Roche J, Lenton TM, Mahowald NM, Marañón E, Marinov I, Moore JK, Nakatsuka T, Oschlies A, Saito MA, Thingstad TF, Tsuda A, Ulloa O (2013) Processes and patterns of oceanic nutrient limitation. Nature Geoscience 6, 701-710.
| Crossref | Google Scholar |
Morel FMM, Milligan AJ, Saito MA (2003) 6.05 – Marine bioinorganic chemistry: the role of trace metals in the oceanic cycles of major nutrients. In ‘Treatise on Geochemistry. Vol. 6’. (Ed. H Elderfield) pp. 113−143. (Pergamon) 10.1016/B0-08-043751-6/06108-9
Paytan A, Mackey KRM, Chen Y, Lima ID, Doney SC, Mahowald N, Labiosa R, Post AF (2009) Toxicity of atmospheric aerosols on marine phytoplankton. Proceedings of the National Academy of Sciences 106(12), 4601-4605.
| Crossref | Google Scholar | PubMed |
Shi Z, Krom MD, Bonneville S, Baker AR, Jickells TD, Benning LG (2009) Formation of iron nanoparticles and increase in iron reactivity in mineral dust during simulated cloud processing. Environmental Science & Technology 43, 6592-6596.
| Crossref | Google Scholar | PubMed |
Shi Z, Krom MD, Bonneville S, Benning LG (2015) Atmospheric processing outside clouds increases soluble iron in mineral dust. Environmental Science & Technology 49, 1472-1477.
| Crossref | Google Scholar | PubMed |
Stockdale A, Tipping E, Lofts S, Mortimer RJG (2016a) Effect of ocean acidification on organic and inorganic speciation of trace metals. Environmental Science & Technology 50(4), 1906-1913.
| Crossref | Google Scholar |
Stockdale A, Krom MD, Mortimer RJG, Benning LG, Carslaw KS, Herbert RJ, Shi Z, Myriokefalitakis S, Kanakidou M, Nenes A (2016b) Understanding the nature of atmospheric acid processing of mineral dusts in supplying bioavailable phosphorus to the oceans. Proceedings of the National Academy of Sciences 113(51), 14639-14644.
| Crossref | Google Scholar |
Suska-Malawska M, Sulwiński M, Wilk M, Otarov A, Mętrak M (2019) Potential eolian dust contribution to accumulation of selected heavy metals and rare earth elements in the aboveground biomass of Tamarix spp. from saline soils in Kazakhstan. Environmental Monitoring and Assessment 191, 57.
| Crossref | Google Scholar | PubMed |
Thangal SH, Nandhinipriya R, Vasuki C, Gayathri V, Anandhan K, Yogeshwaran A, Muralisankar T, Ramesh M, Rajaram R, Santhanam P, Venmathi Maran BA (2023) The impact of ocean acidification and cadmium toxicity in the marine crab Scylla serrata: biological indices and oxidative stress responses. Chemosphere 345, 140447.
| Crossref | Google Scholar | PubMed |
Tomadin L, Lenaz R, Landuzzi V, Mazzucotelli A, Vannucci R (1984) Wind-blown dusts over the central Mediterranean. Oceanologica Acta 7, 13-23.
| Google Scholar |
Weber RJ, Guo H, Russell AG, Nenes A (2016) High aerosol acidity despite declining atmospheric sulfate concentrations over the past 15 years. Nature Geosciences 9, 282-285.
| Crossref | Google Scholar |
Xu Y, Morel FMM (2013) Cadmium in marine phytoplankton. In ‘Cadmium: From Toxicity to Essentiality’. (Eds A Sigel, H Sigel, R Sigel) Metal Ions in Life Sciences 11, 509–528. (Springer: Dordrecht, Netherlands) 10.1007/978-94-007-5179-8_16


